Application of Hyperspectral Imaging for Early Detection of Pathogen-Induced Stress in Cabbage as Case Study
Abstract
1. Introduction
2. Principles of Spectral Methods
3. Spectral Methods in Plant Trait Analysis
3.1. Chlorophyll
3.2. Flavonoids
3.3. Anthocyanin
3.4. Carotenoids
3.5. Xanthophyll
3.6. NPK
3.7. Water (Leaf Moisture)
3.8. Polyphenols
3.9. Mycotoxins Produced by Pathogens
3.10. Sugars
3.11. Lignin/Cellulose/Proteins
4. Hyperspectral Research for Cabbage
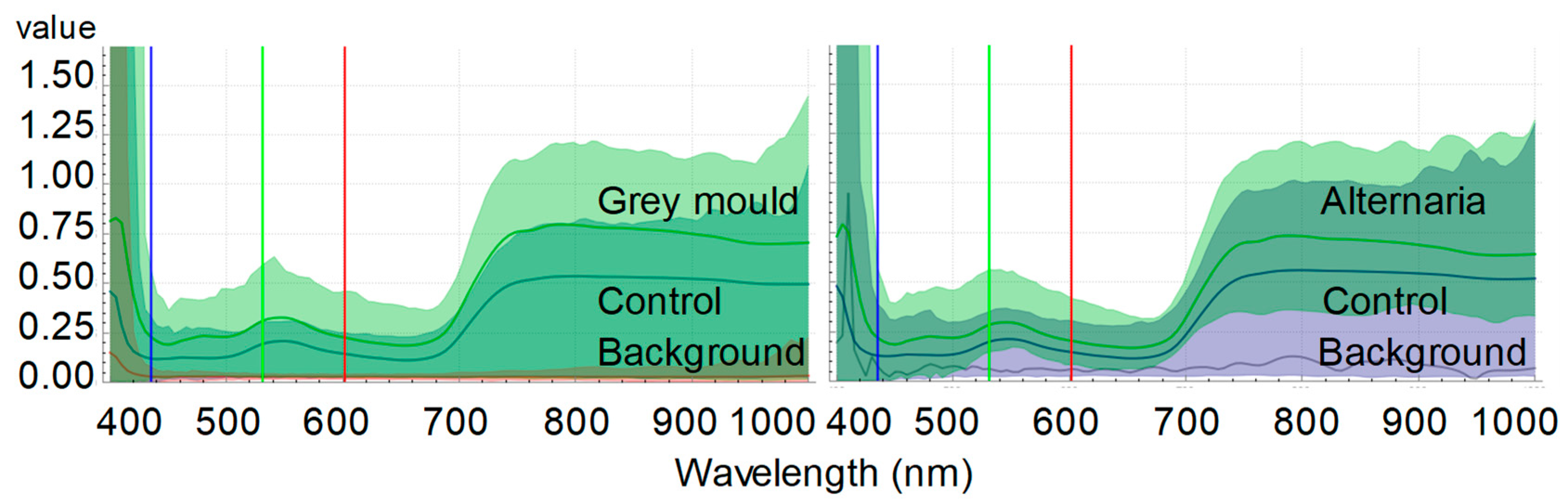
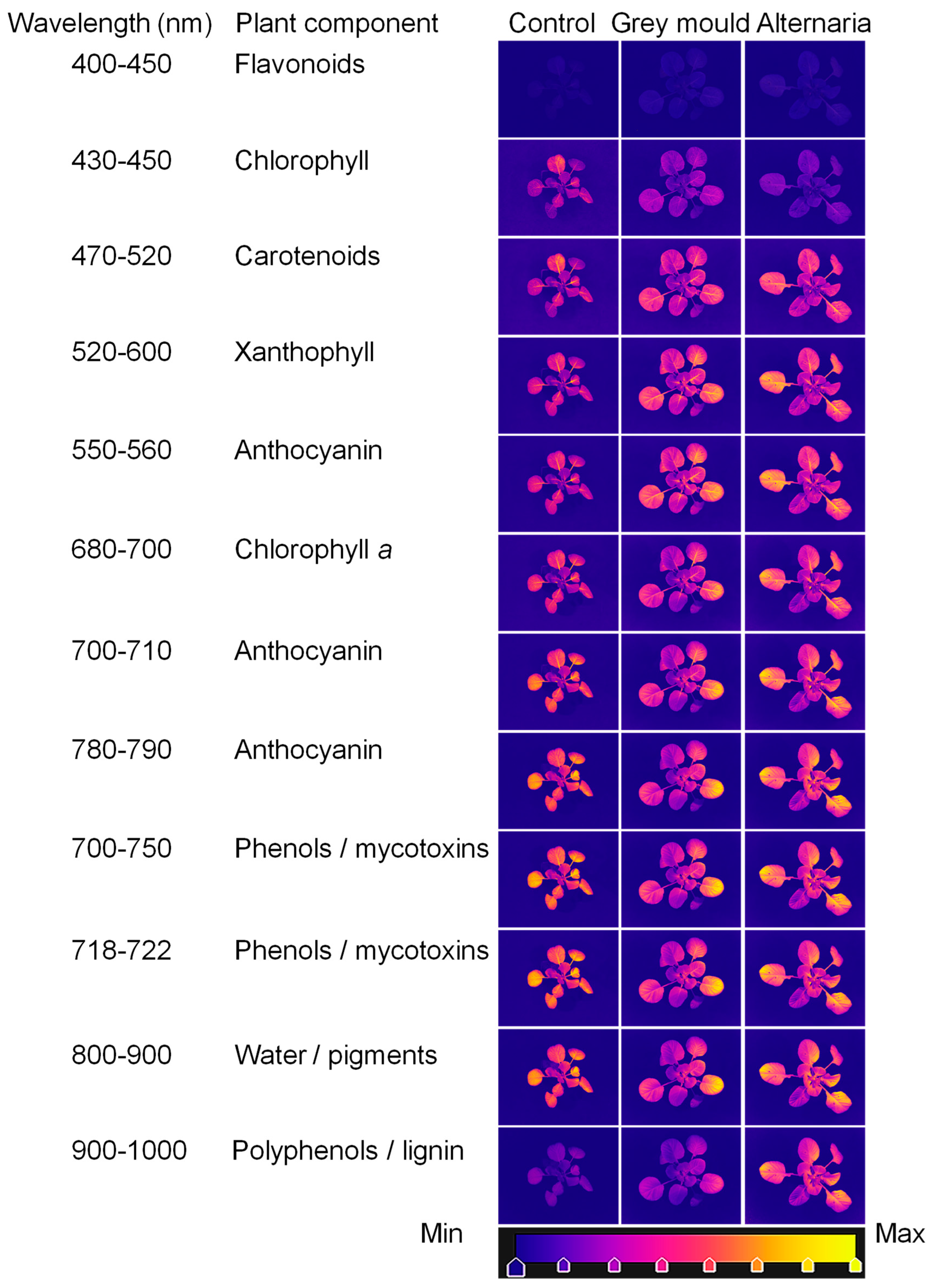
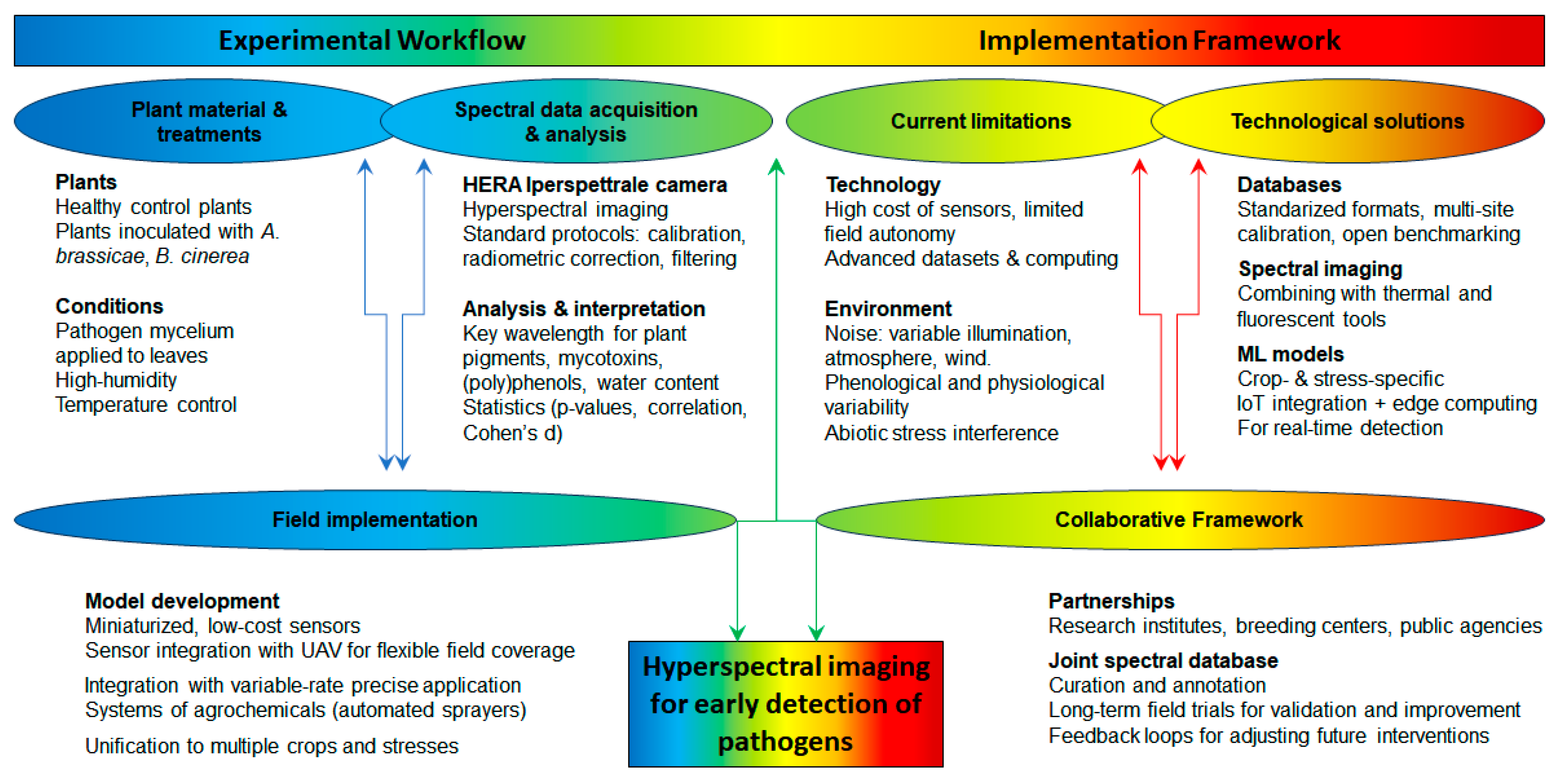
5. An Experimental Case Study: A Novel Contribution to Hyperspectral Imaging for Detecting Cabbage Diseases
6. Conclusions
- ✓
- Hyperspectral sensors are generally expensive, require specialised handling, and are often not readily adaptable for use on standard agricultural machinery or UAVs.
- ✓
- High-dimensional datasets require significant computational power for storage and real-time processing (advanced algorithms and domain expertise), which may not be feasible in remote field settings and without expert training.
- ✓
- Field conditions introduce multiple sources of spectral noise, including the following:
- -
- Variable solar illumination (e.g., cloud cover and diurnal light changes) that affect reflectance measurements.
- -
- Atmospheric interference (e.g., humidity, aerosols) that distorts spectral signals, especially in NIR regions.
- -
- Wind (induced leaf movement and plant geometry variability) that reduces image sharpness and spatial precision.
- -
- Heterogeneous background (soil, debris, weeds) that complicates segmentation and classification.
- -
- Phenological variability (e.g., flowering, senescence) that can mimic or mask symptoms of disease.
- -
- Physiological variability, e.g., different plant species, even within the same botanical family, can exhibit distinct spectral responses to biotic and abiotic stresses due to variations in leaf morphology, pigment composition, metabolic pathways, and stress signalling mechanisms; similarly, the spectral signature of infection varies across pathogen types (fungi, bacteria, viruses) and disease progression stages, further complicating the universal application of spectral indices.
- -
- Abiotic stresses such as drought, nutrient deficiency, or heat can mimic or mask biotic stress symptoms, making differential diagnosis based on spectral data particularly challenging.
- ✓
- Systems are not designed for long-term autonomous operation in field conditions and often depend on external power sources and stable platforms.
- ✓
- Exploring miniaturised, low-cost sensors; recent sensors weigh <200 g and are compact enough for UAV integration, while acceptable spectral resolution (e.g., 5–10 nm) are maintained across key regions (400–1000 nm), sufficient for detecting plant stress and disease; reduced power consumption and thermal management needs, which make them suitable for autonomous operations in field settings; however, mobile spectrometers do not always provide spatial data with enough resolution for large-scale monitoring.
- ✓
- Integration with UAV platforms to provide flexible, rapid, and repeated coverage of large agricultural plots with centimetre-level spatial resolution, which enables monitoring across different phenological stages and under various light and weather conditions.
- ✓
- Linking spectral imaging devices with Internet of Things (IoT) platforms for seamless data sharing and decision-making; hyperspectral nodes can be linked with soil moisture sensors, temperature/humidity loggers, and weather stations to enable multi-dimensional crop environment monitoring, while integration with edge computing allows for on-site processing, reducing the volume of data transmitted and enabling faster decision-making.
- ✓
- Expanding spectral imaging research to include a broader range of crops and pathogens, as well as methodologies across agricultural systems; current models and diagnostic algorithms are often trained and validated on single species (e.g., cabbage) and specific stress types (pathogens), limiting their generalisability (for other species such as cauliflower and broccoli) and stresses (abiotic drought).
- ✓
- Combining spectral imaging with other diagnostic tools, such as thermal imaging and genomics, to create comprehensive crop health assessment frameworks; ultimately, multispectral methods have too low a resolution to detect details of plant stress, while hyperspectral methods are not yet a fully mature field-deployable tool for most farming operations; their integration with other sensor technologies and decision support systems holds significant promise for early disease detection and improved crop health management in precision agriculture.
- ✓
- Technology selection based on spectral and spatial resolution appropriate to the crop, portability, cost-efficiency, and robustness in outdoor conditions.
- ✓
- Standardised data workflows supported by standardised protocols, i.e., calibration, radiometric correction, and filtering to mitigate atmospheric and illumination variability.
- ✓
- Targeted data analysis and pre-trained machine learning models enable real-time crop-specific detection of early stress symptoms.
- ✓
- Decision-support integration to feed spectral outputs into precision management systems (e.g., for fungicide application or selective harvesting), ideally linked with IoT infrastructure for automated response.
- ✓
- The use of common data formats and metadata standards for spectral signatures;
- ✓
- Multi-site calibration campaigns to align sensor outputs under varying environmental and agronomic conditions;
- ✓
- Validation of reflectance-based stress indicators across crop species and regions;
- ✓
- Benchmarking of model performance using open-access reference datasets.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blagojević, J.D.; Vukojević, J.B.; Ivanović, Ž.S. Occurrence and characterization of Alternaria species associated with leaf spot disease in rapeseed in Serbia. Plant Pathol. 2020, 69, 883–900. [Google Scholar] [CrossRef]
- Kumar, D.; Maurya, N.; Bharati, Y.K.; Kumar, A.; Kumar, K.; Srivastava, K.; Chand, G.; Kushwaha, C.; Singh, S.K.; Mishra, R.K.; et al. Alternaria blight of oilseed brassicas: A comprehensive review. Asian J. Med. Res. 2014, 8, 2816–2829. [Google Scholar]
- Schinkovitz, A.; Guillemette, T.; Blond, N.; Pogam-Alluard, P.L.; Jaozara, N.; Kerdia, K.; Boustie, J.; Simoneau, P.; Richomme, P. Antifungal activity of lichen extracts and compounds against Alternaria brassicola. In Proceedings of the Joint Natural Products Conference, Copenhague, Denmark, 9 July 2016; Planta Medica, 81 (S 01). pp. S1–S381. [Google Scholar]
- Padmathilake, K.R.E.; Fernando, W.G.D. Less virulent Leptosphaeria biglobosa immunizes the canola plant to resist highly virulent l. maculans, the blackleg pathogen. Plants 2022, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.S.; Monteiro, A.A.; Lopes, V.R.; Branca, F. New sources of resistance to downy mildew in a collection of wild and cultivated brassicas. Acta Hortic. 2018, 1202, 93–100. [Google Scholar] [CrossRef]
- Ding, Y.; Mei, J.; Chai, Y.; Yu, Y.; Shao, C.; Wu, Q.; Disi, J.O.; Li, Y.; Wan, H.; Qian, W. Simultaneous transcriptome analysis of host and pathogen highlights the interaction between Brassica oleracea and Sclerotinia sclerotiorum. Phytopathology 2019, 109, 542–550. [Google Scholar] [CrossRef]
- Donald, C.; Porter, I. Integrated control of clubroot. J. Plant Growth Regul. 2009, 28, 289–303. [Google Scholar] [CrossRef]
- Borkar, S.G. Threat perception of bacterial plant pathogen Xanthomonas campestris pv. campestris (Pammel) Dowson, causing black rot disease in cabbage fields. Can. J. Agric. Crops 2020, 5, 174–186. [Google Scholar] [CrossRef]
- Lee, D.H.; Lim, J.-A.; Lee, J.; Roh, E.; Jung, K.; Choi, M.; Oh, C.; Ryu, S.; Yun, J.; Heu, S. Characterization of genes required for the pathogenicity of Pectobacterium carotovorum subsp. carotovorum Pcc21 in chinese cabbage. Microbiology 2013, 159, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Mauzey, S.J.; Koike, S.T.; Bull, C.T. First report of bacterial blight of cabbage (Brassica oleracea var. capitata) caused by Pseudomonas cannabina pv. alisalensis in California. Plant Dis. 2011, 95, 71. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.C.; Lyv, X.; Xu, J.W.; Wu, M.D.; Zhang, J.; Yang, L.; Li, G.Q. Large-Scale surveys of blackleg of oilseed rape (Leptosphaeria biglobosa) revealed new insights into epidemics of this disease in China. Plant Dis. 2023, 107, 1408–1417. [Google Scholar] [CrossRef]
- Garrett, K.A.; Forbes, G.A.; Savary, S.; Skelsey, P.; Sparks, A.H.; Valdivia, C.; van Bruggen, A.H.C.; Willocquet, L.; Djurle, A.; Duveiller, E.; et al. Complexity in climate-change impacts: An analytical framework for effects mediated by plant disease. Plant Pathol. 2011, 60, 15–30. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Khanal, S.; Fulton, J.; Shearer, S. An overview of current and potential applications of thermal remote sensing in precision agriculture. Comput. Electr. Agric. 2017, 139, 22–32. [Google Scholar] [CrossRef]
- Kuswidiyanto, L.W.; Noh, H.-H.; Han, X. Plant disease diagnosis using deep learning based on aerial hyperspectral images: A review. Remote Sens. 2022, 14, 6031. [Google Scholar] [CrossRef]
- Omia, E.; Bae, H.; Park, E.; Kim, M.S.; Baek, I.; Kabenge, I.; Cho, B.-K. Remote sensing in field crop monitoring: A comprehensive review of sensor systems, data analyses and recent advances. Remote Sens. 2023, 15, 354. [Google Scholar] [CrossRef]
- Lu, B.; He, Y. Evaluating empirical regression, machine learning, and radiative transfer modelling for estimating vegetation chlorophyll content using bi-seasonal hyperspectral images. Remote Sens. 2019, 11, 1979. [Google Scholar] [CrossRef]
- Bergsträsser, S.; Fanourakis, D.; Schmittgen, S.; Cendrero-Mateo, M.P.; Jansen, M.; Scharr, H.; Rascher, U. HyperART: Non-invasive quantification of leaf traits using hyperspectral absorption-reflectance-transmittance imaging. Plant Meth. 2015, 11, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.M.; Miller, J.R.; Noland, T.L. Leaf chlorophyll content retrieval from airborne hyperspectral remote sensing imagery. Remote Sens. Environ. 2008, 112, 3234–3247. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Kruk, J.; Górecka, M.; Karpińska, B.; Karpiński, S. Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 2010, 22, 2201–2218. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Karpiński, S. Light intensity-dependent retrograde signalling in higher plants. J. Plant Physiol. 2013, 170, 1501–1516. [Google Scholar] [CrossRef]
- Qin, J.; Lu, R. Measurement of the optical properties of fruits and vegetables using spatially resolved hyperspectral diffuse reflectance imaging technique. Posthar. Biol. Technol. 2008, 49, 355–365. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Thomas, S.; Wahabzada, M.; Kuska, M.T.; Rascher, U.; Mahlein, A.-K. Observation of plant–pathogen interaction by simultaneous hyperspectral imaging reflection and transmission measurements. Funct. Plant Biol. 2016, 44, 23–34. [Google Scholar] [CrossRef]
- Karpiński, S.; Szechyńska-Hebda, M.; Wituszyńska, W.; Burdiak, P. Light Acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ. 2013, 36, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Szechyńska-Hebda, M.; Wasek, I.; Gołebiowska, G.; Dubas, E.; Zur, I.; Wedzony, M. Photosynthesis-dependent physiological and genetic crosstalk between cold acclimation and cold-induced resistance to fungal pathogens in triticale (Triticosecale Wittm.). J. Plant Physiol. 2015, 177, 30–43. [Google Scholar] [CrossRef]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Meth. 2017, 13, 80. [Google Scholar] [CrossRef]
- Ferreira, L.d.C.; Carvalho, I.C.B.; Jorge, L.A.d.C.; Quezado-Duval, A.M.; Rossato, M. Hyperspectral imaging for the detection of plant pathogens in seeds: Recent developments and challenges. Front. Plant Sci. 2024, 15, 1387925. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Razzaq, A.; Tariq, W.; Hameed, A.; Rehman, A.; Razzaq, K.; Sarfraz, S.; Rajput, N.A.; Zaki, H.E.M.; Shahid, M.S.; et al. Spectral intelligence: Ai-driven hyperspectral imaging for agricultural and ecosystem applications. Agronomy 2024, 14, 2260. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Neugart, S.; Brestic, M. Assessment of hyperspectral indicators related to the content of phenolic compounds and multispectral fluorescence records in chicory leaves exposed to various light environments. Plant Physiol. Biochem. 2020, 154, 429–438. [Google Scholar] [CrossRef]
- Kim, M.-J.; Yu, W.-H.; Song, D.-J.; Chun, S.-W.; Kim, M.S.; Lee, A.; Kim, G.; Shin, B.-S.; Mo, C. Prediction of soluble-solid content in citrus fruit using visible–near-infrared hyperspectral imaging based on effective-wavelength selection algorithm. Sensors 2024, 24, 1512. [Google Scholar] [CrossRef]
- Feng, L.; Wu, B.; Chen, S.; Zhang, C.; He, Y. Application of visible/near-infrared hyperspectral imaging with convolutional neural networks to phenotype aboveground parts to detect cabbage Plasmodiophora brassicae (clubroot). Infrared Phys. Techn. 2022, 121, 104040. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Ma, C.; Yin, X.; Guo, Y.; Sun, X.; Jin, C. Application of visible-near-infrared hyperspectral imaging technology coupled with wavelength selection algorithm for rapid determination of moisture content of soybean seeds. J. Food Comp. Anal. 2023, 116, 105048. [Google Scholar] [CrossRef]
- Huang, J.; Wei, C.; Zhang, Y.; Blackburn, G.A.; Wang, X.; Wei, C.; Wang, J. Meta-analysis of the detection of plant pigment concentrations using hyperspectral remotely sensed data. PLoS ONE 2015, 10, e0137029. [Google Scholar] [CrossRef] [PubMed]
- Fiļipovics, M. Hyperspectral Imaging for Early Detection of Foliar Fungal Diseases on Small Grain Cereals: A Minireview. Available online: https://rrd.lbtu.lv/node/69 (accessed on 11 January 2025).
- Wan, S.; Yeh, M.-L.; Ma, H.-L. An innovative intelligent system with integrated CNN and SVM: Considering various crops through hyperspectral image data. ISPRS Int. J. Geo-Inf. 2021, 10, 242. [Google Scholar] [CrossRef]
- Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. Reflectance spectroscopy for the classification and prediction of pigments in agronomic crops. Plants 2023, 12, 2347. [Google Scholar] [CrossRef]
- Brugger, A.; Yamati, F.I.; Barreto, A.; Paulus, S.; Schramowsk, P.; Kersting, K.; Steiner, U.; Neugart, S.; Mahlein, A.-K. Hyperspectral imaging in the uv range allows for differentiation of sugar beet diseases based on changes in secondary plant metabolites. Phytopathology 2023, 113, 44–54. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, L.; Chu, B.; Zhang, C. Determination of total polysaccharides and total flavonoids in Chrysanthemum morifolium using near-infrared hyperspectral imaging and multivariate analysis. Molecules 2018, 23, 2395. [Google Scholar] [CrossRef]
- Yoon, H.I.; Lee, H.; Yang, J.-S.; Choi, J.-H.; Jung, D.-H.; Park, Y.J.; Park, J.-E.; Kim, S.M.; Park, S.H. Predicting models for plant metabolites based on PLSR, AdaBoost, XGBoost, and LightGBM algorithms using hyperspectral imaging of Brassica juncea. Agriculture 2023, 13, 1477. [Google Scholar] [CrossRef]
- Jiang, S.; Chang, Q.; Wang, X.; Zheng, Z.; Zhang, Y.; Wang, Q. Estimation of anthocyanins in whole-fertility maize leaves based on ground-based hyperspectral measurements. Remote Sens. 2023, 15, 2571. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Xie, A.; Yu, H.; Yin, Y.; Li, X.; Duan, X. Potential of hyperspectral imaging for rapid prediction of anthocyanin content of purple-fleshed sweet potato slices during drying process. Food Anal. Methods 2017, 10, 3836–3846. [Google Scholar] [CrossRef]
- Kuswidiyanto, L.W.; Han, X.; Wang, P.; Noh, H.-H.; Jung, H.-Y.; Jung, D.-H. Airborne Hyperspectral Imaging for Early Diagnosis of Kimchi Cabbage Downy Mildew Using 3D-Resnet and Leaf Segmentation. 2023. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4535845 (accessed on 9 August 2023).
- Lassalle, G. Monitoring natural and anthropogenic plant stressors by hyperspectral remote sensing: Recommendations and guidelines based on a meta-review. Sci. Total Environ. 2021, 788, 147758. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Kuska, M.T.; Bohnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.-K. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J. Plant Dis. Prot. 2018, 125, 5–20. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Guo, S.; Xue, Y.; Liu, J.; Zhang, X. Application of airborne hyperspectral data for precise agriculture. In Proceedings of the 2004 IEEE International Geoscience and Remote Sensing Symposium (IGARSS 2004), Anchorage, AK, USA, 20–24 September 2004; Volume 6, pp. 4195–4198. [Google Scholar]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sen. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Sonobe, R.; Wang, Q. Assessing the xanthophyll cycle in natural beech leaves with hyperspectral reflectance. Funct. Plant. Biol. 2016, 43, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Sanaeifar, A.; Yang, C.; Min, A.; Jones, C.R.; Michaels, T.E.; Krueger, Q.J.; Barnes, R.; Velte, T.J. Noninvasive early detection of nutrient deficiencies in greenhouse-grown industrial hemp using hyperspectral imaging. Remote Sen. 2024, 16, 187. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Liu, F.; Nie, P.-C.; He, Y.; Bao, Y.-D. Rapid detection of nitrogen content and distribution in oilseed rape leaves based on hyperspectral imaging. Guang Pu Xue Yu Guang Pu Fen Xi 2014, 34, 2513–2518. [Google Scholar]
- Zou, W.; Fang, H.; Bao, Y.D.; He, Y. Detection of nitrogen content changes of rape leaf using hyperspectral imaging. Adv. Materials Res. 2011, 204–210, 131–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, M.; Wang, L.; Cui, X.; Li, T.; Zhang, F. In situ nondestructive detection of nitrogen content in soybean leaves based on hyperspectral imaging technology. Agronomy 2024, 14, 806. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; He, L. A wheat WRKY transcription factor TaWRKY17 Enhances tolerance to salt stress in transgenic arabidopsis and wheat plant. Plant. Mol. Biol. 2023, 113, 171–191. [Google Scholar] [CrossRef]
- Higa, S.; Kobori, H.; Tsuchikawa, S. Mapping of leaf water content using near-infrared hyperspectral imaging. Appl. Spectrosc. 2013, 67, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, G.; He, J.; Kang, N.; Yuan, R.; Fan, N. Determination of sugar content in lingwu jujube by nir–hyperspectral imaging. J. Food Sci. 2021, 86, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Zhang, H.; Zhou, H.; Du, T.; Wu, Q.; Mockler, T.C.; Berezin, M.Y. Highly sensitive image-derived indices of water-stressed plants using hyperspectral imaging in swir and histogram analysis. Sci. Rep. 2015, 5, 15919. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Shao, Y.; Li, X.; He, Y. Detection of early blight and late blight diseases on tomato leaves using hyperspectral imaging. Sci. Rep. 2015, 5, 16564. [Google Scholar] [CrossRef]
- Sun, H.; Liu, N.; Wu, L.; Chen, L.; Yang, L.; Li, M.; Zhang, Q. Water content detection of potato leaves based on hyperspectral image. IFAC-PapersOnLine 2018, 51, 443–448. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Steiner, U. Hyperspectral imaging reveals small-scale water gradients in apple leaves due to minimal cuticle perforation by venturia inaequalis conidiophores. J. Exp. Bot. 2024, 75, 3125–3140. [Google Scholar] [CrossRef] [PubMed]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and future perspectives of multi-/hyperspectral thermal infrared remote sensing for crop water-stress detection: A review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, G.; Pan, Y.; Yang, X.; Chen, L.; Zhao, C. A review of advanced technologies and development for hyperspectral-based plant disease detection in the past three decades. Remote Sens. 2020, 12, 3188. [Google Scholar] [CrossRef]
- Zhao, T.; Nakano, A.; Iwaski, Y.; Umeda, H. Application of hyperspectral imaging for assessment of tomato leaf water status in plant factories. Appl. Sci. 2020, 10, 4665. [Google Scholar] [CrossRef]
- Suratanee, A.; Chutimanukul, P.; Saelao, T.; Chadchawan, S.; Buaboocha, T.; Plaimas, K. Phenolic content discrimination in thai holy basil using hyperspectral data analysis and machine learning techniques. PLoS ONE 2024, 19, e0309132. [Google Scholar] [CrossRef]
- Kang, Y.S.; Ryu, C.S.; Kang, J.G. Presenting a multispectral image sensor for quantification of total polyphenols in low-temperature stressed tomato seedlings using hyperspectral imaging. Sensors 2024, 24, 4260. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yoon, S.-R.; Dang, Y.-M.; Yang, J.-S.; Hwang, I.M.; Ha, J.-H. Nondestructive classification of soft rot disease in napa cabbage using hyperspectral imaging analysis. Sci. Rep. 2022, 12, 14707. [Google Scholar] [CrossRef]
- Khan, I.H.; Liu, H.; Li, W.; Cao, A.; Wang, X.; Liu, H.; Cheng, T.; Tian, Y.; Zhu, Y.; Cao, W.; et al. Early detection of powdery mildew disease and accurate quantification of its severity using hyperspectral images in wheat. Remote Sens. 2021, 13, 3612. [Google Scholar] [CrossRef]
- Van De Vijver, R.; Mertens, K.; Heungens, K.; Somers, B.; Nuyttens, D.; Borra-Serrano, I.; Lootens, P.; Roldán-Ruiz, I.; Vangeyte, J.; Saeys, W. In-field detection of Alternaria solani in potato crops using hyperspectral imaging. Comput. Electron. Agric. 2020, 168, 105106. [Google Scholar] [CrossRef]
- Baranowski, P.; Jedryczka, M.; Mazurek, W.; Babula-Skowronska, D.; Siedliska, A.; Kaczmarek, J. Hyperspectral and thermal imaging of oilseed rape (Brassica napus) response to fungal species of the genus alternaria. PLoS ONE 2015, 10, e0122913. [Google Scholar] [CrossRef] [PubMed]
- Fahrentrapp, J.; Ria, F.; Geilhausen, M.; Panassiti, B. Detection of gray mold leaf infections prior to visual symptom appearance using a five-band multispectral sensor. Front. Plant Sci. 2019, 10, 628. [Google Scholar] [CrossRef]
- Scarboro, C.G.; Ruzsa, S.M.; Doherty, C.J.; Kudenov, M.W. Quantification of gray mold infection in lettuce using a bispectral imaging system under laboratory conditions. Plant Direct. 2021, 5, e00317. [Google Scholar] [CrossRef]
- Liang, K.; Liu, Q.X.; Xu, J.H.; Wang, Y.Q.; Okinda, C.S.; Shena, M.X. Determination and visualization of different levels of deoxynivalenol in bulk wheat kernels by hyperspectral imaging. J. Appl. Spectrosc. 2018, 85, 953–961. [Google Scholar] [CrossRef]
- Wang, W.; Heitschmidt, G.W.; Windham, W.R.; Feldner, P.; Ni, X.; Chu, X. Feasibility of detecting aflatoxin b1 on inoculated maize kernels surface using vis/nir hyperspectral imaging. J. Food Sci. 2015, 80, M116–M122. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Y.; Xu, X. A Novel Algorithm for damage recognition on pest-infested oilseed rape leaves. Comput. Electron. Agric. 2012, 89, 41–50. [Google Scholar] [CrossRef]
- Thulin, S.; Hill, M.J.; Held, A.; Jones, S.; Woodgate, P. Predicting levels of crude protein, digestibility, lignin and cellulose in temperate pastures using hyperspectral image data. Am. J. Plant Sci. 2014, 5, 997–1019. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent Advances on the roles of flavonoids as plant protective molecules after uv and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Brugger, A.; Schramowski, P.; Paulus, S.; Steiner, U.; Kersting, K.; Mahlein, A.-K. Spectral signatures in the UV range can be combined with secondary plant metabolites by deep learning to characterize barley–powdery mildew interaction. Plant Pathol. 2021, 70, 1572–1582. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Gould, K.S. Role of anthocyanins in plant defence. In Anthocyanins: Biosynthesis, Functions, and Applications; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2009; pp. 22–28. [Google Scholar]
- Li, X.; Wei, Z.; Peng, F.; Liu, J.; Han, G. Non-destructive prediction and visualization of anthocyanin content in mulberry fruits using hyperspectral imaging. Front. Plant. Sci. 2023, 14, 1137198. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Karpiński, S.; Szechyńska-Hebda, M. Systemic acquired acclimation, network acquired acclimation and cellular light memory in plants–molecular, biochemical, and physiological mechanisms. Adv. Bot. Res. 2023, 105, 277–310. [Google Scholar]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Yu, K.-Q.; Zhao, Y.-R.; Li, X.-L.; Shao, Y.-N.; Liu, F.; He, Y. Hyperspectral imaging for mapping of total nitrogen spatial distribution in pepper plant. PLoS ONE 2014, 9, e116205. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.; Kumar, M. Phenolics: A key defence secondary metabolite to counter biotic stress. In Plant Phenolics in Sustainable Agriculture: Volume 1; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer: Singapore, 2020; pp. 309–329. [Google Scholar]
- Gautam, A.K.; Singh, P.K.; Aravind, M. Defensive role of plant phenolics against pathogenic microbes for sustainable agriculture. In Plant Phenolics in Sustainable Agriculture: Volume 1; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer: Singapore, 2020; pp. 579–594. [Google Scholar]
- Cocuron, J.-C.; Casas, M.I.; Yang, F.; Grotewold, E.; Alonso, A.P. Beyond the wall: High-throughput quantification of plant soluble and cell-wall bound phenolics by liquid chromatography tandem mass spectrometry. J. Chrom. A 2019, 1589, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Dyda, M.; Wąsek, I.; Tyrka, M.; Wędzony, M.; Szechyńska-Hebda, M. Local and systemic regulation of psii efficiency in triticale infected by the hemibiotrophic pathogen Microdochium nivale. Physiol. Plant. 2019, 165, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Szechyńska-Hebda, M.; Hebda, M.; Mierzwiński, D.; Kuczyńska, P.; Mirek, M.; Wędzony, M.; van Lammeren, A.; Karpiński, S. Effect of cold-induced changes in physical and chemical leaf properties on the resistance of winter triticale (×Triticosecale) to the fungal pathogen microdochium nivale. Plant. Pathol. 2013, 62, 867–878. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Wȩdzony, M.; Tyrka, M.; Gołȩbiowska, G.; Chrupek, M.; Czyczyło-Mysza, I.; Dubas, E.; Zur, I.; Golemiec, E. Identifying QTLs for cold-induced resistance to microdochium nivale in winter triticale. Plant Gen. Res. Charact. Util. 2011, 9, 296–299. [Google Scholar] [CrossRef]
- Kuswidiyanto, L.W.; Kim, D.E.; Fu, T.; Kim, K.S.; Han, X. Detection of black spot disease on kimchi cabbage using hyperspectral imaging and machine learning techniques. Agriculture 2023, 13, 2215. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Seki, H.; Ma, T.; Murakami, H.; Tsuchikawa, S.; Inagaki, T. Visualization of sugar content distribution of white strawberry by near-infrared hyperspectral imaging. Foods 2023, 12, 931. [Google Scholar] [CrossRef]
- Ma, Q.-H. Lignin biosynthesis and its diversified roles in disease resistance. Genes 2024, 15, 295. [Google Scholar] [CrossRef]
- Wang, Z.; Skidmore, A.K.; Wang, T.; Darvishzadeh, R.; Hearne, J. Applicability of the PROSPECT model for estimating protein and cellulose + lignin in fresh leaves. Remote Sen. Environ. 2015, 168, 205–218. [Google Scholar] [CrossRef]
- He, M.; Li, C.; Cai, Z.; Qi, H.; Zhou, L.; Zhang, C. Leafy vegetable freshness identification using hyperspectral imaging with deep learning approaches. Infr. Phys. Technol. 2024, 138, 105216. [Google Scholar] [CrossRef]
- Jiang, R.; Gu, M.; Zhao, Q.; Li, X.; Shen, J.; Su, Z. Identification of pesticide residue types in chinese cabbage based on hyperspectral and convolutional neural network. Spectrosc. Spectral Anal. 2022, 42, 1385. [Google Scholar]
- Galodha, A.; Vashisht, R.; Nidamanuri, R.R.; Ramiya, A.M. Deep convolution neural networks with resnet architecture for spectral-spatial classification of drone borne and ground based high resolution hyperspectral imagery. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2022, XLIII-B2-2022, 577–584. [Google Scholar] [CrossRef]
- Astor, T.; Wachendorf, M. Biomass Estimation of vegetables—Can remote sensing be a tool for it? In The Rural-Urban Interface: An interdisciplinary Research Approach to Urbanisation Processes Around the Indian Megacity Bengaluru; Hoffmann, E., Buerkert, A., von Cramon-Taubadel, S., Umesh, K.B., Pethandlahalli Shivaraj, P., Vazhacharickal, P.J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 95–102. [Google Scholar]
- Na, S.; Park, C.; So, K.; Ahn, H.; Lee, K. Selection on optimal bands to estimateyield of the chinese cabbage using drone-based hyperspectral image. Korean J. Rem. Sens. 2019, 35, 375–387. [Google Scholar]
- Suthar, G.; Huang, J.Y.; Chidangil, S. Optimisation and evaluation of hyperspectral imaging system using machine learning algorithm. In Proceedings of the Emerging Imaging And Sensing Technologies for Security and Defence II, SPIE Security + Defence, Warsaw, Poland, 11–14 September 2017; Volume 10438, pp. 121–127. [Google Scholar]
- Cheng, S.-X.; Kong, W.-W.; Zhang, C.; Liu, F.; He, Y. Variety recognition of Chinese cabbage seeds by hyperspectral imaging combined with machine learning. Guang Pu Xue Yu Guang Pu Fen Xi 2014, 34, 2519–2522. [Google Scholar] [PubMed]
- Chandra, H.; Nidamanuri, R.R. Object-based spectral library for knowledge-transfer-based crop detection in drone-based hyperspectral imagery. Precision Agric. 2024, 26, 6. [Google Scholar] [CrossRef]
- Chen, C.-T.; Chen, S.; Wang, C.-Y.; Yang, I.-C.; Hsiao, S.-C.; Tsai, C.-Y. Evaluation of nitrogen content in cabbage seedlings using hyper-spectral images. Sens. Instrum. Food Qual. 2008, 2, 97–102. [Google Scholar] [CrossRef]
- Munipalle, V.K.; Nelakuditi, U.R.; Kumar, M.; Nidamanuri, R.R. Ultra-High-resolution hyperspectral imagery datasets for precision agriculture applications. Data Brief 2024, 55, 110649. [Google Scholar] [CrossRef]
- Lee, S.-D.; Lee, J.-H.; Kim, J.-H.; Jang, Y.; Moon, J.-H. Evaluation technologies for assessing drought tolerance of kimchi cabbage seedlings using hyperspectral imaging and principal component analysis. Microchem. J. 2024, 206, 111499. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Lee, M.; Lee, D.U.; Choi, J.H.; Lee, M.-A.; Min, S.G.; Park, S.H. Non-Destructive monitoring of qualitative properties of salted cabbage using hyperspectral image analysis. LWT 2024, 203, 116329. [Google Scholar] [CrossRef]
- Song, H.; Kim, M.; Yoo, K.S.; Ha, J.-H. Rapid and non-destructive classification of salinity levels in brined kimchi cabbage using hyperspectral imaging. Heliyon 2024, 10, e40817. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Park, S.H.; Lee, M.-A.; Chung, Y.B.; Yang, J.H.; Cho, J.-S.; Min, S.G. Chemometrics and neural networks for estimating the chilling injury severity of kimchi cabbage (Brassica rapa L. ssp. pekinensis) based on hyperspectral images. LWT 2024, 207, 116601. [Google Scholar] [CrossRef]
- Ofori, S.; Abebrese, D.K.; Klement, A.; Provazník, D.; Tomášková, I.; Růžičková, I.; Wanner, J. Impact of treated wastewater on plant growth: Leaf fluorescence, reflectance, and biomass-based assessment. Water Sci. Technol. 2024, 89, 1647–1664. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Feng, W.; Qiu, Z.; Wang, S. Identification of early peach aphid infestation based on hyperspectral imaging Technology. J. Beijing Inst. Technol. 2023, 32, 374–383. [Google Scholar]
- Zhao, Y.; Yu, K.; Feng, C.; Cen, H.; He, Y. Early detection of aphid (Myzus persicae) Infestation on chinese cabbage by hyperspectral imaging and feature extraction. Trans. ASABE 2017, 60, 1045–1051. [Google Scholar] [CrossRef]
- Hu, Z.; Xiang, Y.; Li, Y.; Long, Z.; Liu, A.; Dai, X.; Lei, X.; Tang, Z. Research on identification technology of field pests with protective color characteristics. Appl. Sci. 2022, 12, 3810. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Qiu, Z.; Cen, H.; He, Y. A novel method for detection of pieris rapae larvae on cabbage leaves using nir hyperspectral imaging. Appl. Eng. Agric. 2016, 32, 311–316. [Google Scholar]
- Wei, D.; Huang, Y.; Chunjiang, Z.; Xiu, W. Identification of seedling cabbages and weeds using hyperspectral imaging. Int. J. Agric. Biol. Eng. 2015, 8, 65–72. [Google Scholar]
- Zu, Q.; Zhang, S.; Cao, Y.; Zhao, H.; Dang, C. Research on identification of cabbages and weeds combining spectral imaging technology and sam taxonomy. Guang Pu Xue Yu Guang Pu Fen Xi 2015, 35, 479–485. [Google Scholar]
- Rostás, M.; Bennett, R.; Hilker, M. Comparative physiological responses in chinese cabbage induced by herbivory and fungal infection. J. Chem. Ecol. 2002, 28, 2449–2463. [Google Scholar] [CrossRef]
- Mahatma, M.K.; Thawait, L.K.; Jadon, K.S.; Rathod, K.J.; Sodha, K.H.; Bishi, S.K.; Thirumalaisamy, P.P.; Golakiya, B.A. Distinguish Metabolic profiles and defense enzymes in alternaria leaf blight resistant and susceptible genotypes of groundnut. Physiol. Mol. Biol. Plants 2019, 25, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, I.; Gilardi, G.; Ortu, G.; Gisi, U.; Gullino, M.L.; Garibaldi, A. Identification and characterization of alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production. Eur. J. Plant. Pathol. 2017, 149, 401–413. [Google Scholar] [CrossRef]
- Siciliano, I.; Ortu, G.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Mycotoxin Production in liquid culture and on plants infected with Alternaria spp. isolated from rocket and cabbage. Toxins 2015, 7, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis Cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Liu, C.-M.; Liu, S.-Y.; Liao, C.-K.; Lo, C.-T.; Lin, K.-C.; Peng, K.-C. Cabbage defense response provoked by trichoderma Th-LAAO. Arch. Microbiol. 2021, 203, 1641–1647. [Google Scholar] [CrossRef]

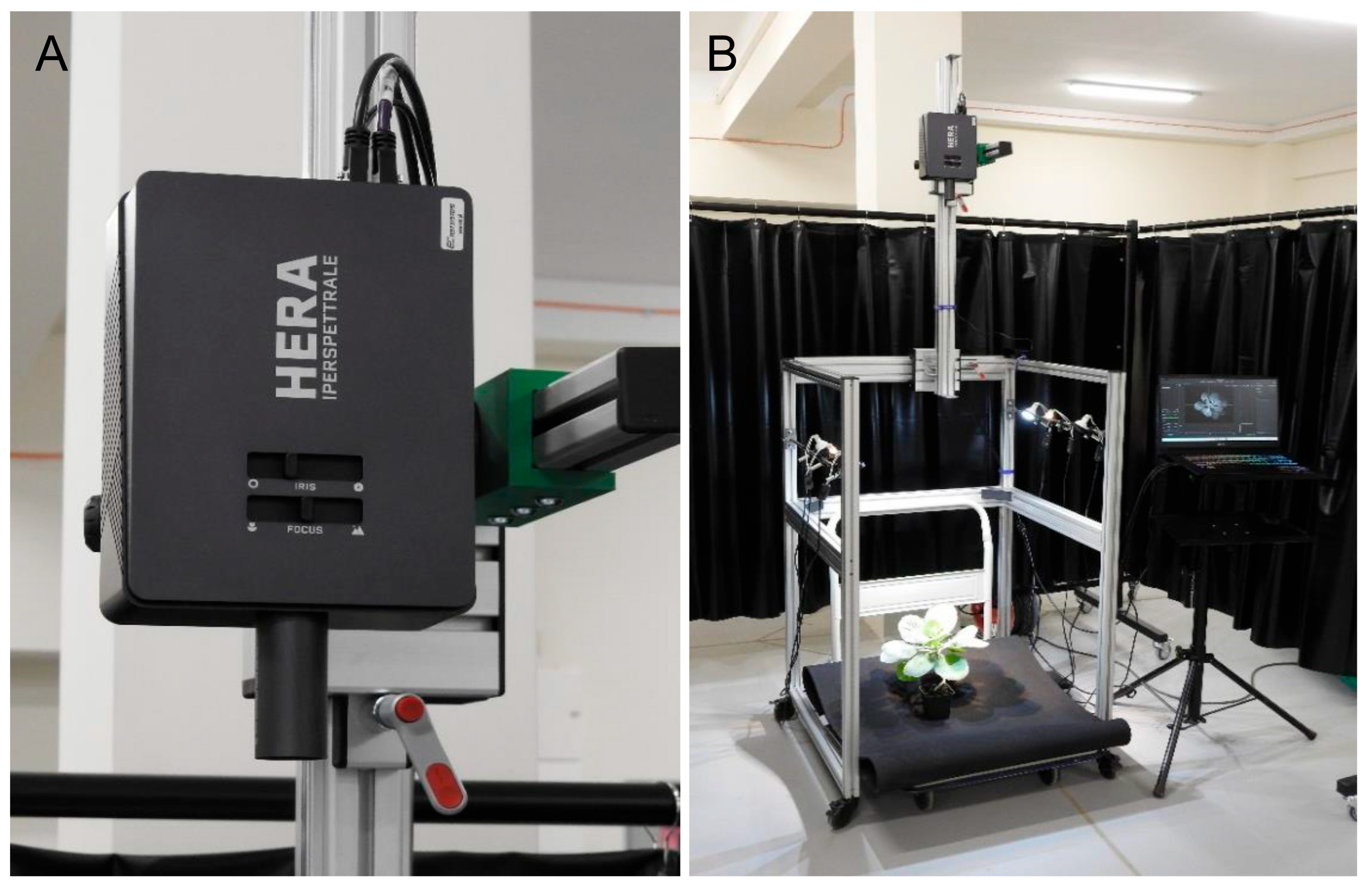


| Aspect | Multispectral Imaging | Hyperspectral Imaging |
|---|---|---|
| Spectral bands | 3–10 broad bands, capturing general reflectance patterns indicative of biotic stress. | Hundreds of contiguous bands allow detailed spectral signatures of pathogens or specific stress markers. |
| Spatial resolution/pathogen specificity | Moderate spatial resolution, identifying stressed areas in fields, like reduced chlorophyll or water content. | High spatial resolution allows detailed mapping of infections at the leaf or plant level, enabling differentiation between pathogen types and biochemical changes. |
| Disease detection stage | Detects stress at early to intermediate stages, but not always pathogen-specific. | Detects biochemical changes at early stages, often before visible symptoms appear. |
| Temporal resolution | Faster data acquisition due to fewer bands; suitable for real-time monitoring. | Slower due to detailed data acquisition, requiring higher computational capacity. |
| Environmental conditions | Moderate performance in varying weather conditions. | Sensitive to environmental variability (e.g., light, humidity), impacting data quality. |
| Integration potential | Easily integrates with drone and satellite systems for real-time data acquisition. | Requires specialised computational tools for processing and analysing large datasets. |
| Integration with models | Produces smaller datasets, is easier to store and analyse, and easily integrates with simple stress detection models. | Generates large datasets, requiring robust computational infrastructure for analysis and advanced machine learning or AI-based models for analysis and prediction. |
| Scalability for farms | Suitable for large-scale, low-cost deployment using drones or satellites. | Best for high-value crops or research settings due to cost and complexity. |
| Users | Relatively simple operation and minimal expertise required; more accessible to farmers and agricultural practitioners. | Requires skilled operators and advanced analytical tools, limiting its use to advanced research or high-value production. |
| Band (nm) | Identification | Monitoring | References |
|---|---|---|---|
| 430–500 550–560 800–810 | Chlorophyll a and b | Leaf health, early disease symptoms, photosynthetic efficiency | [18,23,30,31,32,33,34] |
| 680–750 | Chlorophyll degradation | Photosynthetic efficiency | [35,36] |
| 200–380 400–450 680–800 870–900 940, 1080 1190, 1470 1850, 2245 | 430–500 | Stress response, antioxidant activity | [37,38,39,40] |
| 550–560 700–710 750–770 780–790 910–950 | Anthocyanin | Stress-induced pigmentation, signalling in pathogen defence | [15,23,34,40,41,42,43,44,45] |
| 400–580 510, 700 825 | Carotenoids | Oxidative stress, early infection detection | [23,34,46,47] |
| 500–570 677, 803 | Xanthophyll | Oxidative stress, early infection detection | [34,37,48,49] |
| 495–754 648–650 790, 970 | Nitrogen content | Photosynthetic efficiency, nitrogen availability | [50,51,52,53,54] |
| 542–752 800–1529 | Changes in water/pigment content | Pathogen presence/Abiotic and biotic stress | [24,43,55,56,57,58,59,60,61,62,63] |
| 430–567 601–1002 900–1700 | Polyphenols | Plant defensive response to infections | [30,40,45,64,65,66] |
| 406–489 505–573 636–696 735–780 823–878 901–999 | Mycotoxins by pathogens | Fungal metabolites/plant responses | [43,58,66,67,68,69,70,71,72,73,74] |
| 870–900 972–1450 1052–1254 1940 | Sugar | Carbohydrate storage and translocation | [40,56,61] |
| 970–1450 1600–1800 2100–2300 | Lignin/cellulose/proteins | Structural integrity, mechanical stress | [39,56,73,75] |
| Species | Aim of Study | Bands (nm) | Reference |
|---|---|---|---|
| Yield quantity and quality | |||
| Chinese cabbage | Freshness identification | 874–1734 | [98] |
| Chinese cabbage | Pesticide (chlorpyrifos, dimethoate, methomyl, cypermethrin) | 400–1000 | [99] |
| Cabbage | Spatial crop classification | 450–980 | [100] |
| Cabbage | Biomass estimation | 470–950 | [101] |
| Chinese cabbage | Yield estimation | 403–995 | [102] |
| Cabbage | Physiology, morphology, composition | 400–1000 | [103] |
| Chinese cabbage | Variety recognition (seeds) | 874–1734 | [104] |
| Abiotic stress | |||
| Cabbage | Nitrogen level/content | 400–1090 | [105,106,107] |
| Kimchi cabbage | Drought tolerance | 680–700 | [108] |
| Kimchi cabbage | Salinity level | 874–1734 | [109,110] |
| Kimchi cabbage | Chilling injury | 874–1734 | [111] |
| Napa cabbage | Wastewater treatment | 400–1000 | [112] |
| Biotic stress | |||
| Kimchi cabbage | Alternaria dark spot | 400–1000 | [93] |
| Kimchi cabbage | Downy mildew disease | 400–1000 | [43] |
| Green cabbage/ Chinese cabbage | Aphid infestation | 450–1000/ 380–1030 | [113,114] |
| Napa cabbage | Soft rot disease | 900–1700 | [66] |
| Cabbage | Field pest identification | n/a | [115] |
| Cabbage | Disease clubroot | 400–1000 | [32] |
| Cabbage | Pieris rapae larvae | 1000–1600 | [116] |
| Cabbage | Cabbage seedling vs weed identification | 1000–2500 | [117,118] |
| Wavelength | Control | Grey Mold | Alternaria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (nm) | Avr | STD | Avr | STD | P(0.05) | Cohen’s d | Avr | STD | P(0.05) | Cohen’s d | |||
| Flavonoids | 400–450 | 159.45 | 24.21 | 379.98 | 55.37 | 1.89 × 10−5 | *** | 5.16 | 523.12 | 302.16 | 1.39 × 10−2 | * | 1.70 |
| Chlorophyll | 430–450 | 1.78 | 0.27 | 6.70 | 1.25 | 1.24 × 10−5 | *** | 5.47 | 7.06 | 2.47 | 7.21 × 10−4 | *** | 3.01 |
| Carotenoids | 470–520 | 2.92 | 0.28 | 5.28 | 0.68 | 4.70 × 10−5 | *** | 4.54 | 5.57 | 1.05 | 2.96 × 10−4 | *** | 3.46 |
| Xanthophylls | 520–600 | 6.57 | 0.58 | 10.27 | 1.62 | 6.75 × 10−4 | *** | 3.04 | 10.57 | 2.17 | 7.24 × 10−7 | *** | 2.51 |
| Anthocyanins | 550–560 | 0.89 | 0.09 | 1.36 | 0.16 | 1.14 × 10−8 | *** | 3.61 | 1.45 | 0.29 | 1.53 × 10−3 | ** | 2.65 |
| Chlorophyll a | 680–700 | 1.68 | 0.26 | 2.60 | 0.67 | 2.35 × 10−4 | *** | 1.82 | 2.780 | 0.41 | 4.36 × 10−4 | *** | 3.26 |
| Anthocyanins | 700–710 | 1.03 | 0.06 | 1.78 | 0.47 | 3.44 × 10−1 | 2.23 | 1.89 | 0.19 | 5.65 × 10−6 | *** | 6.09 | |
| Anthocyanins | 780–790 | 1.90 | 0.12 | 3.16 | 0.83 | 5.62 × 10−2 | 2.11 | 3.52 | 0.84 | 1.36 × 10−3 | ** | 2.70 | |
| Resveratrol | 700–750 | 8.98 | 4.66 | 11.85 | 2.86 | 1.37 × 10−1 | 0.74 | 12.54 | 1.43 | 0.07 | 1.03 | ||
| Resveratrol | 718–722 | 0.71 | 0.06 | 1.21 | 0.29 | 2.29 × 10−3 | ** | 2.46 | 1.27 | 0.12 | 7.68 × 10−6 | *** | 5.84 |
| Water | 800–900 | 19.60 | 1.11 | 30.28 | 7.41 | 6.44 × 10−3 | ** | 2.01 | 36.28 | 10.07 | 3.10 × 10−3 | ** | 2.33 |
| Polyphenols/lignins | 900–1000 | 54.91 | 8.94 | 53.49 | 21.56 | 4.47 × 10−1 | −0.09 | 40.75 | 7.07 | 1.20 × 10−2 | * | −1.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szechyńska-Hebda, M.; Hołownicki, R.; Doruchowski, G.; Sas, K.; Puławska, J.; Jarecka-Boncela, A.; Ptaszek, M.; Włodarek, A. Application of Hyperspectral Imaging for Early Detection of Pathogen-Induced Stress in Cabbage as Case Study. Agronomy 2025, 15, 1516. https://doi.org/10.3390/agronomy15071516
Szechyńska-Hebda M, Hołownicki R, Doruchowski G, Sas K, Puławska J, Jarecka-Boncela A, Ptaszek M, Włodarek A. Application of Hyperspectral Imaging for Early Detection of Pathogen-Induced Stress in Cabbage as Case Study. Agronomy. 2025; 15(7):1516. https://doi.org/10.3390/agronomy15071516
Chicago/Turabian StyleSzechyńska-Hebda, Magdalena, Ryszard Hołownicki, Grzegorz Doruchowski, Konrad Sas, Joanna Puławska, Anna Jarecka-Boncela, Magdalena Ptaszek, and Agnieszka Włodarek. 2025. "Application of Hyperspectral Imaging for Early Detection of Pathogen-Induced Stress in Cabbage as Case Study" Agronomy 15, no. 7: 1516. https://doi.org/10.3390/agronomy15071516
APA StyleSzechyńska-Hebda, M., Hołownicki, R., Doruchowski, G., Sas, K., Puławska, J., Jarecka-Boncela, A., Ptaszek, M., & Włodarek, A. (2025). Application of Hyperspectral Imaging for Early Detection of Pathogen-Induced Stress in Cabbage as Case Study. Agronomy, 15(7), 1516. https://doi.org/10.3390/agronomy15071516






