Systemic Effects of Nitrate on Nitrogen Fixation and Sucrose Catabolism in Soybean (Glycine max (L.) Merr.) Nodules
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Climate Conditions
2.2. Plant Materials and Growth Conditions
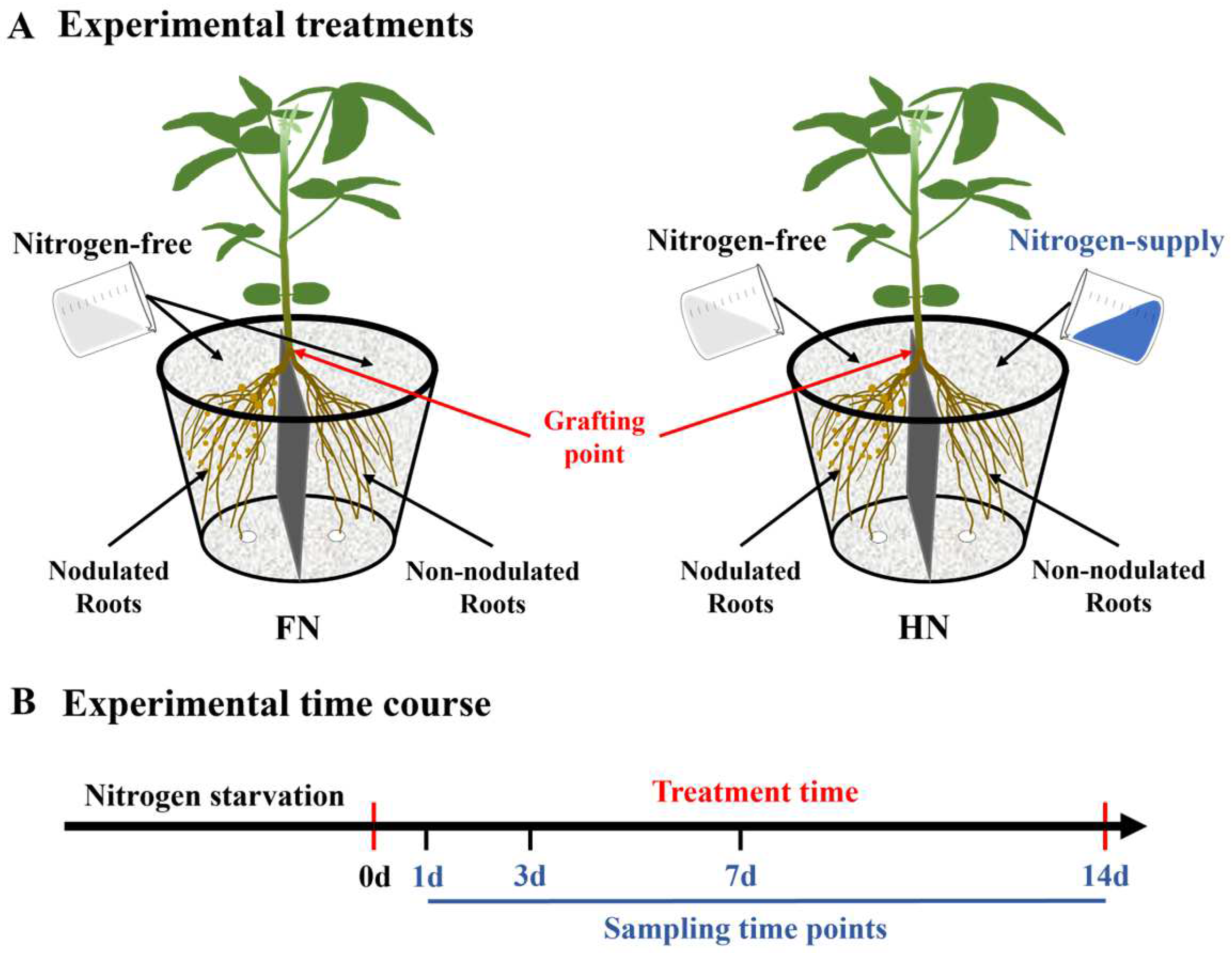
2.3. Experimental Treatments and Sampling Methods
2.4. Analysis Methods
2.5. Data Calculation
2.6. Data Analysis
3. Results
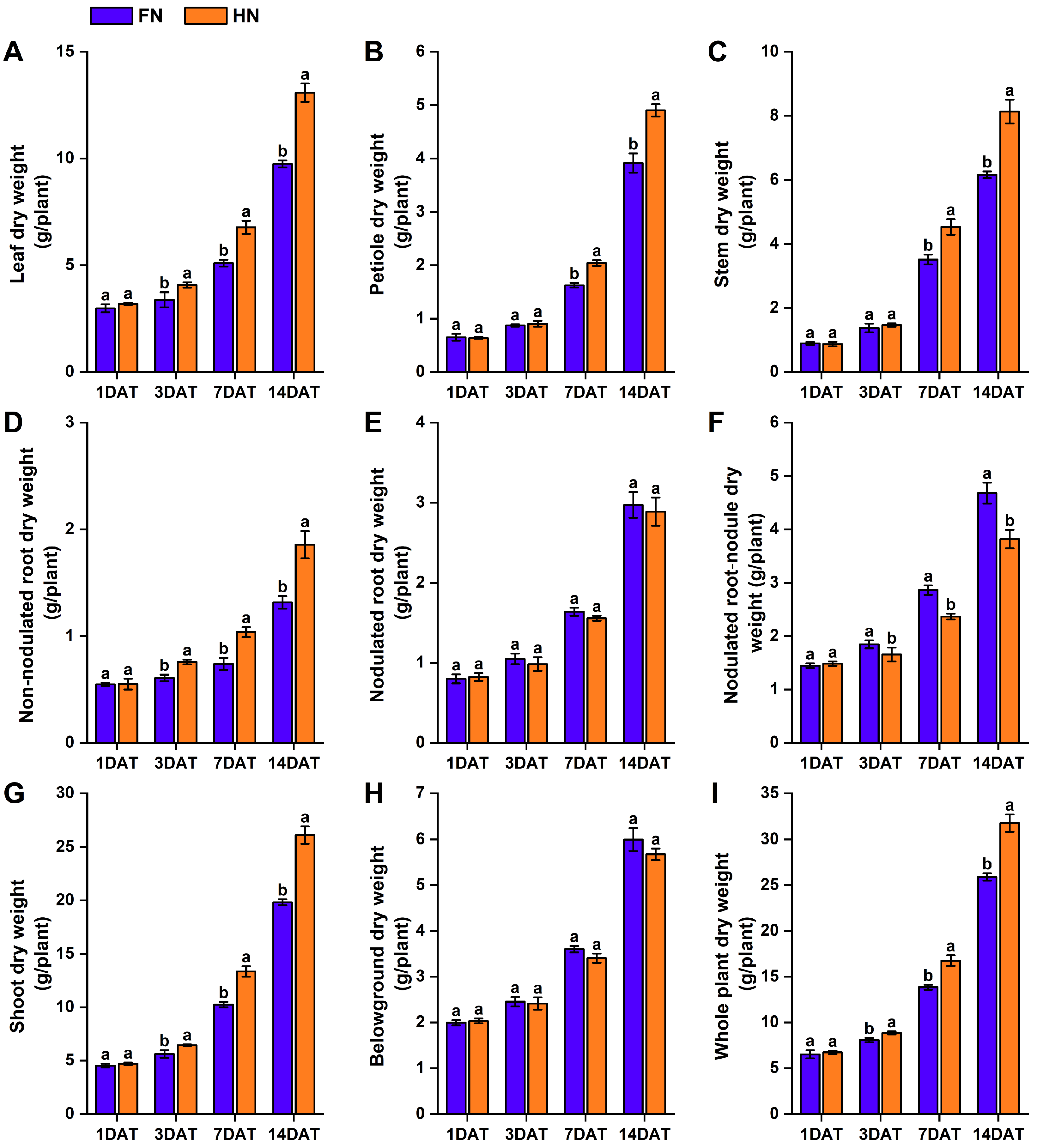
3.1. Effects of Nitrate on Dry Matter Accumulation in Various Organs of Dual-Root Soybean
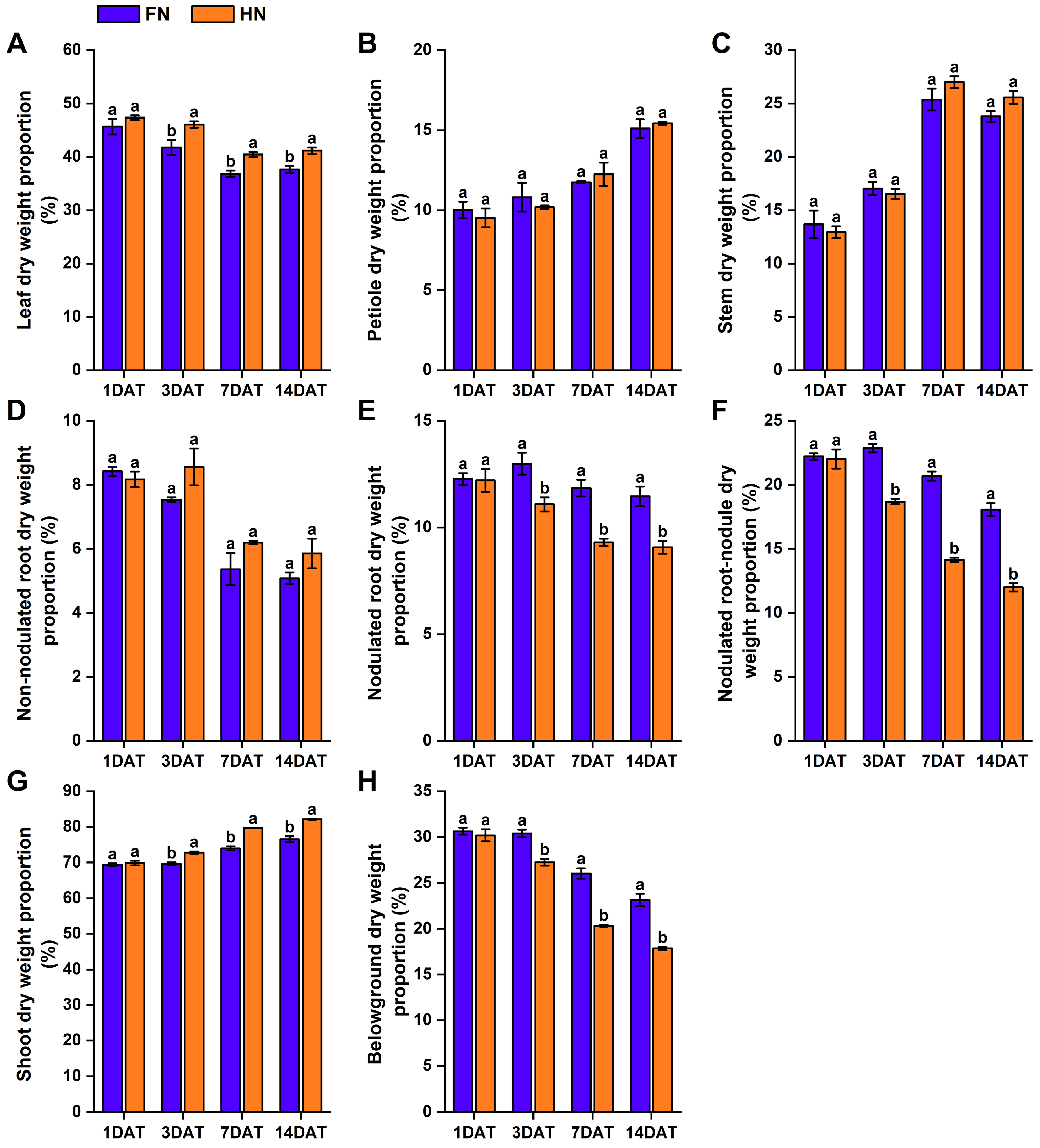
3.2. Effects of Nitrate on Dry Matter Allocation in Various Organs of Dual-Root Soybean
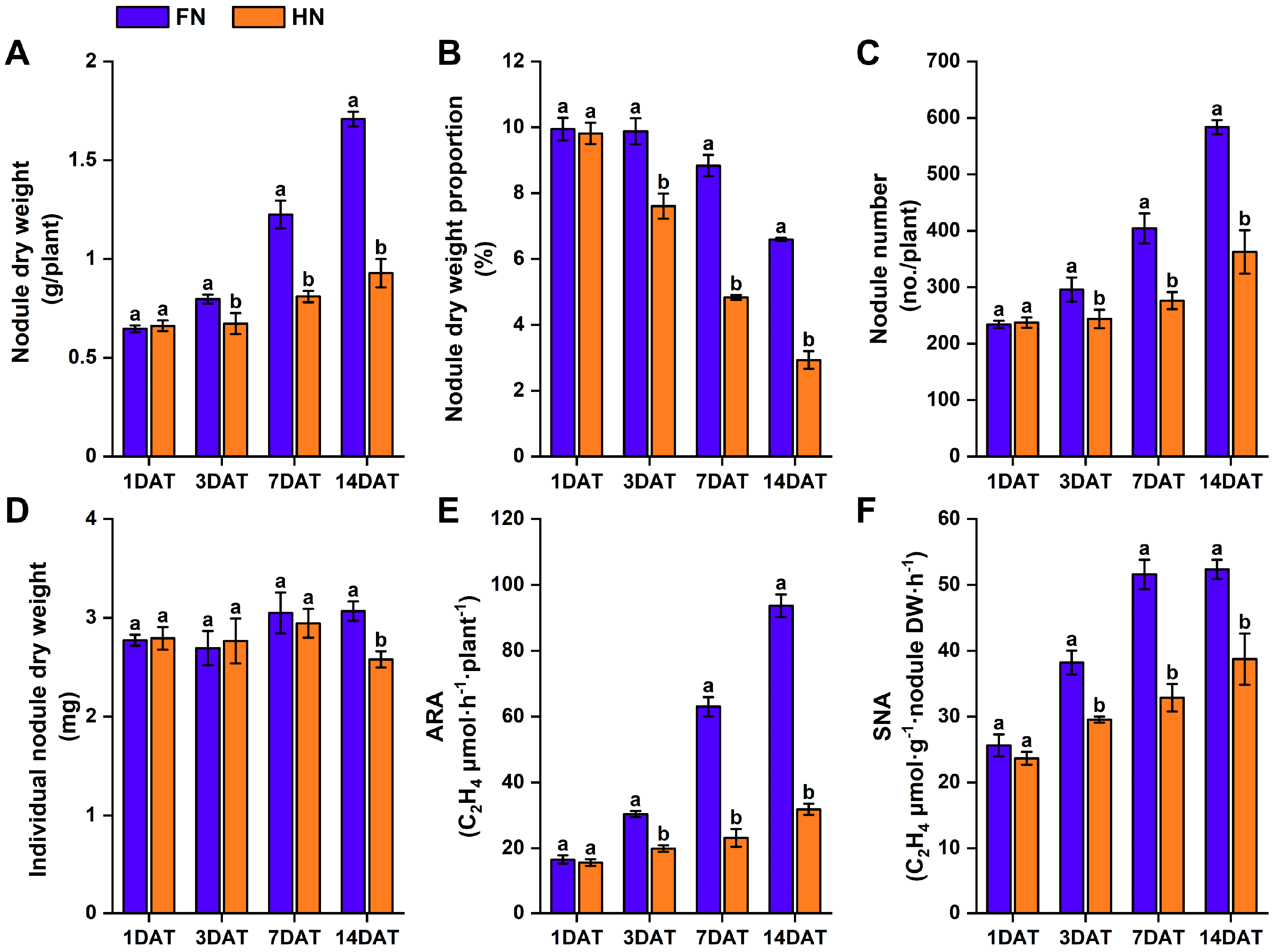
3.3. Effects of Nitrate on Nodulation and Nitrogenase Activity in Dual-Root Soybean
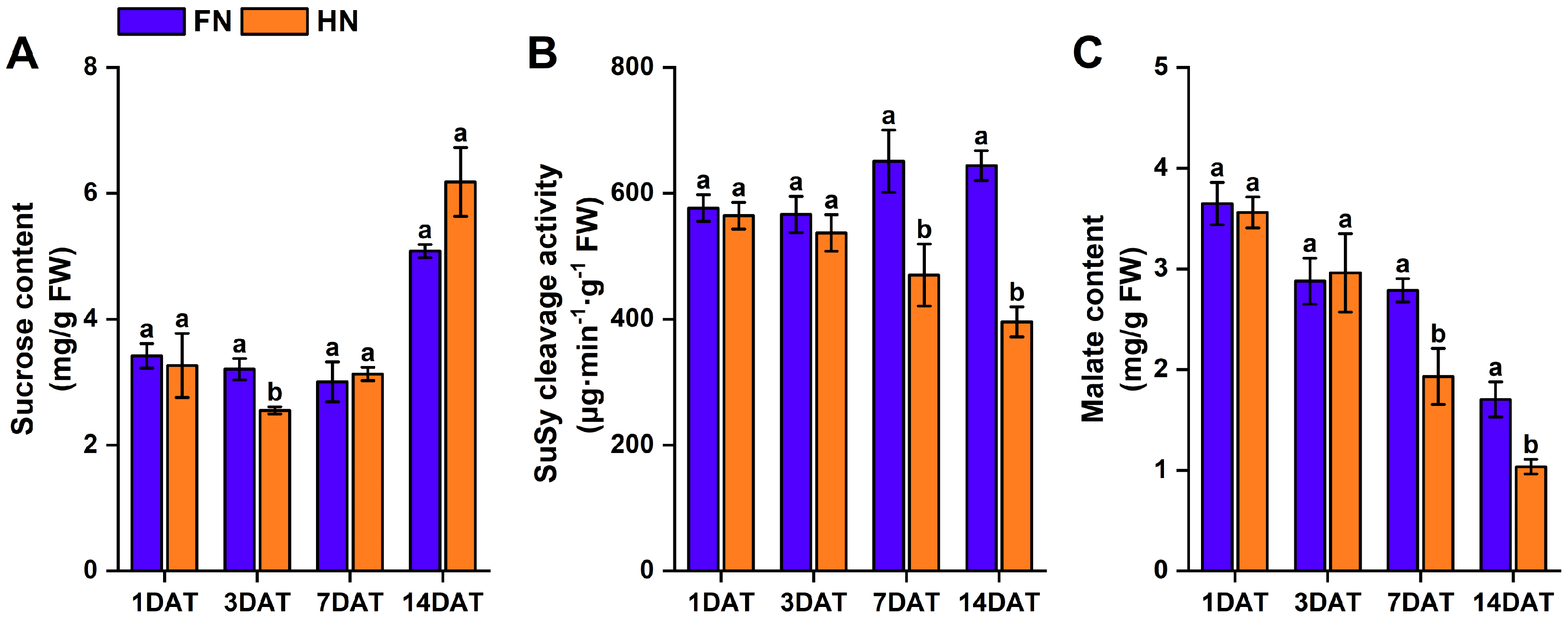
3.4. Effects of Nitrate Supply on Sucrose Content, Sucrose Synthase Cleavage Activity, and Malate Content in Nodules of Dual-Root Soybean
4. Discussion
4.1. Effects of Nitrate on Soybean Dry Matter Accumulation and Allocation
4.2. Effects of Nitrate on Soybean Nodulation, Nitrogen Fixation, and Sucrose Transport and Metabolism in Nodules
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef]
- Nakei, M.D.; Venkataramana, P.B.; Ndakidemi, P.A. Soybean-nodulating rhizobia: Ecology, characterization, diversity, and growth promoting functions. Front. Sustain. Food Syst. 2022, 6, 824444. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Downie, J.A. Calcium, kinases and nodulation signalling in legumes. Nat. Rev. Mol. Cell Biol. 2004, 5, 566–576. [Google Scholar] [CrossRef]
- Oke, V.; Long, S.R. Bacteroid formation in the Rhizobium-legume symbiosis. Curr. Opin. Microbiol. 1999, 2, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Prell, J.; Poole, P. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 2006, 14, 161–168. [Google Scholar] [CrossRef]
- White, J.; Prell, J.; James, E.K.; Poole, P. Nutrient sharing between symbionts. Plant Physiol. 2007, 144, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Streeter, J.G. Carbohydrates in soybean nodules: II. Distribution of compounds in seedlings during the onset of nitrogen fixation. Plant Physiol. 1980, 66, 471–476. [Google Scholar] [CrossRef]
- Nishida, H.; Suzaki, T. Nitrate-mediated control of root nodule symbiosis. Curr. Opin. Plant Biol. 2018, 44, 129–136. [Google Scholar] [CrossRef]
- Voisin, A.S.; Salon, C.; Jeudy, C.; Warembourg, F.R. Root and nodule growth in Pisum sativum L. in relation to photosynthesis: Analysis using 13C-labelling. Ann. Bot. 2003, 92, 557–563. [Google Scholar] [CrossRef]
- Fujikake, H.; Yamazaki, A.; Ohtake, N.; Sueyoshi, K.; Matsuhashi, S.; Ito, T.; Mizuniwa, C.; Kume, T.; Hashimoto, S.; Ishioka, N.S.; et al. Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J. Exp. Bot. 2003, 54, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, C.; Liu, H.; Lyu, X.; Xiao, F.; Zhao, S.; Ma, C.; Yan, C.; Liu, Z.; Li, H.; et al. Systemic regulation of nodule structure and assimilated carbon distribution by nitrate in soybean. Front. Plant Sci. 2023, 14, 1101074. [Google Scholar] [CrossRef] [PubMed]
- Vernon, L.P.; Aronoff, S. Metabolism of soybean leaves. IV. Translocation from soybean leaves. Arch. Biochem. Biophys. 1952, 36, 383–398. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, X.; Wang, N.; Li, S.; Yao, X.; Kuang, H.; Qiu, Z.; Ke, D.; Yang, W.; Guan, Y. Nitrogen inhibition of nitrogenase activity involves the modulation of cytosolic invertase in soybean nodule. J. Genet. Genom. 2024, 51, 1404–1412. [Google Scholar] [CrossRef]
- Streeter, J.G. Effect of nitrate in the rooting medium on carbohydrate composition of soybean nodules. Plant Physiol. 1981, 68, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.J.; Minchin, F.R.; Skot, L.; James, C.L. Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiol. 1997, 114, 937–946. [Google Scholar] [CrossRef]
- Gordon, A.J.; Skot, L.; James, C.L.; Minchin, F.R. Short-term metabolic responses of soybean root nodules to nitrate. J. Exp. Bot. 2002, 53, 423–428. [Google Scholar] [CrossRef]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef]
- Morell, M.; Copeland, L. Sucrose synthase of soybean nodules. Plant Physiol. 1985, 78, 149–154. [Google Scholar] [CrossRef]
- Baier, M.C.; Barsch, A.; Kuster, H.; Hohnjec, N. Antisense repression of the Medicago truncatula nodule-enhanced sucrose synthase leads to a handicapped nitrogen fixation mirrored by specific alterations in the symbiotic transcriptome and metabolome. Plant Physiol. 2007, 145, 1600–1618. [Google Scholar] [CrossRef][Green Version]
- Gordon, A.J.; Minchin, F.R.; James, C.L.; Komina, O. Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol. 1999, 120, 867–878. [Google Scholar] [CrossRef]
- Craig, J.; Barratt, P.; Tatge, H.; Déjardin, A.; Handley, L.; Gardner, C.D.; Barber, L.; Wang, T.; Hedley, C.; Martin, C.; et al. Mutations at the rug4 locus alter the carbon and nitrogen metabolism of pea plants through an effect on sucrose synthase. Plant J. 1999, 17, 353–362. [Google Scholar] [CrossRef]
- Lambert, I.; Pervent, M.; Le Queré, A.; Clément, G.; Tauzin, M.; Severac, D.; Benezech, C.; Tillard, P.; Martin-Magniette, M.L.; Colella, S.; et al. Responses of mature symbiotic nodules to the whole-plant systemic nitrogen signaling. J. Exp. Bot. 2020, 71, 5039–5052. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Schulze, J.; Tran, L.S. N-feedback regulation is synchronized with nodule carbon alteration in Medicago truncatula under excessive nitrate or low phosphorus conditions. J. Plant Physiol. 2014, 171, 407–410. [Google Scholar] [CrossRef]
- Lyu, X.; Sun, C.; Zhang, J.; Wang, C.; Zhao, S.; Ma, C.; Li, S.; Li, H.; Gong, Z.; Yan, C. Integrated proteomics and metabolomics analysis of nitrogen system regulation on soybean plant nodulation and nitrogen fixation. Int. J. Mol. Sci. 2022, 23, 2545. [Google Scholar] [CrossRef]
- Heckmann, M.-O.; Drevon, J.-J.; Saglio, P.; Salsac, L. Effect of oxygen and malate on NO3− inhibition of nitrogenase in soybean nodules. Plant Physiol. 1989, 90, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Vessey, J.K.; Walsh, K.B.; Layzell, D.B. Can a limitation in pholem supply to nodules account for the inhibitory effect of nitrate on nitrogenase activity in soybean? Physiol. Plant. 1988, 74, 137–146. [Google Scholar] [CrossRef]
- Lyu, X.; Li, M.; Li, X.; Li, S.; Yan, C.; Ma, C.; Gong, Z. Assessing the systematic effects of the concentration of nitrogen supplied to dual-root systems of soybean plants on nodulation and nitrogen fixation. Agronomy 2020, 10, 763. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Lyu, X.; Xia, X.; Wang, C.; Ma, C.; Dong, S.; Gong, Z. Effects of changes in applied nitrogen concentrations on nodulation, nitrogen fixation and nitrogen accumulation during the soybean growth period. Soil Sci. Plant Nutr. 2019, 65, 479–489. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Zrenner, R.; Salanoubat, M.; Willmitzer, L.; Sonnewald, U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 1995, 7, 97–107. [Google Scholar] [CrossRef]
- Scherer, R.; Rybka, A.C.P.; Ballus, C.A.; Meinhart, A.D.; Filho, J.T.; Godoy, H.T. Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem. 2012, 135, 150–154. [Google Scholar] [CrossRef]
- Qiang, B.; Chen, S.; Fan, Z.; Cao, L.; Li, X.; Fu, C.; Zhang, Y.; Jin, X. Effects of nitrogen application levels on soybean photosynthetic performance and yield: Insights from canopy nitrogen allocation studies. Field Crops Res. 2025, 326, 109871. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, X.; Zhao, Q.; Zhang, W.; Zhang, B.; Xie, F. Rapid effect of nitrogen supply for soybean at the beginning fowering stage on biomass and sucrose metabolism. Sci. Rep. 2019, 9, 15530. [Google Scholar] [CrossRef] [PubMed]
- Fujikake, H.; Tamura, Y.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Photoassimilate partitioning in hypernodulation mutant of soybean (Glycine max (L.) Merr.) NOD1-3 and its parent williams in relation to nitrate inhibition of nodule growth. Soil Sci. Plant Nutr. 2003, 49, 583–590. [Google Scholar] [CrossRef]
- Qiang, B.; Zhou, W.; Zhong, X.; Fu, C.; Cao, L.; Zhang, Y.; Jin, X. Effect of nitrogen application levels on photosynthetic nitrogen distribution and use efficiency in soybean seedling leaves. J. Plant Physiol. 2023, 287, 154051. [Google Scholar] [CrossRef]
- Hansen, A.P.; Yoneyama, T.; Kouchi, H. Short-term nitrate effects on hydroponically-grown soybean cv. Bragg and its supernodulating mutant: I. carbon, nitrogen and mineral element distribution, respiration and the effect of nitrate on nitrogenase activity. J. Exp. Bot. 1992, 43, 1–7. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, eaba0196. [Google Scholar] [CrossRef]
- Daimon, H.; Yoshioka, M. Responses of root nodule formation and nitrogen fixation activity to nitrate in a split-root system in peanut (Arachis hypogaea L.). J. Agron. Crop Sci. 2001, 187, 89–95. [Google Scholar] [CrossRef]
- Kouchi, H.; Akao, S.; Yoneyama, T. Respiratory utilization of 13C-labelled photosynthate in nodulated root systems of soybean plants. J. Exp. Bot. 1986, 37, 985–993. [Google Scholar] [CrossRef]
- Lyu, X.; Sun, C.; Lin, T.; Wang, X.; Li, S.; Zhao, S.; Gong, Z.; Wei, Z.; Yan, C.; Ma, C. Systemic regulation of soybean nodulation and nitrogen fixation by nitrogen via isoflavones. Front. Plant Sci. 2022, 13, 968496. [Google Scholar] [CrossRef]
- Mbah, G.C.; Dakora, F.D. Nitrate inhibition of N2 fixation and its effect on micronutrient accumulation in shoots of soybean (Glycine max L. Merr.), Bambara groundnut (Vigna subterranea L. Vedc) and Kersting’s groundnut (Macrotyloma geocarpum Harms.). Symbiosis 2018, 75, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.G. Transport and metabolism of carbon and nitrogen in legume nodules. Adv. Bot. Res. 1991, 18, 129–187. [Google Scholar] [CrossRef]
- Hacin, J.I.; Bohlool, B.B.; Singleton, P.W. Partitioning of 14C-labelled photosynthate to developing nodules and roots of soybean (Glycine max). New Phytol. 1997, 137, 257–265. [Google Scholar] [CrossRef]
- Ke, X.; Xiao, H.; Peng, Y.; Xia, X.; Wang, X. Nitrogen deficiency modulates carbon allocation to promote nodule nitrogen fixation capacity in soybean. Exploration 2024, 4, 20230104. [Google Scholar] [CrossRef] [PubMed]
- Thummler, F.; Verma, D.P. Nodulin-100 of soybean is the subunit of sucrose synthase regulated by the availability of free heme in nodules. J. Biol. Chem. 1987, 262, 14730–14736. [Google Scholar] [CrossRef]
- Anthon, G.E.; Emerich, D.W. Developmental regulation of enzymes of sucrose and hexose metabolism in effective and ineffective soybean nodules. Plant Physiol. 1990, 92, 346–351. [Google Scholar] [CrossRef]
- Schulte, C.C.M.; Borah, K.; Wheatley, R.M.; Terpolilli, J.J.; Saalbach, G.; Crang, N.; de Groot, D.H.; Ratcliffe, R.G.; Kruger, N.J.; Papachristodoulou, A.; et al. Metabolic control of nitrogen fixation in rhizobium-legume symbioses. Sci. Adv. 2021, 7, eabh2433. [Google Scholar] [CrossRef]
- Bacanamwo, M.; Harper, J.E. The feedback mechanism of nitrate inhibition of nitrogenase activity in soybean may involve asparagine and/or products of its metabolism. Physiol. Plant. 1997, 100, 371–377. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lian, Z.; Lyu, X.; Yan, C.; Yan, S.; Gong, Z.; Li, S.; Ma, C. Nitrate inhibits nodule nitrogen fixation by accumulating ureide in soybean plants. Plants 2024, 13, 2045. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Guo, T.; Zhang, Y.; Lyu, X.; Yan, S.; Yan, C.; Gong, Z.; Ma, C. Systemic Effects of Nitrate on Nitrogen Fixation and Sucrose Catabolism in Soybean (Glycine max (L.) Merr.) Nodules. Agronomy 2025, 15, 1032. https://doi.org/10.3390/agronomy15051032
Wang X, Guo T, Zhang Y, Lyu X, Yan S, Yan C, Gong Z, Ma C. Systemic Effects of Nitrate on Nitrogen Fixation and Sucrose Catabolism in Soybean (Glycine max (L.) Merr.) Nodules. Agronomy. 2025; 15(5):1032. https://doi.org/10.3390/agronomy15051032
Chicago/Turabian StyleWang, Xuelai, Tong Guo, Yuchen Zhang, Xiaochen Lyu, Shuangshuang Yan, Chao Yan, Zhenping Gong, and Chunmei Ma. 2025. "Systemic Effects of Nitrate on Nitrogen Fixation and Sucrose Catabolism in Soybean (Glycine max (L.) Merr.) Nodules" Agronomy 15, no. 5: 1032. https://doi.org/10.3390/agronomy15051032
APA StyleWang, X., Guo, T., Zhang, Y., Lyu, X., Yan, S., Yan, C., Gong, Z., & Ma, C. (2025). Systemic Effects of Nitrate on Nitrogen Fixation and Sucrose Catabolism in Soybean (Glycine max (L.) Merr.) Nodules. Agronomy, 15(5), 1032. https://doi.org/10.3390/agronomy15051032





