Biophysical and Morphometric Characteristics of Sunflower Achenes: Implications for Industrial Processing and Byproduct Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material and Sample Preparation
2.2. Morphometric Analysis of Achenes, Pericarps, and Seeds
2.3. Biophysical Analysis of Achenes, Pericarp, and Seeds
2.3.1. Pericarp Hardness and Color
2.3.2. Moisture Content
2.3.3. Oil Content
2.3.4. Germination
2.3.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gültekin Subaşı, B.; Vahapoğlu, B.; Capanoglu, E.; Mohammadifar, M.A. A review on protein extracts from sunflower cake: Techno-functional properties and promising modification methods. Crit. Rev. Food Sci. Nutr. 2021, 62, 6682–6697. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, A.; Rodríguez, L.; Riccobene, I.; Nolasco, S. Analysis of the performance of a dehulling system for confectionary sunflower seeds. Food Nutr. Sci. 2014, 5, 541–548. [Google Scholar] [CrossRef][Green Version]
- Morya, S.; Menaa, F.; Jiménez-López, C.; Lourenço-Lopes, C.; BinMowyna, M.N.; Alqahtani, A. Nutraceutical and pharmaceutical behavior of bioactive compounds of miracle oilseeds: An overview. Foods 2022, 11, 1824. [Google Scholar] [CrossRef]
- Demirel, B.; Göl, N.P.; Onay, T.T. Evaluation of heavy metal content in digestate from batch anaerobic co-digestion of sunflower hulls and poultry manure. J. Mater. Cycles Waste Manag. 2013, 15, 242–246. [Google Scholar] [CrossRef]
- Ieremia, V.; Van Caneghem, J.; Ameryckx, A.G. Waste-to-energy treatment of agro-industrial residues: A life cycle assessment of sunflower hulls to generate bioenergy. J. Clean. Prod. 2025, 506, 145465. [Google Scholar] [CrossRef]
- Soldatkina, L.M.; Sagaidak, E.V.; Menchuk, V.V. Adsorption of cationic dyes from aqueous solutions on sunflower husk. J. Water Chem. Technol. 2009, 31, 238–243. [Google Scholar] [CrossRef]
- Witek-Krowiak, A. Analysis of temperature-dependent biosorption of Cu²⁺ ions on sunflower hulls: Kinetics, equilibrium, and mechanism of the process. Chem. Eng. J. 2012, 192, 13–20. [Google Scholar] [CrossRef]
- Tadayon, Y.; Bahrololoom, M.E.; Javadpour, S. An experimental study of sunflower seed husk and zeolite as adsorbents of Ni(II) ion from industrial wastewater. Water Resour. Ind. 2023, 30, 100214. [Google Scholar] [CrossRef]
- Jawad, A.H.; Salleh, N.; ALOthman, Z.A.; Selvasembian, R. Mesoporous activated carbon from sunflower (Helianthus annuus) seed pericarp for crystal violet dye removal: Numerical desirability optimization and mechanism study. Water Air Soil Pollut. 2024, 235, 666. [Google Scholar] [CrossRef]
- Jawad, A.H.; Nafi, M.M.; Awang, H.F.; Wilson, L.D.; ALOthman, Z.A. Numerical parametric optimization with desirability functions for methylene blue dye removal by sunflower seed pericarp activated carbon. Biomass Convers. Biorefinery 2025, 15, 11135–11149. [Google Scholar] [CrossRef]
- Cui, X.; Yang, J.; Shi, X.; Lei, W.; Huang, T.; Bai, C. Pelletization of sunflower seed husks: Evaluating and optimizing energy consumption and physical properties by response surface methodology (RSM). Processes 2019, 7, 591. [Google Scholar] [CrossRef]
- Lunguleasa, A.; Olarescu, A.; Spirchez, C. Pellets obtained from the husks of sunflower seeds and beech sawdust for comparison. Forests 2024, 15, 902. [Google Scholar] [CrossRef]
- Stănescu, M.M.; Bolcu, A. A study of the mechanical properties in composite materials with a dammar based hybrid matrix and reinforcement from crushed shells of sunflower seeds. Polymers 2022, 14, 392. [Google Scholar] [CrossRef]
- Yegorov, B.; Turpurova, T.; Sharabaeva, E.; Bondar, Y. Prospects of using by-products of sunflower oil production in compound feed industry. Food Sci. Technol. 2019, 13, 106–113. [Google Scholar] [CrossRef]
- Zakrzewska, P.; Kuźnia, M.; Zygmunt-Kowalska, B.; Magiera, A.; Magdziarz, A. Utilization of sunflower husk ash in the production of polyurethane materials. Energies 2023, 16, 8080. [Google Scholar] [CrossRef]
- De’Nobili, M.D.; Bernhardt, D.C.; Basanta, M.F.; Rojas, A.M. Sunflower (Helianthus annuus L.) seed hull waste: Composition, antioxidant activity, and filler performance in pectin-based film Composites. Front. Nutr. 2021, 8, 777214. [Google Scholar] [CrossRef] [PubMed]
- Huss, J.; Gierlinger, N. Functional packaging of seeds. New Phytol. 2020, 230, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Del Bel, Z.; Andrade, A.; Lindström, L.; Alvarez, D.; Vigliocco, A.; Alemano, S. The role of the sunflower seed coat and endosperm in the control of seed dormancy and germination: Phytohormone profile and their interaction with seed tissues. Plant Growth Regul. 2024, 102, 51–64. [Google Scholar] [CrossRef]
- Seiler, G.J. Anatomy and morphology of sunflower. In Sunflower Technology and Production; Schneiter, A.A., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1997; Volume 35, Chapter 3; pp. 67–111. [Google Scholar] [CrossRef]
- Evert, R.F. Sclerenchyma. In Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body—Their Structure, Function, and Development, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; Chapter 8; pp. 191–210. [Google Scholar]
- Nathan, R.; Schurr, F.M.; Spiegel, O.; Steinitz, O.; Trakhtenbrot, A.; Tsoar, A. Mechanisms of long-distance seed dispersal. Trends Ecol. Evol. 2008, 23, 638–647. [Google Scholar] [CrossRef]
- Pandey, A.K.; Dhakal, M.R. Phytomelanin in Compositae. Curr. Sci. 2001, 80, 933–940. [Google Scholar]
- Bazhenov, M.S.; Litvinov, D.Y.; Divashuk, M.G. Melanin found in wheat spike husks. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hernandez, L.F.; Belles, P.M. A 3-D finite element analysis of the sunflower (Helianthus annuus L.) fruit. Biomechanical approach for the improvement of its hullability. J. Eng. 2007, 78, 861–869. [Google Scholar] [CrossRef]
- Morrison, W.H. Variation in the wax content of sunflower seed with location and hybrid. J. Am. Oil Chem. Soc. 1983, 60, 1013–1014. [Google Scholar] [CrossRef]
- DeAndrés-Gil, C.; Villoslada-Valbuena, M.; Venegas-Calerón, M.; Moreno-Pérez, A.J.; Beaudoin, F.; Kurup, S.; Martínez-Force, E.; Garcés, R.; Salas, J.J. Wax synthases from sunflower (Helianthus annuus) seeds. Plant Physiol. Biochem. 2025, 222, 109692. [Google Scholar] [CrossRef]
- Hernández, L.; Lindström, L. Cuticle and cuticular wax development in the sunflower (Helianthus annuus L.) pericarp grown at the field under a moderate water deficit. Phyton–Int. J. Exp. Bot. 2010, 79, 153–161. [Google Scholar] [CrossRef]
- Carelli, A.A.; Frizzera, L.M.; Forbito, P.R.; Crapiste, G.H. Wax composition of sunflower seed oils. J. Am. Oil Chem. Soc. 2002, 79, 763–768. [Google Scholar] [CrossRef]
- Givon, V.; Tirtiaux, A. Revisión de los distintos métodos para la eliminación de gomas y ceras. In Libro 10° Aniversario: Recopilación de Artículos Técnicos; Asociación Argentina de Grasas y Aceites: Rosario, Argentina, 2000; Volume 2, pp. 101–110. [Google Scholar]
- Malik, M.A.; Saini, C.S. Engineering properties of sunflower seed: Effect of dehulling and moisture content. Cogent Food Agric. 2016, 2, 1145783. [Google Scholar] [CrossRef]
- Garc’es, R.; Andr’es-Gil, K.; Venegas-Caleron, M.; Martínez-Force, E.; Moreno-P’erez, A.J.; Salas, J.J. Characterization of sunflower seed and oil wax ester composition by GC/MS, a final evaluation. LWT—Food Sci. Technol. 2023, 173, 114365. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, F.; Huang, X.; Liang, M.; Ma, S.; Gafurov, K.; Gu, F.; Guo, Q.; Wang, Q. Properties and characterization of sunflower seeds from different varieties of edible and oil sunflower seeds. Foods 2024, 13, 1188. [Google Scholar] [CrossRef]
- Tranchino, L.; Melle, F.; Sodini, G. Almost complete dehulling of high-oil sunflower seed. J. Am. Oil Chem. Soc. 1984, 61, 1261–1265. [Google Scholar] [CrossRef]
- Subramanian, R.; Shamanthaka Sastry, M.C.; Venkateshmurthy, K. Impact dehulling of sunflower seeds: Effect of operating conditions and seed characteristics. J. Food Eng. 1990, 12, 83–94. [Google Scholar] [CrossRef]
- Merrien, A.; Domínguez, J.; Vannozzi, G.P.; Baldini, M.; Champolivier, L.; Carré, P. Factors affecting the dehulling ability in sunflower. In Proceedings of the 13th International Sunflower Conference, Pisa, Italy, 7–11 September 1992. [Google Scholar]
- Muttagi, G.C.; Joshi, N. A mechanical method for small scale dehulling of sunflower seeds. Int. J. Pure Appl. Biosci. 2017, 5, 379–388. [Google Scholar] [CrossRef]
- Vedmedeva, K.V.; Busarov, P.Y. Trait of sunflower seeds according to the results of studying the phenotype and destructive power of the husk. Sci. Tech. Bull. Inst. Oilseed Crops NAAS 2024, 36, 16–27. [Google Scholar] [CrossRef]
- Dedio, W.; Dorrell, D.G. Factors affecting the hullability and physical characteristics of sunflower achenes. Can. Inst. Food Sci. Technol. J. 1989, 22, 143–146. [Google Scholar] [CrossRef]
- Denis, L.; Dominguez, J.; Baldini, M.; Vear, F. Genetical studies of hullability in comparison with other sunflower seed characteristics. Euphytica 1994, 79, 29–38. [Google Scholar] [CrossRef]
- Sharma, R.; Sogi, D.S.; Saxena, D.C. Dehulling performance and textural characteristics of unshelled and shelled sunflower (Helianthus annuus L.) seeds. J. Food Eng. 2009, 92, 1–7. [Google Scholar] [CrossRef]
- Taradaichenko, M.; Perevalov, L.; Teslenko, S.; Pakhomova, I. Optimal parameters of sunflower seeds dehulling process with freezing. Chem. Eng. Equip. 2013, 52, 374–375. [Google Scholar]
- Mridula, D.; Saha, D.; Gupta, R.K.; Bhadwal, S. Oil expelling of dehulled sunflower: Optimization of screw pressing parameters. J. Food Process. Preserv. 2019, 43, e13852. [Google Scholar] [CrossRef]
- Romanić, R.; Lužaić, T. Dehulling effectiveness of high-oleic and linoleic sunflower oilseeds using air-jet impact dehuller: A comparative study. Food Sci. Technol. 2022, 42, e58620. [Google Scholar] [CrossRef]
- Khodabakhshian, R.; Emadi, B.; Abbaspour Fard, M.H. Some engineering properties of sunflower seed and its kernel. J. Agric. Sci. Technol. 2010, 4, 37–46. [Google Scholar]
- Khodabakhshian, R. Elastic behavior of sunflower seed and its kernel. Agric. Eng. Int. CIGR J. 2012, 14, 173–178. [Google Scholar]
- Biriș, S.S.; Ionescu, M.; Gheorghiță, N.E.; Ungureanu, N.; Vlăduț, N.V. Study of the compression behavior of sunflower seeds using the finite element method. AGROFOR Int. J. 2019, 4, 128–136. [Google Scholar] [CrossRef]
- Mihailović, D.T.; Lalić, B.; Drešković, N.; Mimić, G.; Djurdjević, V.; Jančić, M. Climate change effects on crop yields in Serbia and related shifts of Köppen climate zones under the SRES-A1B and SRES-A2. Int. J. Climatol. 2014, 35, 3320–3334. [Google Scholar] [CrossRef]
- Jancic Tovjanin, M.; Djurdjević, V.; Pejic, B.; Novkovic, N.; Mutavdzic, B.; Markovic, B.; Mackic, K. Modeling the impact of climate change on yield, water requirements, and water use efficiency of maize and soybean grown under moderate continental climate in the Pannonian lowland. Idojárás 2019, 123, 469–486. [Google Scholar] [CrossRef]
- Marinković, J.; Bijelić, D.; Šeremešić, S.; Tintor, B.; Ninkov, J.; Živanov, M.; Vasin, J. Microbial abundance and activity in chernozem under different cropping systems. Field Veg. Crops Res. 2018, 55, 6–11. [Google Scholar] [CrossRef]
- Krstić, Đ.; Vujić, S.; Jaćimović, G.; D’Ottavio, P.; Radanović, Z.; Erić, P.; Ćupina, B. The effect of cover crops on soil water balance in rain-fed conditions. Atmosphere 2018, 9, 492. [Google Scholar] [CrossRef]
- Rebublic Hydrometeorogical Service of Serbia. Available online: https://www.hidmet.gov.rs/data/meteo_godisnjaci/Republika%20Srbija%20-%20Meteorolo%c5%a1ki%20godisnjak%201%20-%20klimatoloki%20podaci%20-%202023.pdf (accessed on 12 April 2025).
- Mohsenin, N.N. Physical Properties of Plant and Animal Materials. Structure, Physical Characteristics and Mechanical Properties, 2nd ed.; Gordon and Breach Science Publishers: New York, NY, USA, 1986; pp. 1–910. [Google Scholar]
- ISO 665:2020; Oilseeds—Determination of Moisture and Volatile Matter Content. (Edition 3). International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 659:2009; Oilseeds—Determination of Oil Content (Reference Method). (Edition 4). International Organization for Standardization: Geneva, Switzerland, 2009.
- ISTA. International Rules for Seed Testing; The International Seed Testing Association: Wallisellen, Switzerland, 2023; Chapter 5. [Google Scholar]
- Aliiev, E. Automatic phenotyping test of sunflower seeds. Helia 2020, 43, 51–66. [Google Scholar] [CrossRef]
- Shevchenko, I.; Aliiev, E. Precise grading and sorting of sunflower plant materials in industrial facilities. J. Cent. Eur. Agric. 2022, 23, 327–341. [Google Scholar] [CrossRef]
- Dedio, W. Association of sunflower achene color with other achene characters and bird preference. Can. J. Plant Sci. 1995, 75, 377–380. [Google Scholar] [CrossRef]
- Nadkarni, S.R.; Goud, I.S.; Sheshaiah, K.C.; Dalawai, N.; Hosamani, M. Genetics of seed colour in sunflower (Helianthus annuus L.). Int. J. Pure Appl. Biosci. 2017, 5, 1207–1214. [Google Scholar] [CrossRef]
- Vaccari, A.; Pifferi, P.G.; Zaccherini, G. Anthocyanins of sunflower (Helianthus annuus). J. Food Sci. 1982, 47, 40–42. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Keleş, Y. Extraction, purification, antioxidant properties and stability conditions of phytomelanin pigment on the sunflower seeds. Int. J. Second. Metab. 2018, 5, 140–148. [Google Scholar] [CrossRef]
- Škorić, D. The genetics of sunflower. In Sunflower Genetics and Breeding; Škorić, D., Sakač, Z., Eds.; Serbian Academy of Science and Arts, Branch in Novi Sad: Novi Sad, Serbia, 2012; pp. 1–163. [Google Scholar]
- Tostain, S.; Chervier, P.; Laulan, A.; Kermorgant, T. Improving energetic independence and environmental impact of a sunflower crushing plant by implementing a dehulling unit in combination with a hull-burning boiler. OCL 2012, 19, 332–340. [Google Scholar] [CrossRef]
- Demir, G.; Nemlioglu, S.; Yazgic, U.; Dogan, E.E.; Bayat, C. Determination of some important emissions of sunflower oil production industrial wastes incineration. J. Sci. Ind. Res. 2005, 64, 226–228. (In French) [Google Scholar]
- Irez, A.B. Development of sunflower husk reinforced polypropylene based sustainable composites: An experimental investigation of mechanical and thermal performance. J. Polym. Sci. 2024, 62, 3471–3484. [Google Scholar] [CrossRef]
- Mantese, A.I.; Medan, D.; Hall, A.J. Achene structure, development and lipid accumulation in sunflower cultivars differing in oil content at maturity. Ann. Bot. 2006, 97, 999–1010. [Google Scholar] [CrossRef]
- Dauguet, S.; Fine, F.; Guillemain, C.; Carré, P.; Merrien, A.; Krouti, M.; Champolivier, L. Impact of pedoclimatic and agricultural conditions on sunflower seeds characteristics in relation to the dehulling process. OCL 2015, 22, D402. [Google Scholar] [CrossRef][Green Version]
- Dimić, E. Cold Pressed Oils; Faculty of Technology, University of Novi Sad: Novi Sad, Serbia, 2005; pp. 1–230. (In Serbian) [Google Scholar]
- de Figueiredo, A.K.; Baumler, E.; Riccobene, I.C.; Nolasco, S.M. Moisture-dependent engineering properties of sunflower seeds with different structural characteristics. J. Food Eng. 2011, 102, 58–65. [Google Scholar] [CrossRef]
- Ahmed, T.A.M.; Mutwalib, E.M.; Salih, E.A. The effect of seed size and burial depth on the germination, growth and yield of sunflower (Helianthus annus L.). Am. Sci. Res. J. Eng. Technol. Sci. 2019, 53, 75–82. [Google Scholar]
- Hernández, L.F.; Orioli, G.A. Imbibition and germination rates of sunflower (Helianthus annuus L.) seeds according to fruit size. Field Crops Res. 1985, 10, 355–360. [Google Scholar] [CrossRef]
- Gupta, R.K.; Arora, G.; Sharma, R. Aerodynamic properties of sunflower seed (Helianthus annuus L.). J. Food Eng. 2007, 79, 899–904. [Google Scholar] [CrossRef]
- Costa, C.; Antonucci, F.; Pallottino, F.; Aguzzi, J.; Sun, D.W.; Menesatti, P. Shape analysis of agricultural products: A review of recent research advances and potential application to computer vision. Food Bioprocess Technol. 2011, 4, 673–692. [Google Scholar] [CrossRef]


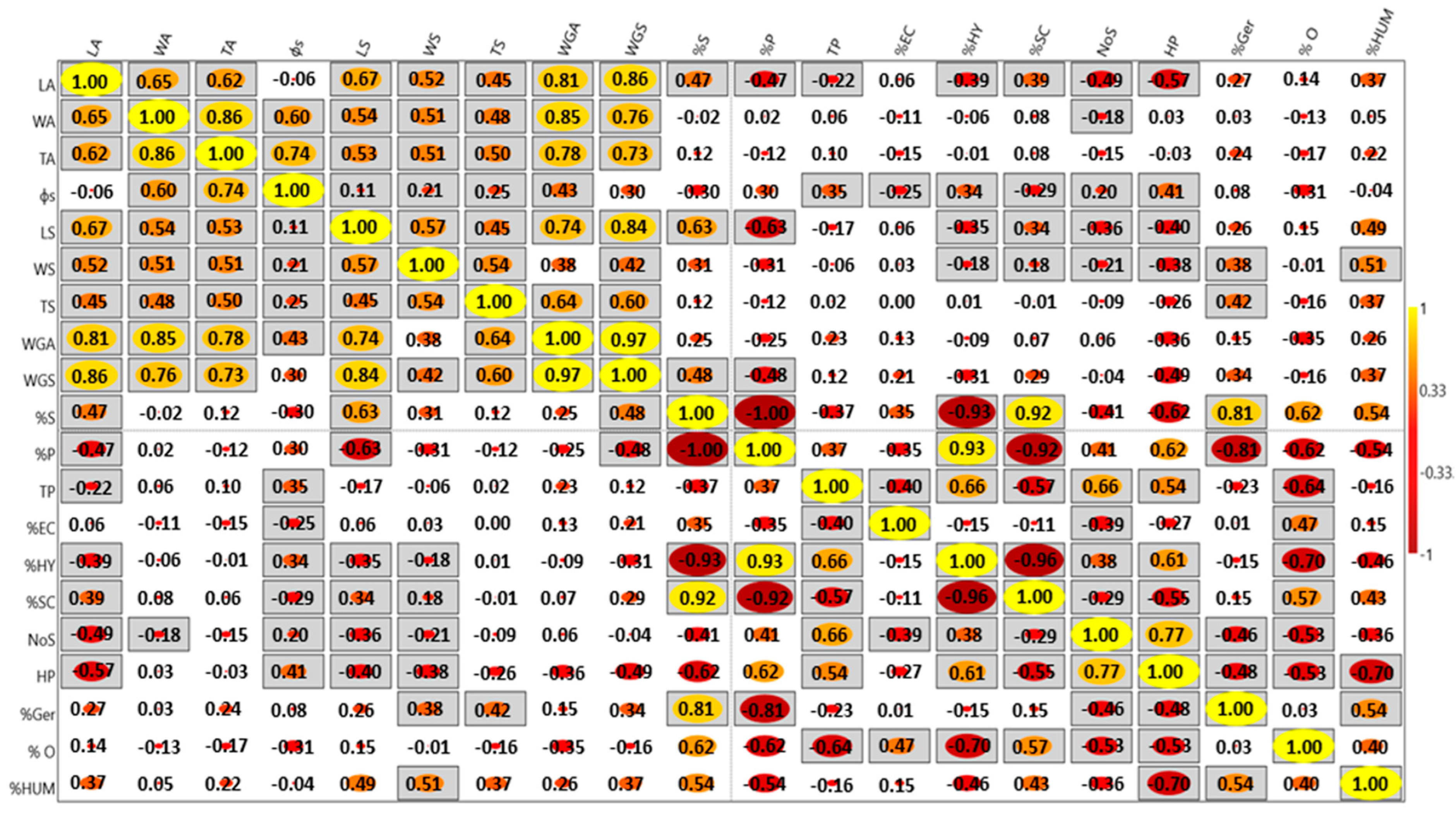
| Genotype | Achene Weight (g) | Moisture Content (%) | Seed | % of Pericarp | |||
|---|---|---|---|---|---|---|---|
| Weight (g) | % | % of Germination | % of Oil | ||||
| L1 | 4.88 ± 0.12 c (4.26) | 4.3 ± 0.1 cd (4.9) | 2.75 ± 0.08 d (5.45) | 56.3 ± 0.98 e (1.76) | 82 ± 1.0 ab (2.5) | 24 ± 0.7 d (5.5) | 43.7 ± 0.57 a (2.27) |
| L2 | 4.91 ± 0.08 c (2.93) | 4.9 ± 0.05 ab (1.5) | 3.68 ± 0.14 c (6.95) | 74.9 ± 1.85 abc (4.29) | 88 ± 1.7 ab (3.9) | 39.2 ± 1.7 ab (8.4) | 25.2 ± 1.85 cde (12.8) |
| L3 | 5.56 ± 0.13 b (4.24) | 5.1 ± 0.1 a (2.8) | 4.13 ± 0.07 b (3.0) | 74.3 ± 1.0 abc (2.47) | 97 ± 0.4 a (0.8) | 49.8 ± 1.7 c (8.7) | 25.7 ± 1.06 cde (7.14) |
| L4 | 4.6 ± 0.03 cd (1.08) | 4.2 ± 0.2 d (5.1) | 3.55 ± 0.10 c (5.0) | 77.2 ± 1.8 a (4.0) | 94 ± 0.5 a (1.0) | 40.7 ± 1.8 bc (8.9) | 22.8 ± 1.8 e (13.7) |
| L5 | 8.5 ± 0.15 a (3.0) | 5.0 ± 0.05 a (1.4) | 6.53 ± 0.11 a (3.09) | 76.9 ± 0.5 ab (1.17) | 96 ± 1.25 a (2.6) | 36.7 ± 1.1 c (5.9) | 23.1 ± 0.52 de (3.9) |
| L6 | 4.28 ± 0.06 de (2.7) | 5.1 ± 0.1 a (2.8) | 2.91 ± 0.04 d (2.61) | 68.1 ± 0.72 d (1.83) | 96 ± 48.7 a (3.9) | 48.2 ± 0.9 a (3.9) | 31.9 ± 0.72 b (3.91) |
| L7 | 4.18 ± 0.14 e (6.0) | 4.9 ± 0.05 ab (1.4) | 2.98 ± 0.04 d (2.56) | 71.5 ± 6.52 cd (3.57) | 95 ± 0.9 a (1.9) | 40.7 ± 0.9 bc (4.6) | 28.6 ± 1.5 bc (8.9) |
| L8 | 3.21 ± 0.04 f (2.37) | 4.6 ± 0.05 bc (1.5) | 2.35 ± 0.05 e (3.68) | 73.0 ± 0.68 bc (1.61) | 87 ± 0.7 b (1.9) | 44.6 ± 1.4 ab (6.2) | 26.9 ± 0.68 cd (4.39) |
| Genotype | Achene Length (mm) | Achene Width (mm) | Achene Thickness (mm) | Achene Sphericity (ϕs) | Seed Length (mm) | Seed Width (mm) | Seed Thickness (mm) |
|---|---|---|---|---|---|---|---|
| L1 | 8.4 ± 0.08 g (8.3) | 4.8 ± 0.07 c (11.4) | 3.33 ± 0.04 c (9.9) | 0.62 ± 0.004 a (5.9) | 6.94 ± 0.06 g (7.5) | 3.12 ± 0.04 c (10.6) | 2.3 ± 0.03 bc (11.8) |
| L2 | 9.7 ± 0.06 c (4.8) | 4.65 ± 0.05 c (9.55) | 3.22 ± 0.03 c (8.2) | 0.56 ± 0.003 b (4.2) | 8.31 ± 0.07 c (6.6) | 3.5 ± 0.04 b (9.8) | 2.4 ± 0.03 b (11.6) |
| L3 | 10.81 ± 0.07 b (5.3) | 4.98 ± 0.05 b (9.3) | 3.47 ± 0.04 b (9.5) | 0.55 ± 0.004 b (6.14) | 9.0 ± 0.09 b (8.2) | 3.42 ± 0.04 b (9.9) | 2.4 ± 0.04 b (14.1) |
| L4 | 9.75 ± 0.07 c (5.9) | 4.65 ± 0.06 c (12.2) | 3.31 ± 0.03 c (7.86) | 0.57 ± 0.003 ab (4.4) | 7.86 ± 0.07 d (6.98) | 3.36 ± 0.05 b (11.7) | 2.37 ± 0.03 b (9.96) |
| L5 | 11.3 ± 0.08 a (5.9) | 6.2 ± 0.05 a (6.9) | 4.3 ± 0.04 a (7.48) | 0.61 ± 0.003 a (4.2) | 9.35 ± 0.09 a (8.0) | 4.13 ± 0.05 a (10.42) | 2.85 ± 0.05 a (14.3) |
| L6 | 9.3 ± 0.09 d (7.5) | 4.2 ± 0.06 d (11.4) | 3.0 ± 0.05 d (13.7) | 0.56 ± 0.004 b (6.1) | 7.34 ± 0.09 ef (10.43) | 3.21 ± 0.05 c (12.7) | 2.23 ± 0.04 c (15.0) |
| L7 | 9.0 ± 0.06 e (5.2) | 3.97 ± 0.06 e (11.7) | 2.85 ± 0.05 e (14.5) | 0.54 ± 0.004 bc (6.8) | 7.4 ± 0.07 c (7.53) | 3.2 ± 0.04 c (10.2) | 2.26 ± 0.03 bc (12.9) |
| L8 | 8.8 ± 0.08 f (7.72) | 4.0 ± 0.06 de (11.5) | 2.7 ± 0.06 f (17.8) | 0.53 ± 0.006 c (9.0) | 7.2 ± 0.09 d (10.6) | 2.97 ± 0.05 d (15.0) | 1.8 ± 0.03 d (15.2) |
| Genotype | Pericarp Thickness (µm) | % of Epidermis with Cuticle | % of Hypodermis | % of Sclerenchyma | No. of Sclerenchyma Layers | Pericarp Hardness (N) |
|---|---|---|---|---|---|---|
| L1 | 298.3 ± 5.2 a (6.8) | 4.3 ± 0.26 c (23.6) | 18.1 ± 0.7 a (14.4) | 78.2 ± 0.7 c (3.2) | 9 ± 0.09 a (4.2) | 2.0 ± 0.20 a (22.2) |
| L2 | 166.4 ± 7.7 b (17.9) | 6.5 ± 0.4 bc (25.9) | 6.2 ± 0.5 bc (34) | 88.3 ± 0.7 ab (3.2) | 7.7 ± 0.4 b (17.9) | 0.89 ± 0.06 cd (15.2) |
| L3 | 203.7 ± 6.9 b (12.2) | 6.3 ± 0.4 ab (21.9) | 5.8 ± 0.5 c (29.1) | 88.6 ± 0.7 b (3.0) | 5.0 ± 0.2 e (15.8) | 0.31 ± 0.04 e (26.7) |
| L4 | 191.1 ± 6.9 b (13.5) | 5.6 ± 0.4 bc (31.5) | 5.4 ± 0.4 bc (26.8) | 89.9 ± 0.8 b (2.5) | 7.6 ± 0.2 b (9.7) | 1.11 ± 0.06 c (13.5) |
| L5 | 185.5± 9.1 b (19.1) | 4.9 ± 0.4 bc (34.8) | 4.8 ± 0.4 c (30.9) | 90.3 ± 0.6 ab (2.5) | 6.2 ± 0.25 cd (15.4) | 0.66 ± 0.05 d (16.7) |
| L6 | 144.1 ± 2.3 c (6.2) | 7.3 ± 0.5 a (25.8) | 7.5 ± 0.6 b (32.1) | 86.5 ± 0.9 bc (4.2) | 5.57 ± 0.2 de (12.0) | 0.29 ± 0.01 e (10.9) |
| L7 | 153.7 ± 11.8 c (29.9) | 6.3 ± 0.4 ab (24.2) | 5.0 ± 0.4 c (48.1) | 89.9 ± 0.7 ab (3.0) | 6.4 ± 0.3 c (20.3) | 0.7 ± 0.05 d (16.3) |
| L8 | 193.6 ± 8.0 b (16.2) | 5.6 ± 0.3 bc (19.8) | 4.9 ± 0.34 c (26.3) | 90.4 ± 0.6 a (2.5) | 8 ± 0.2 b (9.4) | 1.67 ± 0.08 b (10.3) |
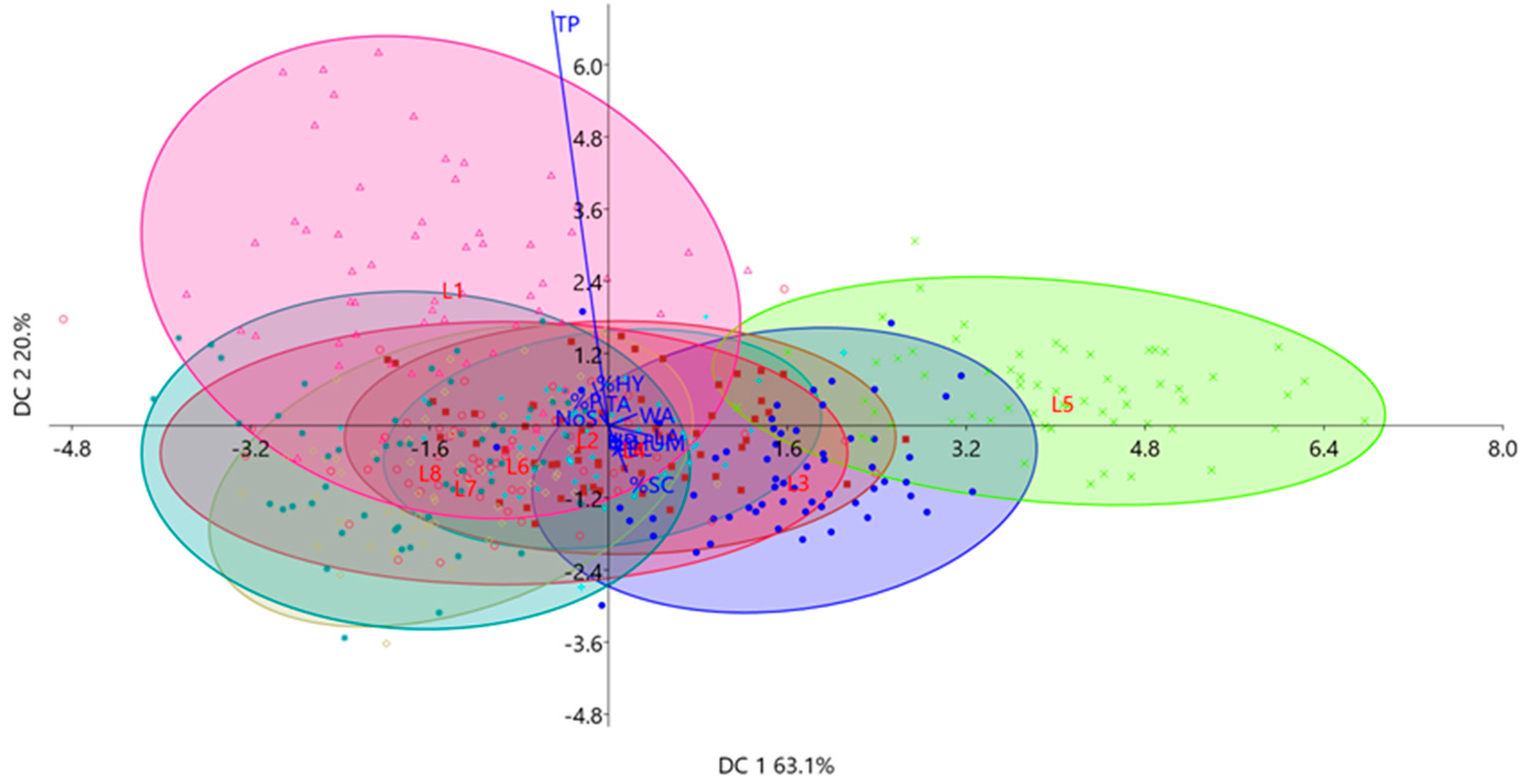
| Characteristics | DC 1 | DC 2 | DC 3 |
|---|---|---|---|
| Achene length (LA) | 0.790 | −0.218 | 0.156 |
| Achene width (WA) | 0.829 | 0.247 | −0.039 |
| Achene thickness (TA) | 0.237 | 0.169 | −0.002 |
| Achene sphericity (ϕs) | 0.005 | 0.025 | −0.001 |
| Pericarp percentage (%P) | −0.094 | 0.226 | 0.095 |
| Pericarp thickness (TP) | −0.655 | 8.994 | 3.625 |
| Epidermis with cuticle percentage (%EC) | 0.005 | −0.128 | 0.048 |
| Hypodermis percentage (%HY) | −0.179 | 0.930 | 0.363 |
| Sclerenchyma percentage (%SC) | 0.213 | −1.003 | −0.468 |
| Number of sclerenchyma layers (NoS) | −0.081 | 0.187 | −0.137 |
| Pericarp hardness (HP) | −0.012 | 0.031 | −0.019 |
| Moisture percentage (% HUM) | 0.002 | −0.005 | 0.0015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovuka, J.; Jocković, J.; Jocković, M.; Jocić, S.; Gvozdenac, S.; Krstić, M.; Jovičić, D. Biophysical and Morphometric Characteristics of Sunflower Achenes: Implications for Industrial Processing and Byproduct Utilization. Agronomy 2025, 15, 1035. https://doi.org/10.3390/agronomy15051035
Ovuka J, Jocković J, Jocković M, Jocić S, Gvozdenac S, Krstić M, Jovičić D. Biophysical and Morphometric Characteristics of Sunflower Achenes: Implications for Industrial Processing and Byproduct Utilization. Agronomy. 2025; 15(5):1035. https://doi.org/10.3390/agronomy15051035
Chicago/Turabian StyleOvuka, Jelena, Jelena Jocković, Milan Jocković, Siniša Jocić, Sonja Gvozdenac, Miloš Krstić, and Dušica Jovičić. 2025. "Biophysical and Morphometric Characteristics of Sunflower Achenes: Implications for Industrial Processing and Byproduct Utilization" Agronomy 15, no. 5: 1035. https://doi.org/10.3390/agronomy15051035
APA StyleOvuka, J., Jocković, J., Jocković, M., Jocić, S., Gvozdenac, S., Krstić, M., & Jovičić, D. (2025). Biophysical and Morphometric Characteristics of Sunflower Achenes: Implications for Industrial Processing and Byproduct Utilization. Agronomy, 15(5), 1035. https://doi.org/10.3390/agronomy15051035






