How Weed Flora Evolves in Cereal Fields in Relation to the Agricultural Environment and Farming Practices in Different Sub-Regions of Eastern Hungary
Abstract
1. Introduction
2. Materials and Methods
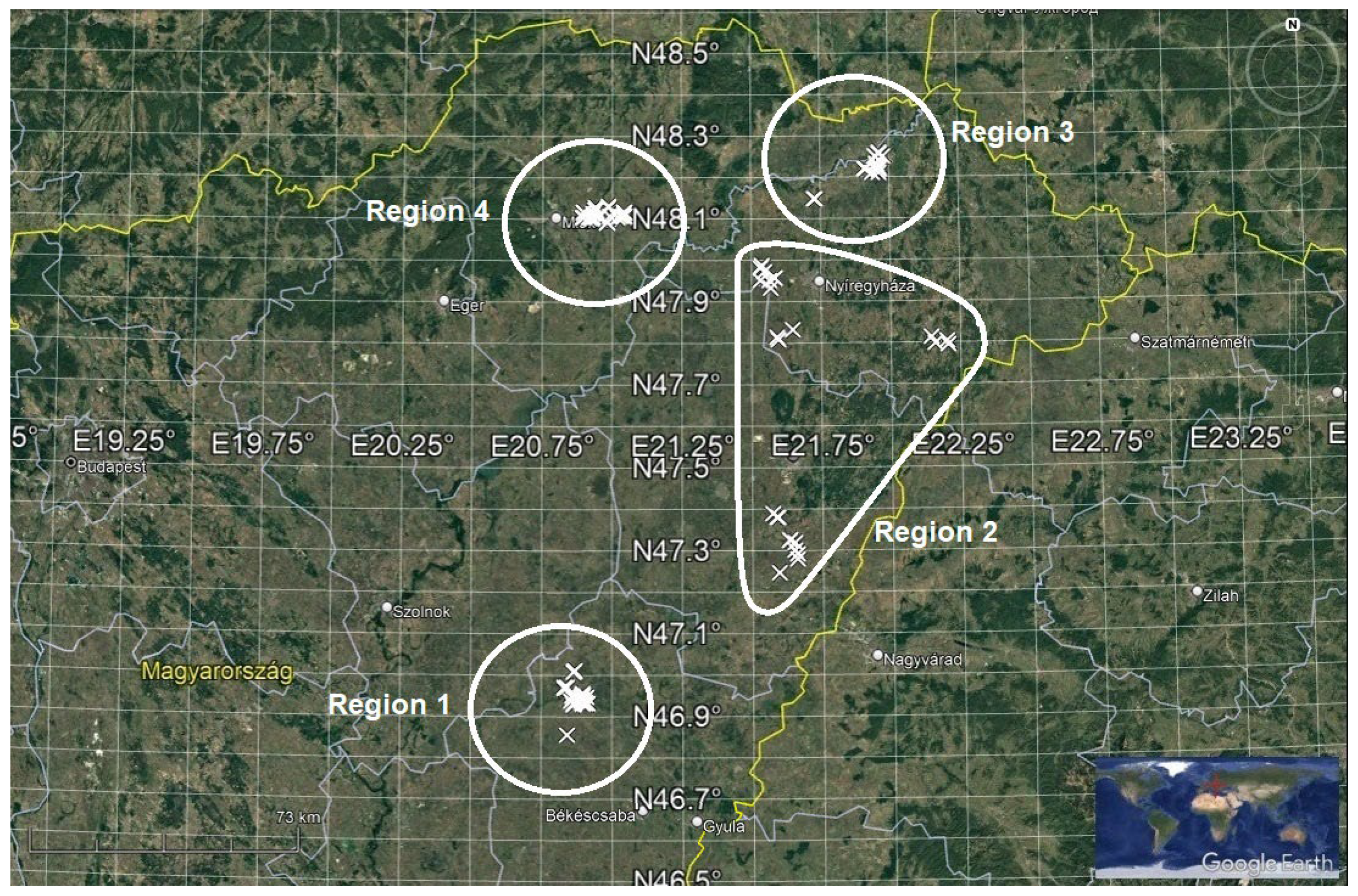
2.1. Description of the Regions Concerned
2.2. Methodology of Data Collection
2.3. Data Preparation
2.4. The Process of Statistical Analysis
3. Results
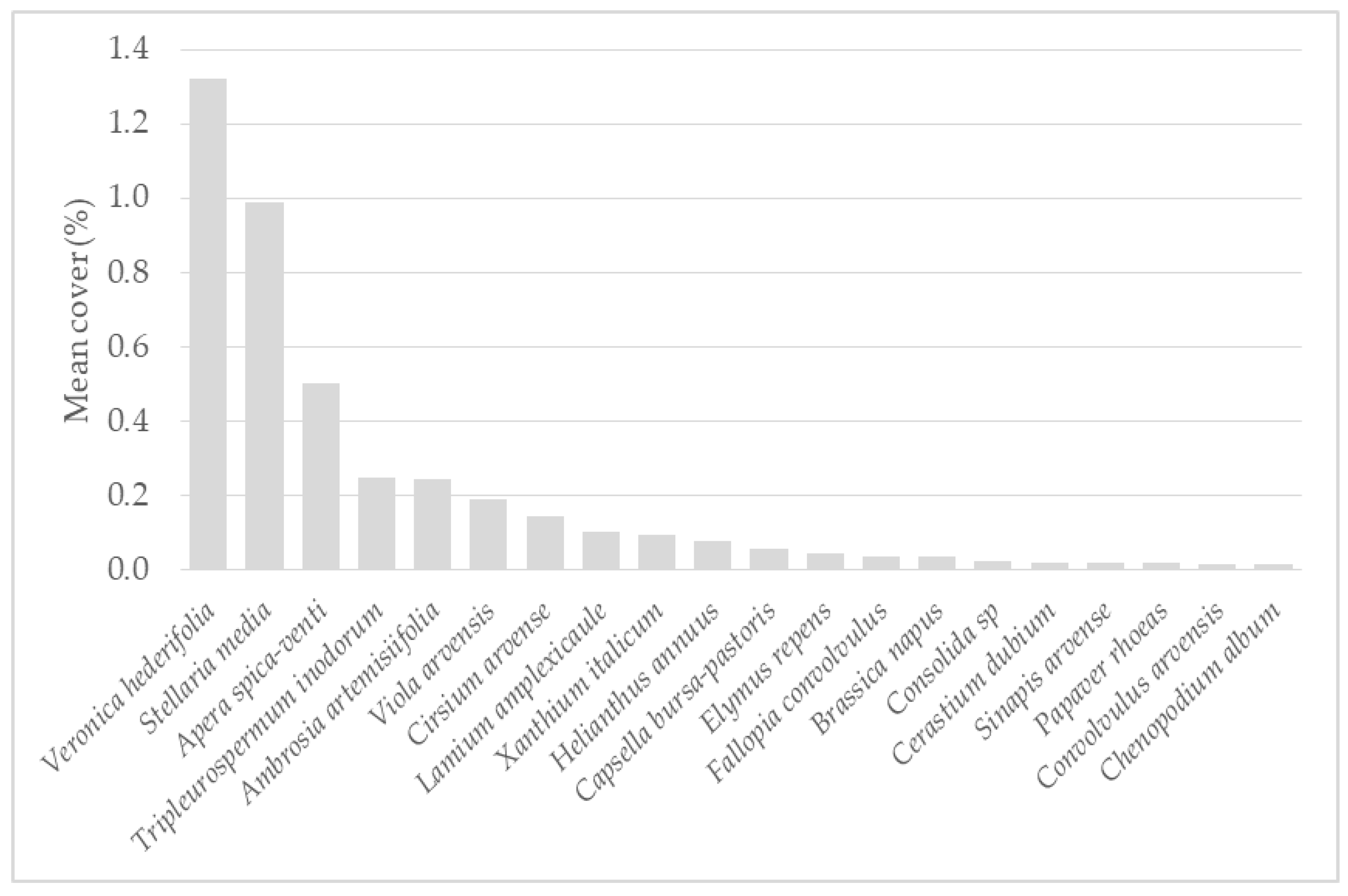
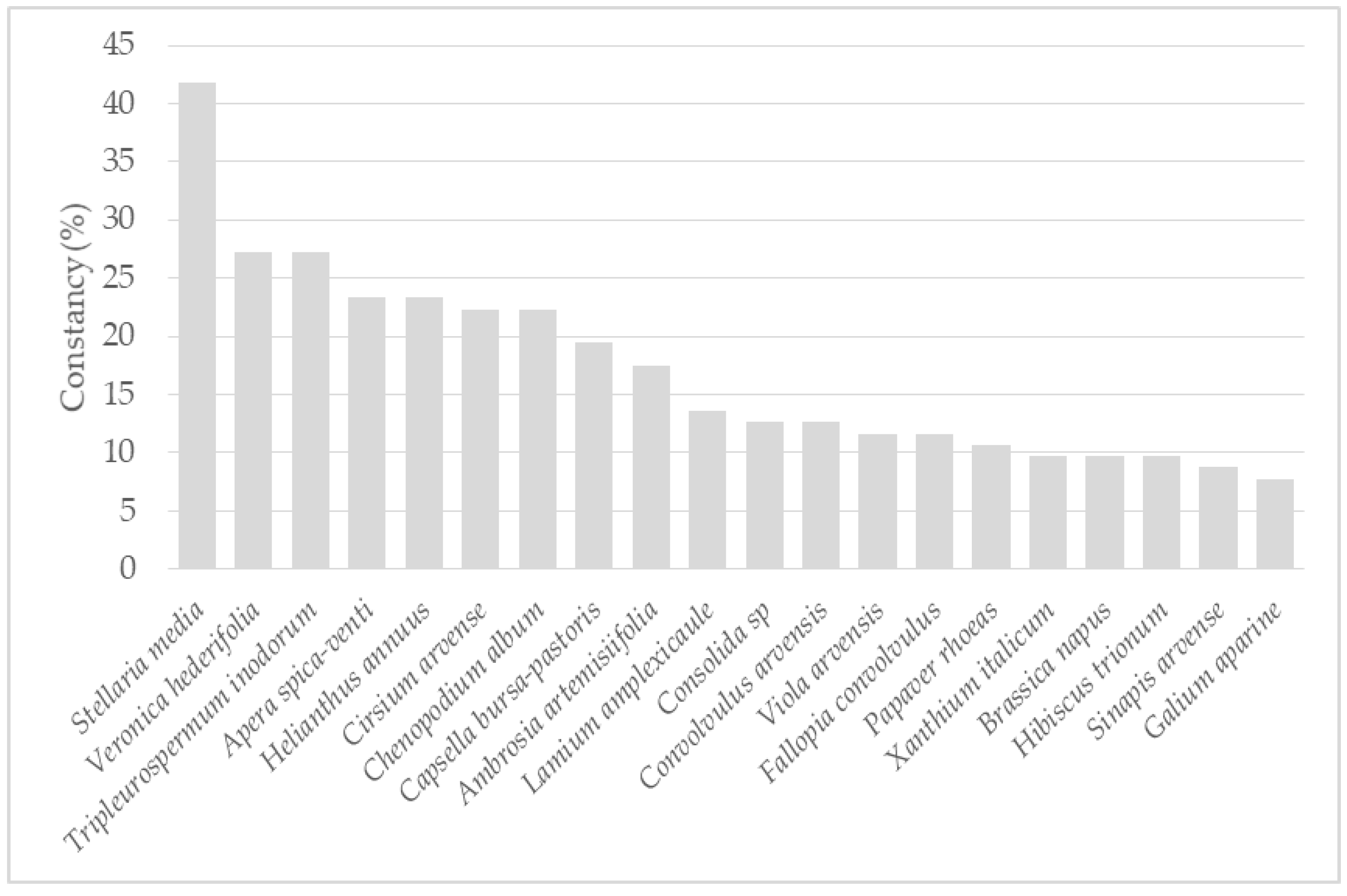
3.1. Weed Vegetation in the Regions Studied
3.2. The Effect of Explanatory Variables on Total Weed Coverage, Species Richness, and Diversity in the Winter Wheat Fields Surveyed
3.3. The Effect of Explanatory Variables on Weed Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Scientific Name A | Taxonomy A | Predominant Photosynthetic Pathway C | Regional Occurrence D | ||||
|---|---|---|---|---|---|---|---|
| Family | Class B | Region 1 | Region 2 | Region 3 | Region 4 | ||
| Amarathus retroflexus | Amaranthaceae | D | C4 | x | x | ||
| Ambrosia artemisiifolia | Asteraceae | D | C3 | x | x | x | |
| Anthemis austriaca | Asteraceae | D | C3 | x | |||
| Apera spica-venti | Poaceae | M | C3 | x | x | x | x |
| Avena fatua | Poaceae | M | C3 | x | |||
| Brassica napus | Brassicaceae | D | C3 | x | x | x | |
| Bromus sterilis | Poaceae | M | C3 | x | |||
| Cannabis sativa | Cannabinaceae | D | C3 | x | x | x | |
| Capsella bursa-pastoris | Brassicaceae | D | C3 | x | x | x | x |
| Cardaria draba | Brassicaceae | D | C3 | x | x | x | |
| Centaurea cyanus | Asteraceae | D | C3 | x | x | ||
| Cerastium dubium | Caryophyllaceae | D | C3 | x | x | ||
| Chenopodium album | Amaranthaceae | D | C3 | x | x | x | x |
| Chenopodium hybridum | Amaranthaceae | D | C3 | x | x | ||
| Chenopodium polyspermum | Amaranthaceae | D | C3 | x | |||
| Cichorium intybus | Asteraceae | D | C3 | x | |||
| Cirsium arvense | Asteraceae | D | C3 | x | x | x | x |
| Consolida sp. | Ranunculaceae | D | C3 | x | x | x | x |
| Convolvulus arvensis | Convolvulaceae | D | C3 | x | x | ||
| Datura stramonium | Solanaceae | D | C3 | x | x | x | |
| Daucus carota | Apiaceae | D | C3 | x | |||
| Descurainia sophia | Brassicaceae | D | C3 | x | x | x | x |
| Elymus repens | Poaceae | M | C3 | x | |||
| Fallopia convolvulus | Polygonaceae | D | C3 | x | x | x | x |
| Fumaria schleicheri | Papaveraceae | D | C3 | x | |||
| Galium aparine | Rubiaceae | D | C3 | x | x | x | |
| Helianthus annuus | Asteraceae | D | C3 | x | x | x | |
| Heliotropium europaeum | Boraginaceae | D | C3 | x | |||
| Hibiscus trionum | Malvaceae | D | C3 | x | x | ||
| Lactuca serriola | Asteraceae | D | C3 | x | |||
| Lamium amplexicaule | Lamiaceae | D | C3 | x | x | x | x |
| Lamium purpureum | Lamiaceae | D | C3 | x | x | x | |
| Lycopus exaltatus | Lamiaceae | D | C3 | x | |||
| Medicago sativa | Fabaceae | D | C3 | x | |||
| Myosurus minimus | Ranunculaceae | D | C3 | x | |||
| Papaver rhoeas | Papaveraceae | D | C3 | x | x | x | x |
| Phragmites australis | Poaceae | M | C3 | x | |||
| Pisum sativum | Fabaceae | D | C3 | x | |||
| Plantago lanceolata | Plantaginaceae | D | C3 | x | |||
| Polygonum aviculare | Polygonaceae | D | C3 | x | x | ||
| Prunus spinosa | Rosaceae | D | C3 | x | |||
| Ranunculus repens | Ranunculaceae | D | C3 | x | x | ||
| Raphanus raphanistrum | Brassicaceae | D | C3 | x | |||
| Sinapis arvense | Brassicaceae | D | C3 | x | x | ||
| Sonchus asper | Asteraceae | D | C3 | x | x | ||
| Stachys annua | Lamiaceae | D | C3 | x | |||
| Stellaria media | Caryophyllaceae | D | C3 | x | x | x | x |
| Taraxacum officinale | Asteraceae | D | C3 | x | |||
| Tripleurospermum inodorum | Asteraceae | D | C3 | x | x | x | x |
| Veronica hederifolia | Scrophulariaceae | D | C3 | x | x | x | |
| Veronica polita | Scrophulariaceae | D | C3 | x | x | ||
| Vicia villosa | Fabaceae | D | C3 | x | |||
| Viola arvensis | Violaceae | D | C3 | x | x | x | |
| Xanthium italicum | Asteraceae | D | C3 | x | |||
References
- FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#compare (accessed on 3 March 2025).
- Rouag, N.; Mekhlouf, A.; Makhlouf, M.; Rouabhi, A. Biological efficacy trial of several active substances against broadleaf weed herbicides Wheat: Case of Veronica species in soft wheat fields (Triticum aestivum L.). Rev. Agric. 2015, 10, 23–30. [Google Scholar]
- Hunyadi, K.; Béres, I.; Kazinczi, G. Gyomnövények, Gyomirtás, Gyombiológia; Mezőgazda Kiadó: Budapest, Hungary, 2011; pp. 24–28. (In Hungarian) [Google Scholar]
- Moss, S. Black-grass (Alopecurus myosuroides): Why has this Weed become such a Problem in Western Europe and what are the Solutions? Outlooks Pest Manag. 2017, 28, 207–212. [Google Scholar] [CrossRef]
- Novák, R.; Magyar, M.; Simon, G.; Kadaravek, B.; Kadaravekné Guttyán, A.; Blazsek, K.; Erdélyi, K.; Farkas, G.; Gyulai, B.; Hornyák, A.; et al. A Hatodik Országos Szántóföldi Gyomfelvételezés előzetes eredményei. Magy. Gyomkutatás Technol. 2019, 20, 55–58, (In Hungarian with an English Summary). [Google Scholar]
- Andersson, T.N.; Milberg, P. Weed flora and the relative importance of site, crop, crop rotation, and nitrogen. Weed Sci. 1998, 46, 30–38. [Google Scholar] [CrossRef]
- Lososova, Z.; Chytry, M.; Cimalova, S.; Kropac, Z.; Otypkova, Z.; Pysek, P.; Tichy, L. Weed vegetation of arable land in Central Europe: Gradients of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- Pysek, P.; Leps, J. Response of a weed community to nitrogen fertilization: A multivariate analysis. Veg. Sci. 1991, 2, 237–244. [Google Scholar] [CrossRef]
- Hallgren, E.; Palmer, M.W.; Milberg, P. Data diving with cross-validation: An investigation of broad-scale gradients in Swedish weed communities. J. Ecol. 1999, 87, 1037–1051. [Google Scholar] [CrossRef]
- Liebman, M.; Dyck, E. Crop rotation and intercropping strategies for weed management. Ecol. Appl. 1993, 3, 92–122. [Google Scholar] [CrossRef]
- Loudyi, M.C.; Godron, M.; El Khyari, D. Influence des variables écologiques sur la distribution des mauvaises herbes des cultures du Sais. Weed Res. 1995, 35, 225–240. [Google Scholar] [CrossRef]
- Jordan, N.; Mortensen, D.A.; Prenzlow, M.; Cox, K.C. Simulation analyses of crop rotation effects on weed seedbanks. Am. J. Bot. 1995, 82, 390–398. [Google Scholar] [CrossRef]
- Fried, G.; Petit, S.; Reboud, X. A specialist-generalist classification of the arable flora and its response to changes in agricultural practices. BMC Ecol. 2010, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Arbhri, B.; Silvestri, N.; Bonari, F. Weed communities of winter wheat as influenced by input level and rotation. Weed Res. 1997, 37, 301–313. [Google Scholar]
- Kauppila, R. Conventional and organic cropping systems at Suitia IV: Weeds. Agric. Food Sci. 1990, 62, 331–337. [Google Scholar] [CrossRef]
- Pallutt, B. Population dynamics and competition of weeds depending on crop rotation and mechanical and chemical control measures in cereals. Proc. Br. Crop Prot. Conf. Weeds 1993, 3, 1197–1204. [Google Scholar]
- Ewald, J.; Aebischer, N.J. Pesticide Use, Avian Food Resources and Bird Densities in Sussex; JNCC Report No. 296; Joint Nature Conservation Committee: Peterborough, UK, 1999; p. 101. [Google Scholar]
- Andersson, T.N.; Milberg, R. Weed performance in crop rotations with and without leys and at different nitrogen levels. Ann. Appl. Biol. 1996, 128, 505–518. [Google Scholar] [CrossRef]
- Patterson, D.T.; Westbrook, J.K.; Joyce, R.J.V.; Lingren, P.D.; Rogasik, J. Weeds, insects, and diseases. Clim. Chang. 1999, 43, 711–727. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Soussana, J.F.; Howden, S.M. Crop and pasture response to climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19686–19690. [Google Scholar] [CrossRef]
- Kismányoky, A. Effect of Agrotechnical Factors to Crop Plants and Weeds. Ph.D. Thesis, University of Pannonia, Keszthely, Hungary, 2010. Available online: http://konyvtar.uni-pannon.hu/doktori/2010/Kismanyoky_Andras_theses_en.pdf (accessed on 6 March 2025).
- Walter, A.M.; Chritensen, S.; Simmelsgaard, S.E. Spatial correlation between weed species densities and soil properties. Weed Res. 2002, 42, 26–38. [Google Scholar] [CrossRef]
- Hoitnik, H.A.J.; Boehm, N.J. Biocontrol within the context of soil icrobial communities: A substrate—Depend phenomeon. Annu. Rev. Phytopatol. 1999, 37, 427–446. [Google Scholar] [CrossRef]
- Nordmeyer, H.; Dunker, M. Variable weed densities and soil properties in a weed mapping concept for patchy weed control. In Proceedings of the Second European Conference on Precision Agriculture, Odense Congress Centre, Odense, Denmark, 11–15 July 1999. [Google Scholar]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Laghari, A.H.; Bhabhan, G.M.; Talpur, K.H. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Ellenberg, H. Zeigerwerte der Gefässpflantzen Mitteleuropeas. Scr. Geobot. 1974, 9, 97. [Google Scholar] [CrossRef]
- Borhidi, A. Social Behavior Types of the Hungarian Flora, Its Naturalness and Relative Ecological Indicator Values; Janus Pannonius University: Pécs, Hungary, 1993; p. 37. [Google Scholar]
- Korres, N.E.; Norsworthy, J.K.; Bryne, K.R.; Skinner, V.; Mauromoustakos, A. Relationship between soil properties and the occurence of the most agronomically important weed species int he field margins of eastern Arkansas—Implications for weed management in field margins. Weed Res. 2017, 57, 159–171. [Google Scholar] [CrossRef]
- Kone, B.; Amadji, G.L.; Toure, A.; Togola, A.; Mariko, M.; Huat, J. A case of Cyperus spp. and Imperata cylindrica occurences on acrisol of the Dahomey Gap in south Beninas affected by soil characteristic: A strategy for soil and weed management. Appl. Environ. Soil Sci. 2013, 7, 601058. [Google Scholar] [CrossRef]
- Weedsmart. Available online: https://www.weedsmart.org.au/content/does-soil-ph-affect-weed-management/ (accessed on 20 February 2025).
- Manna, M.C.; Swarup, A.; Wanjari, R.H.; Mishra, B.; Shahi, D.K. Long-term fertilization, manure and liming effects on soil organic matter and crop yelds. Soil Till Res. 2007, 94, 397–409. [Google Scholar] [CrossRef]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L.) following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils. 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Flowers, J.T.; Comer, D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 3, 327–331. [Google Scholar] [CrossRef]
- James, T.K.; Merfield, C.N. Weed and soil management: A balancing act. Encycl. Soils Environ. 2023, 3, 439–449. [Google Scholar]
- Pekrun, C.; Claupein, W. The implication of stubble tillage for weed population dynamics in organic farming. Weed Res. 2006, 46, 414–423. [Google Scholar] [CrossRef]
- Kovács, E.B.; Dorner, Z.; Csík, D.; Zalai, M. Effect of environmental, soil and management factors on weed flora of field pea in South-East Hungary. Agronomy 2023, 13, 1864. [Google Scholar] [CrossRef]
- Marshall, E.J.P. The impact of landscape structure and sown grass margin strips on weed assemblages in arable crops and their boundaries. Weed Res. 2009, 49, 107–115. [Google Scholar] [CrossRef]
- Fahrig, L.; Girard, J.; Duro, D.; Pasher, J.; Smith, A.; Javorek, S.; King, D.; Freemark, L.K.; Mitchell, S.; Tischendorf, L. Farmlands with smaller crop fields have higher within-field biodiversity. Agric. Ecosyst. Environ. 2015, 200, 219–234. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Botta-Dukát, Z.; Czúcz, B. Relating Ambrosia artemisiifolia and other weeds to the management of Hungarian sunflower crops. J. Pest Sci. 2013, 86, 621–631. [Google Scholar] [CrossRef]
- de Mol, F.; von Redwitz, C.; Gerowitt, B. Weed species composition of maize fields in Germany is influenced by site and crop sequence. Weed Res. 2015, 55, 574–585. [Google Scholar] [CrossRef]
- Kövespataki, E.; Turcsányi-Jérdi, L.; Wichmann, B.; Saláta-Falusi, E. The effect of vintage on the vegetation of Hungarian Grey cattle pasture in 2022 and 2023—Case study. Gyepgazdálkodási Közlemények 2024, 21, 29–36. [Google Scholar] [CrossRef]
- Patterson, D.T. Weeds in a changing climate. Weed Sci. 1995, 43, 685–700. [Google Scholar] [CrossRef]
- Gerencsér, E.E.; Lantos, Z.; Varga-Haszonits, Z.; Varga, Z. Determination of winter barley yield by the aim of multiplicative successive approximation. Időjárás 2011, 115, 167–178. [Google Scholar]
- Varga-Haszonits, Z.; Varga, Z.; Lantos, Z.; Enzsölné, G.E.; Gerencsér, E. Climate Variability and Agroecosystems, 1st ed.; NYME MÉK: Mosonmagyaróvár, Hungary, 2008; 410p. [Google Scholar]
- Fried, G.; Norton, L.R.; Reboud, X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008, 128, 68–76. [Google Scholar] [CrossRef]
- Pál, R.W.; Pinke, G.; Botta-Dukát, Z.; Campetella, G.; Bartha, S.; Kalocsai, R.; Lengyel, A. Can management intensity be more important than environmental factors? A case study along an extreme elevation gradient from central Italian cereal fields. Plant Biosyst. -Int. J. Deal. All Asp. Plant 2013, 147, 343–353. [Google Scholar] [CrossRef]
- Csorba, P. Magyarország Kistájai, 1st ed.; Meridián Táj-és Környezetföldrajzi Alapítvány: Debrecen, Hungary, 2021; 416p. [Google Scholar]
- HungaroMet Meteorological Data Archive: Historical Data of Automatic Meteorological Stations. Available online: https://odp.met.hu/climate/observations_hungary/monthly/historical/ (accessed on 28 February 2025).
- EPPO Global Database. Available online: https://gd.eppo.int/search (accessed on 20 February 2025).
- van der Maarel, E.; Franklin, J. Vegetation ecology: Historical notes and outline. Weed Ecol. 2013, 2, 1–27. [Google Scholar]
- Zalai, M.; Dorner, Z.; Kolozsvári, L.; Szalai, M. What does the precision of weed sampling of maize fields depend on? Növényvédelem 2012, 48, 451–456, (In Hungarian with an English Summary). [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; pp. 34–50. [Google Scholar]
- Fox, J. Applied Regression Analysis and Generalized Linear Models, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2016; pp. 342–358. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.; Heiberger, R.M. Analysis of variance, designed experiments. In Statistical Models in S, 1st ed.; Chambers, J.M., Hastie, T.J., Eds.; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1992; pp. 145–194. [Google Scholar]
- Soper, H.E.; Young, A.W.; Cave, B.M.; Lee, A.; Pearson, K. On the distribution of the correlation coefficient in small samples. Appendix II to the papers of “Student” and R.A. Fisher. A co-operative study. Biometrika 1917, 11, 328–413. [Google Scholar]
- Keselman, H.J.; Rogan, J.C. The Tukey multiple comparison test: 1953–1976. Psychol. Bull. 1977, 84, 1050–1056. [Google Scholar] [CrossRef]
- Sutter, J.M.; Kalivas, J.H. Comparison of Forward Selection, Backward Elimination, and Generalized Simulated Annealing for Variable Selection. Microchem. J. 1993, 47, 60–66. [Google Scholar] [CrossRef]
- Novák, R.; Magyar, M.; Simon, G.; Kadaravek, B.; Kadaravekné Guttyán, A.; Nagy, M.; Blazsek, K.; Erdélyi, K.; Farkas, G.; Gyulai, B.; et al. Change in the spread of common ragweed in Hungary in the light of the National Arable Weed Survey (1947–2019). In Proceedings of the International Ragweed Society Conference, Budapest, Hungary, 8–9 September 2022. [Google Scholar]
- Nagy, K. Investigation of Arable Weed Vegetation in Mures County. Ph.D. Dissertation, Széchenyi István University, Mosonmagyaróvár, Hungary, 2017. [Google Scholar]
- Martin, J.R.; Green, J.D. Weed management, Section 6. In A Comprehensive Guide to Wheat Management in Kentucky, 1st ed.; University of Kentucky: Lexington, KY, USA, 2009; pp. 30–41. [Google Scholar]
- Frou-Williams, R.J. Weed Competition. In Weed Management Handbook, 9th ed.; Blackwell: Malden, MA, USA, 2002; pp. 16–38. [Google Scholar]
- Nagy, K.E.; Pinke, G. Az erdélyi Maros megye gyomnövényzete. I. Kalászos Vetések. Magy. Gyomkutatás Technol. 2014, 15, 33–45. [Google Scholar]
- Schumacher, M.; Ohnmacht, S.; Rosenstein, R.; Gerhards, R. How Management Factors Influence Weed Communities of Cereals, Their Diversity and Endangered Weed Species in Central Europe. Agriculture 2018, 8, 172. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and methods of vegetation ecology. Geogr. Rev. 1976, 66, 114–116. [Google Scholar]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- Dorner, Z.; Kovács, E.B.; Iványi, D.; Zalai, M. How the Management and Environmental Conditions Affect the Weed Vegetation in Canary Grass (Phalaris canariensis L.) Fields. Agronomy 2024, 14, 1169. [Google Scholar] [CrossRef]
- El-Metwally, I.M.; Abd El-Salam, M.S.; Osama, A.M.A. Effect of Zinc Application and Weed Control on Wheat Yield and Its Associated Weeds Grown in Zinc-Deficient Soil. Int. J. ChemTech Res. 2015, 8, 1588–1600. [Google Scholar]
- Morishima, H.; Oka, H.I. The impact of copper pollution on weed communities in Japanese rice fields. Agro-ecosystems 1976, 3, 131–145. [Google Scholar] [CrossRef]
- Strandberg, B.; Axelsen, J.A.; Pedersen, M.B.; Jensen, J.; Attrill, M.J. Effect of a copper gradient on plant community structure. Environ. Toxicol. Chem. 2006, 25, 743–753. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. When herbicides don’t really matter: Weed species composition of oil pumpkin (Cucurbita pepo L.) fields in Hungary. Crop Prot. 2018, 110, 236–244. [Google Scholar] [CrossRef]
- Pinke, G.; Blazsek, K.; Magyar, L.; Nagy, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed species composition of conventional soyabean crops in Hungary is determined by environmental, cultural, weed management and site variables. Weed Res. 2016, 56, 470–481. [Google Scholar] [CrossRef]
- Kristó, I.; Vályi-Nagy, M.; Rácz, A.; Tar, M.; Irmes, K.; Szentpéteri, L.; Ujj, A. Effects of weed control treatments on weed composition and yield components of winter wheat (Triticum aestivum L.) and winter pea (Pisum sativum L.) intercrops. Agronomy 2022, 12, 2590. [Google Scholar] [CrossRef]
- Murphy, C.E.; Lemerle, D. Continuous cropping systems and weed selection. Euphytica 2006, 148, 61–73. [Google Scholar] [CrossRef]
- Lugowska, M.; Pawlonka, Z.; Skrzyczynska, J. The effects of soil conditions and crop types on diversity of weed communities. Acta Agrobot. 2016, 6, 1687. [Google Scholar] [CrossRef]
- Muktamar, Z.; Setyowati, N.; Utami, K.; Haris, H.A.; Nurjanah, U.; Sukisno, S.; Hindarto, K.S. Distribution of weed species and soil nitrogen, phosphorus, and potassium across various land uses in coastal areas. Int. J. Agric. Technol. 2025, 21, 107–124. [Google Scholar]
- Kovács, E.B. Weed Flora Analysis of Canary Grass and Dry Pea in the Area of Gyomaendrőd and Szarvas, in Ecological and Conventional Fields. Ph.D. Dissertation, Magyar Agrár-és Élettudományi Egyetem, Gödöllő, Hungary, 2024. [Google Scholar]
- Cimalová, Š.; Lososová, Z. Arable weed vegetation of the northeastern part of the Czech Republic: Effects of environmental factors on species composition. Plant Ecol. 2009, 203, 45–57. [Google Scholar] [CrossRef]
- Hanzlik, K.; Gerowitt, B. The importance of climate, site and management on weed vegetation in oilseed rape in Germany. Agric. Ecosyst. Environ. 2011, 141, 323–331. [Google Scholar] [CrossRef]
- Yenish, J.P.; Doll, J.D.; Buhler, D.D. Effects of Tillage on Vertical Distribution and Viability of Weed Seed in Soil. Weed Sci. 1992, 40, 429–433. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The role of weeds in supporting biological diversity within crop fields. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef]
- Standovár, T.; Richard, B. Nature Conservation Biology, 1st ed.; Nemzeti Tankönyvkiadó: Budapest, Hungary, 2001; p. 542. [Google Scholar]
- Inouye, R.S.; Tilman, D. Convergence and divergence of old-field vegetation after 11 yr of nitrogen addition. Ecology 1995, 76, 1872–1887. [Google Scholar] [CrossRef]
- Storkey, J.; Moss, S.R.; Cussans, J.W. Using assembly theory to explain changes in a weed flora in response to agricultural intensification. Weed Sci. 2010, 58, 39–46. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Gill, G.S.; Preston, C. Tillage system effects on weed ecology, herbicide activity and persistence: A review. Aust. J. Exp. Agric. 2006, 46, 1557–1570. [Google Scholar] [CrossRef]
- Cardina, J.; Herms, C.P.; Doohan, D.J. Crop rotation and tillage system effects on weed seedbanks. Weed Sci. 2002, 50, 448–460. [Google Scholar] [CrossRef]
- Zubko, V.; Sokolik, S.; Khvorost, T.; Melnyk, V. Factors affecting quality of tillage with disc harrow, engineering for rural development. Eng. Rural Dev. 2021, 26, 1193–1199. [Google Scholar] [CrossRef]
- Williams, J.W.; Webb, T., III; Richard, P.H.; Newby, P. Late quaternary biomes of Canada and the eastern United States. J. Biogeogr. 2000, 27, 585–607. [Google Scholar] [CrossRef]
- Kalapos, T. C3 and C4 grasses of Hungary: Environmental requirements, phenology and role in the vegetation. Abstr. Bot. 1991, 15, 83–88. [Google Scholar]
- Kalapos, T.; Baloghné-Nyakas, A.; Csontos, P. Occurrence and ecological characteristics of C4 dicot and Cyperaceae species in the Hungarian flora. Phytosynthetica 1997, 33, 227–240. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Website. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 18 April 2025).

| Year A | Region 1 B | Region 2 C | Region 3 D | Region 4 E | ||||
|---|---|---|---|---|---|---|---|---|
| Rainfall (mm) | Avg. Temp. (°C) | Rainfall F (mm) | Avg. Temp. (°C) | Rainfall G (mm) | Avg. Temp. (°C) | Rainfall (mm) | Avg. Temp. (°C) | |
| 2018 | 737.5 | 11.9 | 643.4 | 10.6 | 699.3 | 10.9 | 618.6 | 10.7 |
| 2019 | 527.3 | 12.4 | 433.3 | 11.4 | 432.3 | 11.6 | 401.0 | 11.5 |
| 2020 | 589.8 | 12.3 | 623.4 | 11.0 | 540.9 | 11.5 | 572.4 | 11.2 |
| 2021 | 664.8 | 11.5 | 710.6 | 10.3 | 768.3 | 10.5 | 638.0 | 10.4 |
| 2018–2021 on average | 629.9 | 12.0 | 602.7 | 10.8 | 610.2 | 11.1 | 557.5 | 11.0 |
| Variable (Unit) | Range/Recorded or Calculated Values |
|---|---|
| Soil variables | |
| Soil texture (KArany) | 25–67 |
| Soil pH (KCl) | 3.65–7.20 |
| Soil properties | |
| Salt (m/m %) | 0.01–1.13 |
| Humus (m/m %) | 0.70–3.90 |
| N (mg kg−1) | 1.0–213 |
| P2O5 (mg kg−1) | 31–931 |
| K2O (mg kg−1) | 59–604 |
| CaCO3 (m/m %) | 0.04–2.74 |
| Na (mg kg−1) | 2.5–157 |
| Mg (mg kg−1) | 27–1192 |
| S (mg kg−1) | 0.5–67.3 |
| Cu (mg kg−1) | 0.5–12.5 |
| Mn (mg kg−1) | 9–525 |
| Zn (mg kg−1) | 0.4–9.1 |
| Environmental variables | |
| Altitude (m, AMSL) | 78–180 |
| Latitude (° N) | 46.858889–48.263222 |
| Longitude (° E) | 20.835611–22.212083 |
| Region A | Region 1, Region 2, Region 3, Region 4 |
| Year | 2018–2021 |
| Farming variables | |
| Date of weed survey (Julian day) | 78–128 |
| Field size (ha) | 1.5–77 |
| Preceding crops B | |
| Untillaged C | 0–1.0 |
| Spring row crops D | 0–1.0 |
| Cereal crops E | 0–0.6 |
| Other dense crops F | 0–0.6 |
| Tillage system | Disc harrowing, shallow cultivation, ploughing, deep loosening |
| Tillage depth (cm) | 10–40 |
| Amount of nitrogen fertilizer (kg a.i. ha−1) | 36–168 |
| Amount of phosphorus fertilizer (kg a.i. ha−1) | 43–96 |
| Amount of potassium fertilizer (kg a.i. ha−1) | 48–110 |
| Soil Variables | Total Weed Coverage [%] | Species Richness | Shannon Diversity |
|---|---|---|---|
| p-values of ANCOVAs (Pearson correlation coefficients) | |||
| Soil texture | ns | <0.001 (+0.41) | <0.001 (+0.31) |
| Soil reaction | ns | 0.025 (+0.20) | ns |
| Soil properties | |||
| Salt | ns | <0.001 (+0.32) | ns |
| Humus | ns | ns | ns |
| N | ns | ns | ns |
| P2O5 | ns | ns | ns |
| K2O | ns | <0.001 (+0.41) | <0.001 (+0.33) |
| CaCO3 | ns | 0.041 (+0.20) | ns |
| Na | ns | <0.001 (+0.39) | 0.001 (+0.31) |
| Mg | ns | <0.001 (+0.31) | 0.009 (+0.25) |
| S | ns | <0.001 (+0.35) | 0.009 (+0.26) |
| Cu | 0.033 (+0.21) | 0.024 (+0.22) | ns |
| Mn | ns | ns | ns |
| Zn | 0.023 (+0.22) | <0.001 (+0.35) | 0.015 (+0.24) |
| Environmental and Farming Variables | Total Weed Coverage [%] | Species Richness | Shannon Diversity |
|---|---|---|---|
| p-values of ANCOVAs (Pearson correlation coefficients)/[mean and sign. classes of Tukey’s tests A] | |||
| Altitude | ns | 0.003 (−0.29) | ns |
| Latitude | ns | <0.001 (−0.36) | 0.017 (−0.23) |
| Longitude | ns | 0.013 (−0.24) | ns |
| Region | ns | <0.001 [BEK—6.23 a HBB—2.92 b SSB—4.00 b BAZ—3.11 b] | ns |
| Year | 0.033 [2018—5.25 a 2019—3.01 b 2020—4.31 ab 2021—4.56 ab] | <0.001 [2018—7.36 a 2019—3.61 b 2020—3.25 b 2021—2.96 b] | <0.001 [2018—1.13 a 2019—0.85 ab 2020—0.64 bc 2021—0.49 c] |
| Date of weed survey | ns | <0.001 (+0.42) | <0.001 (+0.34) |
| Field size | ns | ns | ns |
| Preceding crops | |||
| Undisturbed | ns | ns | ns |
| Spring row crops | ns | ns | ns |
| Cereal crops | ns | 0.008 (+0.26) | 0.039 (+0.20) |
| Other dense crops | ns | ns | ns |
| Tillage system DH (disc harrowing) SC (shallow cultivation) PL (ploughing) DL (deep loosening) | 0.023 [DH—5.69 ab SC—1.66 c PL—9.75 a DL—1.70 bc] | 0.008 [DH—5.59 a SC—3.10 b PL—3.93 ab DL—3.33 b] | ns |
| Tillage depth | 0.006 (−0.27) | 0.001 (−0.32) | 0.034 (−0.13) |
| Amount of N fertilizer | 0.011 (−0.25) | 0.019 (−0.23) | ns |
| Amount of P fertilizer | ns | <0.001 (−0.41) | 0.006 (−0.27) |
| Amount of K fertilizer | ns | 0.023 (−0.22) | 0.045 (−0.20) |
| Significant Explanatory Variables | Df | Gross Effect | Net Effect | ||||
|---|---|---|---|---|---|---|---|
| Explained Variation (%) | R2adj | Explained Variation (%) | R2adj | F | p-Value | ||
| Soil N content | 1 | 1.24 | 0.003 | 1.75 | 0.007 | 1.721 | 0.035 |
| Soil Mg content | 1 | 2.80 | 0.018 | 1.88 | 0.010 | 2.103 | 0.007 |
| Region | 3 | 9.96 | 0.072 | 7.70 | 0.060 | 3.164 | 0.001 |
| Year | 3 | 4.50 | 0.016 | 6.26 | 0.043 | 2.570 | 0.001 |
| Spring row preceding crop | 1 | 1.37 | 0.004 | 1.58 | 0.009 | 1.950 | 0.019 |
| Other dense preceding crop | 1 | 1.29 | 0.003 | 1.34 | 0.006 | 1.653 | 0.044 |
| Tillage system | 3 | 5.02 | 0.021 | 5.58 | 0.036 | 2.293 | 0.001 |
| Tillage depth | 1 | 1.54 | 0.006 | 1.73 | 0.011 | 2.135 | 0.007 |
| Amount of K fertilizer | 1 | 2.67 | 0.017 | 1.38 | 0.007 | 1.703 | 0.047 |
| Species | Fit | Ax 1 Score | Species | Fit | Ax 1 Score |
|---|---|---|---|---|---|
| Region 1 (+ high; − low) | Region 2 (+ high; − low) | ||||
| Cirsium arvense | 0.140 | 0.292 | Consolida spp. | 0.090 | 0.166 |
| Xanthium italicum | 0.205 | 0.250 | Raphanus raphanistrum | 0.060 | 0.069 |
| Tripleurospermum inodorum | 0.072 | 0.236 | Phragmites australis | 0.031 | 0.053 |
| Helianthus annuus | 0.070 | 0.187 | Vicia villosa | 0.031 | 0.047 |
| Convolvulus arvensis | 0.144 | 0.165 | Fumaria schleicheri | 0.031 | 0.042 |
| Fallopia convolvulus | 0.050 | 0.096 | Lycopus exaltatus | 0.031 | 0.030 |
| Sinapis arvensis | 0.077 | 0.089 | Convolvulus arvensis | 0.028 | −0.073 |
| Cerastium dubium | 0.108 | 0.087 | Xanthium italicum | 0.028 | −0.093 |
| Hibiscus trionum | 0.128 | 0.077 | Helianthus annuus | 0.062 | −0.176 |
| Stellaria media | 0.096 | −0.349 | Tripleurospermum inodorum | 0.048 | −0.193 |
| Region 3 (+ high; − low) | Region 4 (+ high; − low) | ||||
| Viola arvensis | 0.172 | 0.151 | Stellaria media | 0.067 | 0.291 |
| Elymus repens | 0.088 | 0.136 | Chenopodium album | 0.143 | 0.218 |
| Ambrosia artemisiifolia | 0.035 | 0.113 | Capsella bursa-pastoris | 0.071 | 0.178 |
| Medicago sativa | 0.043 | 0.068 | Chenopodium hybridum | 0.077 | 0.115 |
| Bromus sterilis | 0.043 | 0.012 | Plantago lanceolata | 0.045 | 0.074 |
| Daucus carota | 0.043 | 0.008 | Pisum sativum | 0.052 | 0.054 |
| Lactuca serriola | 0.043 | 0.008 | Xanthium italicum | 0.033 | −0.100 |
| Amaranthus retroflexus | 0.029 | 0.008 | Ambrosia artemisiifolia | 0.046 | −0.130 |
| Cichorium intybus | 0.043 | 0.005 | Cirsium arvense | 0.030 | −0.136 |
| Chenopodium album | 0.039 | −0.114 | Veronica hederifolia | 0.112 | −0.365 |
| Species | Fit | Ax 1 Score | Species | Fit | Ax 1 Score |
|---|---|---|---|---|---|
| 2018 (+ high; − low) | 2019 (+ high; − low) | ||||
| Ambrosia artemisiifolia | 0.112 | 0.204 | Chenopodium album | 0.115 | 0.196 |
| Xanthium italicum | 0.124 | 0.195 | Helianthus annuus | 0.029 | 0.120 |
| Lamium amplexicaule | 0.053 | 0.078 | Fallopia convolvulus | 0.078 | 0.120 |
| Cardaria draba | 0.130 | 0.061 | Chenopodium hybridum | 0.060 | 0.101 |
| Cannabis sativa | 0.088 | 0.053 | Pisum sativum | 0.041 | 0.048 |
| Polygonum aviculare | 0.070 | 0.047 | Phragmites australis | 0.021 | 0.044 |
| Datura stramonium | 0.112 | 0.027 | Stachys annua | 0.021 | 0.006 |
| Ranunculus repens | 0.072 | 0.024 | Cerastium dubium | 0.024 | −0.041 |
| Amaranthus retroflexus | 0.064 | 0.011 | Viola arvensis | 0.041 | −0.074 |
| Chenopodium polyspermum | 0.065 | 0.009 | Ambrosia artemisiifolia | 0.024 | −0.094 |
| 2020 (+ high; − low) | 2021 (+ high; − low) | ||||
| Tripleurospermum inodorum | 0.059 | 0.213 | Elymus repens | 0.053 | 0.106 |
| Plantago lanceolata | 0.069 | 0.092 | Consolida spp. | 0.029 | 0.095 |
| Galium aparine | 0.039 | 0.081 | Medicago sativa | 0.026 | 0.053 |
| Viola arvensis | 0.033 | 0.066 | Anthemis austriaca | 0.026 | 0.024 |
| Taraxacum officinale | 0.041 | 0.023 | Cichorium intybus | 0.026 | 0.004 |
| Lamium purpureum | 0.051 | 0.022 | Fallopia convolvulus | 0.036 | −0.082 |
| Bromus sterilis | 0.041 | 0.012 | Xanthium italicum | 0.033 | −0.100 |
| Lamium amplexicaule | 0.032 | −0.061 | Ambrosia artemisiifolia | 0.046 | −0.130 |
| Fallopia convolvulus | 0.023 | −0.066 | Helianthus annuus | 0.034 | −0.131 |
| Chenopodium album | 0.046 | −0.124 | Chenopodium album | 0.053 | −0.133 |
| Species | Fit | Ax 1 Score | Species | Fit | Ax 1 Score |
|---|---|---|---|---|---|
| Deep loosening (+ high; − low) | Disc harrowing (+ high; − low) | ||||
| Veronica hederifolia | 0.027 | 0.177 | Tripleurospermum inodorum | 0.082 | 0.253 |
| Fallopia convolvulus | 0.057 | 0.103 | Xanthium italicum | 0.044 | 0.116 |
| Phragmites australis | 0.038 | 0.059 | Convolvulus arvensis | 0.070 | 0.115 |
| Vicia villosa | 0.038 | 0.052 | Cerastium dubium | 0.082 | 0.076 |
| Hibiscus trionum | 0.027 | 0.035 | Sinapis arvensis | 0.027 | 0.053 |
| Avena fatua | 0.027 | 0.025 | Polygonum aviculare | 0.027 | 0.030 |
| Stachys annua | 0.038 | 0.008 | Ranunculus repens | 0.035 | 0.016 |
| Papaver rhoeas | 0.022 | −0.042 | Amaranthus retroflexus | 0.031 | 0.008 |
| Galium aparine | 0.015 | −0.051 | Chenopodium polyspermum | 0.031 | 0.006 |
| Viola arvensis | 0.022 | −0.054 | Chenopodium album | 0.029 | −0.098 |
| Ploughing (+ high; − low) | Shallow cultivation (+ high; − low) | ||||
| Stellaria media | 0.019 | 0.154 | Chenopodium album | 0.151 | 0.224 |
| Viola arvensis | 0.147 | 0.139 | Consolida spp. | 0.040 | 0.111 |
| Medicago sativa | 0.058 | 0.079 | Chenopodium hybridum | 0.069 | 0.109 |
| Elymus repens | 0.020 | 0.064 | Plantago lanceolata | 0.040 | 0.071 |
| Fumaria schleicheri | 0.058 | 0.057 | Pisum sativum | 0.048 | 0.051 |
| Lycopus exaltatus | 0.058 | 0.041 | Descurainia sophia | 0.039 | 0.048 |
| Bromus sterilis | 0.058 | 0.014 | Cannabis sativa | 0.043 | 0.037 |
| Fallopia convolvulus | 0.017 | −0.055 | Xanthium italicum | 0.036 | −0.105 |
| Chenopodium album | 0.025 | −0.091 | Tripleurospermum inodorum | 0.053 | −0.203 |
| Helianthus annuus | 0.018 | −0.095 | Veronica hederifolia | 0.047 | −0.236 |
| Species | Fit | Ax 1 Score | Species | Fit | Ax 1 Score |
|---|---|---|---|---|---|
| Soil N content (+ high; − low) | Soil Mg content (+ high; − low) | ||||
| Veronica hederifolia | 0.050 | 0.242 | Stellaria media | 0.043 | 0.233 |
| Fumaria schleicheri | 0.482 | 0.165 | Avena fatua | 0.024 | 0.023 |
| Brassica napus | 0.011 | 0.057 | Lamium purpureum | 0.035 | 0.018 |
| Plantago lanceolata | 0.015 | 0.043 | Ranunculus repens | 0.022 | −0.013 |
| Cardaria draba | 0.016 | 0.022 | Hibiscus trionum | 0.064 | −0.055 |
| Hibiscus trionum | 0.013 | −0.024 | Anthemis austriaca | 0.182 | −0.064 |
| Medicago sativa | 0.011 | −0.034 | Xanthium italicum | 0.028 | −0.093 |
| Xanthium italicum | 0.011 | −0.059 | Cirsium arvense | 0.022 | −0.114 |
| Ambrosia artemisiifolia | 0.016 | −0.078 | Galium aparine | 0.079 | −0.115 |
| Stellaria media | 0.012 | −0.120 | Capsella bursa-pastoris | 0.037 | −0.129 |
| Amount of K fertilizer (+ high; − low) | Tillage depth (+ high; − low) | ||||
| Stellaria media | 0.046 | 0.241 | Tripleurospermum inodorum | 0.079 | 0.247 |
| Fumaria schleicheri | 0.053 | 0.055 | Brassica napus | 0.048 | 0.121 |
| Lycopus exaltatus | 0.053 | 0.039 | Consolida spp. | 0.023 | 0.084 |
| Anthemis austriaca | 0.024 | 0.023 | Cannabis sativa | 0.038 | 0.035 |
| Lactuca serriola | 0.021 | 0.006 | Cardaria draba | 0.024 | 0.026 |
| Cichorium intybus | 0.034 | −0.004 | Datura stramonium | 0.070 | 0.022 |
| Cerastium dubium | 0.055 | −0.062 | Veronica polita | 0.036 | 0.019 |
| Viola arvensis | 0.047 | −0.079 | Cichorium intybus | 0.030 | −0.004 |
| Ambrosia artemisiifolia | 0.024 | −0.094 | Cerastium dubium | 0.041 | −0.054 |
| Chenopodium album | 0.046 | −0.124 | Cirsium arvense | 0.032 | −0.139 |
| Spring row preceding crops (+ high; − low) | Other dense preceding crops (+ high; − low) | ||||
| Tripleurospermum inodorum | 0.025 | 0.139 | Veronica hederifolia | 0.024 | 0.169 |
| Elymus repens | 0.029 | 0.078 | Brassica napus | 0.067 | 0.142 |
| Descurainia sophia | 0.054 | 0.057 | Apera spica-venti | 0.026 | 0.106 |
| Papaver rhoeas | 0.039 | 0.056 | Pisum sativum | 0.052 | 0.053 |
| Sinapis arvensis | 0.023 | 0.049 | Cichorium intybus | 0.032 | −0.004 |
| Veronica polita | 0.125 | 0.035 | Datura stramonium | 0.029 | −0.014 |
| Datura stramonium | 0.046 | 0.018 | Cardaria draba | 0.024 | −0.026 |
| Cichorium intybus | 0.071 | 0.006 | Veronica polita | 0.106 | −0.032 |
| Cirsium arvense | 0.028 | −0.131 | Elymus repens | 0.025 | −0.073 |
| Brassica napus | 0.089 | −0.165 | Medicago sativa | 0.107 | −0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, E.; Dorner, Z.; Nagy, J.G.; Zalai, M. How Weed Flora Evolves in Cereal Fields in Relation to the Agricultural Environment and Farming Practices in Different Sub-Regions of Eastern Hungary. Agronomy 2025, 15, 1033. https://doi.org/10.3390/agronomy15051033
Tóth E, Dorner Z, Nagy JG, Zalai M. How Weed Flora Evolves in Cereal Fields in Relation to the Agricultural Environment and Farming Practices in Different Sub-Regions of Eastern Hungary. Agronomy. 2025; 15(5):1033. https://doi.org/10.3390/agronomy15051033
Chicago/Turabian StyleTóth, Erzsébet, Zita Dorner, János György Nagy, and Mihály Zalai. 2025. "How Weed Flora Evolves in Cereal Fields in Relation to the Agricultural Environment and Farming Practices in Different Sub-Regions of Eastern Hungary" Agronomy 15, no. 5: 1033. https://doi.org/10.3390/agronomy15051033
APA StyleTóth, E., Dorner, Z., Nagy, J. G., & Zalai, M. (2025). How Weed Flora Evolves in Cereal Fields in Relation to the Agricultural Environment and Farming Practices in Different Sub-Regions of Eastern Hungary. Agronomy, 15(5), 1033. https://doi.org/10.3390/agronomy15051033





