Linking Almond Yield and Quality to the Production System and Irrigation Strategy Considering the Plantation Age in a Mediterranean Semiarid Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of Experimental Plots, Irrigation Treatments, and Production Systems
2.2. Yield Response and Vegetative Growth for the Different Production Systems and Irrigation Treatments
2.3. Analysis of Kernel Physical Parameters: Weight, Size, Instrumental Color, and Texture
2.4. Kernel Chemical Composition
2.4.1. Antioxidant Activity and Total Phenolic Content
2.4.2. Organic Acids and Sugars
2.4.3. Fatty Acids
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Climatic Conditions, Irrigation Doses Applied, Tree Growth and Yield
3.2. Effects of Production System and Irrigation on Kernel Physical Parameters, Antioxidant Activity, and Total Phenolic Content
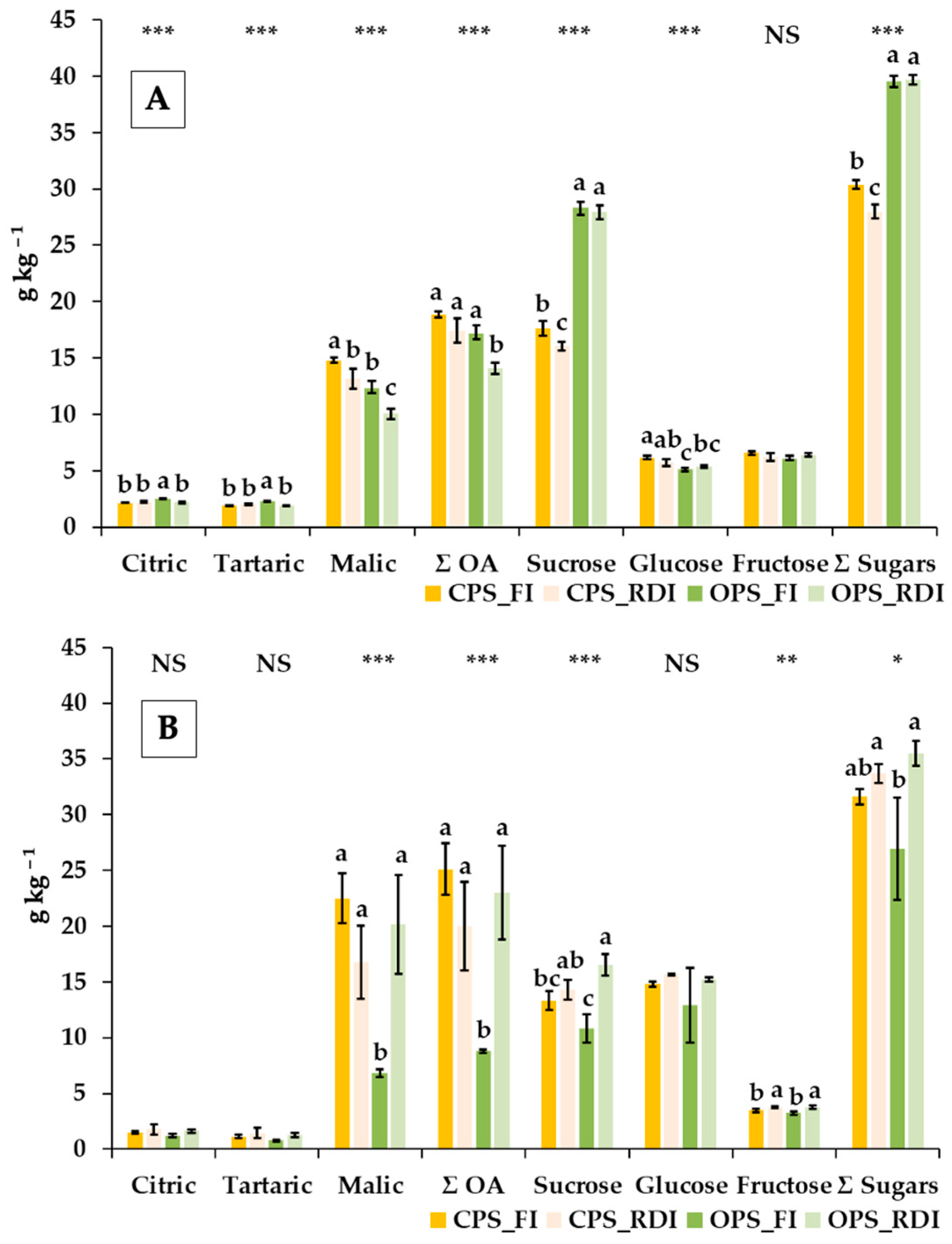
3.3. Effects of Production System and Irrigation Treatments on the Organic Acid and Sugar Contents
3.4. Effects of Production System and Irrigation Treatments on Fatty Acids Profile
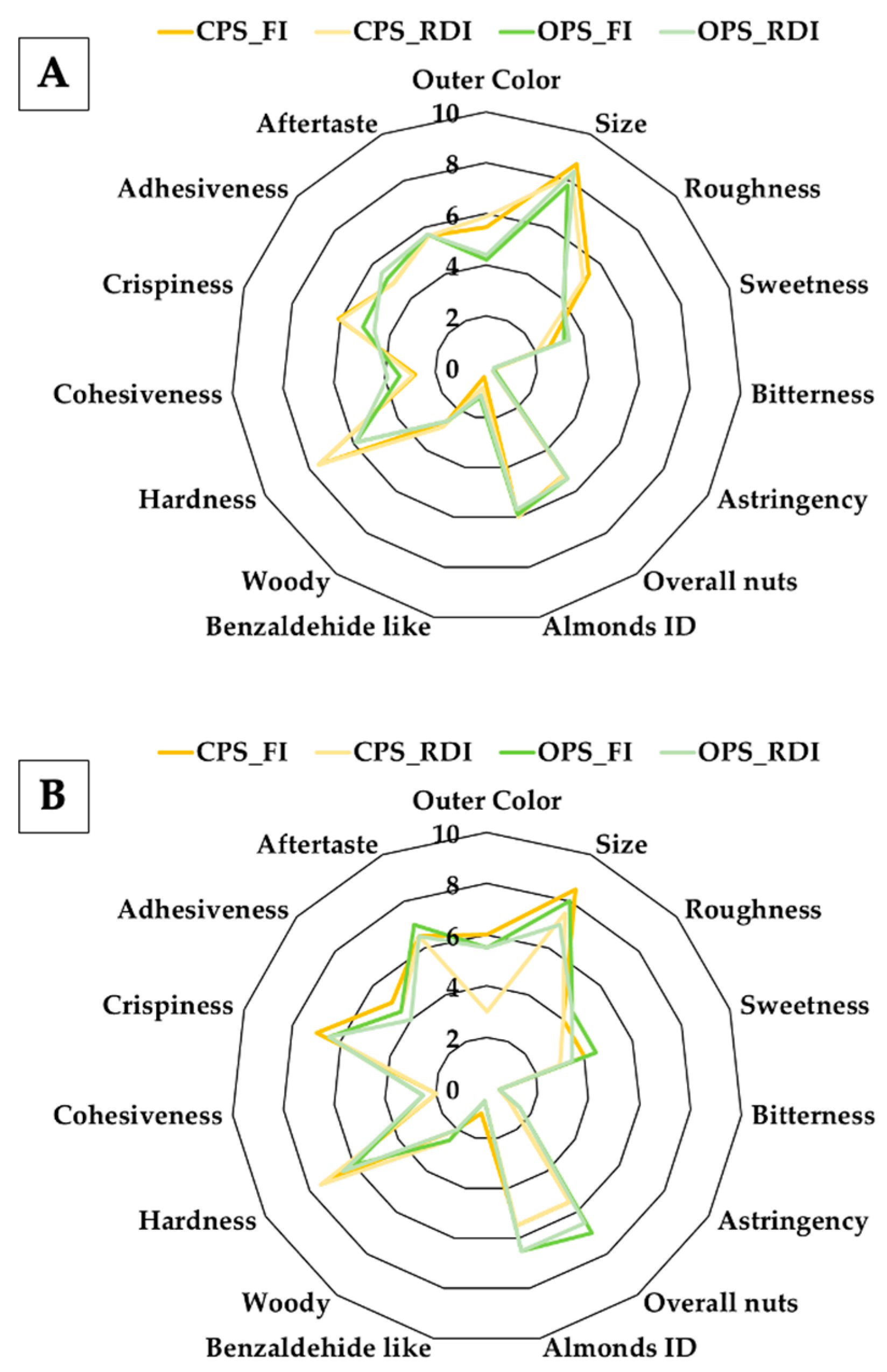
3.5. Descriptive Sensorial Analysis
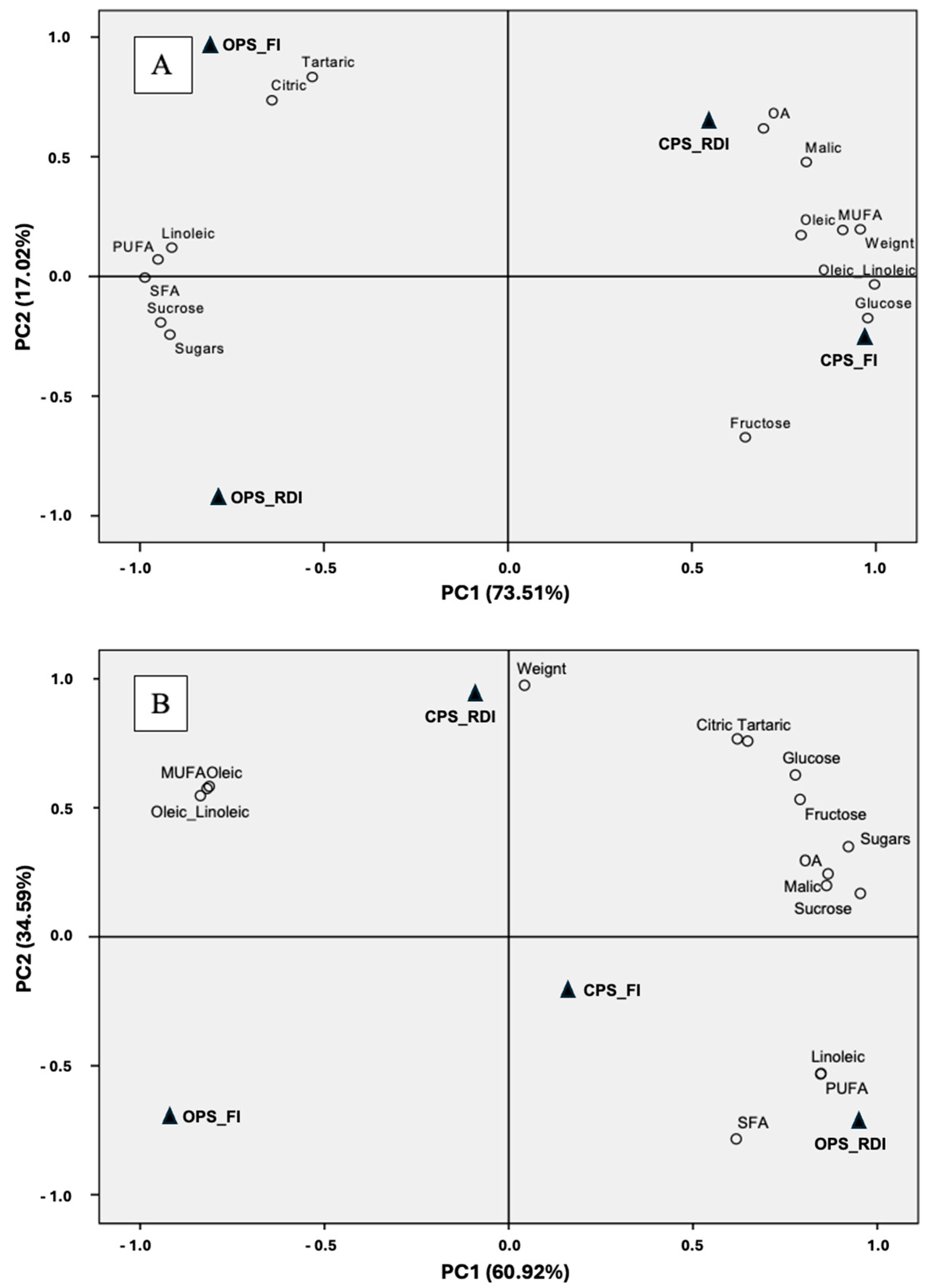
3.6. Disentangling the Effect of Production System and Irrigation Strategy on Almond Quality, Depending on the Tree Age
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organisation of the United Nations. 2025. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 14 April 2025).
- ESYRCE. Encuesta Sobre Superficies y Rendimientos de Cultivos. 2024. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/informe_esyrce_tendencias_rendimiento_agosto_2024_tcm30-692846.pdf (accessed on 1 May 2025).
- Arquero, O. Manual del Almendro; Consejería de Agricultura, Pesca y Desarrollo Rural: Sevilla, Spain, 2013. [Google Scholar]
- Goldhamer, D.A.; Fereres, E. Establishing an almond water production function for California using long-term yield response to variable irrigation. Irrig. Sci. 2016, 35, 169–179. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Gutiérrez-Gordillo, S.; Souza, L.; Cuadros-Tavira, S.; Durán-Zuazo, V.H. Fostering sustainable water use in almond (Prunus dulcis Mill.) orchards in a semiarid Mediterranean environment. Arch. Agron. Soil. Sci. 2019, 65, 164–181. [Google Scholar] [CrossRef]
- Rubio-Asensio, J.S.; Abbatantuono, F.; Ramírez-Cuesta, J.M.; Hortelano, D.; Ruíz, J.L.; Parra, M.; Martínez-Meroño, R.M.; Intrigliolo, D.S.; Buesa, I. Effects of cover crops and drip fertirrigation in a young almond agroecosystem. Agronomy 2022, 12, 2606. [Google Scholar] [CrossRef]
- Durán-Zuazo, V.H.; Cárceles, B.; Gutiérrez, S.; Benítez, M.; Sacristan, P.; Parra, J.; García-Tejero, I.F. Rethinking irrigated almond and pistachio intensification: A shift towards a more sustainable water management paradigm. Rev. Cienc. Agrar. 2021, 43, 24–49. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Santos, R.; Saavedra, M.J.; Aires, A.; Pascual-Seva, N.; Barros, A. Irrigation deficit turns almond by-products into a valuable source of antimicrobial (poly)phenols. Ind. Crops Prod. 2019, 132, 186–196. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Gonzalez-Dugo, V.; García-Tejero, I.F.; López-Urrea, R.; Intrigliolo, D.S.; Egea, G. Quantitative analysis of almond yield response to irrigation regimes in Mediterranean Spain. Agric. Water Manag. 2023, 279, 108208. [Google Scholar] [CrossRef]
- Alcon, F.; Egea, G.; Nortes, P. Financial feasibility of implementing regulated and sustained deficit irrigation in almond orchards. Irrig. Sci. 2012, 31, 931–941. [Google Scholar] [CrossRef]
- González-Gómez, L.; Intrigliolo, D.; Rubio-Asensio, J.S.; Buesa, I.; Ramírez-Cuesta, J. Assessing almond response to irrigation and soil management practices using vegetation indexes time-series and plant water status measurements. Agric. Ecosys. Environ. 2022, 339, 108124. [Google Scholar] [CrossRef]
- MAPA. Análisis de Caracterización de la Producción Ecológica en España. Producción Ecológica. Estadísticas 2024; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2024; Available online: https://www.mapa.gob.es/dam/mapa/contenido/alimentacion/temas/produccion-ecologica/1-estrategias-y-estudios/estudios-de-caracterizacion-de-la-prod.-ecolog./analisisdelacaracterizaciondelaproduccionecologicaenespana2023def.pdf (accessed on 28 February 2025).
- Aznar, S.J.A.; Belmonte, L.; Velasco, M.J. Caracterización del cultivo del almendro en secano en Andalucía y propuestas de reconversión. ITEA—Inf. Tec. Econ. Agrar. 2016, 112, 317–335. [Google Scholar] [CrossRef]
- Cárceles, R.B.; Durán, Z.V.H.; Herencia, G.J.F.; Lipan, L.; Soriano, M.; Hernández, F.; Sendra, E.; Carbonell, B.A.A.; Gálvez, R.B.; García-Tejero, I.F. Soil Management Strategies in Organic Almond Orchards: Implications for Soil Rehabilitation and Nut Quality. Agronomy 2023, 13, 749. [Google Scholar] [CrossRef]
- Iglesias, I.; Foles, P.; Oliveira, C. El almendro en España y Portugal: Situación, innovación tecnológica, costes, rentabilidad y perspectivas. Fruticultura 2021, 81, 6–49. [Google Scholar]
- de-Magistris, T.; Gracia, A. Consumers’ willingness-to-pay for sustainable food products: The case of organically and locally grown almonds in Spain. J. Clean. Prod. 2016, 118, 97–104. [Google Scholar] [CrossRef]
- Egea, G.; González-Real, M.M.; Baille, A.; Nortes, P.A.; Domingo, R. The effects of contrasted deficit irrigation strategies on the fruit growth and kernel quality of mature almond trees. Agric. Water Manag. 2009, 96, 1605–1614. [Google Scholar] [CrossRef]
- Lipan, L.; Martín-Palomo, M.J.; Sánchez-Rodríguez, L.; Cano-Lamadrid, M.; Sendra, E.; Hernández, F.; Burló, F.; Vázquez-Araujo, L.; Andreu, L.; Carbonell-Barrachina, Á.A. Almond fruit quality can be improved by means of deficit irrigation strategies. Agric. Water Manag. 2019, 217, 236–242. [Google Scholar] [CrossRef]
- Lipan, L.; Moriana, A.; López Lluch, D.B.; Cano-Lamadrid, M.; Sendra, E.; Hernández, F.; Carbonell-Barrachina, Á.A. Nutrition quality parameters of almonds as affected by deficit irrigation strategies. Molecules 2019, 24, 2646. [Google Scholar] [CrossRef]
- USDA. Soil Taxonomy. A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; USDA: Washington, DC, USA, 1999. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-06/Soil%20Taxonomy.pdf (accessed on 6 June 2024).
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration. FAO Irrigation and Drainage Paper (56); FAO: Rome, Italy, 1998. [Google Scholar]
- García-Tejero, I.F.; Hernández, A.; Rodríguez, V.; Ponce, J.; Ramos, V.; Muriel, J.; Durán-Zuazo, V. Estimating almond crop coefficients and physiological response to water stress in semiarid environments (SW Spain). J. Agric. Sci. Technol. 2015, 17, 1255–1266. [Google Scholar]
- OJEU. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018. Off. J. Eur. Union L 2018, 150, 1–92. [Google Scholar]
- BOJA. ORDEN de 20 de marzo de 2012, Reglamento específico de producción integrada de almendro. Boletín Oficial de la Junta de Andalucía, 29 March 2012. [Google Scholar]
- Cano-Lamadrid, M.; Hernández, F.; Corell, M.; Burló, F.; Legua, P.; Moriana, A.; Carbonell-Barrachina, Á.A. Antioxidant capacity, fatty acids profile, and descriptive sensory analysis of table olives as affected by deficit irrigation. J. Sci. Food Agric. 2016, 97, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tuberoso, C.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in comercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Klonsky, K.; Tourte, L. Organic Agricultural Production in the United States: Debates and Directions. Am. J. Agric. Econ. 1998, 80, 1119–1124. [Google Scholar] [CrossRef]
- Massantini, R.; Frangipane, M.T. Progress in Almond Quality and Sensory Assessment: An Overview. Agriculture 2022, 12, 710. [Google Scholar] [CrossRef]
- Reganold, J.; Wachter, J. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Viveros, M. Effects of preharvest irrigation cutoff durations and postharvest water deprivation on almond tree performance. Irrig. Sci. 2000, 19, 125–131. [Google Scholar] [CrossRef]
- López-López, M.; Espadafor, M.; Testi, L.; Lorite, I.J.; Orgaz, F.; Fereres, E. Water use of irrigated almond trees when subjected to water deficits. Agric. Water Manag. 2018, 195, 84–95. [Google Scholar] [CrossRef]
- Karat, F.E. Organic vs conventional almond: Market quality, fattuy acid composition and volatile aroma. Appl. Ecol. Environ. Res. 2019, 17, 7783–7793. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.Y.O. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chem. 2010, 122, 819–825. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- García-Martínez, M.D.; Esteve Ciudad, P.; Gómez Tenorio, M.Á.; Raigón Jiménez, M.D. Effect of Organic Farming Techniques on the Quality of Almond Fat. Horticulturae 2025, 11, 135. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.; Sathe, S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, G.S.; Durán-Zuazo, V.H.; Hernández-Santana, V.; Ferrera-Gil, F.; García-Escalera, A.; Amores-Agüera, J.J.; García-Tejero, I.F. Cultivar dependent impact on yield and its components of young almond trees under sustained-deficit irrigation in semi-arid environments. Agronomy 2020, 10, 733. [Google Scholar] [CrossRef]
- Lipan, L.; Cano-Lamadrid, M.; Hernández, F.; Sendra, E.; Corell, M.; Vázquez-Araújo, L.; Moriana, A.; Carbonell-Barrachina, Á.A. Long-Term Correlation between Water Deficit and Quality Markers in HydroSOStainable Almonds. Agronomy 2020, 10, 1470. [Google Scholar] [CrossRef]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañan, G.; Juan, T.; R. Socias i Company. Genetic diversity in an almond germplasm collection: Application of a chemometric approach. Acta Hortic. 2013, 976, 237–242. [Google Scholar] [CrossRef]
- Barreira, J.; Casal, S.; Ferreira, I.; Peres, A.; Pereira, J.; Oliveira, M. Supervised chemical pattern recognition in almond (Prunus dulcis) portuguese PDO cultivars: PCA-and LDA-based triennial study. J. Agric. Food Chem. 2012, 60, 9697–9704. [Google Scholar] [CrossRef]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lapsley, K.; Blumberg, J. A nutrition and health perspective on almonds. J. Sci. Food Agric. 2006, 86, 2245–2250. [Google Scholar] [CrossRef]
- Kalra, A.; Goel, S.; Elias, A.A. Understanding role of roots in plant response to drought: Way forward to climate-resilient crops. Plant Genome 2023, 17, e20395. [Google Scholar] [CrossRef] [PubMed]
| CPS | OPS | |
|---|---|---|
| pH | 8.14 | 8.07 |
| EC (dS m−1) | 0.41 | 0.32 |
| CaCO3 (g kg−1) | 210 | 215 |
| Organic matter (%) | 0.9 | 1.14 |
| N total (g kg−1) | 0.67 | 0.87 |
| Polsen (mg kg−1) | 25.72 | 17.86 |
| K (mg kg−1) | 289.74 | 280.91 |
| B (mg kg−1) | 0.82 | 0.91 |
| Fe (mg kg−1) | 3.08 | 2.06 |
| Zn (mg kg−1) | 0.57 | 0.76 |
| 2019 | 2023 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage I | Stage II | Stages III | Stage IV | Total I–IV | Stage I | Stage II | Stages III | Stage IV | Total I–IV | |
| (mm) | ||||||||||
| Rain | 102 | 180 | 0 | 0 | 282 | 21.2 | 112 | 10 | 75 | 219 |
| ET0 | 89 | 319 | 536 | 136 | 1081 | 92 | 412 | 550 | 141 | 1195 |
| ETC | 0 | 144 | 452 | 54 | 649 | 0 | 271 | 551 | 99 | 921 |
| Treatment | Irrigation doses applied | |||||||||
| FI | 0 | 73 | 413 | 18 | 504 | 0 | 184 | 535 | 10 | 729 |
| RDI | 0 | 56 | 169 | 14 | 239 | 0 | 144 | 277 | 8 | 429 |
| 2019 | 2023 | 2019–2023 | ||||
|---|---|---|---|---|---|---|
| TCS | TCS | ΔTCS | ||||
| CPS | OPS | CPS | OPS | CPS | OPS | |
| (cm2) | ||||||
| FI | 147 a | 63 b | 571 a | 247 b | 424 a | 184 b |
| RDI | 117 a | 50 b | 473 a | 195 b | 356 a | 146 b |
| Irrigation | * | * | ** | * | * | * |
| Treatment | Weight | Size | Kernel Color Coordinates | Texture | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | Length | Width | Thickness | L* | a* | b* | C | Hue | Hardness (N) | Work to Shear | Average Force (N) | Number of Fractures | |
| 2019 | |||||||||||||
| ANOVA test | |||||||||||||
| *** | *** | ** | NS | *** | *** | *** | *** | NS | *** | *** | *** | ** | |
| Tukey’s Multiple Range test | |||||||||||||
| CPS-FI | 1.79 a | 23.71 a | 18.72 a | 9.30 a | 42.92 b | 12.24 b | 24.27 b | 27.23 b | 63.38 a | 82.91 b | 78.04 b | 42.31 c | 14.52 bc |
| CPS-RDI | 1.80 a | 23.52 a | 18.48 ab | 9.35 a | 43.04 b | 12.81 ab | 24.92 b | 28.04 b | 62.81 a | 81.32 b | 79.71 b | 41.04 c | 13.24 c |
| OPS-FI | 1.63 b | 22.03 b | 17.81 b | 9.17 a | 44.58 a | 13.31 a | 27.14 a | 30.23 a | 63.77 a | 114.02 a | 131.24 a | 60.13 a | 19.33 a |
| OPS-RDI | 1.63 b | 22.24 b | 17.84 b | 9.10 a | 44.61 a | 13.28 a | 26.88 a | 30.03 a | 63.72 a | 106.90 a | 103.63 b | 52.31 b | 18.19 ab |
| 2023 | |||||||||||||
| ANOVA test | |||||||||||||
| *** | *** | *** | * | *** | NS | * | NS | *** | NS | NS | NS | NS | |
| Tukey’s Multiple Range test | |||||||||||||
| CPS-FI | 1.39 b | 21.41 ab | 16.32 b | 8.65 a | 41.82 a | 13.64 a | 23.49 ab | 27.21 a | 59.94 a | 84.51 a | 70.53 a | 42.21 a | 7.44 a |
| CPS-RDI | 1.50 a | 21.93 a | 17.14 a | 8.75 a | 42.11 a | 13.62 a | 23.62 a | 27.20 a | 60.02 a | 89.32 a | 73.10 a | 42.47 a | 9.67 a |
| OPS-FI | 1.29 b | 20.92 bc | 16.22 b | 8.24 b | 40.94 b | 13.71 a | 23.26 ab | 26.93 a | 59.51 a | 83.44 a | 66.71 a | 39.52 a | 8.49 a |
| OPS-RDI | 1.30 b | 20.63c | 16.13 b | 8.46 ab | 40.23 b | 13.83 a | 22.53 b | 26.42 a | 58.42 b | 85.09 a | 69.53 a | 39.10 a | 8.59 a |
| ABTS | FRAP | TPC | |
|---|---|---|---|
| (mmol Trolox kg−1) | (g GAE kg−1) | ||
| 2019 | |||
| ANOVA | |||
| *** | *** | *** | |
| Tukey Multiple Range Test | |||
| CPS-FI | 1.95 b | 1.48 d | 0.55 c |
| CPS-RDI | 1.91 b | 2.06 c | 0.67 bc |
| OPS-FI | 2.26 a | 2.96 b | 0.80 b |
| OPS-RDI | 2.96 a | 4.24 a | 1.08 a |
| 2023 | |||
| ANOVA | |||
| * | NS | NS | |
| Tukey Multiple Range Test | |||
| CPS-FI | 0.68 ab | 1.02 a | 0.35 a |
| CPS-RDI | 0.58 b | 0.97 a | 0.29 a |
| OPS-FI | 0.89 a | 1.31 a | 0.34 a |
| OPS-RDI | 0.61 b | 1.08 a | 0.24 a |
| Compounds | ANOVA | CPS_FI | CPS_RDI | OPS_FI | OPS_RDI |
|---|---|---|---|---|---|
| (%) | |||||
| C12:0 (Lauric) | NS | 0.010 | 0.010 | 0.010 | 0.010 |
| C14:0 (Myristic) | NS | 0.120 | 0.130 | 0.130 | 0.130 |
| C14:1 (Myristoleic) | NS | 0.050 | 0.060 | 0.050 | 0.050 |
| C15:0 (Pentadecylic) | ** | 0.021 a | 0.017 bc | 0.015 c | 0.019 ab |
| C15:1 (Pentadecenoic) | NS | 0.030 | 0.030 | 0.020 | 0.030 |
| C16:0 (Palmitic) | NS | 10.750 | 11.130 | 11.040 | 11.080 |
| C16:1c7 | * | 0.086 ab | 0.091 a | 0.083 ab | 0.075 b |
| C16:1c9 (Palmitoleic) | * | 1.81 b | 1.94 ab | 2.05 a | 1.970 ab |
| C16:1c10 | ** | 0.098 b | 0.104 ab | 0.114 a | 0.115 a |
| C17:0 (Margaric acid) | NS | 0.200 | 0.220 | 0.220 | 0.190 |
| C17:1c10 (cis-Heptadecenoic) | NS | 0.350 | 0.370 | 0.350 | 0.340 |
| C18:0 (Stearic) | ** | 3.650 b | 3.870 b | 4.190 a | 4.080 a |
| C18:1t9 (Elaidic) | NS | 0.110 | 0.120 | 0.100 | 0.080 |
| C18:1c9n9 (Oleic) | NS | 60.000 | 60.900 | 58.900 | 59.200 |
| C18:1n7 (cis-Vaccenic) | NS | 5.620 | 5.570 | 5.990 | 5.650 |
| C18:2n6 cis 9,12 (Linoleic) | * | 19.300 b | 20.100 ab | 20.500 a | 20.400 a |
| C20:0 (Arachidic) | NS | 0.170 | 0.150 | 0.170 | 0.180 |
| C20:1c11 (Eicosenoic) | NS | 0.170 | 0.170 | 0.180 | 0.140 |
| C18:3n3c9,12,15 (α-Linolenic) | NS | 0.110 | 0.110 | 0.100 | 0.090 |
| C21:0 (Heneicosylic) | * | 0.021 ab | 0.024a | 0.022 ab | 0.020 b |
| C20:2n6c11,14 (Eicosadienoic) | *** | 0.010 b | 0.014a | 0.013 a | 0.0130 a |
| C22:0 (Behenic) | *** | 0.050 c | 0.070b | 0.090 a | 0.060 c |
| C24:1c15 (Nervonic) | NS | 0.240 | 0.260 | 0.290 | 0.270 |
| C22:6n3 (Docosahexaenoic DHA) | NS | 0.240 | 0.260 | 0.290 | 0.270 |
| Oleic:Linoleic | NS | 3.110 | 3.030 | 2.880 | 2.910 |
| Saturated Fatty Acids (SFA) | NS | 14.620 | 14.850 | 15.160 | 15.130 |
| Monounsaturated Fatty Acids (MUFA) | NS | 66.310 | 66.750 | 64.920 | 65.040 |
| Polyunsaturated Fatty Acids (PUFA) | * | 18.680 b | 19.390 ab | 19.920 a | 19.830 ab |
| PUFA:SFA | NS | 1.310 | 1.310 | 1.310 | 1.310 |
| PUFA:MUFA | NS | 0.290 | 0.300 | 0.310 | 0.310 |
| (MUFA+PUFA)/SFA | ** | 5.850 a | 5.740 ab | 5.580 b | 5.600 b |
| Atherogenic index | NS | 0.130 | 0.130 | 0.130 | 0.130 |
| Thrombogenic index | ** | 0.320 b | 0.330 ab | 0.340 a | 0.340 a |
| Compounds | ANOVA | CPS_FI | CPS_RDI | OPS_FI | OPS_RDI |
|---|---|---|---|---|---|
| (%) | |||||
| C12:0 (Lauric) | NS | 0.000 | 0.000 | 0.000 | 0.000 |
| C14:0 (Myristic) | NS | 0.030 | 0.030 | 0.020 | 0.030 |
| C14:1 (Myristoleic) | NS | 0.000 | 0.000 | 0.000 | 0.000 |
| C15:0 (Pentadecylic) | NS | 0.000 | 0.010 | 0.010 | 0.010 |
| C15:1 (Pentadecenoic) | NS | 0.000 | 0.000 | 0.000 | 0.000 |
| C16:0 (Palmitic) | NS | 6.450 | 6.370 | 6.410 | 6.520 |
| C16:1c7 | NS | 0.020 | 0.020 | 0.02 | 0.020 |
| C16:1c9 (Palmitoleic) | * | 0.513 ab | 0.532 a | 0.498 b | 0.524 ab |
| C16:1c10 | NS | 0.010 | 0.010 | 0.010 | 0.020 |
| C17:0 (Margaric acid) | NS | 0.050 | 0.040 | 0.050 | 0.050 |
| C17:1c10 (cis-Heptadecenoic) | NS | 0.090 | 0.090 | 0.090 | 0.090 |
| C18:0 (Stearic) | ** | 1.408 b | 1.308 b | 1.453 ab | 1.626 a |
| C18:1t9 (Elaidic) | NS | 0.000 | 0.000 | 0.000 | 0.000 |
| C18:1c9n9 (Oleic) | NS | 72.070 | 73.320 | 73.020 | 70.940 |
| C18:1n7 (cis-Vaccenic) | NS | 0.030 | 0.030 | 0.030 | 0.030 |
| C18:2n6 cis 9,12 (Linoleic) | NS | 19.000 | 18.080 | 18.210 | 19.970 |
| C20:0 (Arachidic) | NS | 0.060 | 0.060 | 0.060 | 0.070 |
| C20:1c11 (Eicosenoic) | NS | 0.000 | 0.000 | 0.000 | 0.000 |
| C18:3n3c9,12,15 (α-Linolenic) | NS | 0.080 | 0.070 | 0.080 | 0.080 |
| C21:0 (Heneicosylic) | NS | 0.000 | 0.000 | 0.000 | 0.000 |
| C20:2n6c11,14 (Eicosadienoic) | * | 0.001 ab | 0.000 b | 0.001 ab | 0.001 a |
| C22:0 (Behenic) | NS | 0.010 | 0.010 | 0.020 | 0.020 |
| C24:1c15 (Nervonic) | NS | 0.020 | 0.010 | 0.010 | 0.010 |
| C22:6n3 (Docosahexaenoic DHA) | NS | 0.000 | 0.000 | 0.000 | 0.000 |
| Oleic:Linoleic | NS | 3.810 | 4.060 | 4.020 | 3.560 |
| Saturated Fatty Acids (SFA) | NS | 8.150 | 7.840 | 8.030 | 8.310 |
| Monounsaturated Fatty Acids (MUFA) | NS | 72.750 | 74.010 | 73.680 | 71.640 |
| Polyunsaturated Fatty Acids (PUFA) | * | 19.090 b | 18.150 c | 18.280 c | 20.050 a |
| PUFA:SFA | NS | 2.340 | 2.320 | 2.280 | 2.410 |
| PUFA:MUFA | NS | 0.260 | 0.250 | 0.250 | 0.280 |
| (MUFA+PUFA)/SFA | NS | 11.290 | 11.760 | 11.450 | 11.040 |
| Atherogenic index | NS | 0.070 | 0.070 | 0.070 | 0.070 |
| Thrombogenic index | * | 0.171 ab | 0.167 b | 0.171 ab | 0.177 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Pavón, A.; García-Tejero, I.F.; Noguera-Artiaga, L.; Lipan, L.; Sendra, E.; Hernández, F.; Herencia-Galán, J.F.; Carbonell-Barrachina, Á.A.; Zuazo, V.H.D. Linking Almond Yield and Quality to the Production System and Irrigation Strategy Considering the Plantation Age in a Mediterranean Semiarid Environment. Agronomy 2025, 15, 1448. https://doi.org/10.3390/agronomy15061448
Calderón-Pavón A, García-Tejero IF, Noguera-Artiaga L, Lipan L, Sendra E, Hernández F, Herencia-Galán JF, Carbonell-Barrachina ÁA, Zuazo VHD. Linking Almond Yield and Quality to the Production System and Irrigation Strategy Considering the Plantation Age in a Mediterranean Semiarid Environment. Agronomy. 2025; 15(6):1448. https://doi.org/10.3390/agronomy15061448
Chicago/Turabian StyleCalderón-Pavón, Abel, Iván Francisco García-Tejero, Luis Noguera-Artiaga, Leontina Lipan, Esther Sendra, Francisca Hernández, Juan Francisco Herencia-Galán, Ángel Antonio Carbonell-Barrachina, and Víctor Hugo Durán Zuazo. 2025. "Linking Almond Yield and Quality to the Production System and Irrigation Strategy Considering the Plantation Age in a Mediterranean Semiarid Environment" Agronomy 15, no. 6: 1448. https://doi.org/10.3390/agronomy15061448
APA StyleCalderón-Pavón, A., García-Tejero, I. F., Noguera-Artiaga, L., Lipan, L., Sendra, E., Hernández, F., Herencia-Galán, J. F., Carbonell-Barrachina, Á. A., & Zuazo, V. H. D. (2025). Linking Almond Yield and Quality to the Production System and Irrigation Strategy Considering the Plantation Age in a Mediterranean Semiarid Environment. Agronomy, 15(6), 1448. https://doi.org/10.3390/agronomy15061448











