Nitric Oxide in Plant Cold Stress: Functions, Mechanisms and Challenges
Abstract
1. Introduction
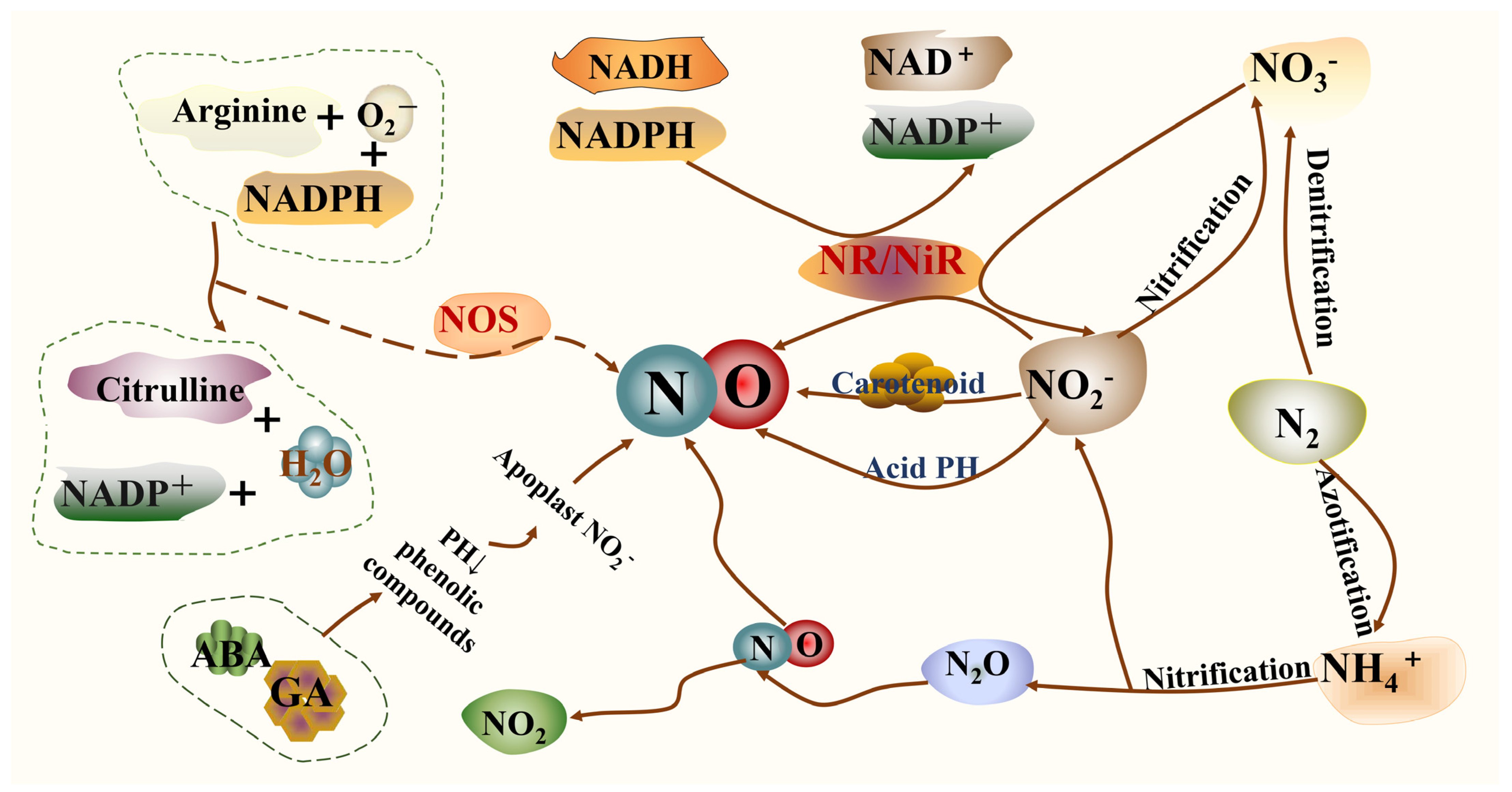
2. Synthesis of NO
3. NO Alleviates Cold Stress in Plants at Different Growth Stages
3.1. NO Promotes Seed Germination Under Cold Stress
3.2. NO Alleviates Cold Injury in Seedling Stage
3.3. NO Enhances Cold Resistance of Fruit
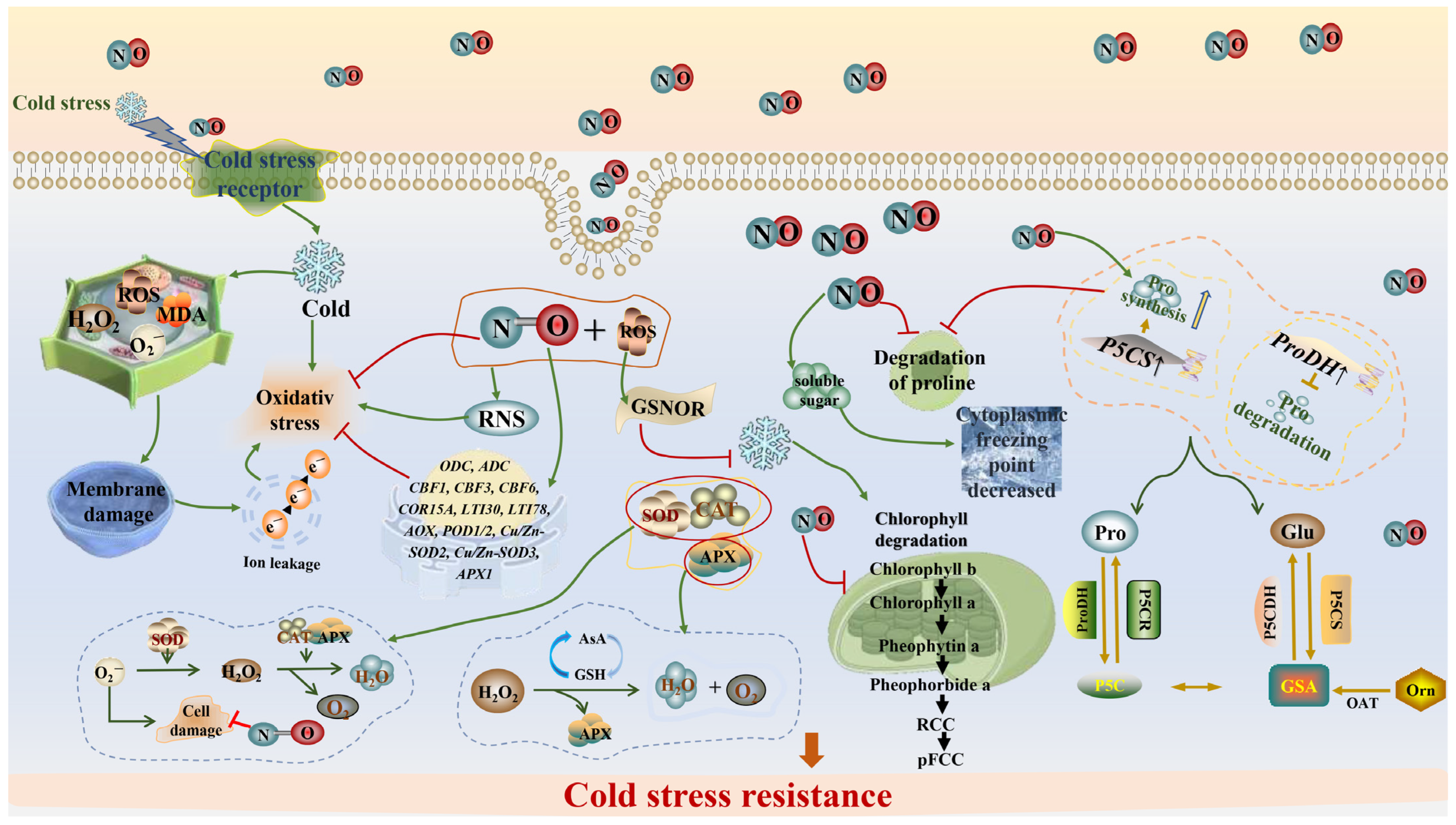
4. Physiological Effects of NO in Alleviating Cold Stress in Plants
4.1. Increase Antioxidant Capacity
4.2. Enhanced Photosynthesis
4.3. Protect Cell Membrane Structures
4.4. Promoting Osmoregulation
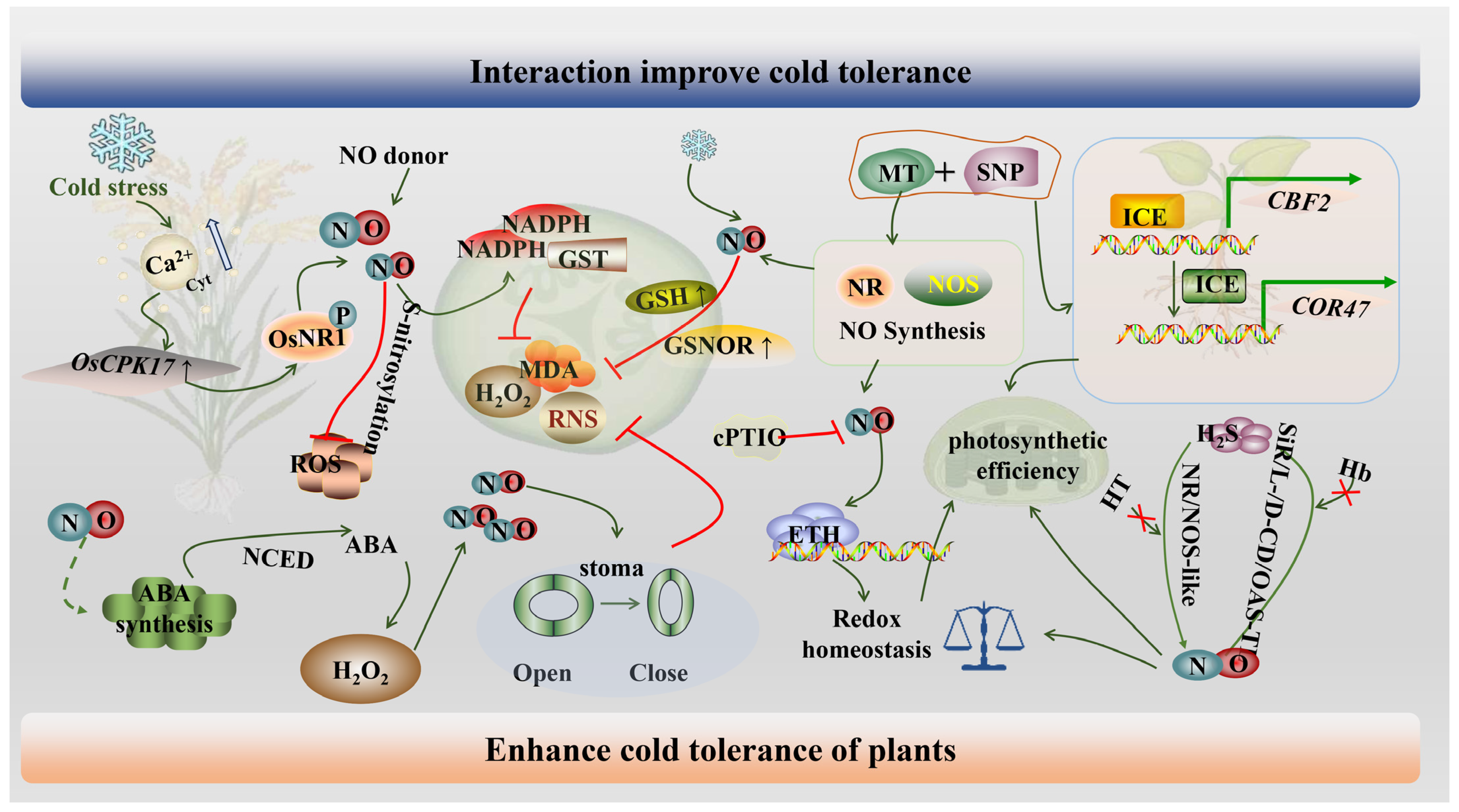
5. Molecular Mechanisms of NO Alleviates Plants Cold Stress
5.1. NO Regulates Gene Expression
5.2. NO Interacts with Signaling Molecules to Alleviate Cold Stress
5.2.1. NO Interacts with Ca2+
5.2.2. NO Interacts with ROS
5.2.3. NO Interacts with GSH
5.2.4. NO Interacts with MT
5.2.5. NO Interacts with ABA
5.2.6. NO Interacts with ETH
5.2.7. NO Interacts with H2S
6. Challenges of NO in Agricultural Production Practices
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Yang, H.; Xu, Q.; Wang, Y.; Sang, Z.; Yuan, H. Comparative metabolomics analysis of the response to cold stress of resistant and susceptible Tibetan hulless barley (Hordeum distichon). Phytochemistry 2020, 174, 112346. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. Sep. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Henderson, S.W.; Gilliham, M. The “Gatekeeper” Concept: Cell-type specific molecular mechanisms of plant adaptation to abiotic stress. In Molecular Mechanisms in Plant Adaptation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 83–115. [Google Scholar]
- Wang, X.; Ge, J.; He, M.; Li, Q.; Cai, J.; Zhou, Q.; Zhong, Y.; Wollenweber, B.; Jiang, D. Enhancing crop resilience: Understand-ing the role of drought priming in wheat stress response. Field Crops Res. 2023, 302, 109083. [Google Scholar] [CrossRef]
- Liang, J.; Lu, L.; Zhang, W.; Chi, M.; Shen, M.; An, C.; Chen, S.; Wang, X.; Liu, R.; Qin, Y.; et al. Comprehensive character-ization and expression analysis of enzymatic antioxidant gene families in passion fruit (Passiflora edulis). iScience 2023, 26, 108329. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; He, L.; Li, F. Understanding cold stress response mechanisms in plants: An overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar] [CrossRef]
- Puyaubert, J.; Baudouin, E. New clues for a cold case: Nitric oxide response to low temperature. Plant Cell Environ. 2014, 37, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Ren, L.; Zhang, J.; Reed, B.M.; Zhang, D.; Shen, X.H. Cryopreservation affects ROS-induced oxidative stress and antioxidant response in Arabidopsis seedlings. Cryobiology 2015, 70, 38–47. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Meng, L.; Feng, Y.; Zhao, M.; Jang, T.; Bi, H.; Ai, X. Hydrogen peroxide mediates melatonin-induced chilling tolerance in cucumber seedlings. Plant Cell Rep. 2024, 43, 279. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Xu, M.J.; Dong, J.F.; Zhang, M.; Xu, X.B.; Sun, L.N. Cold induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 512. [Google Scholar] [CrossRef]
- del Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M.; Gupta, D.K. Plant Superoxide Dismutases: Function Under Abiotic Stress Conditions; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–26. [Google Scholar]
- Sanders, D.; Brownlee, C.; Harper, J.F. Communicating with calcium. Plant Cell 1999, 11, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Wu, X.; Fang, H.; Huang, D.; Pan, X.; Liao, W. Role of protein S-nitrosylation in plant growth and development. Plant Cell Rep. 2024, 43, 204. [Google Scholar] [CrossRef]
- Andronis, E.A.; Moschou, P.N.; Toumi, I.; Roubelakis-Angelakis, K.A. Peroxisomal polyamine oxidase and NADPH-oxidase cross-talk for ROS homeostasis which affects respiration rate in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 132. [Google Scholar] [CrossRef]
- Song, Y.; Diao, Q.; Qi, H. Polyamine metabolism and biosynthetic genes expression in tomato (Lycopersicon esculentum Mill.) seedlings during cold acclimation. Plant Growth Regul. 2015, 75, 21–32. [Google Scholar] [CrossRef]
- Mutlu, S.; Karadağoğlu, Ö.; Atici, Ö.; Nalbantoğlu, B. Protective role of salicylic acid applied before cold stress on antioxidative system and protein patterns in barley apoplast. Biol. Plant. 2013, 57, 507–513. [Google Scholar] [CrossRef]
- Turkyilmaz Unal, B.; Mentis, O.; Akyol, E. Effects of exogenous salicylic acid on antioxidant activity and proline accumulation in apple (Malus domestica L.). Hortic. Environ. Biotechnol. 2015, 56, 606–611. [Google Scholar] [CrossRef]
- Wu, P.; Xiao, C.; Cui, J.; Hao, B.; Zhang, W.; Yang, Z.; Cui, H. Nitric oxide and its interaction with hydrogen peroxide enhance plant tolerance to low temperatures by improving the efficiency of the calvin cycle and the ascorbate-glutathione cycle in cucumber seedlings. J. Plant Growth Regul. 2020, 40, 2390–2408. [Google Scholar] [CrossRef]
- Yemets, A.I.; Karpets, Y.V.; Kolupaev, Y.E.; Blume, Y.B. Emerging Technologies for Enhancing ROS/RNS Homeostasis; Wiley: Hoboken, NJ, USA, 2019; pp. 873–922. [Google Scholar]
- Pandey, S.; Kumari, A.; Shree, M.; Kumar, V.; Singh, P.; Bharadwaj, C.; Gupta, K.J. Nitric oxide accelerates germination via the regulation of respiration in chickpea. J. Exp. Bot. 2019, 70, 4539–4555. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Liao, W.B.; Wang, M.; Niu, L.J.; Xu, Q.Q.; Jin, X. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J. Plant Physiol. 2016, 195, 50–58. [Google Scholar] [CrossRef]
- Seligman, K.; Saviani, E.E.; Oliveira, H.C.; Pinto Maglio, C.A.F.; Salgado, I. Floral transition and nitric oxide emission during flower development in Arabidopsis thaliana is affected in nitrate reductase-deficient plants. Plant Cell Physiol. 2008, 49, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gordo, S.; Bautista, R.; Claros, M.G.; Cañas, A.; Palma, J.M.; Corpas, F.J. Nitric oxide-dependent regulation of sweet pepper fruit ripening. J. Exp. Bot. 2019, 70, 4557–4570. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Du, M.; Jiang, X.; Huang, M.; Zhao, J. Nitric oxide acts as an inhibitor of postharvest senescence in horticultural products. Int. J. Mol. Sci. 2022, 23, 11512. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, S.M.; Zeng, K.F. Exogenous nitric oxide-induced postharvest disease resistance in citrus fruit to colletotrichum gloeosporioides. J. Sci. Food Agric. 2016, 96, 505–512. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.B. Nitric oxide fumigation for postharvest pest control on lettuce. Pest Manag. Sci. 2019, 75, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Modolo, L.; Cunha, F.; Braga, M.; Salgado, I. Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp. meridionalis elicitor. Plant Physiol. 2002, 130, 1288–1297. [Google Scholar] [CrossRef]
- Liu, Y.B. Nitric oxide fumigation for control of western flower thrips and its safety to postharvest quality of fresh fruit and vegetables. J. Asia-Pacific Entomol. 2016, 19, 1191–1195. [Google Scholar] [CrossRef]
- Lazar, E.E.; Wills, R.B.H.; Ho, B.T. Antifungal effect of gaseous nitric oxide on mycelium growth, sporulation and spore germination of the postharvest horticulture pathogens, Aspergillus niger, Monilinia fructicola and Penicillium italicum. Lett. Appl. Microbiol. 2008, 46, 688–692. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Ku, V.V.V.; Leshem, Y.Y. Fumigation with nitric oxide to extend the postharvest life of strawberries. Postharvest Biol. Technol. 2000, 18, 75–79. [Google Scholar] [CrossRef]
- Wang, J.; Higgins, V.J. Nitric oxide has a regulatory effect in the germination of conidia of Colletotrichum codes. Fungal Genet. Biol. 2005, 42, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Wang, Y.; Li, B.; Qin, G.; Tian, S. Defense responses of tomato fruit to exogenous nitric oxide during postharvest storage. Postharvest Biol. Technol. 2011, 62, 127–132. [Google Scholar] [CrossRef]
- Hu, M.; Yang, D.; Huber, D.J.; Jiang, Y.; Li, M.; Gao, Z.; Zhang, Z. Reduction of postharvest anthracnose and enhancement of disease resistance in ripening mango fruit by nitric oxide treatment. Postharvest Biol. Technol. 2014, 97, 115–122. [Google Scholar] [CrossRef]
- Kaya, C.; Polat, T.; Ashraf, M.; Kaushik, P.; Alyemeni, M.N.; Ahmad, P. Endogenous nitric oxide and its potential sources regulate glutathione-induced cadmium stress tolerance in maize plants. Plant Physiol. Bioch. 2021, 167, 723–737. [Google Scholar] [CrossRef]
- Wang, C.L.; Wei, L.J.; Zhang, J.; Hu, D.L.; Gao, R.; Liu, Y.Y.; Feng, L.; Gong, W.T.; Liao, W.B. Nitric oxide enhances salt tolerance in tomato seedlings by regulating endogenous S-nitrosylation levels. J. Plant Growth Regul. 2022, 42, 275–293. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath Kimura, S.D.; Mishra, J.; Arora, N.K. Salttolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Singh, A.; Aftab, T.; Ghosal, P.; Banik, N. Seedling priming with sodium nitroprusside rescues Vigna radiata from salinity stress induced oxidative damages. J. Plant Growth Regul. 2021, 40, 2454–2464. [Google Scholar] [CrossRef]
- Hayat, S.; Yadav, S.; Nasser Alyemeni, M.; Irfan, M.; Wani, A.S.; Ahmad, A. Alleviation of salinity stress with sodium nitroprusside in tomato. Int. J. Veg. Sci. 2013, 19, 164–176. [Google Scholar] [CrossRef]
- Li, Z.G.; Luo, L.J.; Sun, Y.F. Signal crosstalk between nitric oxide and hydrogen sulfide may be involved in hydrogen peroxide induced thermotolerance in maize seedlings. Russ. J. Plant Physiol. 2015, 62, 507514. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Hancock, J.T. Nitric oxide signalling in plants. New Phytol. 2003, 159, 1135. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 2008, 59, 2139. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Belghazi, M.; Filippou, P.; Fotopoulos, V.; Grigorios, D.; Molassiotis, A. Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants. Plant Mol. Biol. 2015, 89, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Chu, X.T.; Fu, J.J.; Yang, L.Y.; Hu, T.M. Crosstalk of nitric oxide with calcium induced tolerance of tall fescue leaves to high irradiance. Biol. Plant. 2016, 60, 376–384. [Google Scholar] [CrossRef]
- Diao, Q.N.; Song, Y.J.; Shi, D.M.; Qi, H.Y. Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) seedlings. Front. Plant Sci. 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lamattina, L.; Spoel, S.H. Nitric oxide function in plant biology: A redox cue in deconvolution. New Phytol. 2014, 202, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Acedo, G.N.; Cristinsin, M.; Conkling, M.A. Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc. Natl. Acad. Sci. USA 1992, 89, 1861–1864. [Google Scholar] [CrossRef]
- Durner, J.; Klessig, D.F. Nitric oxide as a signal in plants. Curr. Opin. Plant Biol. 1999, 2, 369–374. [Google Scholar] [CrossRef]
- Bethke, P.C.; Badger, M.R.; Jones, R.L. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 2004, 16, 332–341. [Google Scholar] [CrossRef]
- Wildt, D.; Kley, A.; Rockel, A.; Rockel, P.; Segschneider, H.J. Emission of no from several higher plant species. J. Geo. Res. 1997, 102, 5919–5927. [Google Scholar] [CrossRef]
- Santolini, J.; André, F.; Jeandroz, S.; Wendehenne, D. Nitric oxide synthase in plants: Where do we stand? Nitric Oxide 2017, 63, 30–38. [Google Scholar] [CrossRef]
- Fatima, A.; Husain, T.; Suhel, M.; Prasad, S.M.; Singh, V.P. Implication of nitric oxide under salinity stress: The possible interaction with other signaling molecules. J. Plant Growth Regul. 2022, 41, 163–177. [Google Scholar] [CrossRef]
- Gu, D.; Yang, J.; Wu, S.; Liao, Y.; Zeng, L.; Yang, Z. Epigenetic regulation of the phytohormone abscisic acid accumulation under dehydration stress during postharvest processing of tea (Camellia sinensis). Agric. Food Chem. 2021, 69, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, S.; Zhao, L.; Tan, J.; Zhang, Q.; Gao, F.; Wang, P.; Hou, H.; Li, L. Histone acetylation associated up-regulation of the cell wall related genes is involved in salt stress induced maize root swelling. BMC Plant Biol. 2014, 14, 105. [Google Scholar] [CrossRef]
- Fejes, G.; Bodor, T.; Szőllősi, R.; Kondak, S.; Kutasi, K.; Fotopoulos, V.; Kolbert, Z. Nitric oxide as integral element in priming- induced tolerance and plant stress memory. J. Exp. Bot. 2025, eraf033. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y.; et al. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS ONE 2021, 16, e0257236. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Nikzad, K. The role of nitric oxide in priming-induced low-temperature tolerance in two genotypes of tomato. Seed. Sci. Res. 2013, 23, 123–131. [Google Scholar] [CrossRef]
- Li, X.N.; Jiang, H.D.; Liu, F.L.; Cai, J.A.; Dai, T.B.; Cao, W.X.; Jiang, D. Induction of chilling tolerance in wheat during germination by presoaking seed with nitric oxide and gibberellin. Plant Growth Regul. 2013, 71, 3140. [Google Scholar] [CrossRef]
- Ning, K.; Sun, T.; Wang, Z.; Li, H.; Fang, P.; Cai, X.; Wu, X.; Xu, M.; Xu, P. Selective penetration of fullerenol through pea seed coats mitigates osmosis-repressed germination via chromatin remodeling and transcriptional reprograming. J. Sci. Food Agric. 2024, 104, 6008–6017. [Google Scholar] [CrossRef]
- Belin, C.; Lopez-Molina, L. Arabidopsis seed germination responses to osmotic stress involve the chromatin modifier PICKLE. Plant Signal Behav. 2008, 3, 478–479. [Google Scholar] [CrossRef][Green Version]
- Bai, X.; Chen, J.; Kong, X.; Todd, C.D.; Yang, Y.; Hu, X.; Li, D. Carbon monoxide enhances the chilling tolerance of recalcitrant Baccaurea ramiflora seeds via nitric oxide-mediated glutathione homeostasis. Free Radic. Biol. Med. 2012, 53, 710–720. [Google Scholar] [CrossRef]
- Zhao, M.G.; Chen, L.; Zhang, L.L.; Zhang, W.H. Nitric reductase dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Zaharah, S.S.; Singh, Z. Postharvest nitric oxide fumigation alleviates chilling injury, delays fruit ripening and maintains quality in cold-stored ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2011, 60, 202–210. [Google Scholar] [CrossRef]
- Liu, L.Q.; Yu, D.; Guan, J.F. Effects of nitric oxide on the quality and pectin metabolism of Yali pears during cold storage. Agric. Sci. China 2011, 10, 1125–1133. [Google Scholar] [CrossRef]
- Ghorbani, B.; Pakkish, Z.; Khezri, M. Nitric oxide increases antioxidant enzyme activity and reduces chilling injury in orange fruit during storage. N. Z. J. Crop Hortic. Sci. 2018, 46, 101–116. [Google Scholar] [CrossRef]
- Saba, M.K.; Moradi, S. Sodium nitroprusside (SNP) spray to maintain fruit quality and alleviate postharvest chilling injury of peach fruit. Sci. Hortic. 2017, 216, 193–199. [Google Scholar] [CrossRef]
- Fan, J.B.; Chen, K.; Amombo, E.; Hu, Z.R.; Chen, L.; Fu, J.M.; Reigosa, M. Physiological and molecular mechanism of nitric oxide (NO) involved in bermudagrass response to cold stress. PLoS ONE 2015, 10, e0132991. [Google Scholar] [CrossRef]
- Dong, N.G.; Li, Y.F.; Qi, J.X.; Chen, Y.H.; Hao, Y.B. Nitric oxide synthase dependent nitric oxide production enhances chilling tolerance of walnut shoots in vitro via involvement chlorophyll fluorescence and other physiological parameter levels. Sci. Hortic. 2018, 230, 68–77. [Google Scholar] [CrossRef]
- Song, C.C.; Zhao, Y.Y.; Li, A.; Qi, S.N.; Lin, Q.; Duan, Y.Q. Postharvest nitric oxide treatment induced the alternative oxidase pathway to enhance antioxidant capacity and chilling tolerance in peach fruit. Plant Physiol. Biochem. 2021, 167, 113–122. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.; Guo, Z.; Lu, S. Nitric oxide regulates glutathione synthesis and cold tolerance in forage legumes. Environ. Exp. Bot. 2019, 167, 103851. [Google Scholar] [CrossRef]
- Fu, J.J.; Chu, X.T.; Sun, Y.F.; Miao, Y.J.; Xu, Y.F.; Hu, T.M. Nitric oxide mediates 5Aminolevulinic acid induced antioxidant defense in leaves of Elymus nutans Griseb. exposed to chilling stress. PLoS ONE 2016, 10, e0130367. [Google Scholar]
- Feng, Y.; Fu, X.; Han, L.; Xu, C.; Liu, C.; Bi, H.; Ai, X. Nitric oxide functions as a downstream signal for melatonin-induced cold tolerance in cucumber seedlings. Front. Plant Sci. 2021, 12, 686545. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Luo, Z.S.; Mao, L.C.; Ying, T.J. Contribution of polyamines metabolism and GABA shunt to chilling tolerance induced by nitric oxide in cold stored banana fruit. Food Chem. 2016, 197, 333–339. [Google Scholar] [CrossRef]
- Esim, N.; Atici, O. Nitric oxide improves chilling tolerance of maize by affecting apoplastic antioxidative enzymes in leaves. Plant Growth Regul. 2014, 72, 2938. [Google Scholar] [CrossRef]
- Mu, X.J. Physiological mechanism of exogenous nitric oxide on alleviating low temperature stress of Phalaenopsis spp. J. Fruit. Sci. 2015, 10, 12240. [Google Scholar]
- Li, Y.; Hoch, G. The sensitivity of root water uptake to cold root temperature follows species-specific upper elevational distribution limits of temperate tree species. Plant Cell Environ. 2024, 47, 2192–2205. [Google Scholar] [CrossRef]
- Plohovska, S.H.; Krasylenko, Y.A.; Yemets, A.I. Nitric oxide modulates actin filament organization in Arabidopsis thaliana primary root cells at low temperatures. Cell Biology Int. 2018, 43, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Leterrier, M.; Valderrama, R.; Airaki, M.; Chaki, M.; Palma, J.M.; Barroso, J.B. Nitric oxide imbal-ance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011, 181, 604–611. [Google Scholar] [CrossRef]
- Singh, D.; Chaudhary, P.; Taunk, J.; Kumar Singh, C.; Sharma, S.; Singh, V.J.; Singh, D.; Chinnusamy, V.; Yadav, R.; Pal, M. Plant epigenomics for extenuation of abiotic stresses: Challenges and future perspectives. J. Exp. Bot. 2021, 72, 6836–6855. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; He, J.; Liu, H.; Liu, G.; Ren, X. Nitric oxide alleviates deterioration and preserves antioxidant properties in ‘Tainong’ mango fruit during ripening. Hortic. Environ. Biotechnol. 2017, 58, 27–37. [Google Scholar] [CrossRef]
- Dong, J.F.; Qin, Y.; Li, L.; Xu, M.J. Effect of yeast saccharide treatment on nitric oxide accumulation and chilling injury in cucumber fruit during cold storage. Postharvest Biol. Technol. 2012, 68, 1–7. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Xu, Y.; Limwachiranon, J.; Li, D.; Ban, Z.; Luo, Z. Effect of exogenous nitro oxide on chilling tolerance, polyamine, proline, and gamma-aminobutyric acid in bamboo shoots (Phyllostachys praecox f. prevernalis). J. Agric. Food Chem. 2017, 65, 5607–5613. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Muñoz, R.; Palma, F.; Carvajal, F.; Castro-Cegrí, A.; Pulido, A.; Jamilena, M.; Garrido, D. Pre-storage nitric oxide treatment enhances chilling tolerance of zucchini fruit (Cucurbita pepo L.) by S-nitrosylation of proteins and modulation of the antioxidant response. Postharvest Biol. Technol. 2021, 171, 111345. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, P.; Singh, P.; Kumar, V.; Yadav, P.; Sharma, A. Nitric oxide: An emerging warrior of plant physiology under abiotic stress. Nitric Oxide 2023, 140–141, 58–76. [Google Scholar] [CrossRef]
- Hyun, T.K. Plant histone modifications in response to cold stress. Bot. Serbica 2022, 46, 1–6. [Google Scholar] [CrossRef]
- Chhatwal, H.; Naik, J.; Pandey, A.; Trivedi, P.K. Broadening the epigenetic horizon of abiotic stress response in plants. Plant Growth Regul. 2024, 103, 491–501. [Google Scholar] [CrossRef]
- Sougrakpam, Y.; Babuta, P.; Deswal, R. Nitric oxide (NO) modulates low temperature-stress signaling via S-nitrosation, a NO PTM, inducing ethylene biosynthesis inhibition leading to enhanced post-harvest shelf-life of agricultural produce. Physiol. Mol. Biol. Plant. 2023, 29, 2051–2065. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Wang, C.; Liao, W. Recent progress in the knowledge on the alleviating effect of nitric oxide on heavy metal stress in plants. Plant Physiol. Biochem. 2022, 147, 161–171. [Google Scholar] [CrossRef]
- Wang, C.L.; Huang, Y.M.; Luo, X. The effects of exogenous ABA on the reactive oxygen metabolism of pitaya seedlings under low temperature stress. Acta Bot. Boreal. Occident. Sin. 2023, 43, 1344–1351. [Google Scholar]
- Sinha, S.; Kukreja, B.; Arora, P.; Sharma, M.; Pandey, G.K.; Agarwal, M.; Pandey, G.K. The Omics of Cold Stress Responses in Plants; Springer: Berlin/Heidelberg, Germany, 2015; pp. 143–194. [Google Scholar]
- Wu, J.C.; Chen, W.J.; Cai, L.Q.; Xie, C.P.; Huang, S.J.; Lin, L.J.; Ye, M.L. Effects of exogenous nitric oxide on anti-oxidation capacities in young loquat fruits under low temperature stress. Scientia 2010, 46, 73–78. [Google Scholar]
- Arora, D.; Jain, P.; Singh, N.; Kaur, H.; Bhatla, S.C. Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free Radic. Res. 2016, 50, 291–303. [Google Scholar] [CrossRef]
- Hancock, J.T.; Whiteman, M. Hydrogen sulfide signaling: Interactions with nitric oxide and reactive oxygen species. Ann. N. Y. Acad. Sci. 2016, 1365, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Chen, J.; EF, A.; Wang, P.; Wang, G.; Hu, X.; Shi, J. Quantitative proteomics analysis reveals that S-nitrosoglutathione reductase (GSNOR) and nitric oxide signaling enhance poplar defense against chilling stress. Planta 2015, 243, 1081. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Ashraf, M. Regulation in plant stress tolerance by a potential plant growth regulator, 5aminolevulinic acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, F.; Nong, S.; Liao, J.; Xing, A.; Shen, Q. Effects of nitric oxide on the GABA, polyamines, and proline in tea (Camellia sinensis) roots under cold stress. Sci. Rep. 2020, 10, 12240. [Google Scholar] [CrossRef] [PubMed]
- Lamsaadi, N.; Farssi, O.; El Moukhtari, A.; Farissi, M. Different approaches to improve the tolerance of aromatic and medicinal plants to salt stressed conditions. J. Appl. Res. Med. Aromat. Plants 2024, 39, 100532. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Wu, X.H.; Lv, C.M.; Feng, J.M. Protective effect of exogenous nitric oxide against oxidative damage in pumpkin seedlings under chilling stress. Acta Pratacult. Sin. 2016, 25, 161–169. [Google Scholar]
- Grgić, M.; Vitko, S.; Drmić, J.; Leljak-Levanić, D. Connecting the dots: Epigenetics, ABA, and plant stress tolerance. Acta Bot. Croat. 2024, 84. [Google Scholar] [CrossRef]
- Ma, L.; Xing, L.; Li, Z.; Jiang, D. Epigenetic control of plant abiotic stress responses. J Genet. Genom. 2025, 52, 129–144. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wang, N.N.; Zhang, Y.H.; Feng, Q.Z.; Yang, C.W.; Liu, B. DNA methylation involved in proline accumulation in response to osmotic stress in rice (Oryza sativa). Genet. Mol. Res. 2013, 12, 1269–1277. [Google Scholar] [CrossRef]
- Liu, Z.; Bi, S.; Meng, J.; Liu, T.; Li, P.; Yu, C.; Peng, X. Arbuscular mycorrhizal fungi enhanced rice proline metabolism under low temperature with nitric oxide involvement. Front. Plant Sci. 2022, 13, 962460. [Google Scholar] [CrossRef] [PubMed]
- Kolupaev, Y.E.; Yemets, A.I.; Yastreb, T.O.; Blume, Y.B. The role of nitric oxide and hydrogen sulfide in regulation of redox homeostasis at extreme temperatures in plants. Front. Plant Sci. 2023, 14, 1128439. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, E.; Jeandroz, S. Nitric Oxide as a Mediator of Cold Stress Response: A Transcriptional Point of View; Springer: Berlin/Heidelberg, Germany, 2015; pp. 129–139. [Google Scholar]
- Cantrel, C.; Vazquez, T.; Puyaubert, J.; Rezé, N.; Lesch, M.; Kaiser, W.M.; Dutilleul, C.; Guillas, I.; Zachowski, A.; Baudouin, E. Nitric oxide participates in coldresponsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011, 189, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.R.; Sheng, J.P.; Lv, S.N.; Zheng, Y.; Zhang, J.; Yu, M.M.; Shen, L. Nitric oxide participates in the regulation of LeCBF1 gene expression and improves cold tolerance in harvested tomato fruit. Postharvest Biol. Technol. 2011, 62, 121–126. [Google Scholar] [CrossRef]
- Zhang, T.; Che, F.; Zhang, H.; Pan, Y.; Xu, M.; Ban, Q.; Han, Y.; Rao, J. Effect of nitric oxide treatment on chilling injury, antioxidant enzymes and expression of the CmCBF1 and CmCBF3 genes in cold-stored Hami melon (Cucumis melo L.) fruit. Postharvest Biol. Technol. 2017, 127, 88–98. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.; Zhao, P.; Guo, Z.; Lu, S. Comparative physiological analysis reveals the role of nr-derived nitric oxide in the cold tolerance of forage legumes. Int. J. Mol. Sci. 2019, 20, 1368. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Defining robust redox signalling within the context of the plant cell. Plant Cell Environ. 2015, 38, 239. [Google Scholar] [CrossRef]
- Rojo, A.; Salinas, M.; Martin, D.; Perona, R.; Cuadrado, A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappa B. J. Neurosci. 2004, 24, 7324–7334. [Google Scholar] [CrossRef]
- Duszyn, M.; Świeżawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Cyclic nucleotide gated channels (CNGCs) in plant signalling-current knowledge and perspectives. J. Plant Physiol. 2019, 241, 153035. [Google Scholar] [CrossRef]
- Zhang, W.L.; Cao, J.K.; Fan, X.G.; Jiang, W.B. Applications of nitric oxide and melatonin in improving postharvest fruit quality and the separate and crosstalk biochemical mechanisms. Trends Food Tech. 2020, 99, 531–541. [Google Scholar] [CrossRef]
- He, H.; He, L.F. Crosstalk between melatonin and nitric oxide in plant development and stress responses. Physiol. Plantarum 2020, 170, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Yang, T.; Poovaiah, B.W. Calcium signaling-mediated plant response to cold stress. Int. J. Mol. Sci. 2018, 19, 3896. [Google Scholar] [CrossRef]
- Lv, X.Z.; Li, H.Z.; Chen, X.X.; Xiang, X.; Guo, Z.X.; Yu, J.Q.; Zhou, Y.H. The role of calcium dependent protein kinase in hydrogen peroxide, nitric oxide and ABA dependent cold acclimation. J. Exp. Bot. 2018, 69, 4127–4139. [Google Scholar] [CrossRef] [PubMed]
- Almadanim, M.C.; Alexandre, B.M.; Rosa, M.T.G.; Sapeta, H.; Leitão, A.E.; Ramalho, J.C.; Lam, T.T.; Negrão, S.; Abreu, I.A.; Oliveira, M.M. Rice calcium-dependent protein kinase OsCPK17 targets plasma membrane intrinsic protein and sucrose-phosphate synthase and is required for a proper cold stress response. Plant Environ. 2017, 40, 1197–1213. [Google Scholar] [CrossRef]
- Ma, Z.G.; Marsolais, F.; Bykova, N.V.; Igamberdiev, A.U. Nitric oxide and reactive oxygen species mediate metabolic changes in barley seed embryo during germination. Front. Plant Sci. 2016, 7, 138. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Sandalio, L.M. Nitric oxide level is self-regulating and also regulates its ROS partners. Front. Plant Sci. 2016, 7, 316. [Google Scholar] [CrossRef]
- Gaupels, F.; Furch, A.C.U.; Zimmermann, M.R.; Chen, F.; Kaever, V.; Buhtz, A. Corrigendum: Systemic induction of no-, redox-, and cgmp signaling in the pumpkin extra fascicular phloem upon local leaf wounding. Front. Plant Sci. 2016, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Du, M.; Zhao, J.; Dong, X. The role of nitric oxide in defending against chilling stress in postharvest crops. Food Bioscience 2023, 56, 10335. [Google Scholar] [CrossRef]
- Sehrawat, A.; Deswal, R. S-nitrosylation analysis in brassica juncea apoplast highlights the importance of nitric oxide in cold-stress signaling. J. Proteome Res. 2014, 13, 2599–2619. [Google Scholar] [CrossRef]
- Qari, S.H.; Hassan, M.; Chattha, M.U.; Mahmood, A.; Naqve, M.; Nawaz, M. Melatonin induced cold tolerance in plants: Physiological and molecular responses. Front. Plant Sci. 2022, 13, 843071. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Bioch. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Hu, M.; Pan, Y.; Jiang, Y.; Zhang, Z.; Jiang, G. Nitric oxide is involved in melatonin-induced cold tolerance in postharvest litchi fruit. Postharvest Biol. Technol. 2023, 196, 112157. [Google Scholar] [CrossRef]
- Sun, C.L.; Liu, L.J.; Lu, L.L.; Jin, C.W.; Lin, X.Y. Nitric oxide acts downstream of hydrogen peroxide in regulating aluminum induced antioxidant defense that enhances aluminum resistance in wheat seedlings. Environ. Exp. Bot. 2018, 145, 95–103. [Google Scholar] [CrossRef]
- Wu, S.W.; Hu, C.X.; Tan, Q.L.; Zhao, X.H.; Xu, S.J.; Xia, Y.T. Nitric oxide acts downstream of abscisic acid in molybdenum induced oxidative tolerance in wheat. Plant Cell Rep. 2018, 37, 112. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.G.; Qi, J.X.; Li, Y.F.; Chen, Y.H.; Hao, Y.B. Effects of abscisic acid and nitric oxide on chilling resistance and activation of the antioxidant system in walnut shoots in vitro. J. Am. Soc. Hortic. Sci. 2017, 142, 376–384. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Geng, B.; Feng, J.; Zhu, S. Interactive effects of abscisic acid and nitric oxide on chilling resistance and active oxygen metabolism in peach fruit during cold storage. J. Sci. Food Agric. 2019, 99, 3367–3380. [Google Scholar] [CrossRef]
- Tian, W.; Huang, D.; Geng, B.; Zhang, Q.; Feng, J.; Zhu, S. Regulation of the biosynthesis of endogenous nitric oxide and abscisic acid in stored peaches by exogenous nitric oxide and abscisic acid. J. Sci. Food Agric. 2020, 100, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Jahed, K.R.; Saini, A.K.; Sherif, S.M. Coping with the cold: Unveiling cryoprotectants, molecular signaling pathways, and strategies for cold stress resilience. Front. Plant Sci. 2023, 14, 1246093. [Google Scholar] [CrossRef]
- Han, S.; Cai, H.; An, X.; Huan, C.; Wu, X.; Jiang, L.; Mingliang, Y.; Ruijuan, M.; Yu, Z. Effect of nitric oxide on sugar metabolism in peach fruit (cv. Xiahui 2018, 6) during cold storage. Postharvest Biol. Technol. 2018, 142, 72–80. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z.; Swinny, E.E. Postharvest nitric oxide fumigation delays fruit ripening and alleviates chilling injury during cold storage of japanese plums (Prunus salicina Lindell). Postharvest Biol. Technol. 2009, 53, 101–108. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Yu, M.; Ma, R.; Yu, Z. Exogenous nitric oxide fumigation promoted the emission of volatile organic compounds in peach fruit during shelf life after long-term cold storage. Food Res. Int. 2020, 133, 109135. [Google Scholar] [CrossRef] [PubMed]
- Barman, K.; Asrey, R.; Pal, R.K.; Jha, S.K.; Bhatia, K. Post-harvest nitric oxide treatment reduces chilling injury and enhances the shelf-life of mango (Mangifera indica L.) fruit during low-temperature storage. J. Hortic. Sci. Biotech. 2014, 89, 253–260. [Google Scholar] [CrossRef]
- Qian, C.; Ji, Z.; Zhu, Q.; Qi, X.; Li, Q.; Yin, J.; Liu, J.; Kan, J.; Zhang, M.; Jin, C. Effects of 1-MCP on proline, polyamine, and nitric oxide metabolism in postharvest peach fruit under chilling stress. Hortic. Plant J. 2021, 7, 188–196. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Hu, S.; Hou, Y.; Wang, J.; Zheng, Y.; Jin, P. Hydrogen sulfide-induced chilling resistance in peach fruit is performed via sustaining the homeostasis of ROS and RNS. Food Chem. 2023, 398, 133940. [Google Scholar] [CrossRef]
- Geng, B.; Huang, D.; Zhu, S. Regulation of hydrogen sulfide metabolism by nitric oxide inhibitors and the quality of peaches during cold storage. Antioxidants 2019, 8, 401. [Google Scholar] [CrossRef]
- Wu, G.; Cai, B.; Zhou, C.; Li, D.; Bi, H.; Ai, X. Hydrogen sulfide-induced chilling tolerance of cucumber and involvement of nitric oxide. J. Plant Biol. Res. 2016, 5, 58–69. [Google Scholar]
- Pei, Y.; Wang, Z.; Yan, W.; Zhou, B. Characterization of ascorbate-glutathione cycle response in Zostera marina seedlings under short-term temperature surge. Front. Mar. Sci. 2024, 11, 1390074. [Google Scholar] [CrossRef]
- Kunert, K.J.; Foyer, C.H. The ascorbate/glutathione cycle. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2023; pp. 77–112. [Google Scholar]
| Temperature | Treatment | Plants Used | Impact | Reference |
|---|---|---|---|---|
| −8 °C | NOC-18 (30 Μm) | Baccaurea ramiflora | It increased the GSH and GSNOR accumulation activities in Bacillus goatensis embryos, enhanced the activities of antioxidant enzymes involved in glutathione ascorbic acid cycle, reduced the contents of H2O2 and RNS, and improved the tolerance of seeds to low temperature stress | [63] |
| −7 °C | SNP (200 μM) | Arabidopsis | SNP effectively increased the survival rate of Arabidopsis seedlings. | [64] |
| 0 °C | Nitric oxide (10/20/40 µL L−1) | Mango | Reduced ETH production during fruit ripening after cold storage, reduced cold storage damage, delayed fruit color development, and significantly reduced softening and ripening of refrigerated mango fruits. | [65] |
| 0 °C | Nitric oxide (20 µL L−1) | Mango | After 60 days of NO fumigation, ETH production and soluble sugar content decreased, and fruit softening and ripening were simultaneously delayed. | [66] |
| 3 °C | SNP (500 μM) | Citrus sinensis L. | Induced antioxidant levels, decreased H2O2 content and lipid peroxidation levels. | [67] |
| 4 °C | SNP (25/50 μM) | Prunus persica L. | Suppressing ETH production, maintaining firmness, antioxidant capacity and vitamin C and enhancing anti-oxidative enzyme activity | [68] |
| 4 °C | SNP (100 μM) | Bermudagrass | The activities of SOD, POD and CAT were increased. | [69] |
| 4 °C | SNP (100 mM) | Walnut | Alleviated the decrease of and chlorophyll fluorescence parameters, reduced ion leakage and lipid peroxidation, improved photosynthetic efficiency, increased GSH and GSH/GSSG ratio, promoted proline accumulation and inhibited proline degradation. | [70] |
| 4 °C | SNP (15 μM) | Prunus persica L. | The alternate oxidase pathway is activated, thereby increasing antioxidant levels. | [71] |
| 5 °C | SNP (100 μM) | Alfalfa | Exogenous addition of 100 μM diethylamine NONOate diethylamine salt (DEA, NO donor) increased total glutathione levels in mesophyll cells of both cold tolerant and cold intolerant alfalfa. | [72] |
| 5 °C | SNP (100 μM) | Elymus nutans | Elymus nutans seedlings showed significant increases in root surface area, root volume, root diameter, and root tip number and the activities of SOD, CAT, APX and GR were increased. | [73] |
| 5 °C | SNP (75 μM) | Cucumber | MT and SNP interacted to reduce electrolyte leakage (EL), MDA and ROS accumulation in cucumber seedlings by activating antioxidant system. | [74] |
| 7 °C | SNP (50 μM) | Banana | The chilling index was decreased, and the accumulation of PAs, GABA and proline was enhanced. | [75] |
| 10/7 °C | SNP (0.1 μM) | Maize | The CI of leaves was decreased in the SNP treatment and the activities of SOD and POD were increased, and the accumulation of ROS and MDA was reduced. | [76] |
| 10 °C | SNP (200 μM) | Tomato | SNP treatment significantly increased the amylase activity and soluble sugar content of tomato seeds, and improved the low temperature tolerance of tomato seeds. | [59] |
| 12 °C/7 °C | SNP (200 μM) | Phalanthi orchid | SNP can inhibit electrolyte leakage caused by cold stress, maintain intracellular and extracellular ion balance, prevent cell dehydration and death, and protect the cell membrane system. | [77] |
| 12 ± 0.5 °C | SNP (100 μM) | Winter wheat | SNP effectively alleviated the inhibition of cold stress on seed germination and increased germination rate, germination index, radicle and coleoptile length of seeds under cold stress. | [60] |
| 15 °C | SNP (300 μM) | Brassica napus L. | The cold resistance of rapeseed was significantly improved, thus the seed germination rate was increased | [58] |
| Gas Molecule | Species | Gene | Expression | Function | Reference |
|---|---|---|---|---|---|
| NO | Arabidopsis | P5CS | ↑ | Positive | [108] |
| Arabidopsis | ProDH | ↓ | Positive | [98] | |
| Tomato | CBF1 | ↑ | Positive | [107] | |
| Arabidopsis | CBF1, CBF3, | ↑ | Positive | [108] | |
| COR15A, LTI30, LTI78 | |||||
| Tomato | ODC, ADC, ADC1 | ↑ | Positive | [47] | |
| Cantaloupe | CmCBF1, CmCBF3 | ↑ | Positive | [110] | |
| Medicago falcata | Cu/Zn-SOD2, Cu/Zn-SOD3, CAT APX1 | ↑ | Positive | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Huang, M.; Qi, J.; Yu, W.; Li, C. Nitric Oxide in Plant Cold Stress: Functions, Mechanisms and Challenges. Agronomy 2025, 15, 1072. https://doi.org/10.3390/agronomy15051072
Cui J, Huang M, Qi J, Yu W, Li C. Nitric Oxide in Plant Cold Stress: Functions, Mechanisms and Challenges. Agronomy. 2025; 15(5):1072. https://doi.org/10.3390/agronomy15051072
Chicago/Turabian StyleCui, Jing, Mengxiao Huang, Jin Qi, Wenjin Yu, and Changxia Li. 2025. "Nitric Oxide in Plant Cold Stress: Functions, Mechanisms and Challenges" Agronomy 15, no. 5: 1072. https://doi.org/10.3390/agronomy15051072
APA StyleCui, J., Huang, M., Qi, J., Yu, W., & Li, C. (2025). Nitric Oxide in Plant Cold Stress: Functions, Mechanisms and Challenges. Agronomy, 15(5), 1072. https://doi.org/10.3390/agronomy15051072







