Effects of Tropical Typical Organic Materials on Soil Physicochemical Properties and Microbial Community Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Pot Experiment in the Field
2.3. Analysis of Soil pH/EC, Ca/Mg, Total C, and N
2.4. Soil Organic Acids Analysis
2.5. Soil Aggregate Structure
2.6. FTIR Analysis
2.7. Methods of Microbial Amplicon Sequencing Experiment
2.8. Statistics
3. Results
3.1. Soil pH/EC and Organic Acid Content Changes
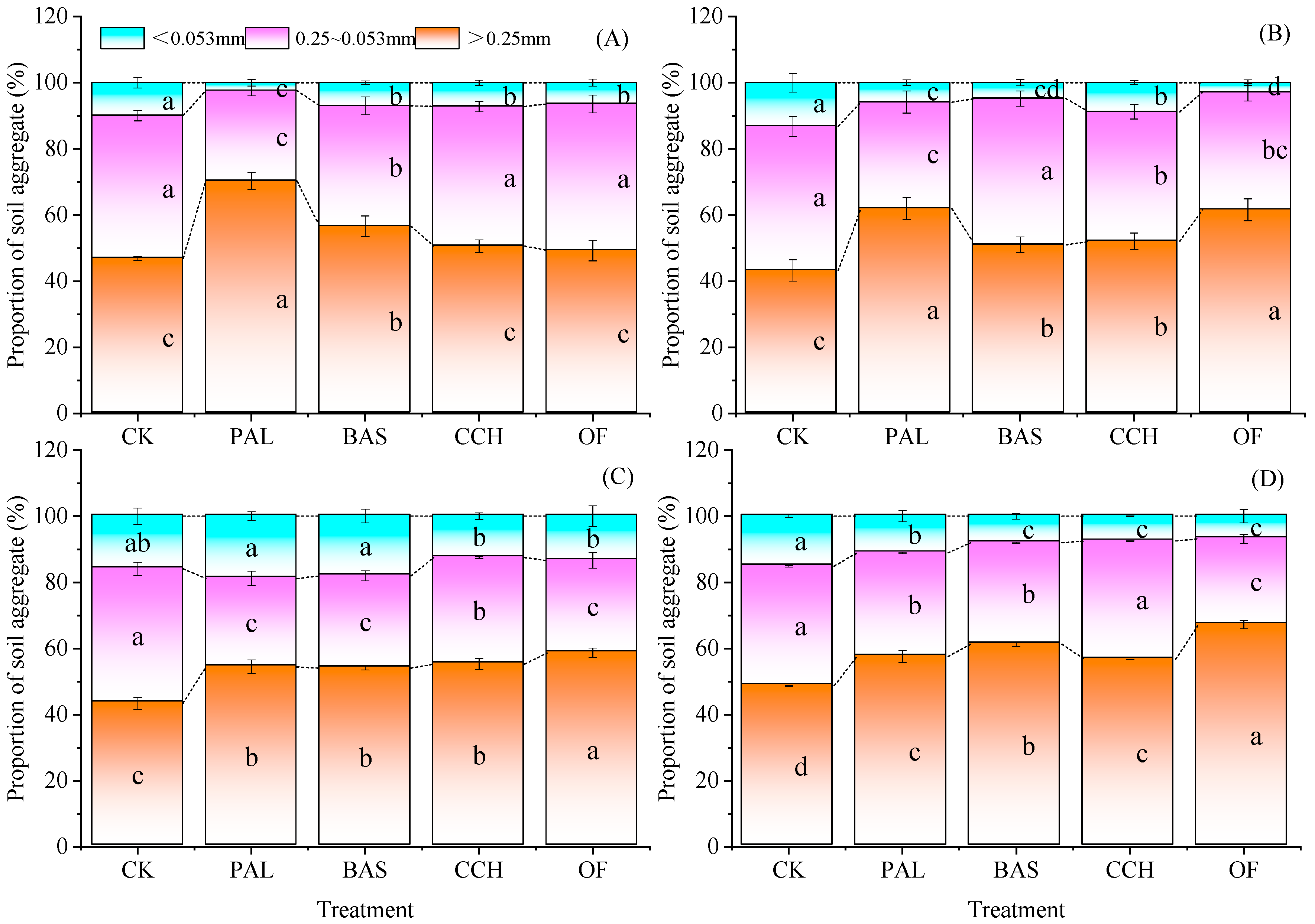
3.2. Changes of Aggregate Structure
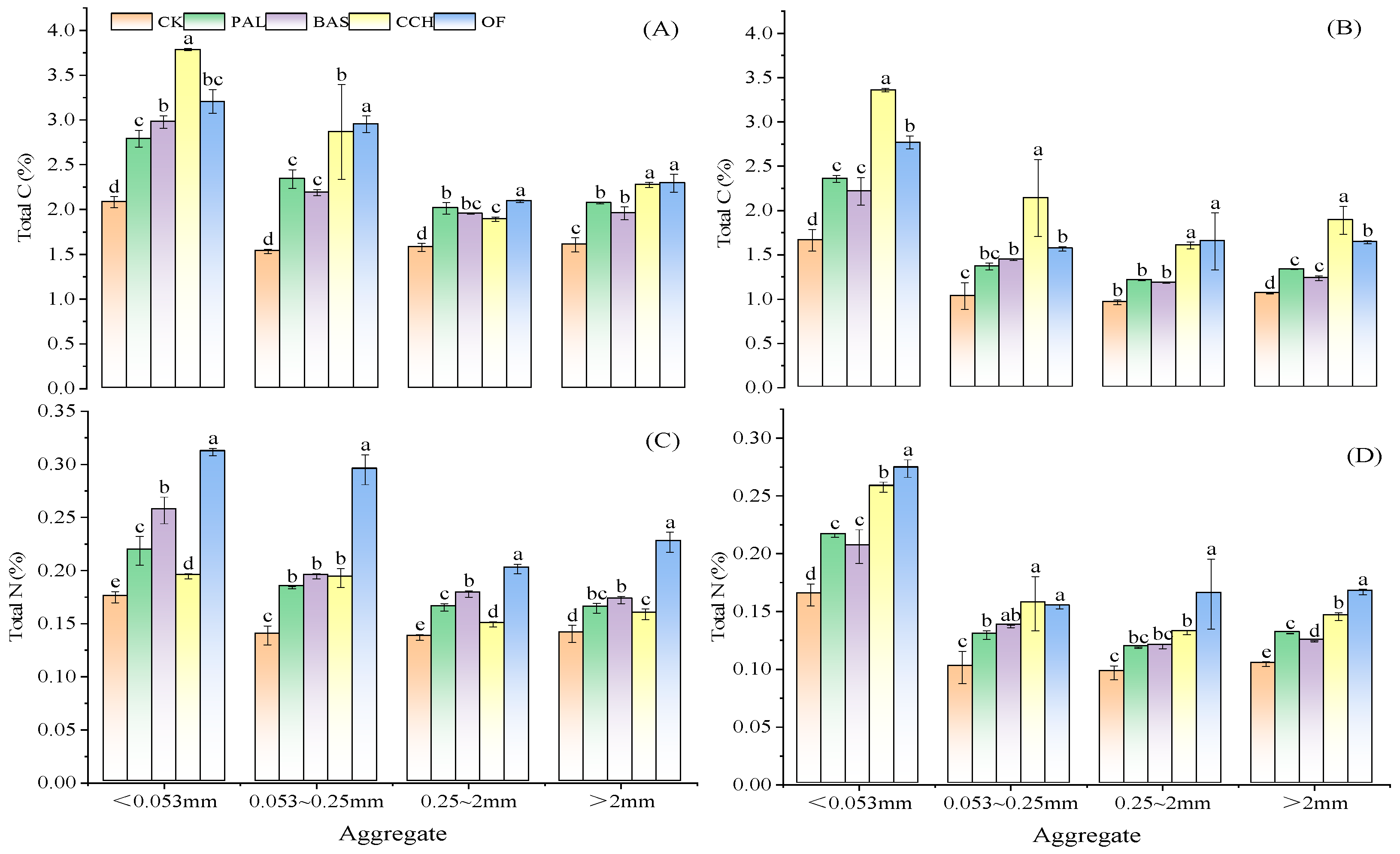
3.3. Distribution of C and N in Soil Aggregates
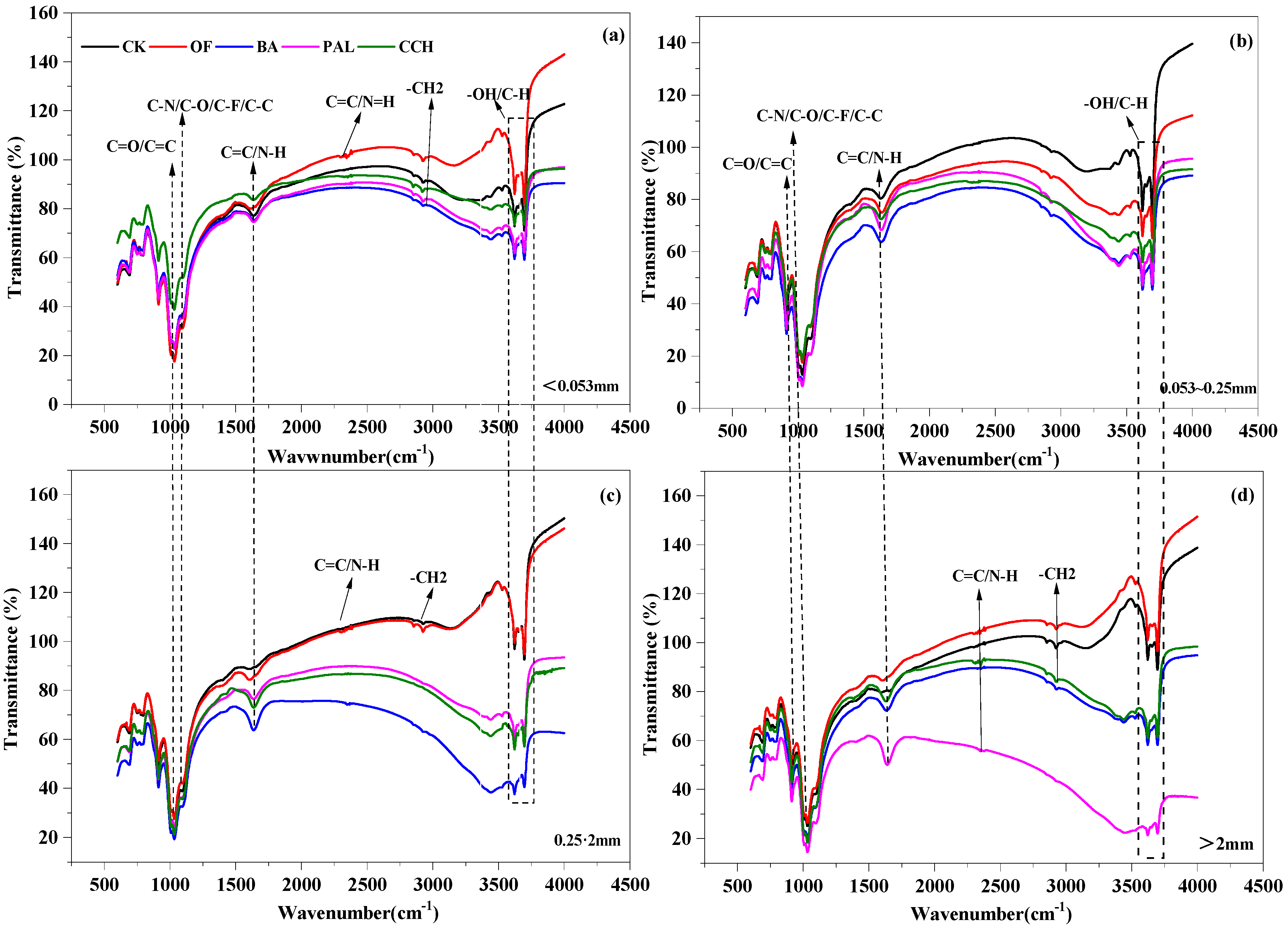
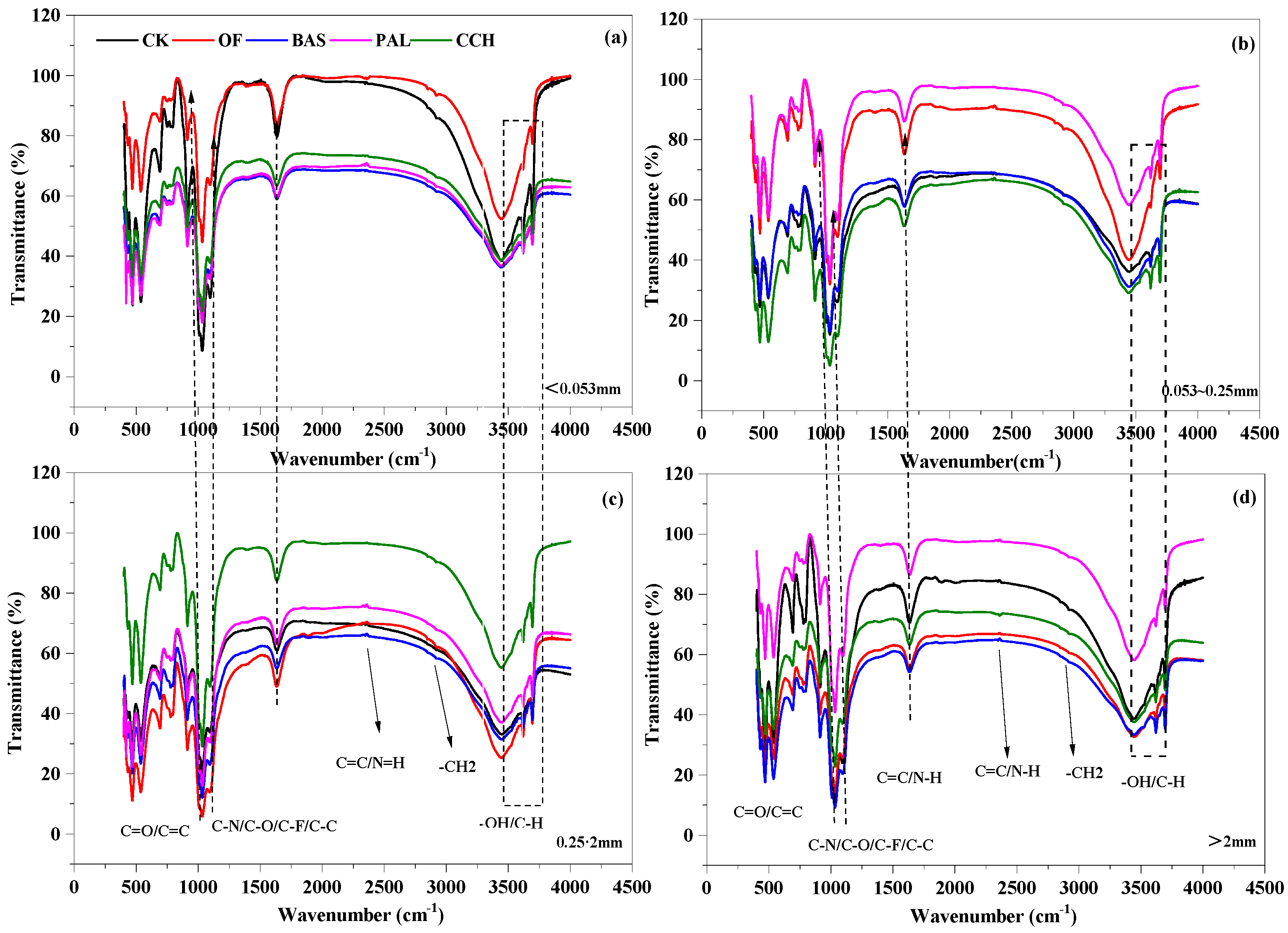
3.4. Infrared Structure of Aggregates
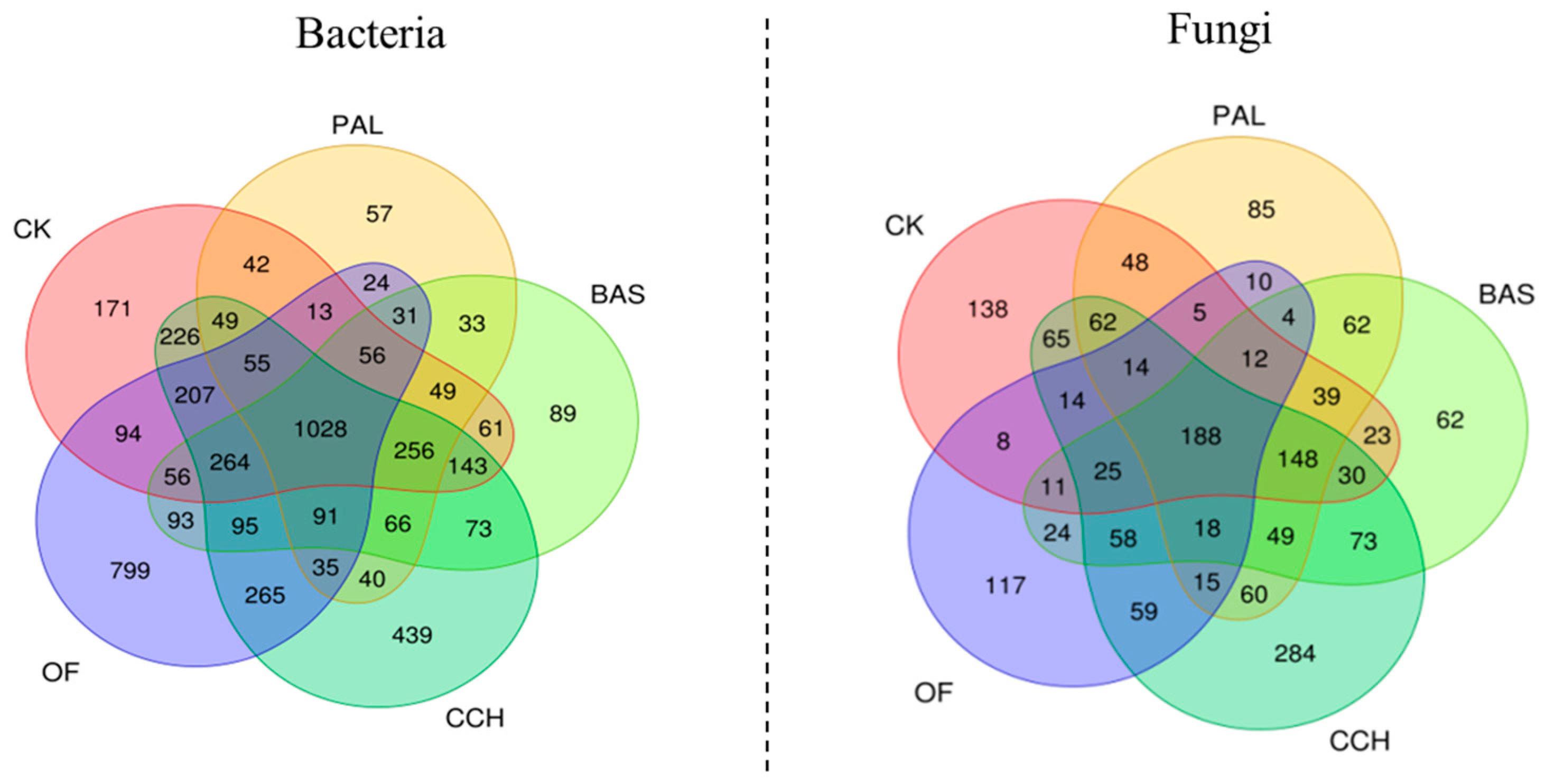
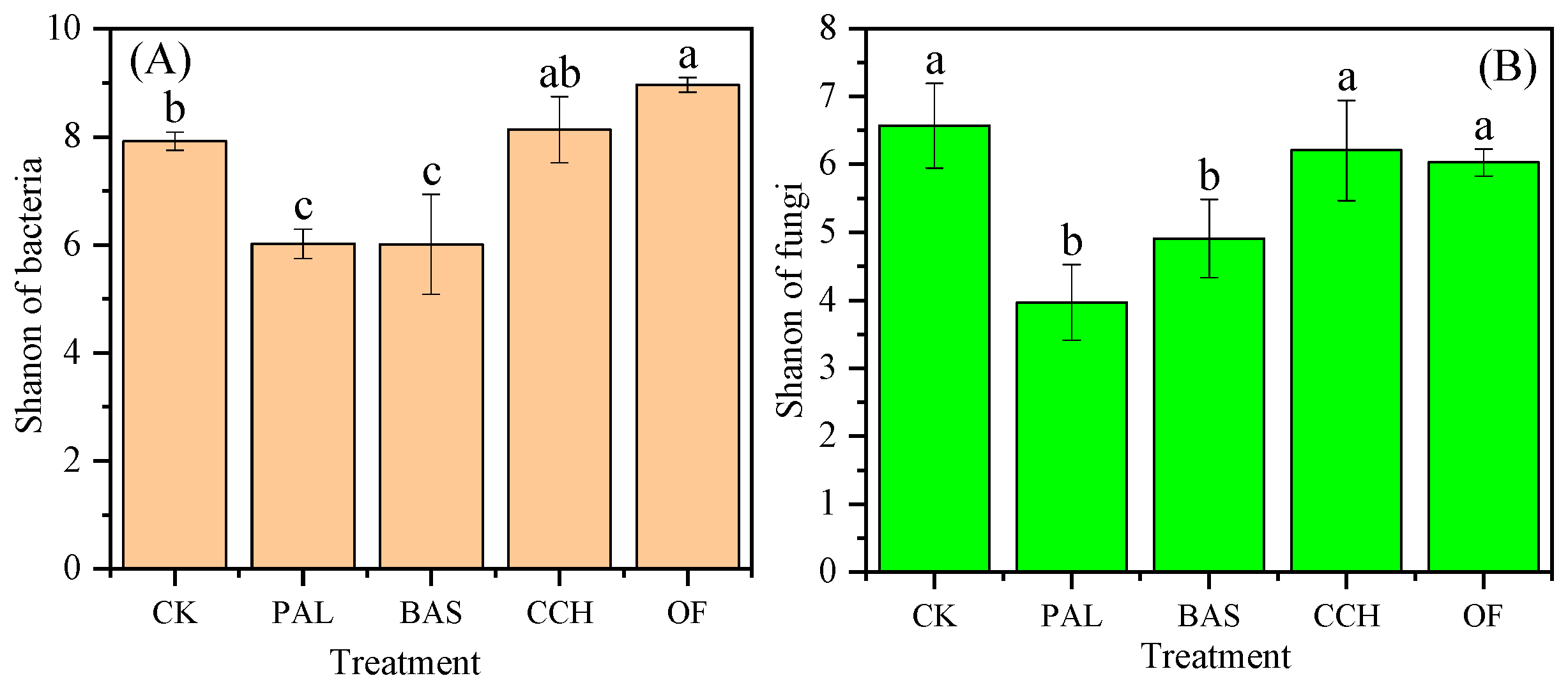
3.5. Microbial Species
4. Discussion
4.1. Effects of Organic Material Composition on Soil pH and Organic Acid Content
4.2. Effect of Organic Materials on Aggregate Formation and the Distribution of Carbon and Nitrogen Content in Aggregates
4.3. Effect of Organic Material Input on Organic Carbon Structure of Soil Aggregates
4.4. Effects of Differences in Organic Material Composition on Microbial Groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Six, J.A.E.T.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Bailey, B.A. Role of cover crops in improving soil and row crop productivity. Commun. Soil Sci. Plant Anal. 2005, 36, 2733–2757. [Google Scholar] [CrossRef]
- Wu, G.; Liang, F.; Wu, Q.; Feng, X.G.; Shang, W.D.; Li, H.W.; Li, X.; Che, Z.; Dong, Z.; Song, H. Soil pH differently affects N2O emissions from soils amended with chemical fertilizer and manure by modifying nitrification and denitrification in wheat-maize rotation system. Biol. Fertil. Soils 2024, 60, 101–113. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.J.; Banerjee, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef]
- Hue, N.V. Alleviating soil acidity with crop residues. Soil Sci. 2011, 176, 543–549. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental factors affecting the mineralization of crop residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Wendeborn, S. The chemistry, biology, and modulation of ammonium nitrification in soil. Angew. Chem. Int. Ed. 2020, 59, 2182–2202. [Google Scholar] [CrossRef]
- Bolan, N.S.; Hedley, M.J.; White, R.E. Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant Soil 1991, 134, 53–63. [Google Scholar] [CrossRef]
- Daba, A. Review on Minerals Transformation and Cycling of Basic Cations in Soil Systems. 2024. Available online: https://ssrn.com/abstract=5167028 (accessed on 5 May 2024).
- Rosariastuti, R.; Shafa, A.N.; Widijanto, H.; Cahyani, V.R. Improving soil fertility in shallot (Allium ascalonicum L.) fields through a bioremediation process using microorganisms and biochar. IOP Conf. Ser. Earth Environ. Sci. 2025, 1438, 012079. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Matłok, N.; Ilek, A.; Słowik, K.; Kuboń, M. Acidity and salinization of soil following the application of ashes from biomass combustion under different crop plant species cultivation. Appl. Sci. 2024, 14, 9812. [Google Scholar] [CrossRef]
- Yu, Q.G.; Hu, X.; Ma, J.W.; Ye, J.; Sun, W.C.; Wang, Q.; Lin, H. Effects of long-term organic material applications on soil carbon and nitrogen fractions in paddy fields. Soil Tillage Res. 2020, 196, 104483. [Google Scholar] [CrossRef]
- Cao, Y.; He, Z.; Zhu, T.; Zhao, F. Organic-C quality as a key driver of microbial nitrogen immobilization in soil: A meta-analysis. Geoderma 2021, 383, 114784. [Google Scholar] [CrossRef]
- Roberts, P.; Stockdale, R.; Khalid, M.; Iqbal, Z.; Jones, D.L. Carbon-to-nitrogen ratio is a poor predictor of low molecular weight organic nitrogen mineralization in soil. Soil Biol. Biochem. 2009, 41, 1750–1752. [Google Scholar] [CrossRef]
- Soman, C.; Li, D.; Wander, M.M.; Kent, A.D. Long-term fertilizer and crop-rotation treatments differentially affect soil bacterial community structure. Plant Soil 2017, 413, 145–159. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef]
- Sun, R.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef]
- Vida, C.; de Vicente, A.; Cazorla, F.M. The role of organic amendments to soil for crop protection: Induction of suppression of soilborne pathogens. Ann. Appl. Biol. 2020, 176, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Zhu, W.; Pang, X.; Wang, Z. Effects of organic materials on soil bacterial community structure in long-term continuous cropping of tomato in greenhouse. Open Life Sci. 2022, 17, 381–392. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.K. Agrochemical Analysis of Soil; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Han, J.; Gagnon, S.; Eckle, T.; Borchers, C.H. Metabolomic analysis of key central carbon metabolism carboxylic acids as their 3-nitrophenylhydrazones by UPLC/ESI-MS. Electrophoresis 2013, 34, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Shariat Ullah, S.; Ikram, M.; Ismail, I.; Babar, M.A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot 2020, 52, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, S.; Kitunen, V.; Kiikkilä, O.; Loponen, J.; Smolander, A. Response of soil C and N transformations to tannin fractions originating from Scots pine and Norway spruce needles. Soil Biol. Biochem. 2006, 38, 1364–1374. [Google Scholar] [CrossRef]
- Shi, R.Y.; Liu, Z.D.; Li, Y.; Jiang, T.; Xu, M.; Li, J.Y.; Xu, R.K. Mechanisms for increasing soil resistance to acidification by long-term manure application. Soil Tillage Res. 2019, 185, 77–84. [Google Scholar] [CrossRef]
- Song, S.; Cong, P.; Wang, C.; Li, P.; Liu, S.; He, Z.; Zhou, C.; Liu, Y.; Yang, Z. Properties of biochar obtained from tropical crop wastes under different pyrolysis temperatures and its application on acidic soil. Agronomy 2023, 13, 921. [Google Scholar] [CrossRef]
- Citak, S.; Sonmez, S. Effects of chemical fertilizer and different organic manure application on soil pH, EC and organic matter content. J. Food Agric. Environ. 2011, 9, 739–741. [Google Scholar]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Sustainable energy from waste organic matters via efficient microbial processes. Sci. Total Environ. 2020, 722, 137927. [Google Scholar] [CrossRef]
- Yudina, A.; Kuzyakov, Y. Dual nature of soil structure: The unity of aggregates and pores. Geoderma 2023, 434, 116478. [Google Scholar] [CrossRef]
- Zhong, P.; Chen, P.; Huo, P.; Ma, L.; Xu, Z.; Li, F.; Cai, C. Characterization of cotton stalk as a lignocellulosic feedstock for single-cell protein production. Bioresour. Technol. 2025, 417, 131797. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Yin, Y.; Mu, M.; Liu, Y.; Zhou, D.; Wang, W.; Zou, X.; Yang, J. A novel green biorefinery strategy for corn stover by pretreatment with weak alkali-assisted deep eutectic solvents. Green Chem. 2024, 26, 2300–2312. [Google Scholar] [CrossRef]
- Ma, S.; Kan, Z.; Qi, J.; Zhang, H. Effects of straw return mode on soil aggregates and associated carbon in the north China plain. Agronomy 2020, 10, 61. [Google Scholar] [CrossRef]
- Du, S.; Ma, Z.; Chen, J.; Xue, L.; Tang, C.; Shareet, T.M.E. Effects of organic fertilizer proportion on the distribution of soil aggregates and their associated organic carbon in a field mulched with gravel. Sci. Rep. 2022, 12, 11513. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Q.; Zhang, H.; Zhang, Y.; Wei, W.; Jiang, W.; Xu, X.; Liu, S. Long-term manure fertilization increases rill erosion resistance by improving soil aggregation and polyvalent cations. Catena 2023, 223, 106909. [Google Scholar] [CrossRef]
- Xu, H.; Mustafa, A.; Saeed, Q.; Jiang, G.; Sun, N.; Liu, K.; Kucerik, J.; Yang, X.; Xu, M. Combined application of chemical and organic fertilizers enhances soil organic carbon sequestration. Chem. Biol. Technol. Agric. 2025, 12, 1. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, H.; Zhang, M.; Shao, Y.; Wang, J.; Liu, Y.; Li, C. Straw returning combined with controlled-release nitrogen fertilizer affected organic carbon storage and crop yield by changing humic acid composition and aggregate distribution. J. Clean. Prod. 2023, 415, 137783. [Google Scholar] [CrossRef]
- Liu, L.; Tan, Z.; Gong, H.; Huang, Q. Migration and transformation mechanisms of nutrient elements (N, P, K) within biochar in straw–biochar–soil–plant systems: A review. ACS Sustain. Chem. Eng. 2018, 7, 22–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Cheng, C.; Li, Y.; Li, Z. Effects of combined application proportion of cow manure and chemical fertilizer on soil organic carbon pool and enzyme activity in apple orchard. Sci. Agric. Sin. 2024, 57, 4107–4118. [Google Scholar]
- Dong, X.; Guan, T.; Li, G.; Lin, Q.; Zhao, X. Long-term effects of biochar amount on the content and composition of organic matter in soil aggregates under field conditions. J. Soils Sediments 2016, 16, 1481–1497. [Google Scholar] [CrossRef]
- Ndung’u, M.; Ngatia, L.W.; Onwonga, R.N.; Mucheru-Muna, M.W.; Fu, R.; Moriasi, D.N.; Ngetich, K.F. The influence of organic and inorganic nutrient inputs on soil organic carbon functional groups content and maize yields. Heliyon 2021, 7, e07881. [Google Scholar] [CrossRef]
- Sun, J.; Lu, X.; Chen, G.; Luo, N.; Zhang, Q.; Li, X. Biochar promotes soil aggregate stability and associated organic carbon sequestration and regulates microbial community structures in Mollisols from northeast China. Soil 2023, 9, 261–275. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated carbon and nitrogen dynamics during 9 years after conversion from native prairie to no-till and conventional-till corn. Soil Sci. Soc. Am. J. 2006, 70, 42–50. [Google Scholar]
- Marschner, B.; Kalbitz, K. Biodegradability of dissolved organic matter: A critical review. Biogeochemistry 2003, 64, 277–304. [Google Scholar]
- Lehmann, J.; Kinyangi, J.; Solomon, D. Organic matter stabilization in soil microaggregates: Implications from spatial heterogeneity of organic carbon contents and carbon forms. Biogeochemistry 2007, 85, 45–57. [Google Scholar] [CrossRef]
- Wan, X.; Huang, Z.; He, Z.; Yu, Z.; Wang, M.; Davis, M.R.; Yang, Y. Soil C: N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
- Nivelle, E.; Verzeaux, J.; Habbib, H.; Kuzyakov, Y.; Decocq, G.; Roger, D.; Jérôme, L.; Duclercq, J.; Spicher, F.; Nava-Saucedo, J.; et al. Functional response of soil microbial communities to tillage, cover crops and nitrogen fertilization. Appl. Soil Ecol. 2016, 108, 147–155. [Google Scholar] [CrossRef]
- Sharma, P.; Sangwan, S.; Kaur, H.; Patra, A.; Mehta, S. Microbial remediation of agricultural residues. In Sustainable Agriculture Reviews 60: Microbial Processes in Agriculture; Springer Nature: Cham, Switzerland, 2023; pp. 325–358. [Google Scholar]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011, 28, 1883–1896. [Google Scholar] [CrossRef]


| Organic Material | C Content/% | N Content/% | C/N | δ13C | δ15N | Amount of Organic Materials/g | Amount of (NH4)2SO4/g |
|---|---|---|---|---|---|---|---|
| PAL | 41.79 | 0.57 | 73.31 | −14.63 | 0.22 | 11.96 | 0.62 |
| BAS | 37.78 | 0.34 | 111.12 | −24.05 | 5.05 | 13.23 | 0.73 |
| CCH | 27.93 | 0.19 | 147.00 | −27.42 | 4.18 | 17.90 | 0.78 |
| OF | 27.47 | 2.35 | 11.69 | −21.09 | 6.12 | 18.20 | 0.00 |
| Treatment | pH | EC | Ca | Mg | |
|---|---|---|---|---|---|
| μs/cm | mg/kg | ||||
| 90 d | PAL | 4.76 ± 0.02 d | 174.87 ± 42.7 a | 35.00 ± 5.77 b | 6.10 ± 0.01 b |
| BAS | 5.13 ± 0.12 b | 166.17 ± 26.97 a | 47.50 ± 5.00 a | 15.25 ± 4.31 a | |
| CCH | 4.44 ± 0.08 e | 189.64 ± 31.57 a | 32.50 ± 5.00 bc | 15.25 ± 4.31 a | |
| OF | 6.93 ± 0.04 a | 116.84 ± 7.17 b | 27.50 ± 5.00 c | 10.68 ± 3.05 ab | |
| CK | 4.94 ± 0.06 c | 63.31 ± 7.21 c | 20.00 ± 0.01 d | 6.10 ± 0.01 b | |
| 1080 d | PAL | 4.80 ± 0.04 bc | 46.82 ± 2.70 b | 25.00 ± 4.08 a | 9.18 ± 2.49 a |
| BAS | 4.76 ± 0.11 c | 76.32 ± 3.59 a | 23.33 ± 4.71 a | 8.13 ± 2.88 ab | |
| CCH | 5.23 ± 0.16 a | 42.00 ± 2.25 b | 10.00 ± 0.01 c | 6.10 ± 0.01 b | |
| OF | 5.28 ± 0.02 a | 58.03 ± 23.16 ab | 15.00 ± 4.08 bc | 6.10 ± 0.01 b | |
| CK | 4.92 ± 0.09 b | 50.38 ± 19.82 b | 16.68 ± 4.71 b | 6.10 ± 0.01 b | |
| Treatment | Bacterial Biomarkers | Fungi Biomakers |
|---|---|---|
| PAL | Koribacteraceae | Chrysozymaceae, Trimorphomycetaceae, and Tremellales |
| BAS | Caulobacteraceae | Aspergillaceae, Onygenales, and Ceratostomataceae |
| CCH | Solirubrobacterales, Rhizobiales, Rhodospirillales, and Alphaproteobacteria | Botryosphaeriales, Didymellaceae, Xylariales, and Sebacinaceae |
| OF | Blastocatellaceae, Gaiellaceae, Bacillaceae, Paenibacillaceae, et al. | Dothideomycetes, Diaporthaceae, Stephanosporaceae, et al. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Liu, S.; Liu, Y.; Shi, W.; Ma, H. Effects of Tropical Typical Organic Materials on Soil Physicochemical Properties and Microbial Community Structure. Agronomy 2025, 15, 1073. https://doi.org/10.3390/agronomy15051073
Song S, Liu S, Liu Y, Shi W, Ma H. Effects of Tropical Typical Organic Materials on Soil Physicochemical Properties and Microbial Community Structure. Agronomy. 2025; 15(5):1073. https://doi.org/10.3390/agronomy15051073
Chicago/Turabian StyleSong, Shuhui, Siru Liu, Yanan Liu, Weiqi Shi, and Haiyang Ma. 2025. "Effects of Tropical Typical Organic Materials on Soil Physicochemical Properties and Microbial Community Structure" Agronomy 15, no. 5: 1073. https://doi.org/10.3390/agronomy15051073
APA StyleSong, S., Liu, S., Liu, Y., Shi, W., & Ma, H. (2025). Effects of Tropical Typical Organic Materials on Soil Physicochemical Properties and Microbial Community Structure. Agronomy, 15(5), 1073. https://doi.org/10.3390/agronomy15051073







