Overexpression of AgDREBA6b Gene Significantly Increases Heat Tolerance in Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. The AgDREBA6b Gene Overexpression Vector Agrobacterium Transformation
2.2. Cultivation of Arabidopsis thaliana
2.3. Genetic Transformation of Arabidopsis thaliana
2.4. Screening of T1 Generation Positive Plants
2.5. T2 Generation Positive Test of Transgenic Plants
2.6. Phenotype Observation of Arabidopsis Positive Plants
2.7. Determination of Leaf Structural Properties of Arabidopsis Positive Plants
2.8. Measurement of Physiological and Biochemical Indices in Leaves of Arabidopsis Positive Plants
2.9. Validation of Quantitative Expression in Arabidopsis Positive Plants
2.10. Data Analysis
3. Results
3.1. Identification of Transgenic Arabidopsis thaliana
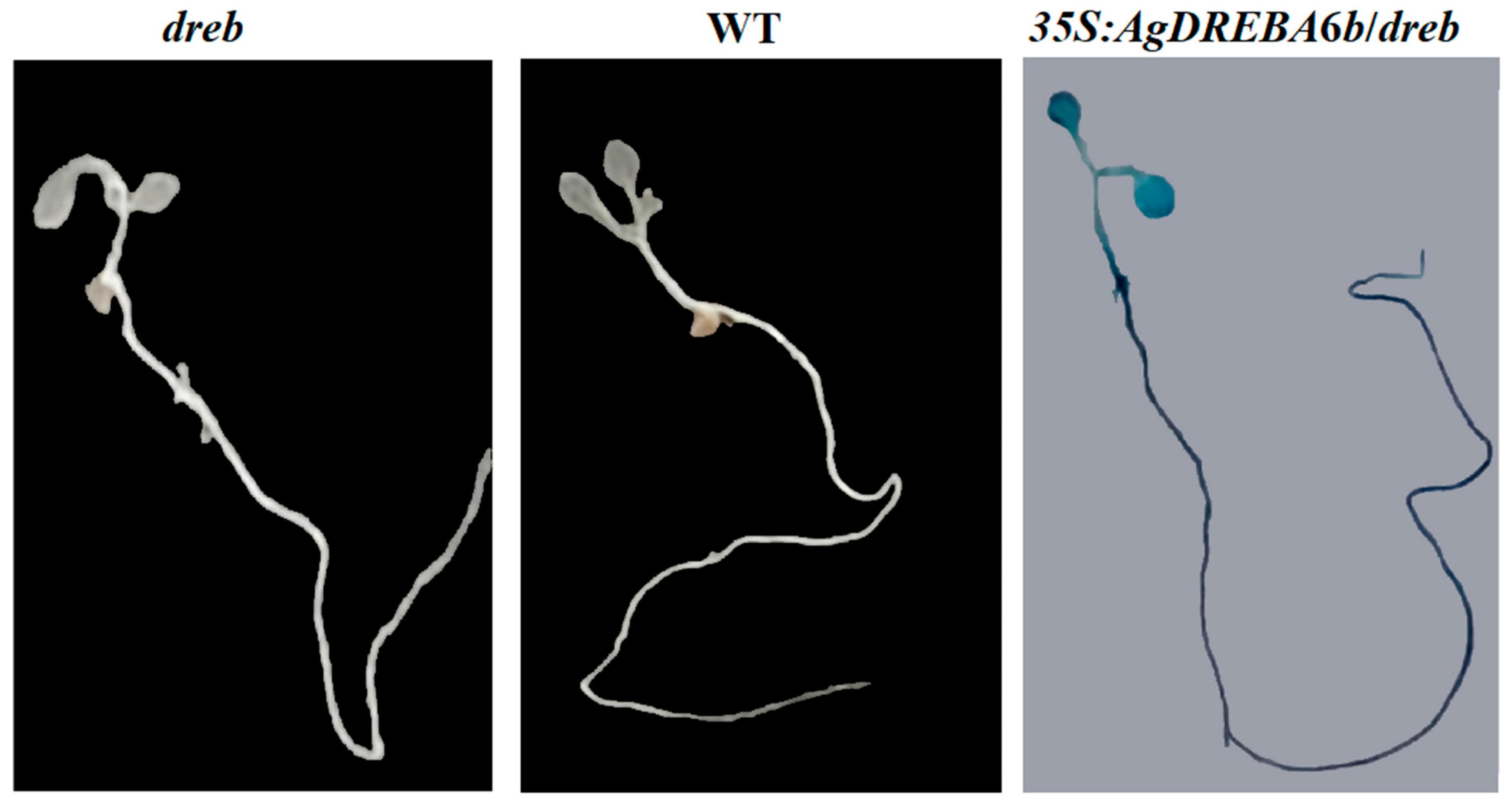
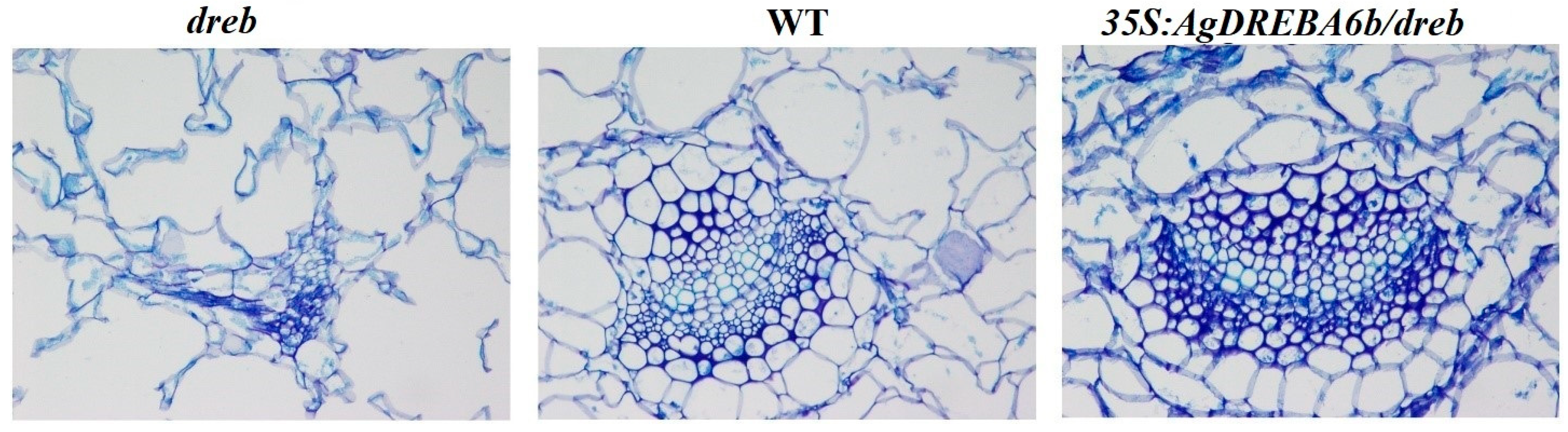
3.2. The Phenotype and Leaf Anatomy of Arabidopsis Plants Under Heat Stress
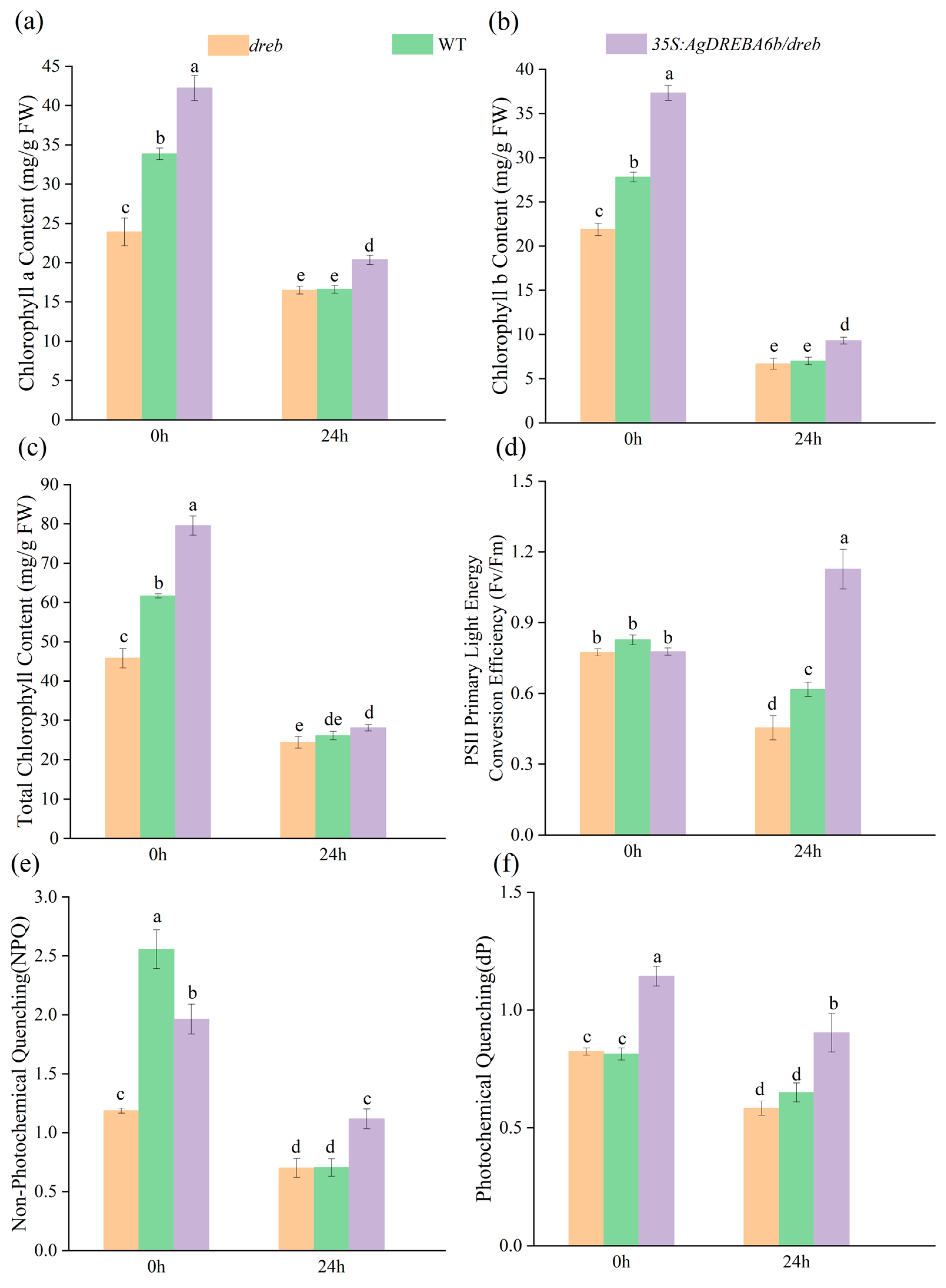
3.3. Effect of Heat Stress on Chlorophyll Content and Chlorophyll Fluorescence Properties of Leaves of Transgenic Plants
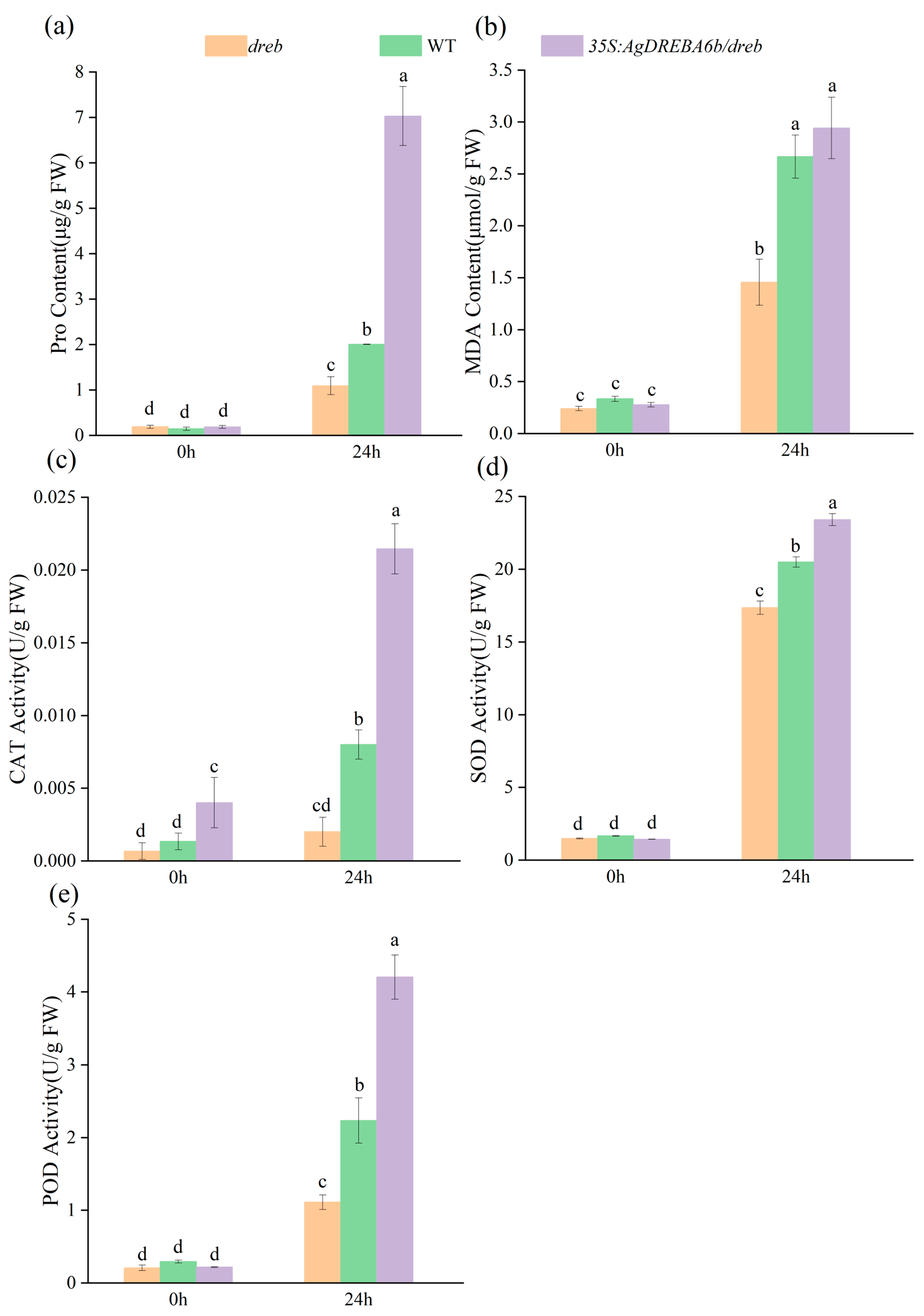
3.4. Effects of Heat Stress on Proline and Antioxidant Enzyme Activity in Transgenic Plants
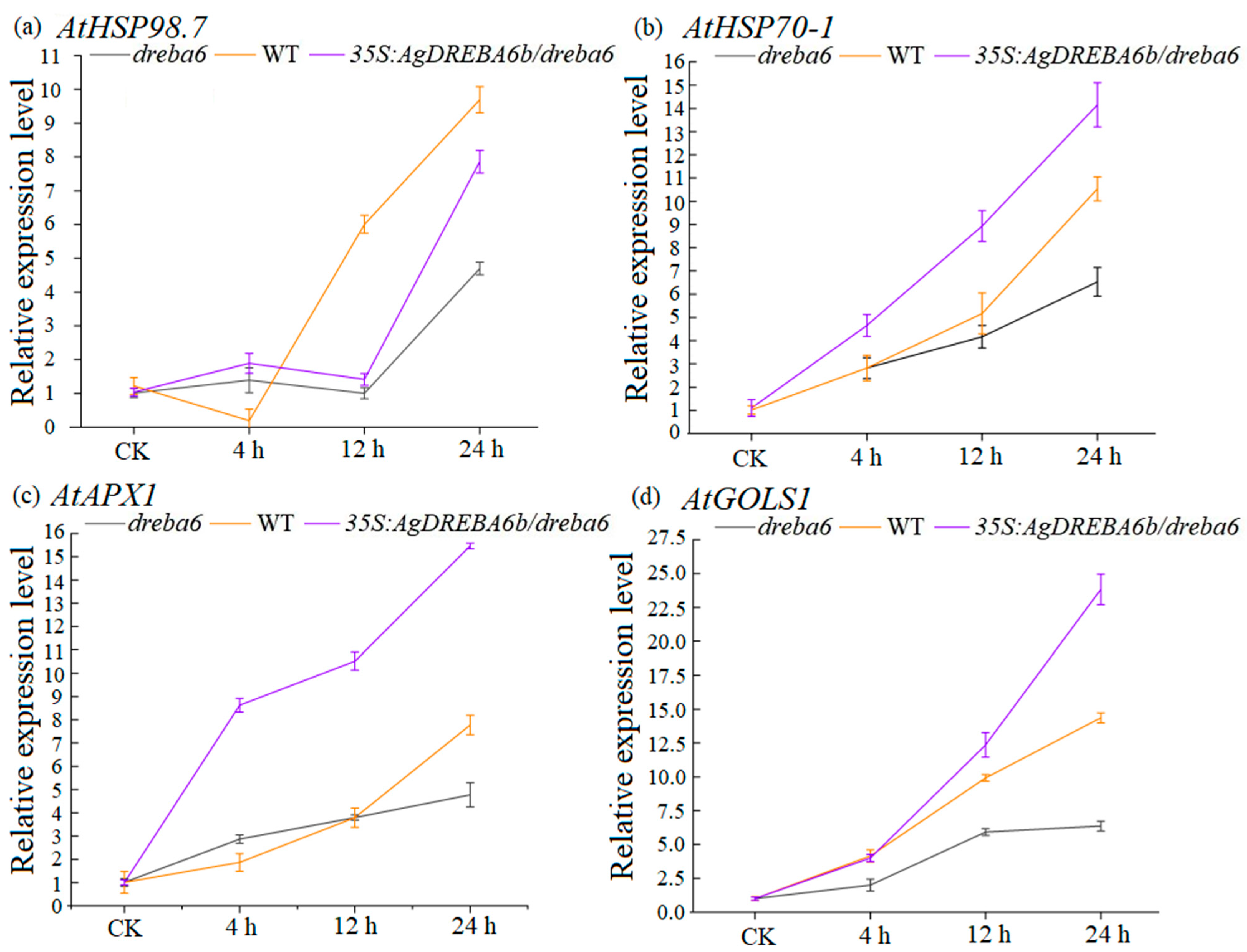
3.5. Expression Patterns of Four High-Temperature-Associated Genes Under Heat Stress
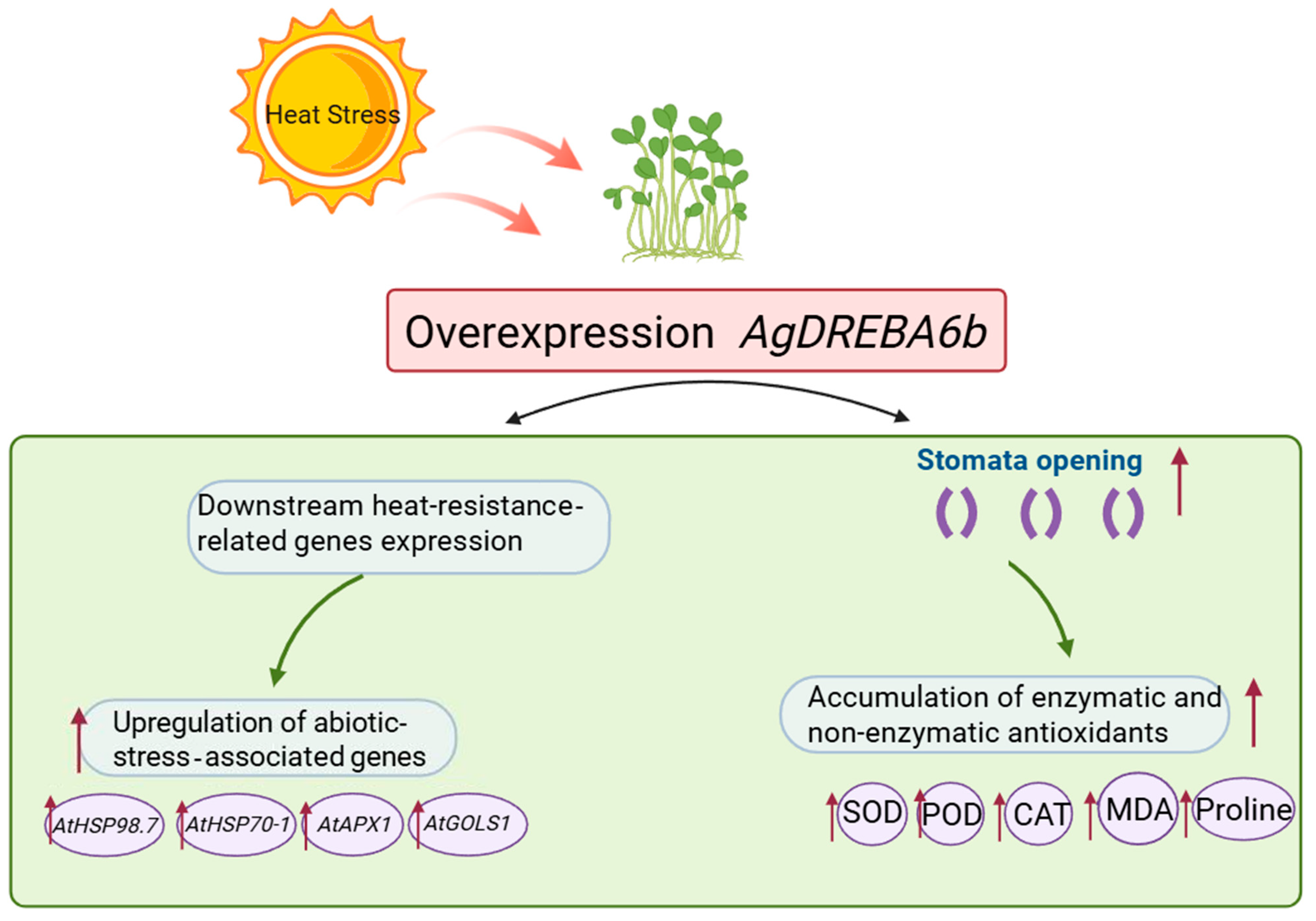
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muthuramalingam, P.; Muthamil, S.; Shilpha, J.; Venkatramanan, V.; Priya, A.; Kim, J.; Shin, Y.; Chen, J.T.; Baskar, V.; Park, K.; et al. Molecular insights into abiotic stresses in mango. Plants 2023, 12, 1939. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Wu, Y.; Lv, X.; Li, J.; Liu, Y.; Du, G.; Chen, J.; Liu, L. Refactoring transcription factors for metabolic engineering. Biotechnol. Adv. 2022, 57, 107935. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription factors interact with ABA through gene expression and signaling pathways to mitigate drought and salinity stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef]
- Lee, S.; Jeon, D.; Choi, S.; Kang, Y.; Seo, S.; Kwon, S.; Lyu, J.; Ahn, J.; Seo, J.; Kim, C. Expression profile of sorghum genes and cis-regulatory elements under salt-stress conditions. Plants 2022, 11, 869. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhou, W.; Liu, H.; Liu, P.; Li, Z.G. Genome-wide analysis of the soybean DREB gene family: Identification, genomic organization and expression profiles in response to drought stress. Plant Breed. 2020, 139, 1158–1167. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Khadr, A.; Wang, Y.H.; Xu, Z.S.; Xiong, A.S. DcDREB1A, a DREB-binding transcription factor from Daucus carota, enhances drought tolerance in transgenic Arabidopsis thaliana and modulates lignin levels by regulating lignin biosynthesis-related genes. Environ. Exp. Bot. 2020, 169, 103896. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Qin, F.; Kakimoto, M.; Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 2007, 50, 54–69. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Wang, M.L.; Lynch, T.J.; Rao, S.; Goodman, H.M. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 1998, 10, 1043–1054. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, P. The DREB transcription factor, a biomacromolecule, responds to abiotic stress by regulating the expression of stress-related genes. Int. J. Biol. Macromol. 2023, 243, 125231. [Google Scholar] [CrossRef] [PubMed]
- Azeem, S.; Munir, F.; Gul, A.; Amir, R. An A-6 subgroup member of DREB gene family positively regulates cold stress tolerance by modulating an antioxidant defense system in transgenic potato. Sci. Rep. 2025, 15, 15421. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Luo, T.; Zhao, H.; Su, Y.; Ji, W.; Li, H. Identification of wheat DREB genes and functional characterization of TaDREB3 in response to abiotic stresses. Gene 2020, 740, 144514. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.-O.; Wu, Z. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ. Exp. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Zhou, N.; Huang, J.; Jiang, F.; Hu, E.; Song, X.; Zhou, R.; Wu, Z. SOS3-3 enhances the salt tolerance of tomato plants by regulating ROS balance. Agronomy 2024, 14, 3044. [Google Scholar] [CrossRef]
- Ban, Q.; Liu, G.; Wang, Y. A DREB gene from Limonium bicolor mediates molecular and physiological responses to copper stress in transgenic tobacco. J. Plant Physiol. 2011, 168, 449–458. [Google Scholar] [CrossRef]
- Singh, S.; Chopperla, R.; Shingote, P.; Chhapekar, S.S.; Deshmukh, R.; Khan, S.; Padaria, J.C.; Sharma, T.R.; Solanke, A.U. Overexpression of EcDREB2A transcription factor from finger millet in tobacco enhances tolerance to heat stress through ROS scavenging. J. Biotechnol. 2021, 336, 10–24. [Google Scholar] [CrossRef]
- Du, X.; Li, W.; Sheng, L.; Deng, Y.; Wang, Y.; Zhang, W.; Yu, K.; Jiang, J.; Fang, W.; Guan, Z.; et al. Over-expression of chrysanthemum CmDREB6 enhanced tolerance of chrysanthemum to heat stress. BMC Plant Biol. 2018, 18, 178. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, J.; Xie, F.J.; Zhou, J.; Sun, Y.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; Lin, Y.X.; et al. Combined evaluation of agronomic and quality traits to explore heat germplasm in celery (Apium graveolens L.). Sci. Hortic. 2023, 317, 112039. [Google Scholar] [CrossRef]
- Xie, F.J.; Hu, J.F.; Zhang, W.J.; Wang, X.R.; Zhou, S.H.; Yang, G.Z.; Lv, R.H. Cloning and functional characterization of the AgDREBA6b gene in response to abiotic stress in celery (Apium graveolens L.). Jiangsu Agric. Sci. 2025, 53, 233–241. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Wijewardene, I.; Mishra, N.; Sun, L.; Smith, J.; Zhu, X.; Payton, P.; Shen, G.; Zhang, H. Improving drought-, salinity-, and heat-tolerance in transgenic plants by co-overexpressing Arabidopsis vacuolar pyrophosphatase gene AVP1 and Larrea Rubisco activase gene RCA. Plant Sci. 2020, 296, 110499. [Google Scholar] [CrossRef]
- Tokić, M.; Levanić, D.L.; Ludwig-Müller, J.; Bauer, N. Growth and molecular responses of tomato to prolonged and short-term heat exposure. Int. J. Mol. Sci. 2023, 24, 4456. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dai, Y.; Yue, L.; Chen, G.; Yuan, L.; Zhang, S.; Li, F.; Zhang, H.; Li, G.; Zhu, S.; et al. Heat stress response in Chinese cabbage (Brassica rapa L.) revealed by transcriptome and physiological analysis. PeerJ 2022, 10, e13427. [Google Scholar] [CrossRef]
- Bapela, T.; Shimelis, H.; Tsilo, T.; Mathew, I. Genetic improvement of wheat for drought tolerance: Progress, challenges and opportunities. Plants 2022, 11, 1331. [Google Scholar] [CrossRef]
- Zha, Q.; Yin, X.; Xi, X.; Jiang, A. Heterologous VvDREB2c expression improves heat tolerance in Arabidopsis by inducing photoprotective responses. Int. J. Mol. Sci. 2023, 24, 5989. [Google Scholar] [CrossRef]
- Mao, L.; Deng, M.; Jiang, S.; Zhu, H.; Yang, Z.; Yue, Y.; Zhao, K. Characterization of the DREBA4-type transcription Factor (SlDREBA4), which contributes to heat tolerance in tomatoes. Front. Plant Sci. 2020, 11, 554520. [Google Scholar] [CrossRef]
- Kumar, S.; Muthuvel, J.; Sadhukhan, A.; Kobayashi, Y.; Koyama, H.; Sahoo, L. Enhanced osmotic adjustment, antioxidant defense, and photosynthesis efficiency under drought and heat stress of transgenic cowpea overexpressing an engineered DREB transcription factor. Plant Physiol. Biochem. 2022, 193, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Wang, R.; Li, X.Y.; Li, Y.Y.; Wang, T.Y.; Guo, Z.H.; Li, Y.; Qiu, W.X.; Guan, S.Y.; et al. Overexpression of the GmPM35 gene significantly enhances drought tolerance in transgenic Arabidopsis and soybean. Agronomy 2025, 15, 1. [Google Scholar] [CrossRef]
- Ren, M.; Yang, W.; Zhang, J.; Zhao, L.; Quan, Y.; He, Z.; Xu, Y.; Zhang, F.; Yin, M.; Wang, Y.; et al. Overexpression of ClRAP2.4 in Chrysanthemum enhances tolerance to cold stress. Funct. Plant Biol. 2023, 50, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zeng, Y.; Yang, X.; Nie, S. Characterization of DREB family genes in Lotus japonicus and LjDREB2B overexpression increased drought tolerance in transgenic Arabidopsis. BMC Plant Biol. 2024, 24, 497. [Google Scholar] [CrossRef]
- Chaudhari, R.S.; Jangale, B.L.; Krishna, B.; Sane, P.V. Improved abiotic stress tolerance in Arabidopsis by constitutive active form of a banana DREB2 type transcription factor, MaDREB20.CA, than its native form, MaDREB20. Protoplasma 2023, 260, 671–690. [Google Scholar] [CrossRef]
- Mizoi, J.; Kanazawa, N.; Kidokoro, S.; Takahashi, F.; Qin, F.; Morimoto, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Heat-induced inhibition of phosphorylation of the stress-protective transcription factor DREB2A promotes thermotolerance of Arabidopsis thaliana. J. Biol. Chem. 2019, 294, 902–917. [Google Scholar] [CrossRef]
- Zhao, K.; Shen, X.; Yuan, H.; Liu, Y.; Liao, X.; Wang, Q.; Liu, L.; Li, F.; Li, T. Isolation and characterization of dehydration-responsive element-binding factor 2C (MsDREB2C) from Malus sieversii Roem. Plant Cell Physiol. 2013, 54, 1415–1430. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Y.; Lan, W.; Zhou, Q.; Li, F.; Chen, F.; Bao, M.; Liu, G. LIDREB1G, a novel DREB subfamily gene from Lilium longiflorum, can enhance transgenic Arabidopsis tolerance to multiple abiotic stresses. Plant Cell Tissue Organ. Cult. 2019, 138, 489–506. [Google Scholar] [CrossRef]

| Gene Name | Forward Primer (5′ → 3′) | Reverse Primers (5′ → 3′) |
|---|---|---|
| AtHSP98.7 | F: TGCTTGGACGAGGTGAACTGAG | R: CGTTCGGTGATGTAGCGGTCTG |
| AtHSP70-1 | F: TGCGTGAGATTGCTGAGGCTTA | R: CGGCTGTAGGCTCGTTGATGAT |
| AtAPX1 | F: ACTACCCAACCGTGAGCGAAGA | R: TGCCATGCGAGTCGGACCAT |
| AtGOLS1 | F: ATGGAGTCACACGCCGCAATAC | R: TGACGCCATAACATCGCAAGGA |
| AtACT2 | F: GAAATCACAGCACTTGCACC | R: AAGCCTTTGATCTTGAGAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.; Yang, S.; Peng, Z.; Li, Y.; Yang, Z.; Lv, R. Overexpression of AgDREBA6b Gene Significantly Increases Heat Tolerance in Arabidopsis thaliana. Agronomy 2025, 15, 1565. https://doi.org/10.3390/agronomy15071565
Xie F, Yang S, Peng Z, Li Y, Yang Z, Lv R. Overexpression of AgDREBA6b Gene Significantly Increases Heat Tolerance in Arabidopsis thaliana. Agronomy. 2025; 15(7):1565. https://doi.org/10.3390/agronomy15071565
Chicago/Turabian StyleXie, Fangjie, Shengyan Yang, Zexi Peng, Yonglu Li, Zhenchao Yang, and Ruiheng Lv. 2025. "Overexpression of AgDREBA6b Gene Significantly Increases Heat Tolerance in Arabidopsis thaliana" Agronomy 15, no. 7: 1565. https://doi.org/10.3390/agronomy15071565
APA StyleXie, F., Yang, S., Peng, Z., Li, Y., Yang, Z., & Lv, R. (2025). Overexpression of AgDREBA6b Gene Significantly Increases Heat Tolerance in Arabidopsis thaliana. Agronomy, 15(7), 1565. https://doi.org/10.3390/agronomy15071565







