Response of the Stabilization of Organic Carbon to Straw Incorporation and Nitrogen Application: Evidence from Carbon Fractions and Bacterial Survival Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Soil Sampling
2.4. Soil Physiochemical Analysis
2.5. Scanning Electron Microscopy (SEM) and Fourier-Transform Infrared Spectrometer (FTIR)
2.6. Soil C Fraction Analysis
2.7. Soil Bacterial Community Analysis
2.8. Calculation and Data Analyses
3. Results
3.1. Soil Physical and Chemical Properties
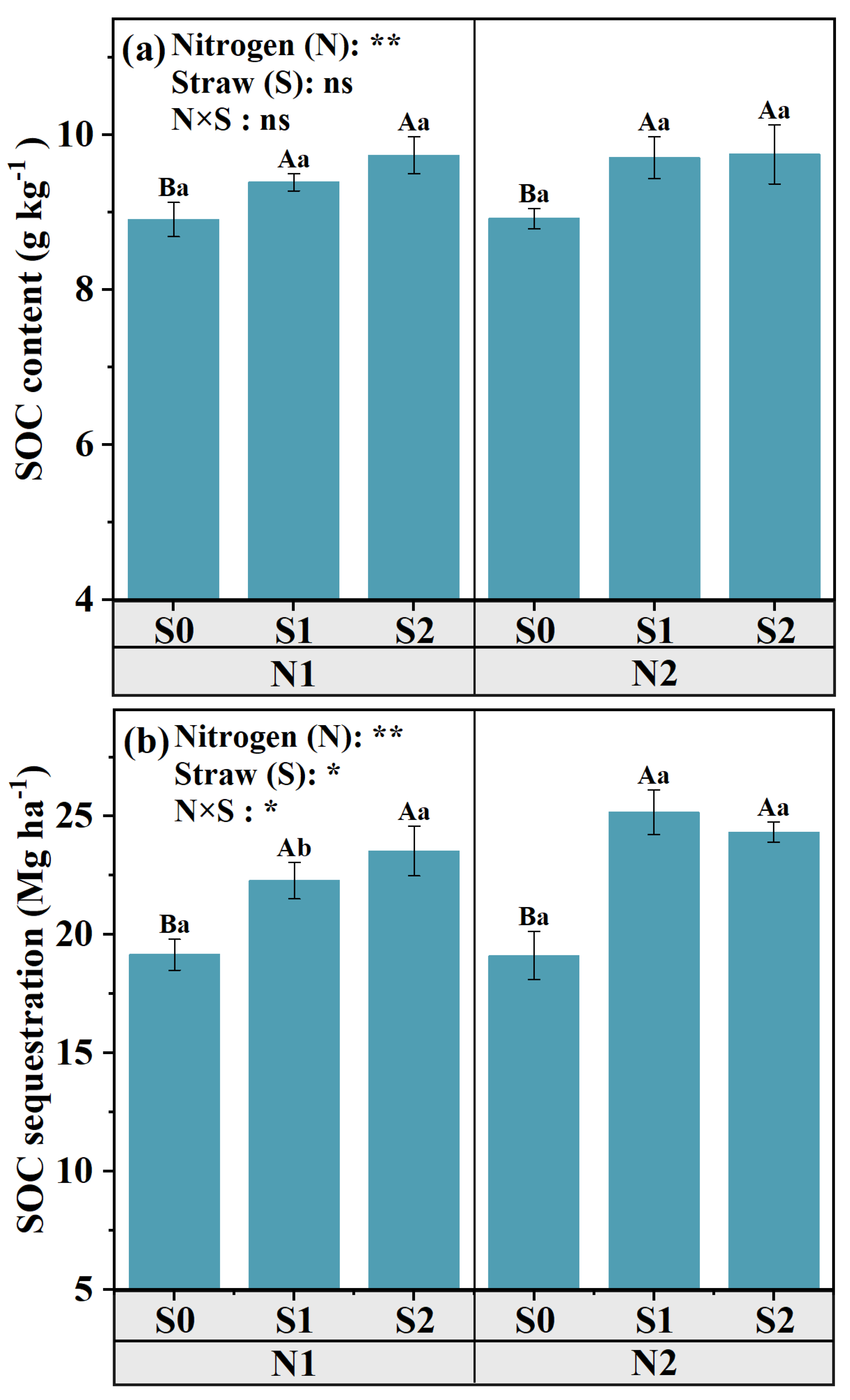
3.2. The Fractions of Soil Labile C and SOC Sequestration
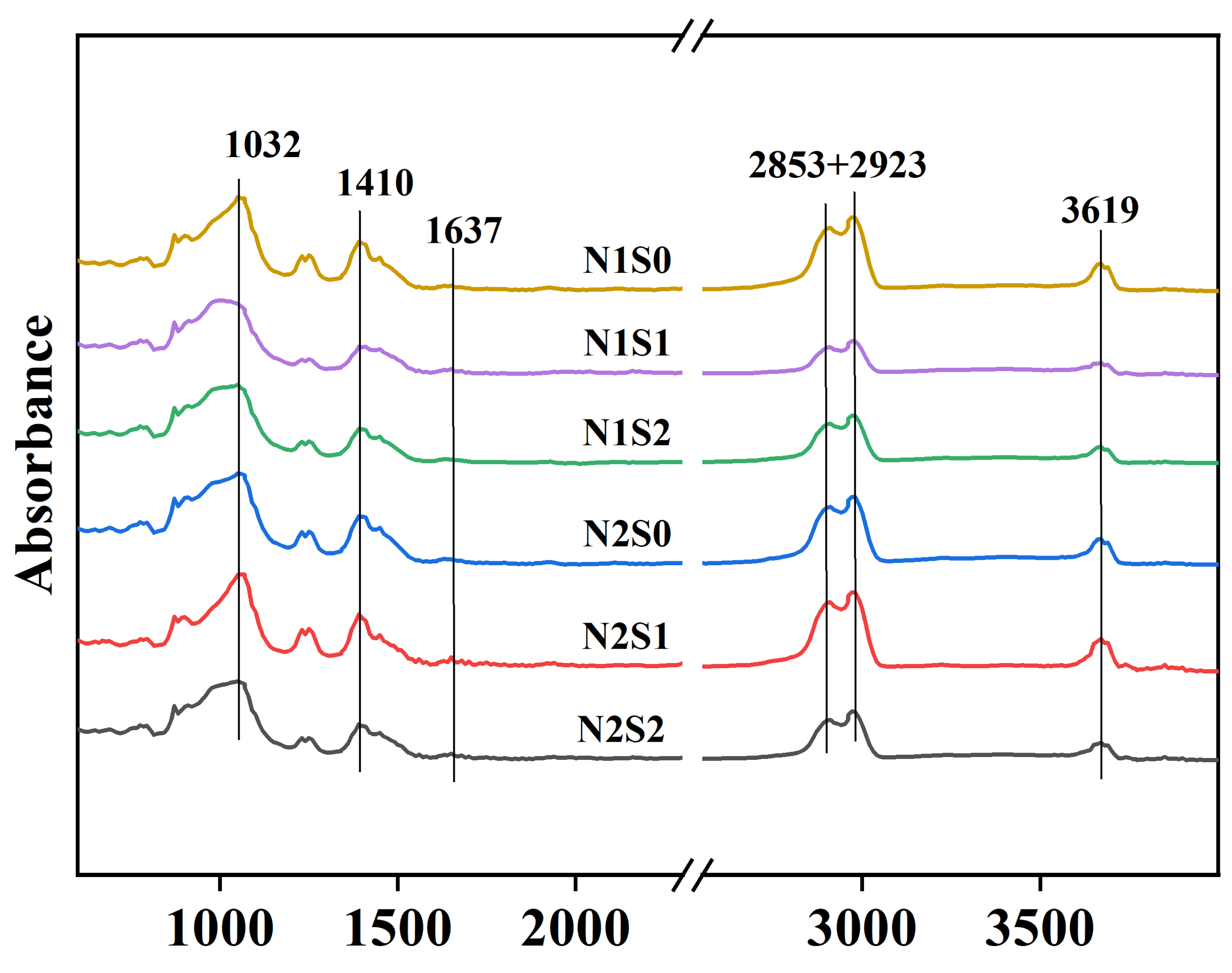
3.3. FTIR of Soil
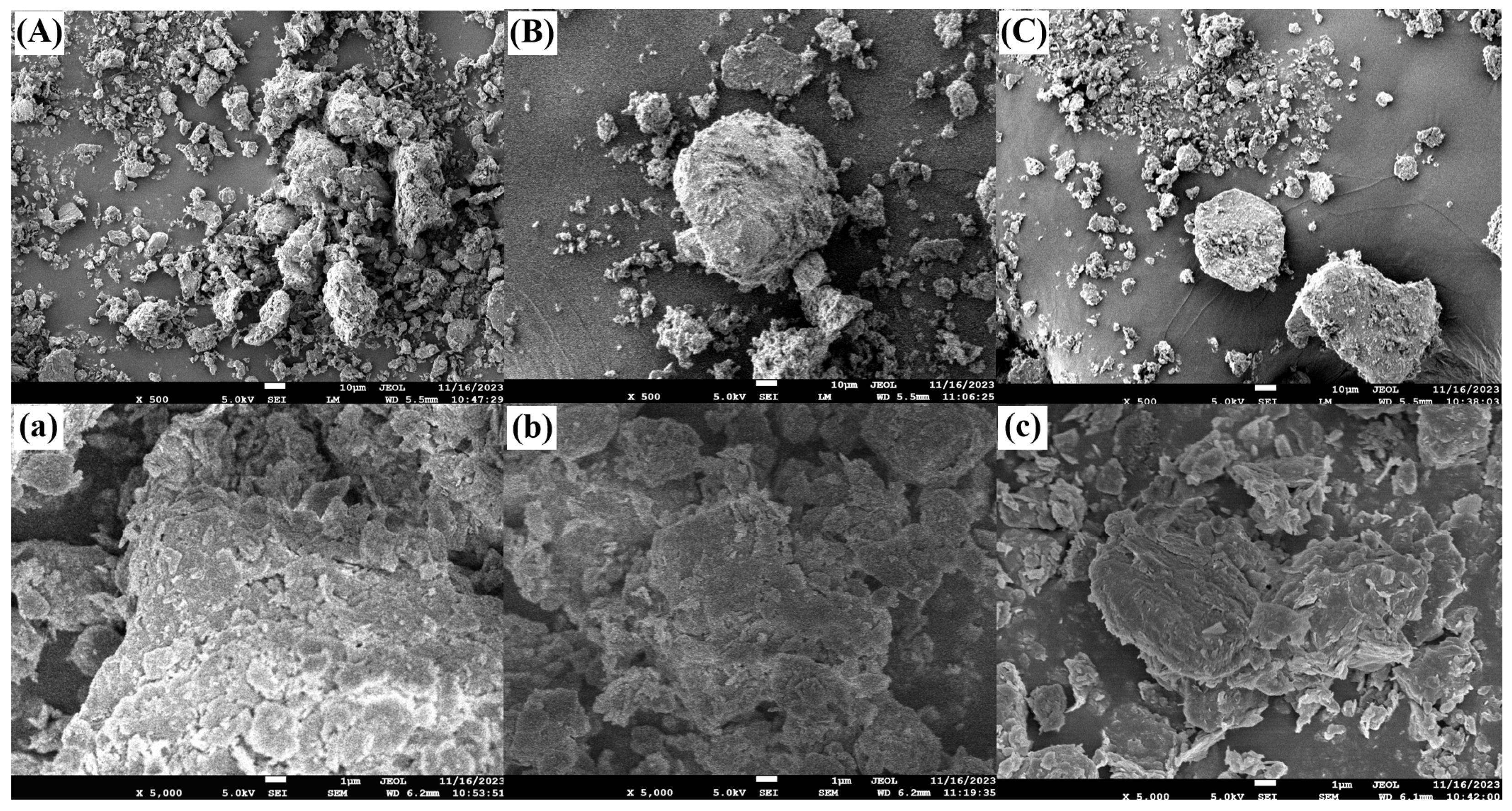
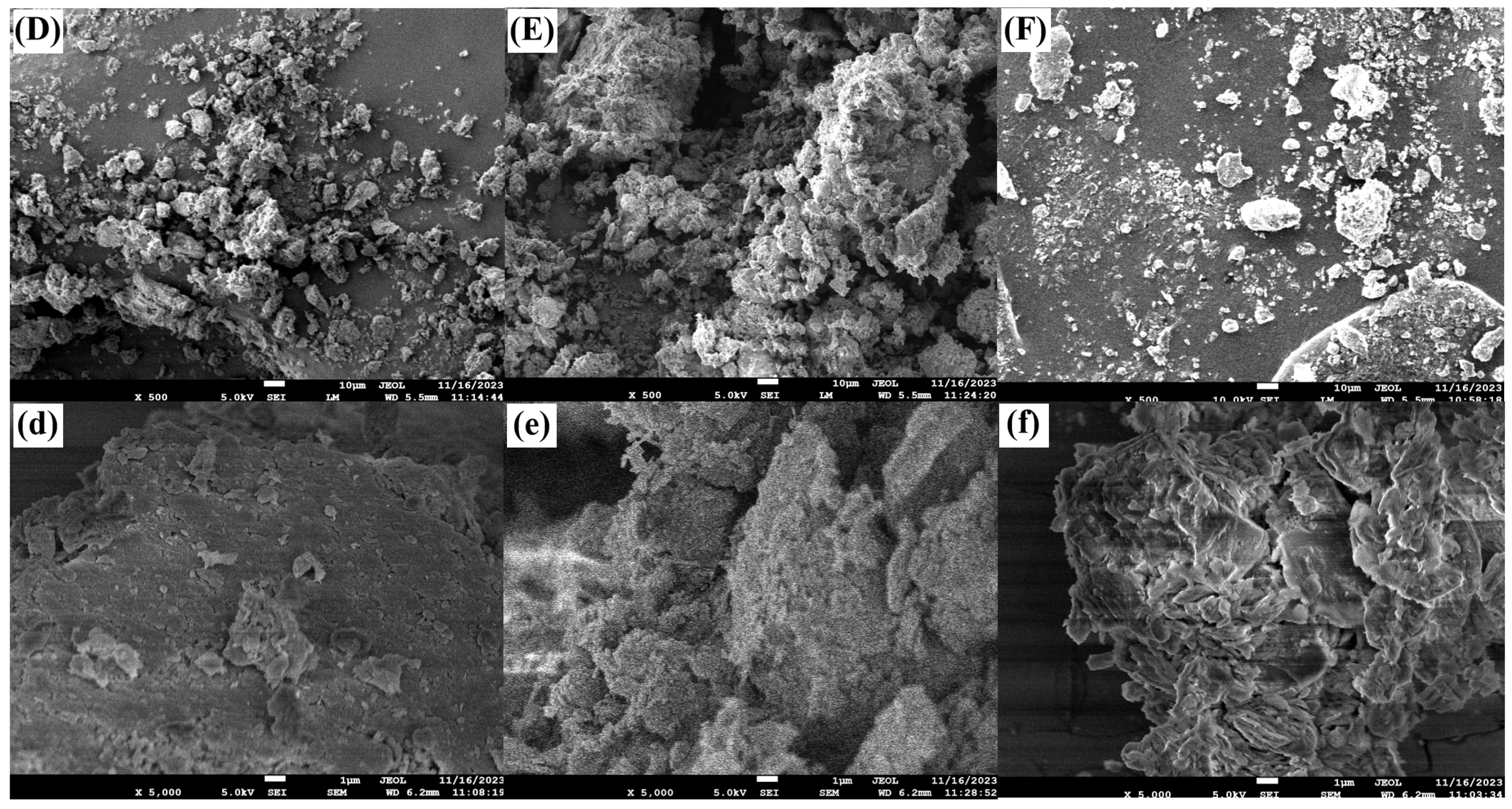
3.4. Soil SEM Analysis
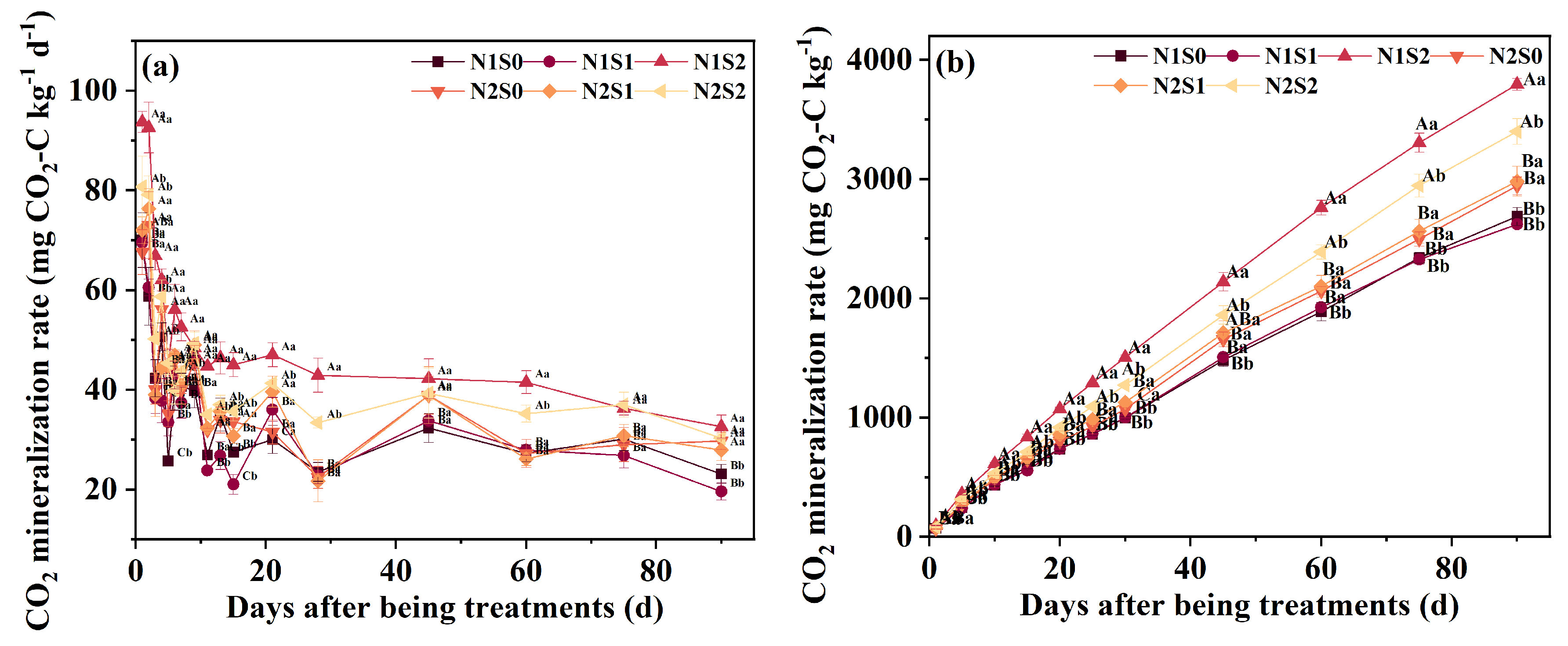
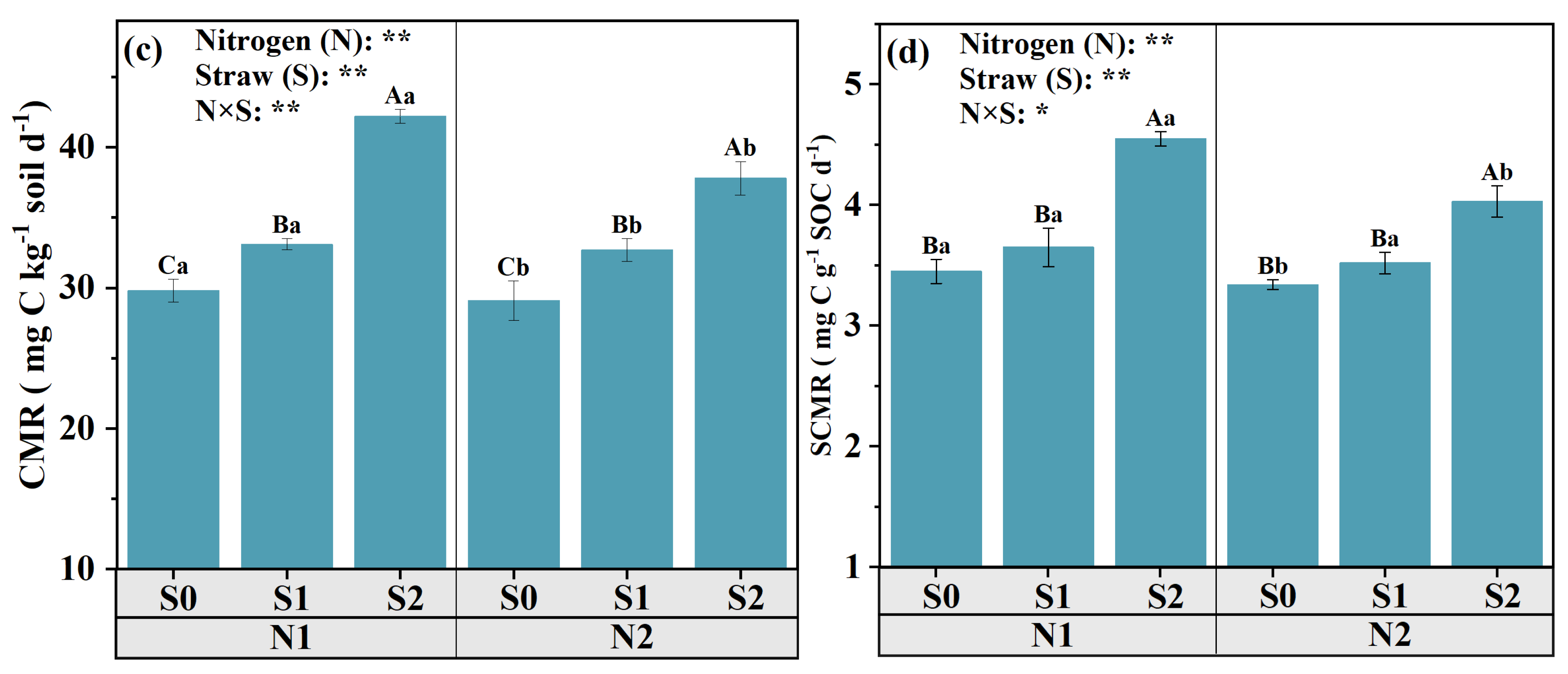
3.5. CO2 Efflux and OC Mineralization Index
3.6. Dynamic Changes in Bacteria
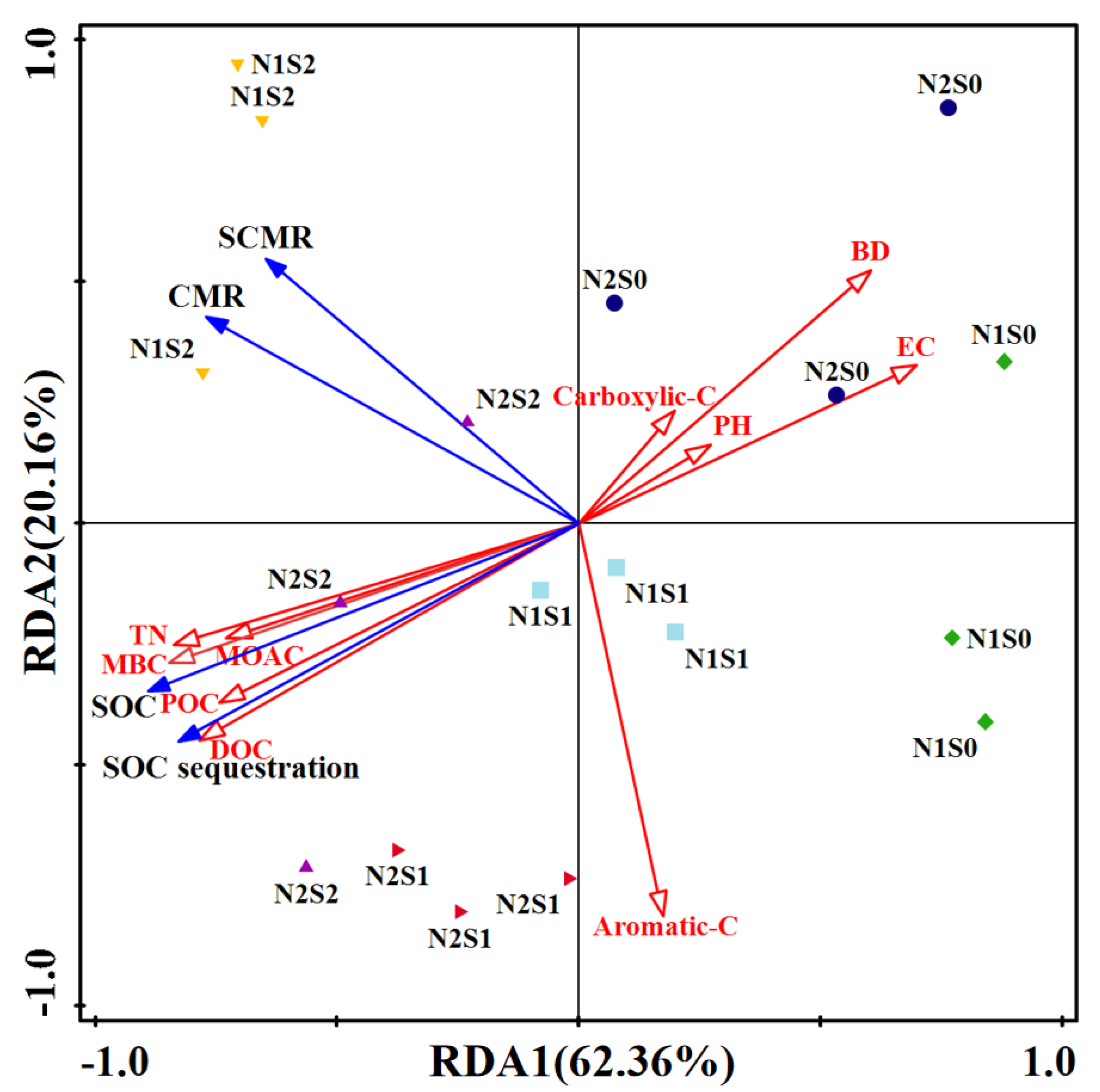
3.7. Correlation Analysis
4. Discussion
4.1. Responses of SOC Components to Straw Incorporation and N Application
4.2. Reactivity of SOC Mineralization to Straw Incorporation and N Application
4.3. Improvement Mechanism of SOC with Straw Incorporation and N Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, W.; Wang, Y.; Sun, Y.; Liu, Z.; Wang, W.; Wu, D.; Zhang, Y.; Sun, W.; Wang, X.; Feng, Z.; et al. Assessing the formation and stability of paddy soil aggregate driven by organic carbon and Fe/Al oxides in rice straw cyclic utilization strategies: Insight from a six-year field trial. Sci. Total Environ. 2024, 951, 175607. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Chen, L.; Jia, Z.; Zou, H.; Ma, L.; Feng, B.; Li, J.; Zhou, G.; Zhang, C.; Ma, D.; et al. Joint regulation of the soil organic carbon accumulation by mineral protection and microbial properties following conservation practices. Catena 2024, 245, 108298. [Google Scholar] [CrossRef]
- Jing, Z.; Zhang, S.; Sun, Z.; Liu, Z.; Liu, S.; Ding, X. Straw incorporation and nitrogen fertilization enhance soil organic carbon sequestration by promoting aggregate stability and iron oxide transformation. Agronomy 2025, 15, 871. [Google Scholar] [CrossRef]
- Ji, X.; Jiang, J.; Wang, Y.; Colinet, G.; Feng, W. Small straw addition enhances straw decomposition and carbon stabilized in soil aggregates over time. Soil Tillage Res. 2024, 238, 106022. [Google Scholar] [CrossRef]
- Ma, W.; Ma, L.; Jiao, J.; Fahim, A.M.; Wu, J.; Tao, X.; Lian, Y.; Li, R.; Li, Y.; Yang, G.; et al. Impact of straw incorporation on the physicochemical profile and fungal ecology of saline-alkaline soil. Microorganisms 2024, 12, 277. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, X.; Li, Y.; Jiao, Y.; Fan, L.; Jiang, Y.; Qu, C.; Filimonenko, E.; Jiang, Y.; Tian, X.; et al. Nitrogen fertilizer builds soil organic carbon under straw return mainly via microbial necromass formation. Soil Biol. Biochem. 2024, 188, 109223. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Fei, C.; Ding, X. Impacts of straw returning and N application on NH4+-N loss, microbially reducible Fe(III) and bacterial community composition in saline-alkaline paddy soils. Appl. Soil Ecol. 2021, 168, 104115. [Google Scholar] [CrossRef]
- Dong, H.; Fan, S.; Sun, H.; Chen, C.; Wang, A.; Jiang, L.; Ma, D. Rhizosphere-associated microbiomes of rice (Oryza sativa L.) under the effect of increased nitrogen fertilization. Front. Microbiol. 2021, 12, 730506. [Google Scholar] [CrossRef]
- Song, Y.; Song, T.; An, Y.; Shan, L.; Su, X.; Yu, S. Soil ecoenzyme activities coupled with soil properties and plant biomass strongly influence the variation in soil organic carbon components in semi-arid degraded wetlands. Sci. Total Environ. 2024, 922, 171361. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Cha, X.; Yang, W.; Zheng, M.; Liu, S.; Wang, Y.; Cai, A.; Han, X.; Yang, G.; et al. Nitrogen addition-driven soil organic carbon stability depends on the fractions of particulate and mineral-associated organic carbon. Nutr. Cycl. Agroecosystems 2024, 128, 269–281. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Wu, J. The effect of land degradation and restoration on particulate and mineral-associated organic carbon. Appl. Soil Ecol. 2024, 196, 105322. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, J.; Jin, W.; Liu, Z.; Wang, Q.; Zha, L.; Zhou, Z.; Meng, Y. Responses of SOC, labile SOC fractions, and amino sugars to different organic amendments in a coastal saline-alkali soil. Soil Tillage Res. 2024, 239, 106051. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, W.; Li, Y.; Liu, S.; Khan, A.; Yan, S.; Hu, T.; Xiong, Y. Effects of reduced tillage with stubble remaining and nitrogen application on soil aggregation, soil organic carbon and grain yield in maize-wheat rotation system. Eur. J. Agron. 2023, 149, 126920. [Google Scholar] [CrossRef]
- Chu, H.; Ni, H.; Su, W.; Fan, S.; Long, Y.; Sun, Y. Enhanced nitrogen fertilizer input alters soil carbon dynamics in moso bamboo forests, impacting particulate organic and mineral-associated carbon pools. Forests 2023, 14, 2460. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, M.; Jin, J.; Fu, M. Effects of different nitrogen applications and straw return depth on straw microbial and carbon and nitrogen cycles in paddy fields in the cool zone. Sci. Rep. 2024, 14, 6424. [Google Scholar] [CrossRef]
- Xiong, S.; Zhu, J.; Yang, J.; Guo, Y.; Meng, X.; Ren, S.; Du, J.; Wen, S.; Jiang, Y.; Shi, J.; et al. Straw return plus zinc fertilization increased the accumulations and changed the chemical compositions of mineral-associated soil organic carbon. Agric. Ecosyst. Environ. 2023, 357, 108699. [Google Scholar] [CrossRef]
- Sun, T.; Mao, X.; Han, K.; Wang, X.; Cheng, Q.; Liu, X.; Zhou, J.; Ma, Q.; Ni, Z.; Wu, L. Nitrogen addition increased soil particulate organic carbon via plant carbon input whereas reduced mineral−associated organic carbon through attenuating mineral protection in agroecosystem. Sci. Total Environ. 2023, 899, 165705. [Google Scholar] [CrossRef]
- Tang, B.; Rocci, K.S.; Lehmann, A.; Rillig, M.C. Nitrogen increases soil organic carbon accrual and alters its functionality. Glob. Change Biol. 2023, 29, 1971–1983. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Ma, R.; Chen, M.; Wei, W.; Ding, X. Carbon sequestration under different organic amendments in saline-alkaline soils. Catena 2021, 196, 104882. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Y.; Xiang, L.; Zhu, Z.; Fu, W.; Hao, G.; Geng, Z.; Chen, S.; Li, Y.; Han, D. Assembly of rhizosphere microbial communities in Artemisia annua: Recruitment of plant growth-promoting microorganisms and inter-kingdom interactions between bacteria and fungi. Plant Soil 2021, 470, 127–139. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, M.; Yang, L.; Gu, X.; Jin, J.; Fu, M. Regulation of straw decomposition and its effect on soil function by the amount of returned straw in a cool zone rice crop system. Sci. Rep. 2023, 13, 15673. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Pan, W.; Tang, S.; Sun, X.; Xie, Y.; Chadwick, D.R.; Hill, P.W.; Si, L.; Wu, L.; Jones, D.L. Maize and soybean experience fierce competition from soil microorganisms for the uptake of organic and inorganic nitrogen and sulphur: A pot test using 13C, 15N, 14C, and 35S labelling. Soil Biol. Biochem. 2021, 157, 108260. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, S.; Song, Y.; Wang, X.; Jin, F. Biochar application reduces saline–alkali stress by improving soil functions and regulating the diversity and abundance of soil bacterial community in highly saline–alkali paddy field. Sustainability 2024, 16, 1001. [Google Scholar] [CrossRef]
- Bach, E.M.; Williams, R.J.; Hargreaves, S.K.; Yang, F.; Hofmockel, K.S. Greatest soil microbial diversity found in micro-habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Hu, J.; Chen, H.; Yue, L.; Liu, S.; Wu, L.; Wang, B.; Chen, D. Elevational gradient regulates the effects of short-term nutrient deposition on soil microorganisms and SOM decomposition in subtropical forests. Plant Soil 2023, 489, 225–238. [Google Scholar] [CrossRef]
- Wang, X.; Bian, Q.; Jiang, Y.; Zhu, L.; Chen, Y.; Liang, Y.; Sun, B. Organic amendments drive shifts in microbial community structure and keystone taxa which increase C mineralization across aggregate size classes. Soil Biol. Biochem. 2021, 153, 108062. [Google Scholar] [CrossRef]
- Ma, S.; Kan, Z.; Qi, J.; Zhang, H. Effects of straw return mode on soil aggregates and associated carbon in the North China Plain. Agronomy 2020, 10, 61. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, S.; Liu, L.; Wu, L.; Ding, X. Combined organic amendments and mineral fertilizer application increase rice yield by improving soil structure, P availability and root growth in saline-alkaline soil. Soil Tillage Res. 2021, 212, 105060. [Google Scholar] [CrossRef]
- Luo, Z.; Luo, Y.; Wang, G.; Xia, J.; Peng, C. Warming-induced global soil carbon loss attenuated by downward carbon movement. Glob. Change Biol. 2020, 26, 7242–7254. [Google Scholar] [CrossRef]
- Badia, D.; Marti, C.; Aguirre, A.J. Straw management effects on CO2 efflux and C storage in different Mediterranean agricultural soils. Sci. Total Environ. 2013, 465, 233–239. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Feng, H.; Xu, C.; Xu, Q.; Fu, B.; Azwar, E.; Wei, Y.; Lam, S.S.; Liu, G. Exogenous application and interaction of biochar with environmental factors for improving functional diversity of rhizosphere’s microbial community and health. Chemosphere 2022, 294, 133710. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Zhang, W.; Sheng, R.; Liu, Y.; Hou, H.; Liu, F.; Ma, G.; Wei, W.; Qin, H. Long-term partial replacement of mineral fertilizer with in situ crop residues ensures continued rice yields and soil fertility: A case study of a 27-year field experiment in subtropical China. Sci. Total Environ. 2021, 787, 147523. [Google Scholar] [CrossRef] [PubMed]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of long-term straw return on soil organic carbon storage and sequestration rate in North China upland crops: A meta-analysis. Glob. Change Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bei, S.; Li, X.; Kuyper, T.W.; Chadwick, D.R.; Zhang, J. Nitrogen availability mediates the priming effect of soil organic matter by preferentially altering the straw carbon-assimilating microbial community. Sci. Total Environ. 2022, 815, 152882. [Google Scholar] [CrossRef]
- Chen, M.; Song, X.; Liu, L.; Jing, Z.; Miao, J.; Ding, X.; Li, Y.; Zhang, S. Response of soil organic carbon stability and sequestration to long-term phosphorus application: Insight from a 9-year field experiment in saline alkaline paddy soil. Plant Soil 2023, 496, 415–429. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2010, 108, 4516–4522. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Cha, X.; Zhang, P.; Li, Y.; Cai, A.; Zhou, Z.; Yang, G.; Han, X.; Ren, C. A global meta-analysis of land use change on soil mineral-associated and particulate organic carbon. Glob. Change Biol. 2025, 31, e70111. [Google Scholar] [CrossRef]
- Liu, B.; Xia, H.; Jiang, C.; Riaz, M.; Yang, L.; Chen, Y.; Fan, X.; Xia, X. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Li, Y.; Yuan, Z.; Wen, Z.; Shi, L.; Chen, Y. Ectomycorrhizal plant-mediated carbon fixation and microbial mechanisms of organic carbon stabilization in coastal saline soils. J. Soils Sediments 2025, 25, 811–824. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, D.; Zhang, S.; Wei, R.; Ding, X. Volatile fatty acids changed the microbial community during feammox in coastal saline-alkaline paddy soil. Environ. Sci. Pollut. Res. 2023, 30, 41755–41765. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Su, F.; Nie, Y.; Zhong, B.; Zheng, Y.; Mo, J.; Xiong, B.; Lu, X. Divergent responses of soil microbial functional groups to long-term high nitrogen presence in the tropical forests. Sci. Total Environ. 2022, 821, 153251. [Google Scholar] [CrossRef] [PubMed]
- Abbott, K.M.; Quirk, T.; Fultz, L.M. Soil microbial community development across a 32-year coastal wetland restoration time series and the relative importance of environmental factors. Sci. Total Environ. 2022, 821, 153359. [Google Scholar] [CrossRef]
- Connell, R.K.; Zeglin, L.H.; Blair, J.M. Plant legacies and soil microbial community dynamics control soil respiration. Soil Biol. Biochem. 2021, 160, 108350. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, S.; Xue, S.; Hu, Y.; Wang, X. Long-term tillage and cropping systems affect soil organic carbon components and mineralization in aggregates in semiarid regions. Soil Tillage Res. 2023, 231, 105742. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Yang, L. Continuous straw returning enhances the carbon sequestration potential of soil aggregates by altering the quality and stability of organic carbon. J. Environ. Manag. 2024, 358, 120903. [Google Scholar] [CrossRef]
- Kang, L.; Wu, J.; Zhang, C.; Zhu, B.; Chu, G. Alterations of soil aggregates and intra-aggregate organic carbon fractions after soil conversion from paddy soils to upland soils: Distribution, mineralization and driving mechanism. Pedosphere 2024, 34, 121–135. [Google Scholar] [CrossRef]
- Li, W.; Zhong, M.; Wang, H.; Shi, X.; Song, J.; Wang, J.; Zhang, W. Exogenous carbon inputs alleviated salt-induced oxidative stress to cotton in salinized field by improving soil aggregate structure and microbial community. Front. Plant Sci. 2025, 16, 1522534. [Google Scholar] [CrossRef]
- Shu, Q.; Gao, S.; Liu, X.; Yao, Z.; Wu, H.; Qi, L.; Zhang, X. Soil enzyme activities and microbial carbon pump promote carbon storage by influencing bacterial communities under nitrogen-rich conditions in tea plantation. Agriculture 2025, 15, 238. [Google Scholar] [CrossRef]
- Zhu, Y.; An, M.; Anwar, T.; Wang, H. Differences in soil bacterial community structure during the remediation of Cd-polluted cotton fields by biochar and biofertilizer in Xinjiang, China. Front. Microbiol. 2024, 15, 1288526. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Niu, J.; Dang, K.; Zhang, S.; Wang, S.; Wang, Z. Changes in physicochemical properties, enzymatic activities, and the microbial community of soil significantly influence the continuous cropping of Panax quinquefolius L. (American ginseng). Plant Soil 2021, 463, 427–446. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Zhang, W.; Wang, Q.; Guo, P.; Wang, Q. Nitrogen deposition caused higher increases in plant-derived organic carbon than microbial-derived organic carbon in forest soils. Sci. Total Environ. 2024, 925, 171752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, S.; Hui, D.; Vancov, T.; Fang, Y.; Tang, C.; Jiang, Z.; Ge, T.; Cai, Y.; Yu, B.; et al. Pyrogenic organic matter decreases while fresh organic matter increases soil heterotrophic respiration through modifying microbial activity in a subtropical forest. Biol. Fertil. Soils 2024, 60, 509–524. [Google Scholar] [CrossRef]



| Information | |
|---|---|
| Location | 37°18′35″ N, 118°39′20″ E |
| Altitude (m) | −1.2 |
| Annual average precipitation (mm) | 555.9 |
| Annual average sunshine duration (h) | 2234.0 |
| Average daily temperature (°C) | 12.3 |
| pH (soil: water = 1:5) | 8.79 ± 0.12 |
| SOC (g kg−1) | 8.98 ± 0.13 |
| EC (μS m−1) | 453.3 ± 21.1 |
| Total N (TN, g kg−1) | 0.97 ± 0.03 |
| Olsen-P (AP, mg kg−1) | 9.50 ± 0.31 |
| Available potassium (AK, mg kg−1) | 254.48 ± 14.1 |
| Treatments | pH | BD | EC | TN | AP | AK | SOC | |
|---|---|---|---|---|---|---|---|---|
| (g cm−3) | (μS m−1) | (g kg−1) | (mg kg−1) | (mg kg−1) | (g kg−1) | |||
| N1 | S0 | 8.98 ± 0.11 Aa | 1.30 ± 0.07 Aa | 452.13 ± 15.66 Aa | 0.98 ± 0.07 Ba | 9.43 ± 0.15 Ba | 247.40 ± 13.01 Ba | 8.91 ± 0.22 Ba |
| S1 | 8.81 ± 0.05 Aa | 1.25 ± 0.03 Aa | 429.47 ± 11.40 ABa | 1.05 ± 0.03 ABa | 9.92 ± 0.29 Aa | 265.16 ± 8.61 ABa | 9.39 ± 0.11 Aa | |
| S2 | 8.77 ± 0.24 Aa | 1.21 ± 0.05 Aa | 420.87 ± 8.08 Ba | 1.10 ± 0.04 Aa | 10.31 ± 0.12 Aa | 277.69 ± 18.74 Aa | 9.74 ± 0.24 Aa | |
| N2 | S0 | 9.01 ± 0.50 Aa | 1.19 ± 0.03 Aa | 446.63 ± 11.43 Aa | 0.99 ± 0.05 Ba | 9.57 ± 0.13 Ba | 254.28 ± 17.15 Aa | 9.01 ± 0.53 Aa |
| S1 | 8.60 ± 0.14 Aa | 1.06 ± 0.03 Ba | 405.22 ± 5.48 Bb | 1.08 ± 0.04 ABa | 10.47 ± 0.65 ABa | 275.41 ± 14.83 Aa | 9.71 ± 0.27 Aa | |
| S2 | 8.68 ± 0.28 Aa | 1.07 ± 0.03 Ba | 409.53 ± 12.46 Ba | 1.11 ± 0.05 Aa | 10.66 ± 0.55 Aa | 275.27 ± 4.63 Aa | 9.75 ± 0.38 Aa | |
| N | ns | ns | * | ns | ns | ns | ns | |
| S | ns | * | ** | ** | ** | ** | ** | |
| S × N | ns | ns | ns | ns | ns | ns | ns | |
| Treatments | OC Functional Groups (%) | |||||
|---|---|---|---|---|---|---|
| Polysaccharide-C | Carboxylic-C | Aromatic-C | Aliphatic-C | OH-C | ||
| N1 | S0 | 55.26 ± 2.04 Aa | 12.93 ± 1.18 Aa | 2.05 ± 0.25 Aa | 25.18 ± 2.33 Ba | 4.58 ± 0.25 Aa |
| S1 | 52.83 ± 2.99 Ba | 12.52 ± 0.80 Aa | 1.98 ± 0.28 Aa | 27.66 ± 2.20 Aa | 5.02 ± 0.49 Aa | |
| S2 | 55.22 ± 1.46 ABa | 12.44 ± 0.83 Aa | 1.47 ± 0.08 Ba | 28.05 ± 0.80 Aa | 2.82 ± 0.23 Bb | |
| N2 | S0 | 54.13 ± 3.07 Aa | 12.98 ± 0.47 Aa | 1.57 ± 0.15 Cb | 26.39 ± 2.58 Ba | 4.93 ± 0.21 Aa |
| S1 | 53.96 ± 1.42 Aa | 11.26 ± 0.34 Bb | 2.55 ± 0.17 Aa | 27.67 ± 1.78 Ba | 4.57 ± 0.20 Aa | |
| S2 | 48.36 ± 3.32 Bb | 12.66 ± 0.42 Aa | 1.82 ± 0.16 Ba | 32.20 ± 2.99 Aa | 4.95 ± 0.33 Aa | |
| N | * | * | ** | * | * | |
| S | * | * | * | ns | * | |
| S × N | ns | * | * | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Ding, X.; Sun, Z.; Liu, Z.; Du, R.; Jing, Z.; Zhang, S. Response of the Stabilization of Organic Carbon to Straw Incorporation and Nitrogen Application: Evidence from Carbon Fractions and Bacterial Survival Strategies. Agronomy 2025, 15, 1034. https://doi.org/10.3390/agronomy15051034
Liu S, Ding X, Sun Z, Liu Z, Du R, Jing Z, Zhang S. Response of the Stabilization of Organic Carbon to Straw Incorporation and Nitrogen Application: Evidence from Carbon Fractions and Bacterial Survival Strategies. Agronomy. 2025; 15(5):1034. https://doi.org/10.3390/agronomy15051034
Chicago/Turabian StyleLiu, Shenglin, Xiaodong Ding, Zeqiang Sun, Zhaohui Liu, Runxiang Du, Zhichang Jing, and Shirong Zhang. 2025. "Response of the Stabilization of Organic Carbon to Straw Incorporation and Nitrogen Application: Evidence from Carbon Fractions and Bacterial Survival Strategies" Agronomy 15, no. 5: 1034. https://doi.org/10.3390/agronomy15051034
APA StyleLiu, S., Ding, X., Sun, Z., Liu, Z., Du, R., Jing, Z., & Zhang, S. (2025). Response of the Stabilization of Organic Carbon to Straw Incorporation and Nitrogen Application: Evidence from Carbon Fractions and Bacterial Survival Strategies. Agronomy, 15(5), 1034. https://doi.org/10.3390/agronomy15051034






