Biostimulant Extracts Obtained from the Brown Seaweed Cystoseira barbata Enhance the Growth, Yield, Quality, and Nutraceutical Value of Soil-Grown Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Storage of C. barbata
2.2. Preparation and Composition of C. barbata Extracts
2.3. Plant Material and Growing Conditions
2.4. Experimental Design
2.5. Plant Growth Measurements, Fruit Harvest, and Sample Storage
2.6. Determination of Total Soluble Solids (TSS, Brix), EC, and pH of Tomato Fruits
2.7. Mineral Analysis
2.8. Determination of Total Phenolic Content Using the Folin–Ciocalteu Assay
2.9. Determination of Total Antioxidant Capacity
2.10. Determination of Lycopene and β-Carotene Concentrations
- -
- Lycopene (mg per 100 g FW) = –0.0458 A663 + 0.204 A645 + 0.372 A505 − 0.0806 A453
- -
- β-Carotene (mg per 100 g FW) = 0.2160 A663 − 1.220 A645 − 0.304 A505 + 0.452 A453
2.11. Vitamin C Assay
2.12. Determination of Reduced Glutathione (GSH) Concentration
2.13. Determination of Total Soluble Carbohydrate Concentration
2.14. Statistical Analysis
2.15. Generative AI
3. Results
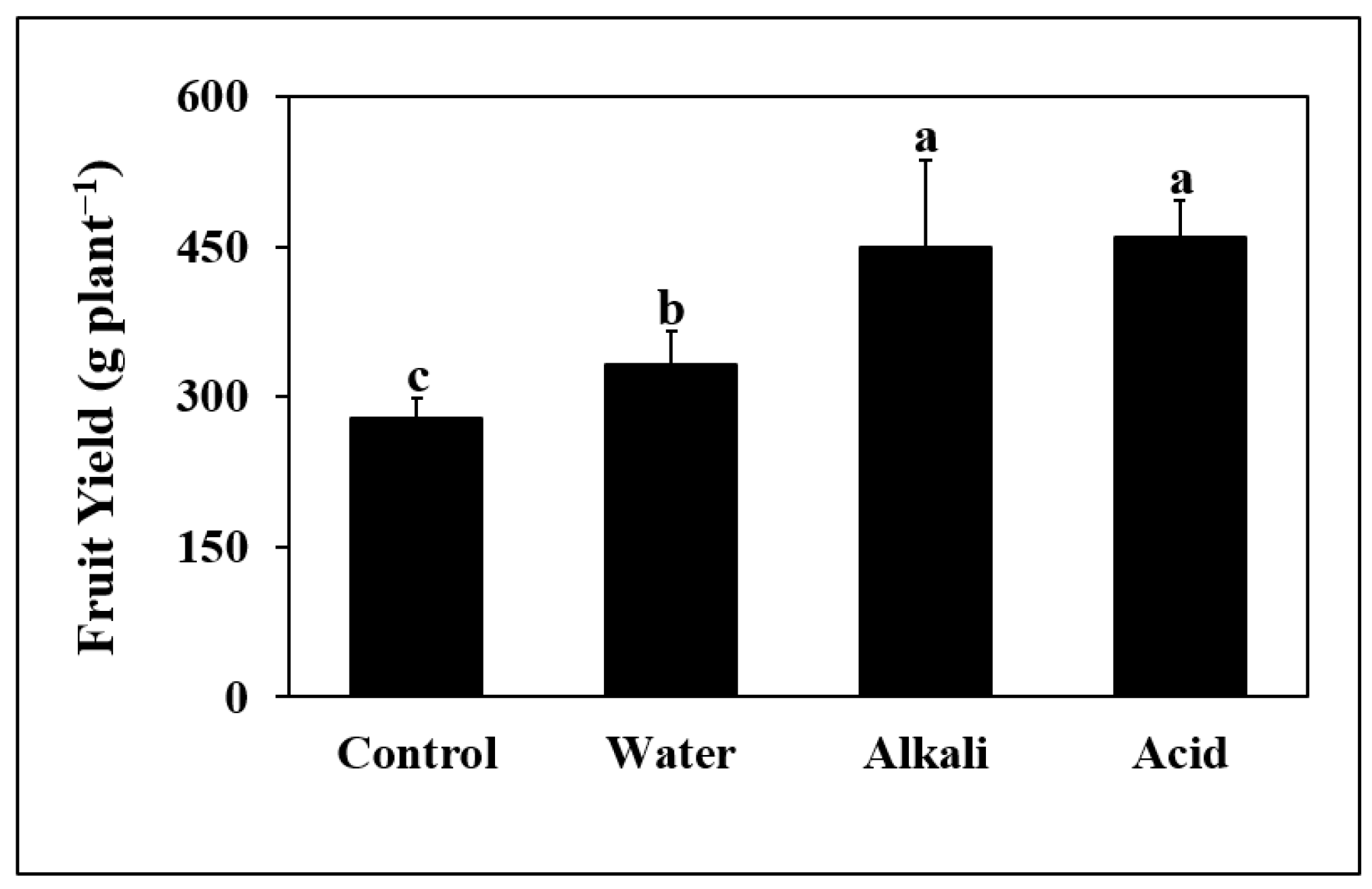
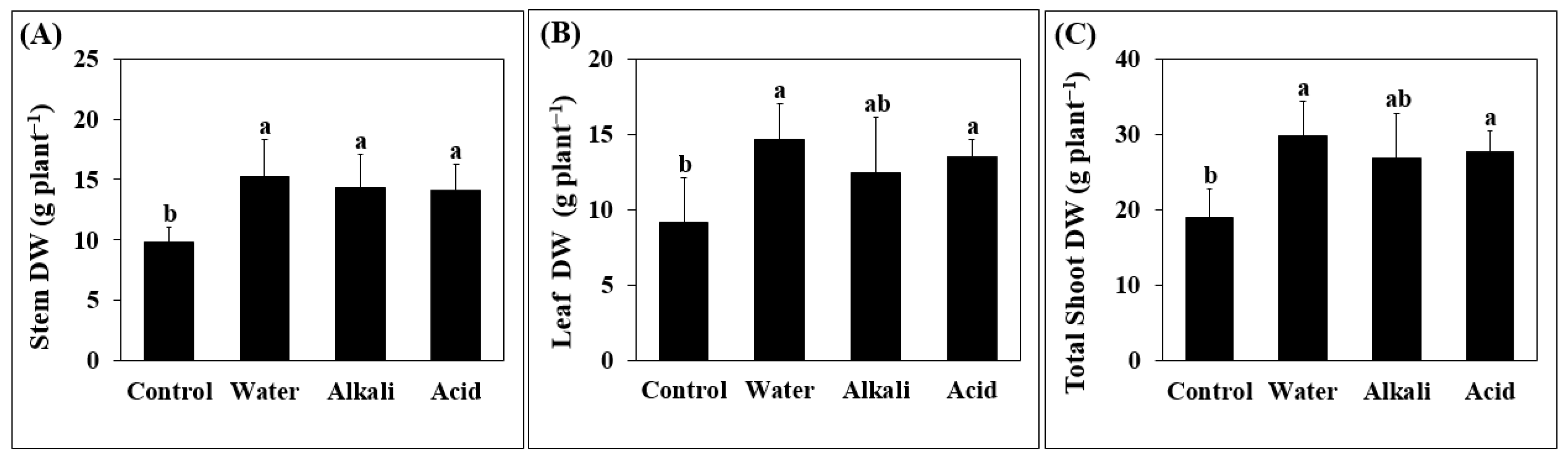
3.1. Plant Growth and Yield Parameters
3.2. Brix, EC, and pH
3.3. Mineral Concentration
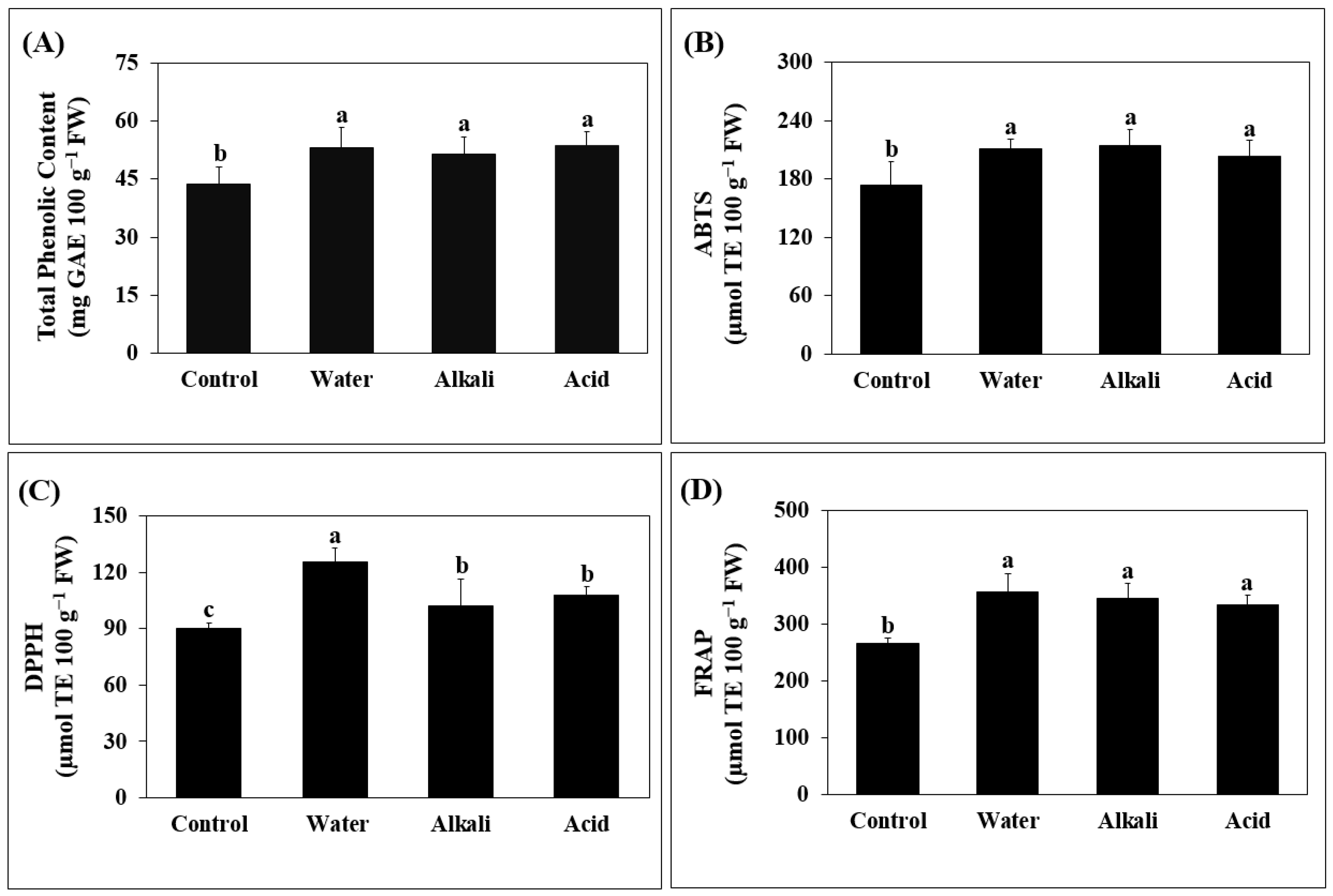
3.4. Phenolic and Antioxidant Activity
3.5. Carotenoids
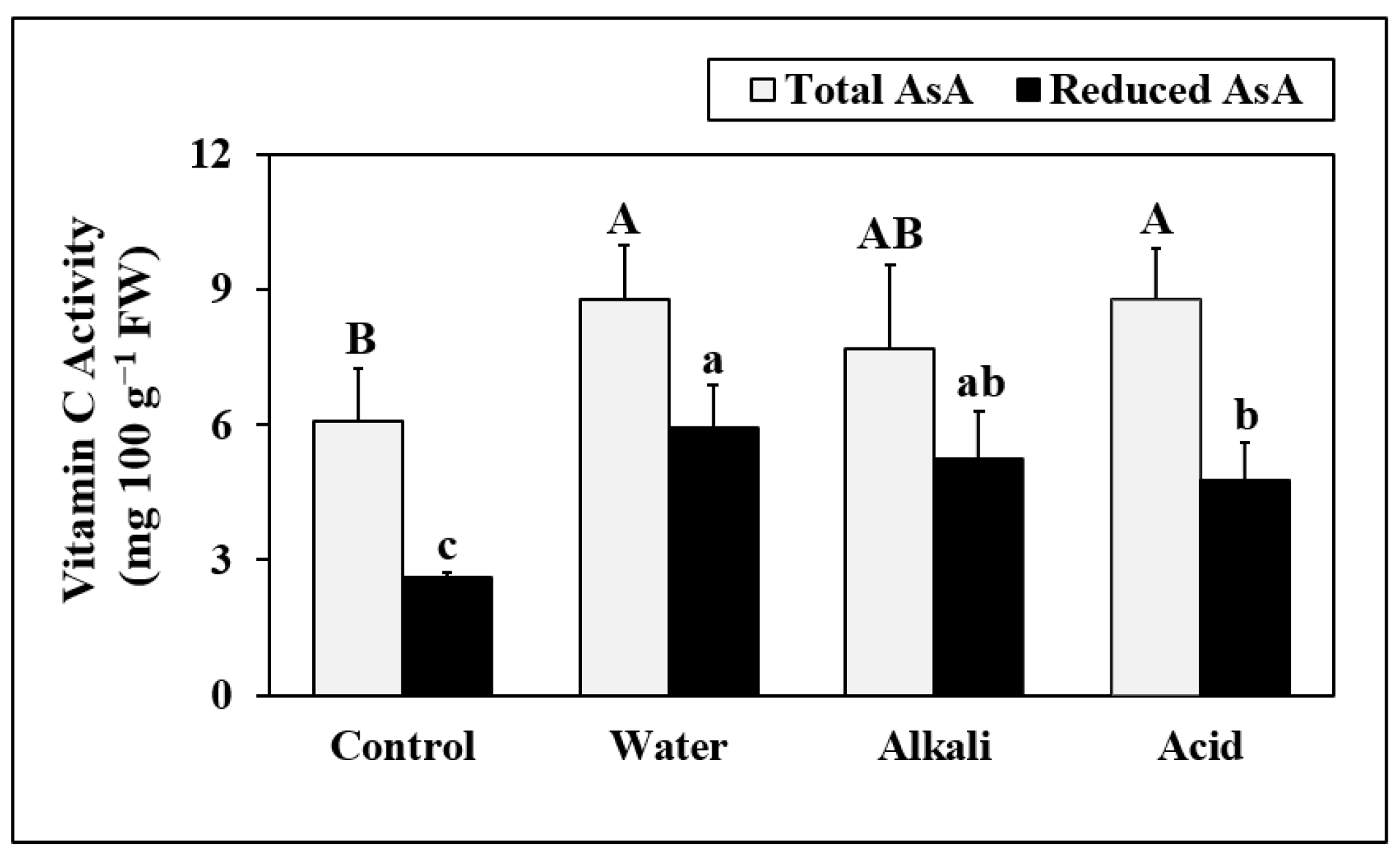
3.6. Vitamin C
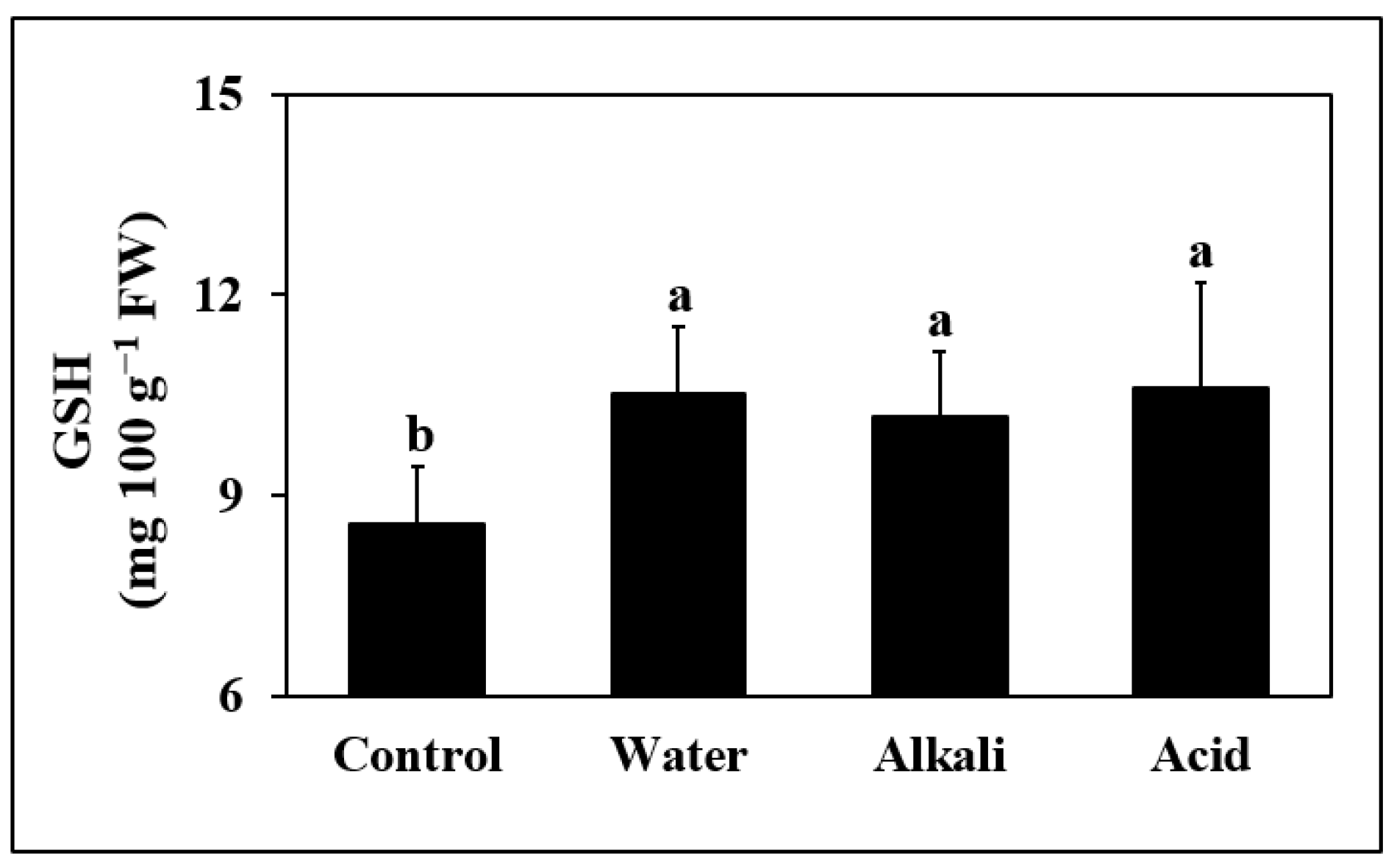
3.7. Reduced Glutathione
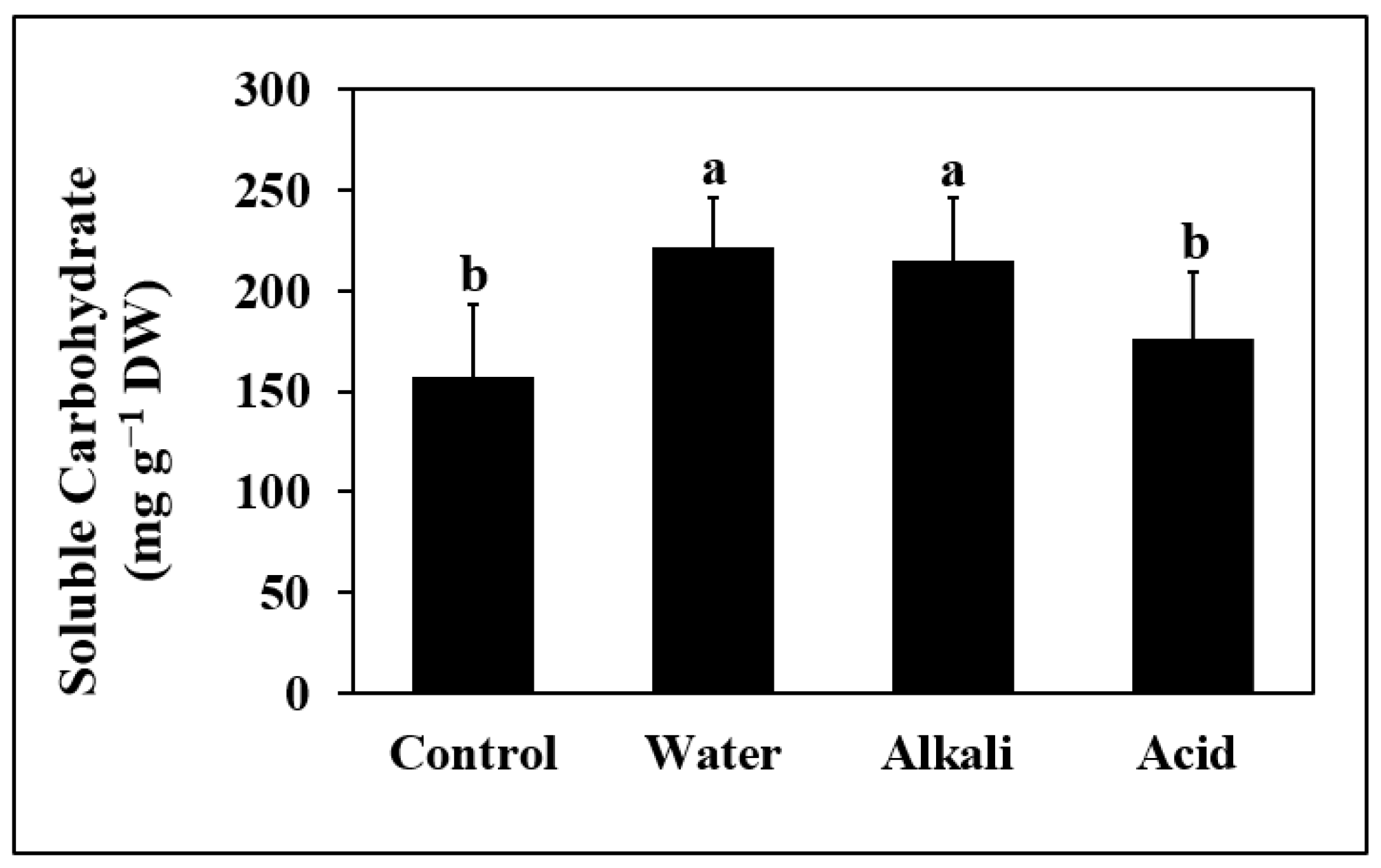
3.8. Soluble Carbohydrate
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C. barbata | Cystoseira barbata |
| SWE | Seaweed extract |
| TSS | Total soluble solids |
| EC | Electrical conductivity |
| TPC | Total phenolic content |
| GAE | Gallic acid equivalent |
| FW | Fresh weight |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | Ferric-reducing antioxidant power |
| Trolox | (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid |
| TE | Trolox equivalent |
| AsA | Ascorbic acid |
| GSH | Reduced glutathione |
| DW | Dry weight |
| TUBITAK | Scientific and Technological Research Council of Turkey |
| YOK | Council of Higher Education |
References
- United Nations. Peace, Dignity and Equality on a Healthy Planet. 2023. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 17 February 2025).
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y.A. Meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Wake, B. Economics of adaptation. Nat. Clim. Change 2023, 13, 764. [Google Scholar] [CrossRef]
- Kabir, A.H.; Baki, M.Z.I.; Ahmed, B.; Mostofa, M.G. Current, faltering, and future strategies for advancing microbiome-assisted sustainable agriculture and environmental resilience. New Crops 2024, 1, 100013. [Google Scholar] [CrossRef]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for production, characterization, and application of protein-based biostimulants in agriculture: A review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Bauza-Kaszewska, J.; Breza-Boruta, B.; Lemańczyk, G.; Lamparski, R. Effects of Eco-Friendly Product Application and Sustainable Agricultural Management Practices on Soil Properties and Phytosanitary Condition of Winter Wheat Crops. Sustainability 2022, 14, 15754. [Google Scholar] [CrossRef]
- Ávila-Pozo, P.; Parrado, J.; Caballero, P.; Tejada, M. Use of a Biostimulant Obtained from Slaughterhouse Sludge in a Greenhouse Tomato Crop. Horticulturae 2022, 8, 622. [Google Scholar] [CrossRef]
- EC. REGULATION (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 8 April 2025).
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Islam, M.T.; Tran, T.L.C.; Weisser, M.; Winberg, P.; Cahill, D.M. Applications of seaweed extracts in agriculture: An Australian perspective. J. Appl. Phycol. 2024, 36, 713–726. [Google Scholar] [CrossRef]
- Di Stasio, E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. (Osmo-priming with seaweed extracts enhances yield of salt-stressed tomato plants. Agronomy 2020, 10, 1559. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Seaweed carbohydrates. In The Chemical Biology of Plant Biostimulants; Ghent University: Gent, Belgium, 2020; pp. 57–95. [Google Scholar]
- Vaghela, P.; Gandhi, G.; Trivedi, K.; Anand, K.G.V.; Chavda, D.; Manna, M.; Seth, T.; Seth, A.; Shanmugam, M.; Ghosh, A. Underpinning beneficial maize response to application of minimally processed homogenates of red and brown seaweeds. Front. Plant Sci. 2023, 14, 1273355. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Stirk, W.A.; Rengasamy, K.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed: An overview. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 31–55. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; El Arroussi, H.; Khouloud, M.; Dhiba, D.; Kadmiri, I.M.; Bamouh, A. Polysaccharides extracted from Moroccan seaweed: A promising source of tomato plant growth promoters. J. Appl. Phycol. 2018, 30, 2953–2962. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products/Regions(World+(Total))/Elements(Area harvested and Product Quantity)/Items(Tomatoes). 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 5 May 2025).
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W., Jr. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [CrossRef]
- Natali, P.G.; Piantelli, M.; Sottini, A.; Eufemi, M.; Banfi, C.; Imberti, L. A step forward in enhancing the health-promoting properties of whole tomato as a functional food to lower the impact of non-communicable diseases. Front. Nutr. 2025, 12, 1519905. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. [Google Scholar]
- Zodape, S.T.; Gupta, A.; Bhandari, S.C.; Rawat, U.S.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J. Sci. Ind. Res. 2011, 7, 215–219. [Google Scholar]
- Subramaniyan, L.; Veerasamy, R.; Prabhakaran, J.; Selvaraj, A.; Algarswamy, S.; Karuppasami, K.M.; Nalliappan, S. Biostimulation Effects of Seaweed Extract (Ascophyllum nodosum) on Phytomorpho-Physiological, Yield, and Quality Traits of Tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 348. [Google Scholar] [CrossRef]
- Zhang, L.; Freschi, G.; Rouphael, Y.; De Pascale, S.; Lucini, L. The differential modulation of secondary metabolism induced by a protein hydrolysate and a seaweed extract in tomato plants under salinity. Front. Plant Sci. 2023, 13, 1072782. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. Hortic. Sci. 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Hussain, H.I.; Kasinadhuni, N.; Arioli, T. The effect of seaweed extract on tomato plant growth, productivity and soil. J. Appl. Phycol. 2021, 33, 1305–1314. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Sánchez-Hernández, C.V.; Palmeros-Suárez, P.A.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Meza-Canales, I.D.; Becerril-Espinosa, A. Seaweed extract improves growth and productivity of tomato plants under salinity stress. Agronomy 2022, 12, 2495. [Google Scholar] [CrossRef]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Hernández-Herrera, R.M. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; Benhima, R.; Khouloud, M.; Boulif, R.; Douira, A.; Bamouh, A.; Meftah Kadmiri, I. Biostimulants derived from Moroccan seaweeds: Seed germination metabolomics and growth promotion of tomato plant. J. Plant Growth Regul. 2021, 40, 353–370. [Google Scholar] [CrossRef]
- Pei, B.; Zhang, Y.; Liu, T.; Cao, J.; Ji, H.; Hu, Z.; Wu, X.; Wang, F.; Lu, Y.; Chen, N.; et al. Effects of seaweed fertilizer application on crops’ yield and quality in field conditions in China-A meta-analysis. PLoS ONE 2024, 19, e0307517. [Google Scholar] [CrossRef] [PubMed]
- Savonitto, G.; De La Fuente, G.; Tordoni, E.; Ciriaco, S.; Srijemsi, M.; Bacaro, G.; Falace, A.; Chiantore, M.; Falace, A. Addressing reproductive stochasticity and grazing impacts in the restoration of a canopy-forming brown alga by implementing mitigation solutions. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1611–1623. [Google Scholar] [CrossRef]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Thibaut, T. Severe decline of Gongolaria Barbata (Fucales) along most of the French Mediterranean coast. Sci. Rep. 2025, 15, 5701. [Google Scholar] [CrossRef]
- El Khattabi, O.; El Hasnaoui, S.; Toura, M.; Henkrar, F.; Collin, B.; Levard, C.; Colin, F.; Merghoub, N.; Smouni, A.; Fahr, M. Seaweed extracts as promising biostimulants for enhancing lead tolerance and accumulation in tomato (Solanum lycopersicum). J. Appl. Phycol. 2023, 35, 459–469. [Google Scholar] [CrossRef]
- Morales-Sierra, S.; Luis, J.C.; Jiménez-Arias, D.; Rancel-Rodríguez, N.M.; Coego, A.; Rodriguez, P.L.; Cueto, M.; Borges, A.A. Biostimulant activity of Galaxaura rugosa seaweed extracts against water deficit stress in tomato seedlings involves activation of ABA signaling. Front. Plant Sci. 2023, 14, 1251442. [Google Scholar] [CrossRef] [PubMed]
- Krid, A.; Ennoury, A.; Kchikich, A.; Oumassi, F.; Oualid, J.A.; Roussi, Z.; Nhiri, M.; Aberkani, K.; El Imache, A.; Bouhcain, B.; et al. Cystoseira tamariscifolia Aqueous Extract Mitigates Salinity Stress in Tomato Plants by Mediating Their Physiology and Biochemistry. Int. J. Environ. Res. 2024, 18, 58. [Google Scholar] [CrossRef]
- Demir, N.; Dural, B.; Yıldırım, K. Effect of seaweed suspensions on seed germination of tomato, pepper and aubergine. J. Biol. Sci. 2006, 6, 1130–1133. [Google Scholar]
- Mutlu-Durak, H.; Arikan-Algul, Y.; Bayram, E.; Haznedaroglu, B.Z.; Kutman, U.B.; Kutman, B.Y. Various extracts of the brown seaweed Cystoseira barbata with different compositions exert biostimulant effects on seedling growth of wheat. Physiol. Plant. 2024, 176, e14503. [Google Scholar] [CrossRef]
- Ceylan, Y.; Kutman, U.B.; Mengutay, M.; Cakmak, I. Magnesium applications to growth medium and foliage affect the starch distribution, increase the grain size and improve the seed germination in wheat. Plant Soil 2016, 406, 145–156. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The application of a plant biostimulant based on seaweed and yeast extract improved tomato fruit development and quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. A simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J. Jpn. Soc. Food Sci. Technol. 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Fernández-Garcí, N.; Martínez, V.; Cerdá, A.; Carvajal, M. Fruit quality of grafted tomato plants grown under saline conditions. J. Hortic. Sci. Biotechnol. 2004, 79, 995–1001. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Phytochemical composition and antioxidant activity of high-lycopene tomato (Solanum lycopersicum L.) cultivars grown in Southern Italy. Sci. Hortic. 2011, 127, 255–261. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Wang, W.; Lv, H.; Liang, B.; Li, J. Exogenous pig blood-derived protein hydrolysates as a promising method for alleviation of salt stress in tomato (Solanum lycopersicum L.). Sci. Hortic. 2022, 294, 110779. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef]
- Mengutay, M.; Ceylan, Y.; Kutman, U.B.; Cakmak, I. Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant Soil 2013, 368, 57–72. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; Khouloud, M.; Farrie, Y.; Boulif, R.; Kadmiri, I.M.; Bamouh, A.; Douira, A. Improving growth, yield, and quality of Tomato plants (Solanum lycopersicum L) by the application of Moroccan seaweed-based biostimulants under greenhouse conditions. Agronomy 2021, 11, 1373. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Chen, B.; Zhang, M.; Ma, J. Seaweed extract improved yields, leaf photosynthesis, ripening time, and net returns of tomato (Solanum lycopersicum Mill.). ACS Omega 2020, 5, 4242–4249. [Google Scholar] [CrossRef]
- Uzun, S. The quantitative effects of temperature and light on the number of leaves preceding the first fruiting inflorescence on the stem of tomato (Lycopersicon esculentum, Mill.) and aubergine (Solanum melongena L.). Sci. Hortic. 2006, 109, 142–146. [Google Scholar] [CrossRef]
- Dookie, M.; Ali, O.; Ramsubhag, A.; Jayaraman, J. Flowering gene regulation in tomato plants treated with brown seaweed extracts. Sci. Hortic. 2021, 276, 109715. [Google Scholar] [CrossRef]
- Zhu, Y.; Sims, C.A.; Klee, H.J.; Sarnoski, P.J. Sensory and flavor characteristics of tomato juice from garden gem and roma tomatoes with comparison to commercial tomato juice. J. Food Sci. 2018, 83, 153–161. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Zhu, X.; Chang, Y.; Wang, C.; Ma, N.; Wang, J.; Zhang, X.; Lyu, J.; Xie, J. A comprehensive evaluation of tomato fruit quality and identification of volatile compounds. Plants 2023, 12, 2947. [Google Scholar] [CrossRef]
- Iglesias, M.J.; García-López, J.; Collados-Luján, J.F.; López-Ortiz, F.; Díaz, M.; Toresano, F.; Camacho, F. Differential response to environmental and nutritional factors of high-quality tomato varieties. Food Chem. 2015, 176, 278–287. [Google Scholar] [CrossRef]
- Hernández Suárez, M.; Rodríguez Rodríguez, E.; Díaz Romero, C. Analysis of organic acid content in cultivars of tomato harvested in Tenerife. Eur. Food Res. Technol. 2008, 226, 423–435. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Pectin methylesterase activity and other factors affecting pH and titratable acidity in processing tomatoes. Food Chem. 2012, 132, 915–920. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Wang, B.; Huang, S.; Hu, J.; Yang, T.; Yu, Q. Identification of the carbohydrate and organic acid metabolism genes responsible for brix in tomato fruit by transcriptome and metabolome analysis. Front. Genet. 2021, 12, 714942. [Google Scholar] [CrossRef] [PubMed]
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, F.; Naumann, M.; Pawelzik, E.; Smit, I. Improvement of cocktail tomato yield and consumer-oriented quality traits by potassium fertilization is driven by the cultivar. J. Sci. Food Agric. 2019, 99, 3350–3358. [Google Scholar] [CrossRef] [PubMed]
- Kutman, U.B. Mineral nutrition and crop quality. In Marschner’s Mineral Nutrition of Plants; Academic Press: Cambridge, MA, USA, 2023; pp. 419–444. [Google Scholar]
- World Health Organization Guideline. Potassium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012; pp. 1–52. [Google Scholar]
- Hernández-Pérez, O.I.; Valdez-Aguilar, L.A.; Alia-Tejacal, I.; Cartmill, A.D.; Cartmill, D.L. Tomato fruit yield, quality, and nutrient status in response to potassium: Calcium balance and electrical conductivity in the nutrient solution. J. Soil Sci. Plant Nutr. 2020, 20, 484–492. [Google Scholar] [CrossRef]
- Jauregui, J.I.; Lumbreras, M.; Chavarri, M.J.; Macua, J.I. Dry weight and brix degree correlation in different varieties of tomatoes intended for industrial processing. In Proceedings of the VI International Symposium on Processing Tomato & Workshop on Irrigation & Fertigation of Processing Tomato, Pamplona, Spain, 25–29 May 1998. [Google Scholar]
- Bouissil, S.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; El Modafar, C.; Delattre, C. Use of alginate extracted from Moroccan brown algae to stimulate natural defense in date palm roots. Molecules 2020, 25, 720. [Google Scholar] [CrossRef]
- Nivetha, N.; Shukla, P.S.; Nori, S.S.; Kumar, S.; Suryanarayan, S. A red seaweed Kappaphycus alvarezii-based biostimulant (AgroGain®) improves the growth of Zea mays and impacts agricultural sustainability by beneficially priming rhizosphere soil microbial community. Front. Microbiol. 2024, 15, 1330237. [Google Scholar] [CrossRef]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Du, X.; Song, M.; Baldwin, E.; Rouseff, R. Identification of sulphur volatiles and GC-olfactometry aroma profiling in two fresh tomato cultivars. Food Chem. 2015, 171, 306–314. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Yang, A. Sulfur homeostasis in plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef] [PubMed]
- Decros, G.; Dussarrat, T.; Baldet, P.; Cassan, C.; Cabasson, C.; Dieuaide-Noubhani, M.; Destailleur, A.; Flandin, A.; Prigent, S.; Mori, K.; et al. Enzyme-based kinetic modelling of ASC–GSH cycle during tomato fruit development reveals the importance of reducing power and ROS availability. New Phytol. 2023, 240, 242–257. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A review of dietary (phyto) nutrients for glutathione support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, P.; Yuan, L.; Chen, X.; Hu, X. Effects of application methods of boron on tomato growth, fruit quality and flavor. Horticultura 2021, 7, 223. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE 2019, 14, e0216710. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Khanday, A.H.; Badroo, I.A.; Wagay, N.A.; Rafiq, S. Role of phenolic compounds in disease resistance to plants. In Plant Phenolics in Biotic Stress Management; Springer Nature: Singapore, 2024; pp. 455–479. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Correlations of antioxidant activity against phenolic content revisited: A new approach in data analysis for food and medicinal plants. J. Food Sci. 2009, 74, R107–R113. [Google Scholar] [CrossRef]
- Bouissil, S.; Alaoui-Talibi, Z.E.; Pierre, G.; Rchid, H.; Michaud, P.; Delattre, C.; El Modafar, C. Fucoidans of Moroccan brown seaweed as elicitors of natural defenses in date palm roots. Mar. Drugs 2020, 18, 596. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Phytoelicitor activity of Sargassum vulgare and Acanthophora spicifera extracts and their prospects for use in vegetable crops for sustainable crop production. J. Appl. Phycol. 2021, 33, 639–651. [Google Scholar] [CrossRef]
- Stra, A.; Almarwaey, L.O.; Alagoz, Y.; Moreno, J.C.; Al-Babili, S. Carotenoid metabolism: New insights and synthetic approaches. Front. Plant Sci. 2023, 13, 1072061. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, V.; Nandwani, D.; Wang, L.; Wu, Y. A study on organic tomatoes: Effect of a biostimulator on phytochemical and antioxidant activities. J. Food Qual. 2017, 1, 5020742. [Google Scholar] [CrossRef]
- Sariñana-Aldaco, O.; Benavides-Mendoza, A.; Robledo-Olivo, A.; González-Morales, S. The biostimulant effect of hydroalcoholic extracts of Sargassum spp. in tomato seedlings under salt stress. Plants 2022, 11, 3180. [Google Scholar] [CrossRef] [PubMed]

| Seaweed Extracts | Stem Length (cm) | Number of Inflorescences (plant−1) | Number of Flowers (plant−1) |
|---|---|---|---|
| Control | 60 ± 10 b | 3.8 ± 0.4 b | 9 ± 2 b |
| Water | 65 ± 12 b | 4.8 ± 1.5 b | 14 ± 3 a |
| Alkali | 84 ± 7 a | 5.5 ± 1.3 ab | 10 ± 2 b |
| Acid | 97 ± 12 a | 6.8 ± 1.7 a | 14 ± 2 a |
| Seaweed Extracts | pH | EC (μS cm−1) | Brix (°Bx) |
|---|---|---|---|
| Control | 4.8 ± 0.1 a | 3409 ± 110 d | 5.4 ± 0.5 c |
| Water | 4.2 ± 0.1 b | 5739 ± 140 a | 7.5 ± 0.5 a |
| Alkali | 4.3 ± 0.0 b | 4913 ± 219 b | 6.9 ± 0.7 ab |
| Acid | 4.3 ± 0.1 b | 4381 ± 155 c | 6.2 ± 0.3 b |
| Seaweed Extracts | P (mg g−1) | K (mg g−1) | Ca (mg g−1) | Mg (mg g−1) | S (mg g−1) |
|---|---|---|---|---|---|
| Control | 3.0 ± 0.3 a | 25 ± 1 b | 3.1 ± 0.2 a | 1.2 ± 0.1 a | 0.8 ± 0.1 c |
| Water | 2.6 ± 0.1 a | 27 ± 0 a | 2.1 ± 0.5 b | 1.2 ± 0.1 a | 1.2 ± 0.1 b |
| Alkali | 3.0 ± 0.2 a | 27 ± 1 a | 2.3 ± 0.1 b | 1.3 ± 0.1 a | 1.4 ± 0.1 a |
| Acid | 2.9 ± 0.3 a | 25 ± 1 b | 2.4 ± 0.1 b | 1.2 ± 0.1 a | 1.2 ± 0.1 b |
| Seaweed Extracts | Fe (mg kg−1) | Zn (mg kg−1) | Mn (mg kg−1) | Cu (mg kg−1) | B (mg kg−1) |
|---|---|---|---|---|---|
| Control | 32 ± 2 a | 5.6 ± 0.5 a | 8.6 ± 0.7 b | 3.2 ± 0.2 a | 8.8 ± 0.6 b |
| Water | 31 ± 4 a | 5.5 ± 0.6 a | 8.8 ± 0.9 b | 3.1 ± 0.4 a | 11.1 ± 1.9 a |
| Alkali | 35 ± 3 a | 5.7 ± 0.7 a | 11.0 ± 0.5 a | 3.5 ± 0.6 a | 9.0 ± 0.9 b |
| Acid | 33 ± 3 a | 5.6 ± 0.5 a | 10.4 ± 0.6 a | 3.2 ± 0.3 a | 9.5 ± 0.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arikan-Algul, Y.; Mutlu-Durak, H.; Kutman, U.B.; Yildiz Kutman, B. Biostimulant Extracts Obtained from the Brown Seaweed Cystoseira barbata Enhance the Growth, Yield, Quality, and Nutraceutical Value of Soil-Grown Tomato. Agronomy 2025, 15, 1138. https://doi.org/10.3390/agronomy15051138
Arikan-Algul Y, Mutlu-Durak H, Kutman UB, Yildiz Kutman B. Biostimulant Extracts Obtained from the Brown Seaweed Cystoseira barbata Enhance the Growth, Yield, Quality, and Nutraceutical Value of Soil-Grown Tomato. Agronomy. 2025; 15(5):1138. https://doi.org/10.3390/agronomy15051138
Chicago/Turabian StyleArikan-Algul, Yagmur, Hande Mutlu-Durak, Umit Baris Kutman, and Bahar Yildiz Kutman. 2025. "Biostimulant Extracts Obtained from the Brown Seaweed Cystoseira barbata Enhance the Growth, Yield, Quality, and Nutraceutical Value of Soil-Grown Tomato" Agronomy 15, no. 5: 1138. https://doi.org/10.3390/agronomy15051138
APA StyleArikan-Algul, Y., Mutlu-Durak, H., Kutman, U. B., & Yildiz Kutman, B. (2025). Biostimulant Extracts Obtained from the Brown Seaweed Cystoseira barbata Enhance the Growth, Yield, Quality, and Nutraceutical Value of Soil-Grown Tomato. Agronomy, 15(5), 1138. https://doi.org/10.3390/agronomy15051138








