Characterization of Selenium Enrichment in Soybean and Its Relationship with Rhizosphere Microbial Communities in Se-Enriched Saline Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Processing
2.3. Analytical Test Analyses and Methods
2.3.1. Soil and Plant Composition Assays
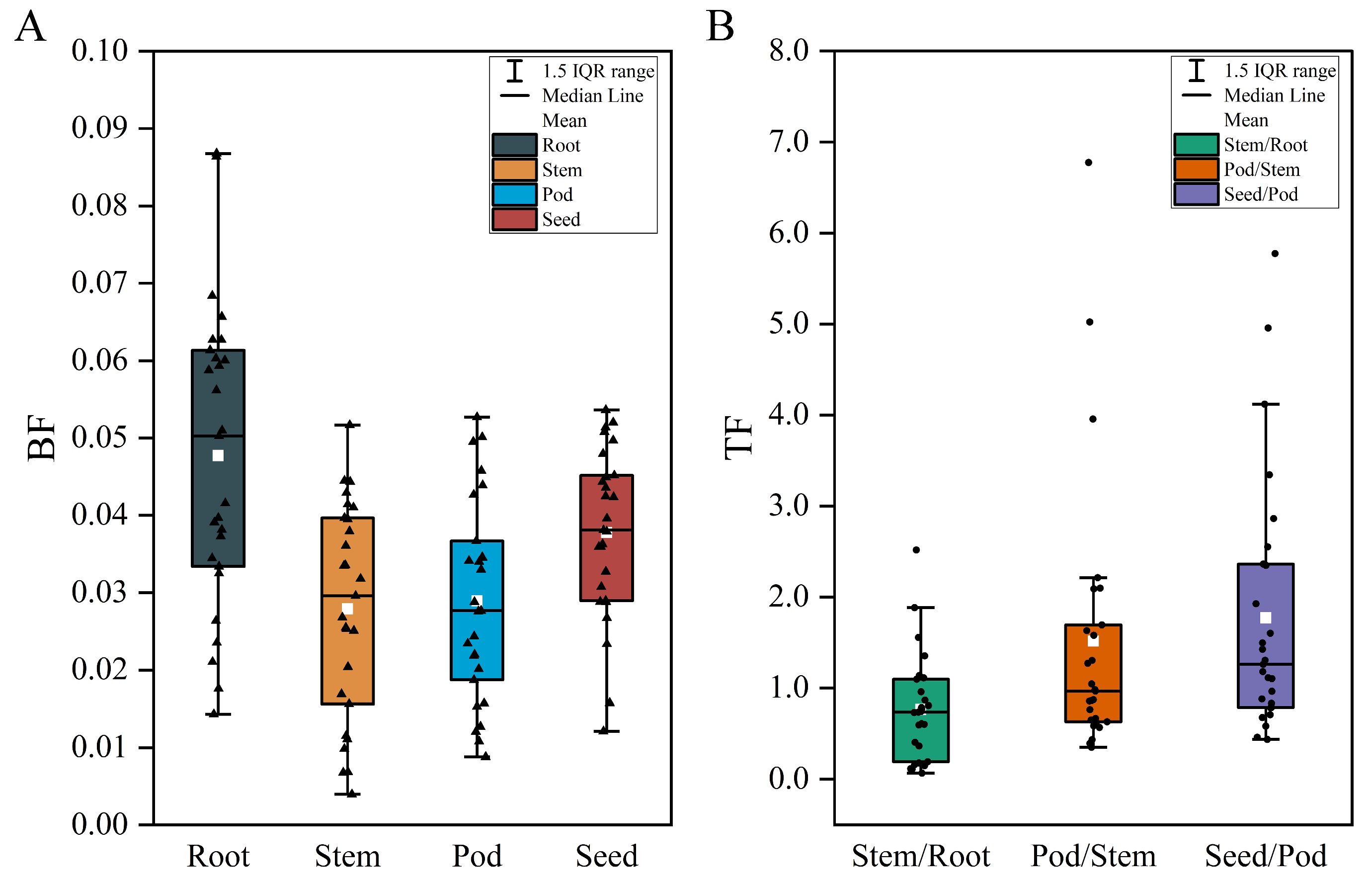
2.3.2. Se Bioconcentration and Translocation Coefficients
2.3.3. DNA Extraction and 16S rRNA Sequencing
2.4. Data Processing and Statistical Analysis
3. Results
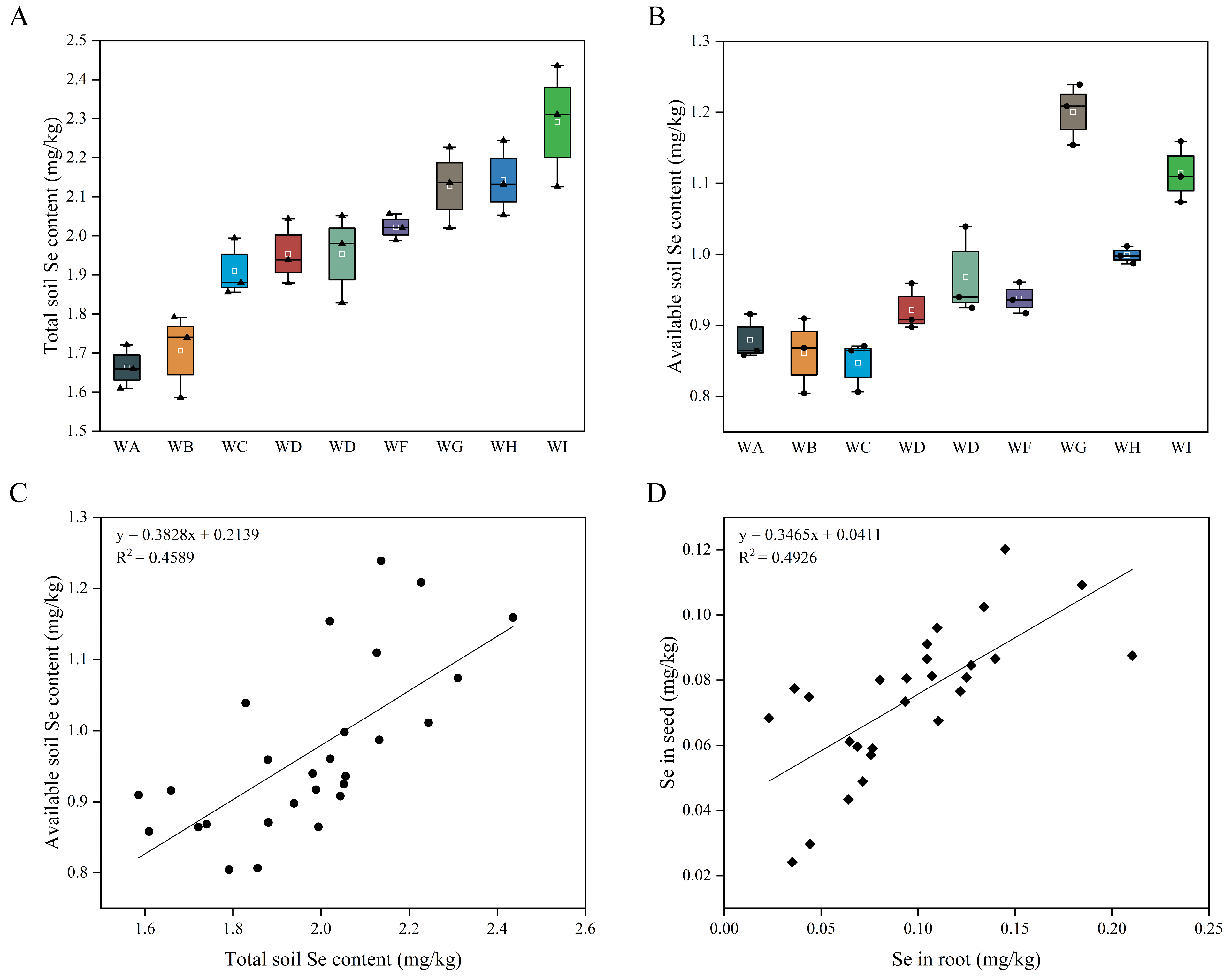
3.1. Se Content in Different Organs of Soybean and Soil Se Content
3.2. Characterization of Se Enrichment and Translocation in Soybean
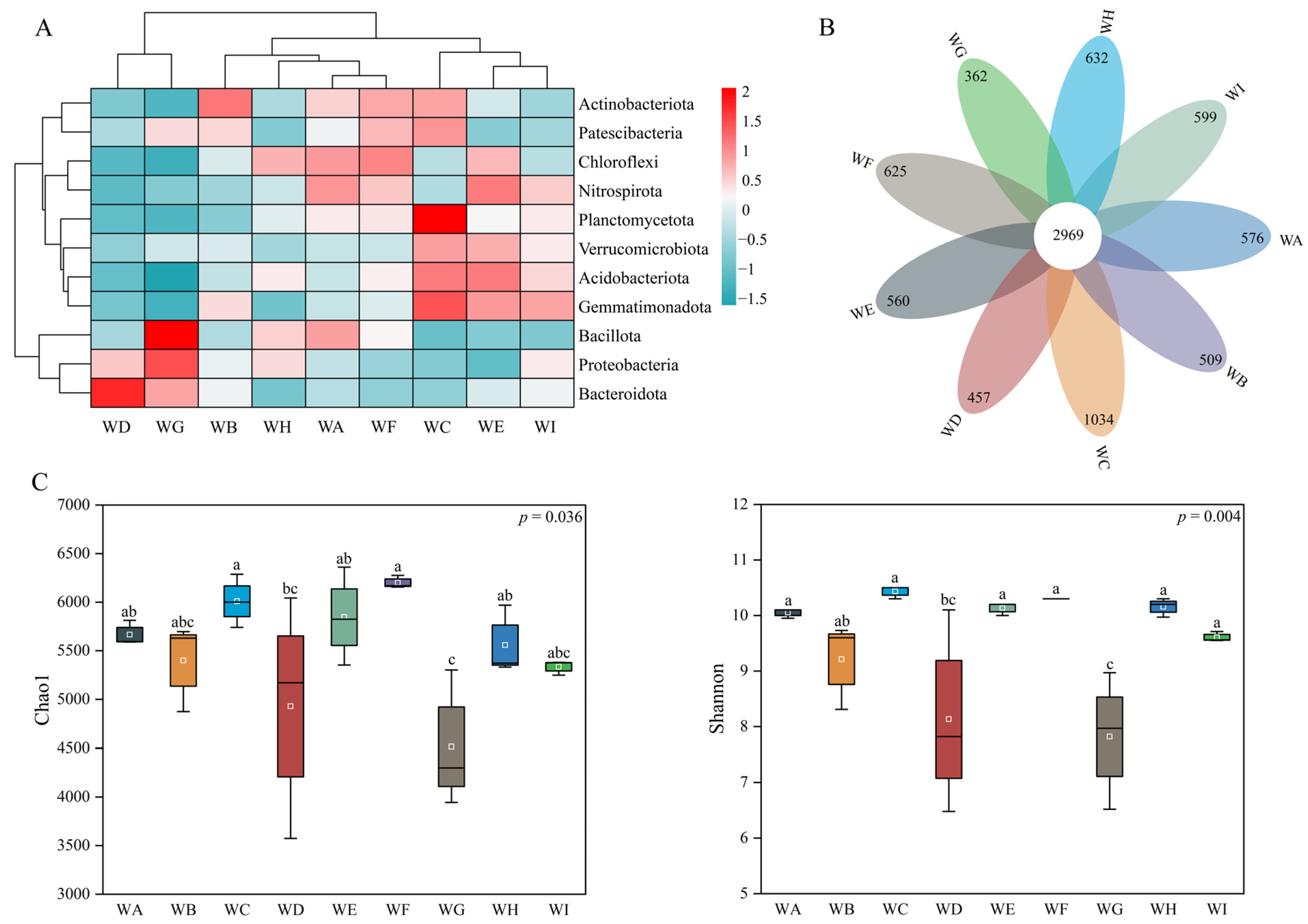
3.3. Bacterial Amplicon Structure Analysis
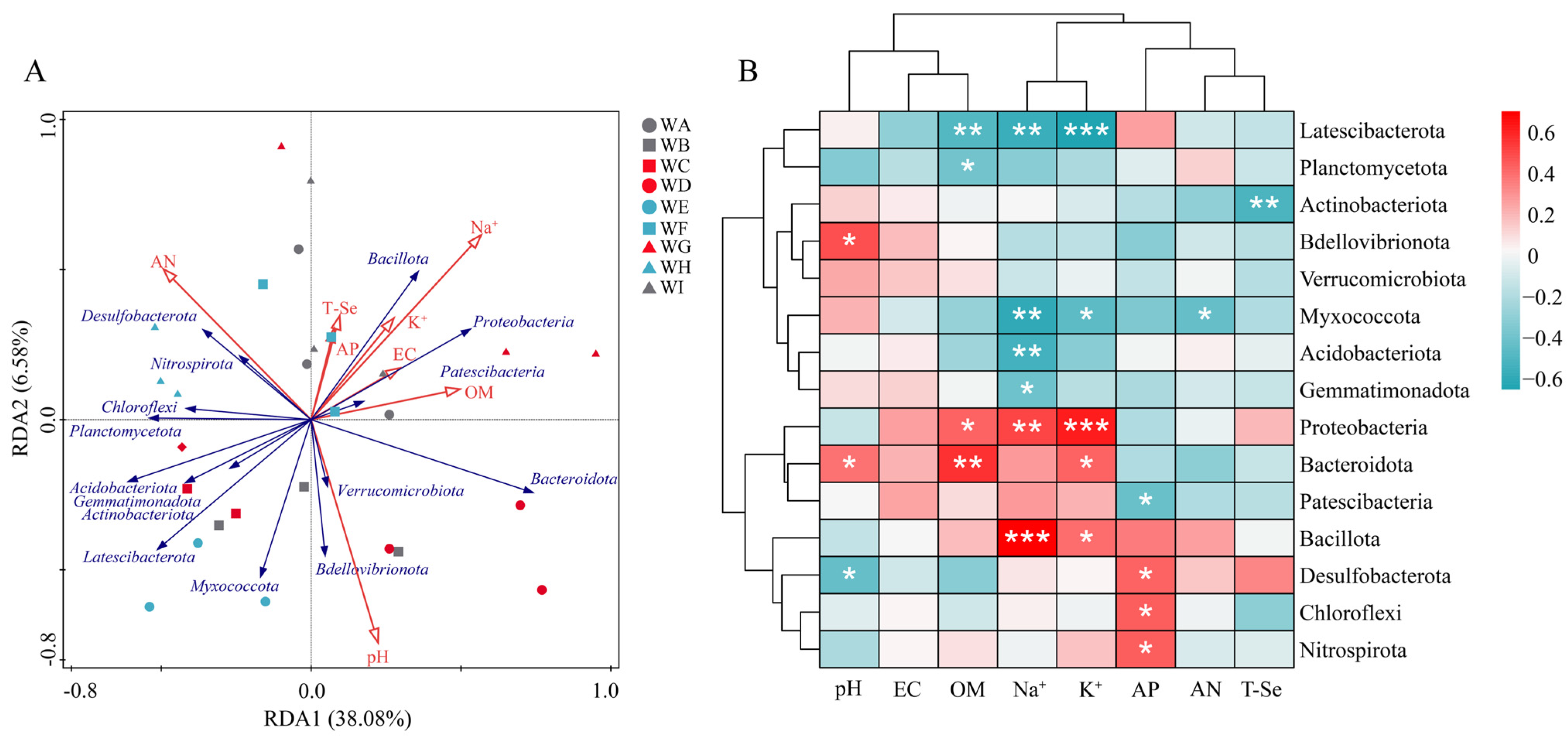
3.4. Differences in Microbial Communities Among Different Rhizosphere Soil Environments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burk, R.F.; Hill, K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Q.; Gao, J.; Lin, Z.; Banuelos, G.S.; Yuan, L.; Yin, X. Daily dietary selenium intake in a high selenium area of Enshi, China. Nutrients 2013, 5, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.H.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef]
- Zhang, L.; Song, H.; Guo, Y.; Fan, B.; Huang, Y.; Mao, X.; Liang, K.; Hu, Z.; Sun, X.; Fang, Y. Benefit–risk assessment of dietary selenium and its associated metals intake in China (2017–2019): Is current selenium-rich agro-food safe enough? J. Hazard. Mater. 2020, 398, 123224. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, Q.; Wang, C.; Tang, Z.; Li, Y. Study on the Migration and Accumulation of Selenium between Soil and Vegetations. Sustainability 2022, 15, 592. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.-D.A.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, G.; Qin, H.; Guan, Y.; Wang, T.; Ni, W.; Xie, H.; Xing, Y.; Tian, G.; Lyu, M. Metabolomics combined with physiology and transcriptomics reveal key metabolic pathway responses in apple plants exposure to different selenium concentrations. J. Hazard. Mater. 2024, 464, 132953. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Qi, M.; Peng, Q.; Wang, M.; Bañuelos, G.S.; Miao, S.; Li, Z.; Dinh, Q.T.; Liang, D. Insights into uptake, accumulation, and subcellular distribution of selenium among eight wheat (Triticum aestivum L.) cultivars supplied with selenite and selenate. Ecotoxicol. Environ. Saf. 2021, 207, 111544. [Google Scholar] [CrossRef]
- Chilimba, A.D.; Young, S.D.; Black, C.R.; Meacham, M.C.; Lammel, J.; Broadley, M.R. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crops Res. 2012, 125, 118–128. [Google Scholar] [CrossRef]
- Kápolna, E.; Hillestrøm, P.R.; Laursen, K.H.; Husted, S.; Larsen, E.H. Effect of foliar application of selenium on its uptake and speciation in carrot. Food Chem. 2009, 115, 1357–1363. [Google Scholar] [CrossRef]
- Fordyce, F.; Guangdi, Z.; Green, K.; Xinping, L. Soil, grain and water chemistry in relation to human selenium-responsive diseases in Enshi District, China. Appl. Geochem. 2000, 15, 117–132. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; De Sousa, G.F.; Corguinha, A.P.B.; de Lima Lessa, J.H.; Dinali, G.S.; Oliveira, C.; Lopes, G.; Amaral, D.; Brown, P.; Guilherme, L.R.G. Selenium biofortification of soybean genotypes in a tropical soil via Se-enriched phosphate fertilizers. Front. Plant Sci. 2022, 13, 988140. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Ahkami, A.H.; White, R.A., III; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Deng, M.; Wang, X.; Wang, Z.; Yang, L.; Liu, L. Allocation and turnover of rhizodeposited carbon in different soil microbial groups. Soil Biol. Biochem. 2020, 150, 107973. [Google Scholar] [CrossRef]
- Spohn, M. Element cycling as driven by stoichiometric homeostasis of soil microorganisms. Basic Appl. Ecol. 2016, 17, 471–478. [Google Scholar] [CrossRef]
- Han, Q.; Ma, Q.; Chen, Y.; Tian, B.; Xu, L.; Bai, Y.; Chen, W.; Li, X. Variation in rhizosphere microbial communities and its association with the symbiotic efficiency of rhizobia in soybean. ISME J. 2020, 14, 1915–1928. [Google Scholar] [CrossRef]
- Meng, T.; Wang, Q.; Abbasi, P.; Ma, Y. Deciphering differences in the chemical and microbial characteristics of healthy and Fusarium wilt-infected watermelon rhizosphere soils. Appl. Microbiol. Biotechnol. 2019, 103, 1497–1509. [Google Scholar] [CrossRef]
- Brockett, B.F.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Jin, H.; Ju, Q.; Yu, Z.; Hao, J.; Gu, H.; Gu, H.; Li, W. Simulation of snowmelt runoff and sensitivity analysis in the Nyang River Basin, southeastern Qinghai-Tibetan Plateau, China. Nat. Hazards 2019, 99, 931–950. [Google Scholar] [CrossRef]
- Stark, S.; Eskelinen, A.; Männistö, M.K. Regulation of microbial community composition and activity by soil nutrient availability, soil pH, and herbivory in the tundra. Ecosystems 2012, 15, 18–33. [Google Scholar] [CrossRef]
- Stefanello, L.; Schwalbert, R.; Schwalbert, R.; Tassinari, A.; Garlet, L.; De Conti, L.; Ciotta, M.; Ceretta, C.; Ciampitti, I.; Brunetto, G. Phosphorus critical levels in soil and grapevine leaves for South Brazil vineyards: A Bayesian approach. Eur. J. Agron. 2023, 144, 126752. [Google Scholar] [CrossRef]
- Silva, M.A.; de Sousa, G.F.; Bañuelos, G.; Amaral, D.; Brown, P.H.; Guilherme, L.R.G. Selenium speciation in se-enriched soybean grains from biofortified plants grown under different methods of selenium application. Foods 2023, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Arvy, M.P. Translocation of selenium in the bean plant (Phaseolus vulgaris) and the field bean (Vicia faba). Physiol. Plant. 1982, 56, 299–302. [Google Scholar] [CrossRef]
- Nouri, H.; Borujeni, S.C.; Nirola, R.; Hassanli, A.; Beecham, S.; Alaghmand, S.; Saint, C.; Mulcahy, D. Application of green remediation on soil salinity treatment: A review on halophytoremediation. Process Saf. Environ. Prot. 2017, 107, 94–107. [Google Scholar] [CrossRef]
- Lei, Z.; Li, Q.; Tang, Y.; Zhang, H.; Han, C.; Wang, X.; Zhao, X.; Shi, G. Selenium enhanced nitrogen accumulation in legumes in soil with rhizobia bacteria. J. Clean. Prod. 2022, 380, 134960. [Google Scholar] [CrossRef]
- Ekanayake, L.J.; Thavarajah, D.; McGee, R.; Thavarajah, P. Will selenium fertilization improve biological nitrogen fixation in lentils? J. Plant Nutr. 2017, 40, 2392–2401. [Google Scholar] [CrossRef]
- Ni, G.; Shi, G.; Hu, C.; Wang, X.; Nie, M.; Cai, M.; Cheng, Q.; Zhao, X. Selenium improved the combined remediation efficiency of Pseudomonas aeruginosa and ryegrass on cadmium-nonylphenol co-contaminated soil. Environ. Pollut. 2021, 287, 117552. [Google Scholar] [CrossRef]
- Liu, K.; Cai, M.; Hu, C.; Sun, X.; Cheng, Q.; Jia, W.; Yang, T.; Nie, M.; Zhao, X. Selenium (Se) reduces Sclerotinia stem rot disease incidence of oilseed rape by increasing plant Se concentration and shifting soil microbial community and functional profiles. Environ. Pollut. 2019, 254, 113051. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Wang, Y.; Du, X.; Wuriyanghan, H.; Wang, X.; Xie, J.; Zhao, X.; Jia, W. Comparison of selenium accumulation in edible parts of wheat and broad bean. Agronomy 2023, 13, 1939. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, H.; Qin, Y.; Zhou, Y.; Zhu, H.; Yao, Q. A synthetic community of siderophore-producing bacteria increases soil selenium bioavailability and plant uptake through regulation of the soil microbiome. Sci. Total Environ. 2023, 871, 162076. [Google Scholar] [CrossRef] [PubMed]
- Keskinen, R.; Räty, M.; Yli-Halla, M. Selenium fractions in selenate-fertilized field soils of Finland. Nutr. Cycl. Agroecosyst. 2011, 91, 17–29. [Google Scholar] [CrossRef]
- Kowalska, I.; Smoleń, S.; Czernicka, M.; Halka, M.; Kęska, K.; Pitala, J. Effect of selenium form and salicylic acid on the accumulation of selenium speciation forms in hydroponically grown lettuce. Agriculture 2020, 10, 584. [Google Scholar] [CrossRef]
- Qin, H.-B.; Zhu, J.-M.; Liang, L.; Wang, M.-S.; Su, H. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ. Int. 2013, 52, 66–74. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R.; Woch, W.; Hasanuzzaman, M. Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol. Biochem. 2018, 127, 446–456. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Dresler, S.; Wójcik, M. Selenium affects physiological parameters and phytochelatins accumulation in cucumber (Cucumis sativus L.) plants grown under cadmium exposure. Sci. Hortic. 2014, 172, 10–18. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Hasanuzzaman, M.; Matraszek-Gawron, R. Mechanisms of selenium-induced enhancement of abiotic stress tolerance in plants. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 269–295. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, J.; Liu, J.; Gao, Z.; Yang, Y.; Zhu, B.; Yao, F.; Ye, Q. Selenium Alleviates Cadmium Toxicity in Pepper (Capsicum annuum L.) by Reducing Accumulation, Enhancing Stress Resistance, and Promoting Growth. Plants 2025, 14, 1291. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yu, Z.; Shen, G.; Cheng, H.; Tao, S. Composition and diversity of soil microbial communities in the alpine wetland and alpine forest ecosystems on the Tibetan Plateau. Sci. Total Environ. 2020, 747, 141358. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Iqbal, A.; He, L.; Khan, A.; Wei, S.; Akhtar, K.; Ali, I.; Ullah, S.; Munsif, F.; Zhao, Q.; Jiang, L. Organic manure coupled with inorganic fertilizer: An approach for the sustainable production of rice by improving soil properties and nitrogen use efficiency. Agronomy 2019, 9, 651. [Google Scholar] [CrossRef]
- Ade, L.; Hu, L.; Zi, H.; Wang, C.; Lerdau, M.; Dong, S. Effect of snowpack on the soil bacteria of alpine meadows in the Qinghai-Tibetan Plateau of China. Catena 2018, 164, 13–22. [Google Scholar] [CrossRef]
- Peng, J.; Ji, W.; Ma, Z.; Li, S.; Chen, S.; Zhou, L.; Shi, Z. Predicting total dissolved salts and soluble ion concentrations in agricultural soils using portable visible near-infrared and mid-infrared spectrometers. Biosyst. Eng. 2016, 152, 94–103. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Duan, B.; Hu, C.; Zhao, X.; Guo, Z. Effect of applied sulphur on the uptake by wheat of selenium applied as selenite. Plant Soil 2015, 386, 35–45. [Google Scholar] [CrossRef]
- Wang, S.; Liang, D.; Wang, D.; Wei, W.; Fu, D.; Lin, Z. Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci. Total Environ. 2012, 427, 159–164. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Liu, F.; Liao, R.; Hu, Y. Accumulation of heavy metals in soil-crop systems: A review for wheat and corn. Environ. Sci. Pollut. Res. 2017, 24, 15209–15225. [Google Scholar] [CrossRef]
- Sasmaz, M.; Akgül, B.; Sasmaz, A. Distribution and accumulation of selenium in wild plants growing naturally in the Gumuskoy (Kutahya) Mining Area, Turkey. Bull. Environ. Contam. Toxicol. 2015, 94, 598–603. [Google Scholar] [CrossRef]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Gao, C.-H.; Yu, G.; Cai, P. ggVennDiagram: An intuitive, easy-to-use, and highly customizable R package to generate Venn diagram. Front. Genet. 2021, 12, 706907. [Google Scholar] [CrossRef]
- Wu, K.; Xu, W.; Yang, W. Effects of precipitation changes on soil bacterial community composition and diversity in the Junggar desert of Xinjiang, China. PeerJ 2020, 8, e8433. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.; Li, J.; Zhao, X.; Han, E.; Qu, H.; Ma, X.; Lian, J. Increasing precipitation weakened the negative effects of simulated warming on soil microbial community composition in a semi-arid sandy grassland. Front. Microbiol. 2023, 13, 1074841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, H.; Liu, R.; Tang, M.; Nie, C.; Han, X.; Zhao, X.; Wei, P.; Wen, F. Spatial and Temporal Variations of Soil pH in Farmland in Xinjiang, China over the Past Decade. Agriculture 2024, 14, 1048. [Google Scholar] [CrossRef]
- Moulick, D.; Santra, S.C.; Ghosh, D. Seed priming with Se mitigates As-induced phytotoxicity in rice seedlings by enhancing essential micronutrient uptake and translocation and reducing As translocation. Environ. Sci. Pollut. Res. 2018, 25, 26978–26991. [Google Scholar] [CrossRef]
- Nie, M.; Hu, C.; Shi, G.; Cai, M.; Wang, X.; Zhao, X. Selenium restores mitochondrial dysfunction to reduce Cr-induced cell apoptosis in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) root tips. Ecotoxicol. Environ. Saf. 2021, 223, 112564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, X.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Li, M.; Yuan, L. Indications of selenium protection against cadmium and lead toxicity in oilseed rape (Brassica napus L.). Front. Plant Sci. 2016, 7, 1875. [Google Scholar] [CrossRef]
- Huang, B.; Xin, J.; Dai, H.; Zhou, W. Effects of interaction between cadmium (Cd) and selenium (Se) on grain yield and Cd and Se accumulation in a hybrid rice (Oryza sativa) system. J. Agric. Food Chem. 2017, 65, 9537–9546. [Google Scholar] [CrossRef]
- Qin, X.; Nie, Z.; Liu, H.; Zhao, P.; Qin, S.; Shi, Z. Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ. Exp. Bot. 2018, 150, 232–239. [Google Scholar] [CrossRef]
- Yang, F.; Chen, L.; Hu, Q.; Pan, G. Effect of the application of selenium on selenium content of soybean and its products. Biol. Trace Elem. Res. 2003, 93, 249–256. [Google Scholar] [CrossRef]
- Mansoor, T.A.; Borralho, P.M.; Dewanjee, S.; Mulhovo, S.; Rodrigues, C.M.; Ferreira, M.-J.U. Monoterpene bisindole alkaloids, from the African medicinal plant Tabernaemontana elegans, induce apoptosis in HCT116 human colon carcinoma cells. J. Ethnopharmacol. 2013, 149, 463–470. [Google Scholar] [CrossRef]
- Jin, C.; Genxing, P.; Yaling, W. Effect of soil se level on selenium uptake and transformation by two spring soybean cultivars. Sci. Agric. Sin. 2005, 38, 428–432. [Google Scholar]
- Song, T.; Su, X.; He, J.; Liang, Y.; Zhou, T.; Liu, C. Selenium (Se) uptake and dynamic changes of Se content in soil–plant systems. Environ. Sci. Pollut. Res. 2018, 25, 34343–34350. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Han, M.; Liang, K.; Liu, H.; Feng, B. Profiling of farmland microorganisms in maize and minor-grain crops under extreme drought conditions. Appl. Soil Ecol. 2024, 204, 105743. [Google Scholar] [CrossRef]
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Conway, J.M.; Law, T.F.; Teixeira, P.J.P.L.; Wilson, E.D.; Fitzpatrick, C.R.; Jones, C.D.; Dangl, J.L. A single bacterial genus maintains root growth in a complex microbiome. Nature 2020, 587, 103–108. [Google Scholar] [CrossRef]
- Gavande, P.V.; Basak, A.; Sen, S.; Lepcha, K.; Murmu, N.; Rai, V.; Mazumdar, D.; Saha, S.P.; Das, V.; Ghosh, S. Functional characterization of thermotolerant microbial consortium for lignocellulolytic enzymes with central role of Firmicutes in rice straw depolymerization. Sci. Rep. 2021, 11, 3032. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Liu, G.; Ma, X.; Mu, C.; Zhao, L. Bacterial communities in the upper soil layers in the permafrost regions on the Qinghai-Tibetan plateau. Appl. Soil Ecol. 2017, 120, 81–88. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Chu, H.; Sun, H.; Tripathi, B.M.; Adams, J.M.; Huang, R.; Zhang, Y.; Shi, Y. Bacterial community dissimilarity between the surface and subsurface soils equals horizontal differences over several kilometers in the western Tibetan Plateau. Environ. Microbiol. 2016, 18, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ge, A.H.; Tóth, T.; An, F.; Guo, L.; Nie, Z.; Liu, J.; Yang, F.; Wang, Z. Soil bacterial microbiota predetermines rice yield in reclaiming saline-sodic soils leached with brackish ice. J. Sci. Food Agric. 2021, 101, 6472–6483. [Google Scholar] [CrossRef] [PubMed]
| Sample | pH | EC (µS/cm) | OM (g/kg) | AP (mg/kg) | AN (mg/kg) | Na+ (mg/kg) | K+ (mg/kg) |
|---|---|---|---|---|---|---|---|
| WA | 8.40 ± 0.20 a | 857.00 ± 29.46 a | 23.58 ± 0.74 cd | 7.93 ± 0.04 e | 53.06 ± 2.84 bcd | 3.86 ± 0.01 c | 4.14 ± 0.21 ab |
| WB | 8.07 ± 0.15 cd | 387.33 ± 6.51 d | 24.12 ± 1.66 cd | 11.81 ± 0.16 b | 59.69 ± 5.92 ab | 4.16 ± 0.02 a | 4.08 ± 0.05 ab |
| WC | 8.07 ± 0.06 cd | 225.67 ± 7.02 g | 24.01 ± 0.92 cd | 7.76 ± 0.15 e | 42.64 ± 1.64 e | 3.92 ± 0.03 bc | 4.21 ± 0.25 a |
| WD | 8.10 ± 0.10 bcd | 285.33 ± 18.04 f | 25.54 ± 0.83 abc | 9.43 ± 0.02 d | 42.64 ± 7.15 e | 4.12 ± 0.03 a | 4.21 ± 0.27 a |
| WE | 8.10 ± 0.10 bcd | 336.00 ± 24.52 e | 22.83 ± 0.32 d | 9.89 ± 0.04 cd | 67.27 ± 5.68 a | 3.86 ± 0.01 c | 3.91 ± 0.02 b |
| WF | 8.23 ± 0.06 abc | 546.00 ± 21.52 b | 27.29 ± 0.15 a | 9.41 ± 0.08 d | 56.85 ± 7.15 bc | 3.87 ± 0.01 c | 3.88 ± 0.06 b |
| WG | 8.30 ± 0.10 ab | 464.33 ± 32.01 c | 26.81 ± 1.45 ab | 10.13 ± 0.04 c | 55.90 ± 7.52 bc | 4.08 ± 0.22 ab | 3.96 ± 0.08 ab |
| WH | 8.30 ± 0.10 ab | 173.07 ± 37.41 h | 25.41 ± 0.96 abc | 13.86 ± 0.96 a | 48.32 ± 3.28 cde | 4.02 ± 0.13 abc | 4.04 ± 0.01 ab |
| WI | 8.00 ± 0.10 d | 375.00 ± 8.54 de | 25.25 ± 1.35 bc | 9.61 ± 0.26 cd | 45.48 ± 4.34 de | 4.02 ± 0.15 abc | 4.01 ± 0.05 ab |
| Sample | Root (mg/kg) | Stem (mg/kg) | Pod (mg/kg) | Seed (mg/kg) |
| WA | 0.034 ± 0.010 d | 0.065 ± 0.006 ab | 0.029 ± 0.009 ef | 0.074 ± 0.005 bc |
| WB | 0.103 ± 0.008 bc | 0.013 ± 0.007 d | 0.018 ± 0.004 f | 0.088 ± 0.008 ab |
| WC | 0.048 ± 0.015 d | 0.044 ± 0.009 bc | 0.060 ± 0.005 cd | 0.032 ± 0.010 d |
| WD | 0.126 ± 0.015 b | 0.018 ± 0.005 cd | 0.091 ± 0.005 ab | 0.080 ± 0.010 abc |
| WE | 0.075 ± 0.003 cd | 0.089 ± 0.012 a | 0.051 ± 0.017 cde | 0.055 ± 0.005 cd |
| WF | 0.071 ± 0.008 cd | 0.051 ± 0.009 b | 0.098 ± 0.009 a | 0.067 ± 0.011 bc |
| WG | 0.127 ± 0.006 b | 0.082 ± 0.008 a | 0.070 ± 0.007 bc | 0.087 ± 0.014 ab |
| WH | 0.102 ± 0.007 bc | 0.084 ± 0.008 a | 0.060 ± 0.013 cd | 0.082 ± 0.009 abc |
| WI | 0.180 ± 0.033 a | 0.055 ± 0.015 b | 0.044 ± 0.007 def | 0.106 ± 0.017 a |
| Sample | Root | Stem | Pod | Seed |
|---|---|---|---|---|
| WA | 0.021 ± 0.006 d | 0.039 ± 0.003 ab | 0.017 ± 0.006 ef | 0.044 ± 0.001 ab |
| WB | 0.060 ± 0.001 ab | 0.007 ± 0.004 f | 0.011 ± 0.002 f | 0.051 ± 0.002 a |
| WC | 0.025 ± 0.009 d | 0.023 ± 0.005 de | 0.032 ± 0.004 cd | 0.017 ± 0.006 d |
| WD | 0.064 ± 0.005 ab | 0.009 ± 0.002 ef | 0.046 ± 0.003 ab | 0.041 ± 0.004 abc |
| WE | 0.038 ± 0.001 cd | 0.045 ± 0.006 a | 0.026 ± 0.008 cde | 0.028 ± 0.001 cd |
| WF | 0.035 ± 0.004 cd | 0.025 ± 0.005 bcd | 0.049 ± 0.005 a | 0.033 ± 0.006 bc |
| WG | 0.060 ± 0.003 b | 0.039 ± 0.006 abc | 0.033 ± 0.004 bc | 0.041 ± 0.006 abc |
| WH | 0.048 ± 0.005 bc | 0.039 ± 0.005 ab | 0.028 ± 0.006 cde | 0.038 ± 0.006 abc |
| WI | 0.079 ± 0.014 a | 0.024 ± 0.008 cd | 0.019 ± 0.003 def | 0.046 ± 0.009 ab |
| Sample | Stem/Root | Pod/Stem | Seed/Pod |
|---|---|---|---|
| WA | 1.986 ± 0.489 a | 0.440 ± 0.116 b | 2.711 ± 0.720 b |
| WB | 0.124 ± 0.063 d | 1.613 ± 0.584 b | 4.951 ± 0.827 a |
| WC | 0.944 ± 0.203 b | 1.403 ± 0.198 b | 0.536 ± 0.150 d |
| WD | 0.142 ± 0.025 d | 5.252 ± 1.424 a | 0.877 ± 0.090 d |
| WE | 1.188 ± 0.145 b | 0.565 ± 0.118 b | 1.147 ± 0.298 d |
| WF | 0.710 ± 0.087 bc | 1.960 ± 0.231 b | 0.685 ± 0.103 d |
| WG | 0.646 ± 0.079 bcd | 0.864 ± 0.101 b | 1.227 ± 0.100 d |
| WH | 0.821 ± 0.043 bc | 0.717 ± 0.137 b | 1.400 ± 0.263 cd |
| WI | 0.318 ± 0.120 cd | 0.826 ± 0.177 b | 2.421 ± 0.113 bc |
| Sample | pH | EC (µS/cm) | OM (g/kg) | AP (mg/kg) | AN (mg/kg) | Na+ (mg/kg) | K+ (mg/kg) |
|---|---|---|---|---|---|---|---|
| WA | 8.07 ± 0.15 c | 93.33 ± 3.06 d | 22.23 ± 1.68 a | 10.16 ± 0.06 a | 55.90 ± 5.68 ab | 4.44 ± 0.06 b | 4.05 ± 0.06 b |
| WB | 8.63 ± 0.06 a | 463.67 ± 34.08 a | 27.80 ± 1.94 a | 6.86 ± 0.20 d | 49.27 ± 7.15 b | 4.17 ± 0.04 c | 4.06 ± 0.03 b |
| WC | 8.27 ± 0.06 bc | 199.53 ± 36.83 c | 15.13 ± 6.40 b | 3.62 ± 0.08 e | 56.85 ± 4.34 ab | 3.88 ± 0.02 d | 3.85 ± 0.02 b |
| WD | 8.40 ± 0.10 ab | 255.33 ± 38.00 b | 22.60 ± 1.09 a | 8.24 ± 0.16 c | 37.90 ± 5.92 c | 4.07 ± 0.04 c | 4.00 ± 0.05 b |
| WE | 8.60 ± 0.10 a | 289.00 ± 14.42 b | 23.21 ± 2.76 a | 10.25 ± 0.12 a | 55.90 ± 5.68 ab | 3.85±0.00 d | 3.91 ± 0.02 b |
| WF | 8.30 ± 0.10 bc | 488.33 ± 8.50 a | 23.82 ± 6.25 a | 9.06 ± 0.08 b | 52.11 ± 3.28 ab | 4.26 ± 0.13 bc | 4.09 ± 0.04 b |
| WG | 8.40 ± 0.26 ab | 463.67 ± 2.52 a | 28.29 ± 1.45 a | 9.05 ± 0.04 b | 63.48 ± 7.15 a | 4.81 ± 0.08 a | 4.10 ± 0.01 b |
| WH | 8.10 ± 0.17 c | 194.30 ± 55.25 c | 14.89 ± 3.59 b | 10.21 ± 0.11 a | 63.48 ± 4.34 a | 3.94 ± 0.03 d | 3.93 ± 0.00 b |
| WI | 8.10 ± 0.20 c | 442.67 ± 20.60 a | 25.95 ± 0.41 a | 9.18 ± 0.34 b | 60.64 ± 9.14 ab | 4.15 ± 0.28 c | 4.49 ± 0.41 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Xu, C.; Wang, Y.; Qin, M.; Xiang, Z.; Yu, X.; Zhao, X. Characterization of Selenium Enrichment in Soybean and Its Relationship with Rhizosphere Microbial Communities in Se-Enriched Saline Soil. Agronomy 2025, 15, 1320. https://doi.org/10.3390/agronomy15061320
Feng T, Xu C, Wang Y, Qin M, Xiang Z, Yu X, Zhao X. Characterization of Selenium Enrichment in Soybean and Its Relationship with Rhizosphere Microbial Communities in Se-Enriched Saline Soil. Agronomy. 2025; 15(6):1320. https://doi.org/10.3390/agronomy15061320
Chicago/Turabian StyleFeng, Tianyuan, Chao Xu, Yin Wang, Mingze Qin, Zequn Xiang, Xi Yu, and Xiaohu Zhao. 2025. "Characterization of Selenium Enrichment in Soybean and Its Relationship with Rhizosphere Microbial Communities in Se-Enriched Saline Soil" Agronomy 15, no. 6: 1320. https://doi.org/10.3390/agronomy15061320
APA StyleFeng, T., Xu, C., Wang, Y., Qin, M., Xiang, Z., Yu, X., & Zhao, X. (2025). Characterization of Selenium Enrichment in Soybean and Its Relationship with Rhizosphere Microbial Communities in Se-Enriched Saline Soil. Agronomy, 15(6), 1320. https://doi.org/10.3390/agronomy15061320






