Field Inoculation of Pleurotus tuoliensis in Natural Habitat Promotes Microbial Communities That Enhance Its Growth
Abstract
1. Introduction
2. Materials and Methods
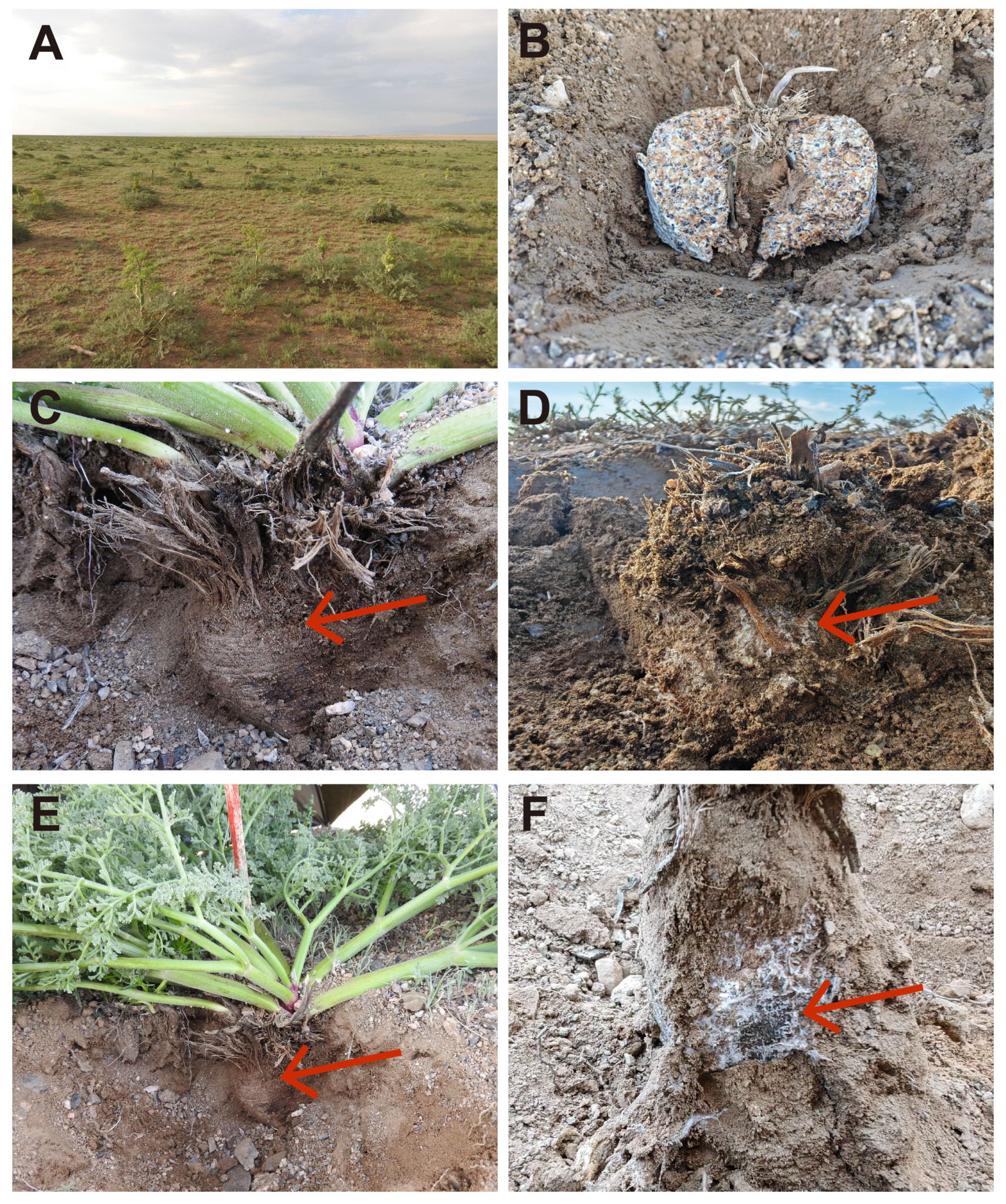
2.1. Experimental Sites
2.2. Experimental Design and Soil Sampling
2.3. Analysis of the Physical and Chemical Properties of Soil
2.4. DNA Extraction and Amplification
2.5. Isolation of MGPB and Analysis of Their Mushroom Growth-Promoting Performance
2.6. Analysis of Growth-Promoting Effects of Isolated Bacteria on P. tuoliensis
2.7. Identification of the Isolated MGPB
2.8. Statistical Analysis
3. Results
3.1. Effect of P. tuoliensis Colonization on the Chemical Properties of Soil in F. feruloides Rhizosphere
3.2. Effects of P. tuoliensis Colonization on the Diversity and Composition of the Bacterial Community in F. feruloides Rhizosphere Soil
3.3. Effects of P. tuoliensis Colonization on the Diversity and Composition of Fungal Community in F. feruloides Rhizosphere Soil
3.4. Correlations Between the Chemical Properties of F. feruloides Rhizosphere Soil and Microbial Community
3.5. Effect of Bacillus on the Growth of P. tuoliensis Hyphae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galappaththi, M.C.A.; Dauner, L.; Madawala, S.; Karunarathna, S.C. Nutritional and medicinal benefits of Oyster (Pleurotus) mushrooms: A review. Fungal Biotec 2021, 1, 65–87. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.-Y.; Oh, Y.-L.; Oh, M.; Im, J.-H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2020, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Chen, Q.; Wu, X.; Gao, W.; Deng, W.; Huang, C. The famous cultivated mushroom Bailinggu is a separate species of the Pleurotus eryngii species complex. Sci. Rep. 2016, 6, 33066. [Google Scholar] [CrossRef]
- Jia, P.; Nurziya, Y.; Luo, Y.; Jia, W.; Zhu, Q.; Tian, M.; Sun, L.; Zhang, B.; Qi, Z.; Zhao, Z.; et al. Evolution and Genetic Differentiation of Pleurotus tuoliensis in Xinjiang, China, Based on Population Genomics. J. Fungi 2024, 10, 472. [Google Scholar] [CrossRef]
- Zhang, J.X.; Huang, C.Y.; Ng, T.B.; Wang, H.X. Genetic polymorphism of ferula mushroom growing on Ferula sinkiangensis. Appl. Microbiol. Biotechnol. 2006, 71, 304–309. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, C.; Chen, Q.; Wu, X.; Qu, J.; Zhang, J. Genetic variability and population structure of the mushroom Pleurotus eryngii var. tuoliensis. PLoS ONE 2013, 8, e83253. [Google Scholar] [CrossRef]
- Ren, Z.; Li, J.; Xu, N.; Zhang, J.; Song, X.; Wang, X.; Gao, Z.; Jing, H.; Li, S.; Zhang, C.; et al. Anti-hyperlipidemic and antioxidant effects of alkali-extractable mycelia polysaccharides by Pleurotus eryngii var. tuolensis. Carbohydr. Polym. 2017, 175, 282–292. [Google Scholar] [CrossRef]
- Xu, N.; Gao, Z.; Zhang, J.; Jing, H.; Li, S.; Ren, Z.; Wang, S.; Jia, L. Hepatoprotection of enzymatic-extractable mycelia zinc polysaccharides by Pleurotus eryngii var. tuoliensis. Carbohydr. Polym. 2017, 157, 196–206. [Google Scholar] [CrossRef]
- Yan, B.; Jing, L.; Wang, J. A polysaccharide (PNPA) from Pleurotus nebrodensis offers cardiac protection against ischemia–reperfusion injury in rats. Carbohydr. Polym. 2015, 133, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Cui, H.; Wang, Y.; Wang, Z.; Li, Z.; Chen, M.; Li, F. Bidirectional Immunomodulatory Activities of Polysaccharides Purified From Pleurotus nebrodensis. Inflammation 2014, 37, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, C.; Zhou, F.; Luo, X.; Li, J.; Zhao, J.; He, J.; Li, X.; Li, J. Chemical composition, antioxidant and antitumor activities of sub-fractions of wild and cultivated Pleurotus ferulae ethanol extracts. PeerJ 2018, 6, e6097. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, S.; Huang, Z.; Yin, L.; Hu, J.; Li, J.; Liu, Y.; Rong, C. Development of a highly productive strain of Pleurotus tuoliensis for commercial cultivation by crossbreeding. Sci. Hortic. 2018, 234, 110–115. [Google Scholar] [CrossRef]
- Gao, W.; Qu, J.; Zhang, J.; Sonnenberg, A.; Chen, Q.; Zhang, Y.; Huang, C. A genetic linkage map of Pleurotus tuoliensis integrated with physical mapping of the de novo sequenced genome and the mating type loci. BMC Genom. 2018, 19, 18. [Google Scholar] [CrossRef]
- Braat, N.; Koster, M.C.; Wösten, H.A.B. Beneficial interactions between bacteria and edible mushrooms. Fungal Biol. Rev. 2022, 39, 60–72. [Google Scholar] [CrossRef]
- Carrasco, J.; Preston, G.M. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef]
- Kumari, S.; Naraian, R. Enhanced growth and yield of oyster mushroom by growth-promoting bacteria Glutamicibacter arilaitensis MRC119. J. Basic Microb. 2021, 61, 45–54. [Google Scholar] [CrossRef]
- Zarenejad, F.; Yakhchali, B.; Rasooli, I. Evaluation of indigenous potent mushroom growth promoting bacteria (MGPB) on Agaricus bisporus production. World J. Microbiol. Biotechnol. 2012, 28, 99–104. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, G.; Wen, Y.; Li, T.; Gao, Y.; Meng, F.; Qiu, L.; Ai, Y. Pseudomonas sp. UW4 acdS gene promotes primordium initiation and fruiting body development of Agaricus bisporus. World J. Microbiol. Biotechnol. 2019, 35, 163. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, J.S.; Crowley, D.E.; Cho, B.G. Growth promotion of the edible fungus Pleurotus ostreatus by fluorescent pseudomonads. FEMS Microbiol. Lett. 2003, 218, 271–276. [Google Scholar] [CrossRef]
- Park, Y.; Park, Y.; Jang, M. Growth Characteristics of Pseudomonas putida and Pleurotus ostreatus After Co-Cultivation. Mycobiology 2025, 53, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Math, R.K.; Cho, K.M.; Shin, K.J.; Kim, J.O.; Ryu, J.S.; Lee, Y.H.; Yun, H.D. Effect of Pseudomonas sp. P7014 on the growth of edible mushroom Pleurotus eryngii in bottle culture for commercial production. Bioresour. Technol. 2008, 99, 3306–3308. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Yue, Y.; Wang, Q.; Xiao, T.; Zhao, Z.; Zhang, J.; Chen, H. Effects of the rice-mushroom rotation pattern on soil properties and microbial community succession in paddy fields. Front. Microbiol. 2024, 15, 1449922. [Google Scholar] [CrossRef] [PubMed]
- Pandin, C.; Védie, R.; Rousseau, T.; Le Coq, D.; Aymerich, S.; Briandet, R. Dynamics of compost microbiota during the cultivation of Agaricus bisporus in the presence of Bacillus velezensis QST713 as biocontrol agent against Trichoderma aggressivum. Biol. Control. 2018, 127, 39–54. [Google Scholar] [CrossRef]
- Xu, W.; Sun, T.; Du, J.; Jin, S.; Zhang, Y.; Bai, G.; Li, W.; Yin, D. Structure and ecological function of the soil microbiome associated with ‘Sanghuang’ mushrooms suffering from fungal diseases. BMC Microbiol. 2023, 23, 218. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Bockle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Cai, W.; Yao, H.; Feng, W.; Jin, Q.; Liu, Y.; Li, N.; Zheng, Z. Microbial Community Structure of Casing Soil During Mushroom Growth. Pedosphere 2009, 19, 446–452. [Google Scholar] [CrossRef]
- García-Montero, L.G.; Monleón, V.J.; Valverde-Asenjo, I.; Menta, C.; Kuyper, T.W. Niche construction by two ectomycorrhizal truffle species (Tuber aestivum and T. melanosporum). Soil Biol. Biochem. 2024, 189, 109276. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, J.; Xiong, C.; Li, X.; Chen, Z.; Li, P.; Huang, W. Tuber indicum shapes the microbial communities of ectomycorhizosphere soil and ectomycorrhizae of an indigenous tree (Pinus armandii). PLoS ONE 2017, 12, e175720. [Google Scholar] [CrossRef]
- Jo, J.; Oh, J.; Park, C. Microbial community analysis using high-throughput sequencing technology: A beginner’s guide for microbiologists. J. Microbiol. 2020, 58, 176–192. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Purahong, W.; Wubet, T.; Hyde, K.D.; Zhang, W.; Xu, H.; Zhang, G.; Fu, C.; Liu, M.; Xing, Q.; et al. Direct comparison of culture-dependent and culture-independent molecular approaches reveal the diversity of fungal endophytic communities in stems of grapevine (Vitis vinifera). Fungal Divers. 2018, 90, 85–107. [Google Scholar] [CrossRef]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Põlme, S.; Riit, T.; Liiv, I.; Kõljalg, U.; Kisand, V.; Nilsson, H.; Hildebrand, F.; et al. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. Mycokeys 2015, 10, 1–43. [Google Scholar] [CrossRef]
- Zeng, Q.; An, S. Identifying the Biogeographic Patterns of Rare and Abundant Bacterial Communities Using Different Primer Sets on the Loess Plateau. Microorganisms 2021, 9, 139. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Khashi, U.R.M.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Dubey, A.; Kumar, K.; Srinivasan, T.; Kondreddy, A.; Kumar, K. An invasive weed-associated bacteria confers enhanced heat stress tolerance in wheat. Heliyon 2022, 8, e9893. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Zhao, Q.; Yang, X.; Li, Y.; Zhou, H.; Zhao, M.; Zheng, H. Whole-genome analysis revealed the growth-promoting and biological control mechanism of the endophytic bacterial strain Bacillus halotolerans Q2H2, with strong antagonistic activity in potato plants. Front. Microbiol. 2023, 14, 1287921. [Google Scholar] [CrossRef]
- Chen, L.; Yan, M.; Qian, X.; Yang, Z.; Xu, Y.; Wang, T.; Cao, J.; Sun, S. Bacterial Community Composition in the Growth Process of Pleurotus eryngii and Growth-Promoting Abilities of Isolated Bacteria. Front. Microbiol. 2022, 13, 787628. [Google Scholar] [CrossRef]
- Dos Santos, H.R.M.; Argolo, C.S.; Argôlo-Filho, R.C.; Loguercio, L.L. A 16S rDNA PCR-based theoretical to actual delta approach on culturable mock communities revealed severe losses of diversity information. BMC Microbiol. 2019, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Xu, N.; Lei, C.; Zhang, Q.; Zhang, Z.; Sun, L.; He, F.; Zhou, N.; Peñuelas, J.; Zhu, Y.; et al. Bacterial biogeography in China and its association to land use and soil organic carbon. Soil Ecol. Lett. 2023, 5, 230172. [Google Scholar] [CrossRef]
- Tan, H.; Liu, T.; Yu, Y.; Tang, J.; Jiang, L.; Martin, F.M.; Peng, W. Morel Production Related to Soil Microbial Diversity and Evenness. Microbiol. Spectr. 2021, 9, e22921. [Google Scholar] [CrossRef]
- Vos, A.M.; Heijboer, A.; Boschker, H.T.S.; Bonnet, B.; Lugones, L.G.; Wösten, H.A.B. Microbial biomass in compost during colonization of Agaricus bisporus. Amb Express. 2017, 7, 12. [Google Scholar] [CrossRef]
- Mcgee, C.F.; Byrne, H.; Irvine, A.; Wilson, J. Diversity and dynamics of the DNA and cDNA-derived bacterial compost communities throughout the Agaricus bisporus mushroom cropping process. Ann Microbiol. 2017, 67, 751–761. [Google Scholar] [CrossRef]
- Snajdr, J.; Baldrian, P. Production of lignocellulose-degrading enzymes and changes in soil bacterial communities during the growth of Pleurotus ostreatus in soil with different carbon content. Folia Microbiol. 2006, 51, 579–590. [Google Scholar] [CrossRef]
- Banfi, R.; Pohner, Z.; Szabo, A.; Herczeg, G.; Kovacs, G.M.; Nagy, A.; Marialigeti, K.; Vajna, B. Succession and potential role of bacterial communities during Pleurotus ostreatus production. FEMS Microbiol. Ecol. 2021, 97, fiab125. [Google Scholar] [CrossRef]
- Shamugam, S.; Kertesz, M.A. Bacterial interactions with the mycelium of the cultivated edible mushrooms Agaricus bisporus and Pleurotus ostreatus. J. Appl. Microbiol. 2023, 134, lxac018. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P. The fungal holobiont: Evidence from early diverging fungi. Environ. Microbiol. 2017, 19, 2919–2923. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Q.; Chen, Y.; Gao, Y.; Yang, Y.; Liu, Q.; Kong, W.; Chai, H.; Sun, B.; Li, Y.; et al. The Mechanism of Ammonia-Assimilating Bacteria Promoting the Growth of Oyster Mushrooms (Pleurotus ostreatus). J. Fungi 2025, 11, 130. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Liu, H.; Jia, W.; Yalimaimaiti, N.; Zhu, Q.; Jia, P.; Huang, Y.; Shi, W.; Sun, C.; Guan, J. Field Inoculation of Pleurotus tuoliensis in Natural Habitat Promotes Microbial Communities That Enhance Its Growth. Agronomy 2025, 15, 1136. https://doi.org/10.3390/agronomy15051136
Luo Y, Liu H, Jia W, Yalimaimaiti N, Zhu Q, Jia P, Huang Y, Shi W, Sun C, Guan J. Field Inoculation of Pleurotus tuoliensis in Natural Habitat Promotes Microbial Communities That Enhance Its Growth. Agronomy. 2025; 15(5):1136. https://doi.org/10.3390/agronomy15051136
Chicago/Turabian StyleLuo, Ying, Hanbing Liu, Wenjie Jia, Nuerziya Yalimaimaiti, Qi Zhu, Peisong Jia, Yilin Huang, Wenting Shi, Chunhua Sun, and Jianhua Guan. 2025. "Field Inoculation of Pleurotus tuoliensis in Natural Habitat Promotes Microbial Communities That Enhance Its Growth" Agronomy 15, no. 5: 1136. https://doi.org/10.3390/agronomy15051136
APA StyleLuo, Y., Liu, H., Jia, W., Yalimaimaiti, N., Zhu, Q., Jia, P., Huang, Y., Shi, W., Sun, C., & Guan, J. (2025). Field Inoculation of Pleurotus tuoliensis in Natural Habitat Promotes Microbial Communities That Enhance Its Growth. Agronomy, 15(5), 1136. https://doi.org/10.3390/agronomy15051136





