1. Introduction
Parasitic nematodes are integral components of soil ecosystems, playing crucial roles in regulating herbivore populations and contributing to natural pest suppression [
1,
2,
3]. Among these, entomopathogenic nematodes (EPNs) from the families Heterorhabditidae and Steinernematidae have garnered significant attention as effective biological control agents against insect pests [
3]. Species such as
Steinernema feltiae Filipjev,
Steinernema carpocapsae [Weiser],
Heterorhabditis bacteriophora Poinar, and
Phasmarhabditis hermaphrodita (Schneider) are widely integrated into pest management programs due to their ability to infect and eliminate agricultural pests while offering an environmentally sustainable alternative to chemical pesticides [
3,
4].
Beyond insect control, recent research has highlighted the potential of
Phasmarhabditis papillosa (Schneider) Andrassy and
Oscheius myriophilus (Poinar) in managing mollusk pests, particularly slugs, which pose a serious threat to crop yields [
4,
5]. These nematodes establish a parasitic relationship with their slug hosts by entering through natural openings or penetrating the body wall. Once inside, they invade internal tissues such as the mantle cavity, digestive tract, or reproductive organs, leading to a disruption of vital physiological functions. Infected slugs typically cease feeding within a few days—an important factor in reducing crop damage even before mortality occurs. Death usually follows within 4 to 21 days, depending on environmental conditions and the nematode load. The nematodes then continue to reproduce inside the host cadaver, and newly formed infective juveniles later emerge and disperse into the soil to seek new hosts [
6]. This life cycle not only contributes to effective suppression of slug populations but also enables the persistence of nematodes in the environment. Incorporating these nematodes into integrated pest management (IPM) strategies can significantly reduce reliance on synthetic pesticides, enhance biodiversity, and support sustainable agricultural practices by preserving ecological balance.
A fundamental aspect of nematode foraging and host-seeking behavior involves the detection of chemical cues, including carbon dioxide (CO
2) and volatile organic compounds (VOCs) emitted by plants and associated organisms [
7,
8,
9,
10,
11]. Plant-emitted VOCs serve as crucial belowground signals that mediate interactions between plants, herbivores, and natural enemies, influencing nematode host-finding strategies [
9,
10,
11]. In the case of potato (
Solanum tuberosum L.), a diverse array of VOCs is released from tubers, which can play a pivotal role in tritrophic interactions involving herbivores and nematodes [
12]. While VOC emissions are well-established as attractants for natural enemies as part of indirect plant defense mechanisms [
13,
14], the extent to which slug-parasitic nematodes respond to these cues remains largely unexplored.
Potato tubers produce a complex mixture of secondary metabolites that function in both direct and indirect defense, including aldehydes (e.g., decanal, nonanal, and octanal), hydrocarbons (e.g., undecane), and aromatic or ketone compounds (e.g., 1,2,4-trimethylbenzene and 6-methyl-5-hepten-2-one) [
13,
14]. Some of these compounds, such as 2-ethyl-1-hexanol, are known to attract soil-dwelling organisms and may play a key role in nematode chemotaxis [
12]. Notably, plants exhibit remarkable specificity in their VOC emissions depending on the identity of the herbivore, fine-tuning their defense responses to repel pests while simultaneously recruiting beneficial organisms such as predatory insects and nematodes [
9,
10]. Specific compounds such as (E)-β-caryophyllene and 4,5-dimethylthiazole have been shown to attract EPNs [
9,
15], whereas others, such as hexanal and terpinolene, act as deterrents [
10,
12]. The ability of nematodes to respond to these chemical gradients further underscores the complexity of belowground multitrophic interactions [
16].
The response of EPNs to plant-emitted VOCs is well documented, yet the chemotactic behaviors of slug-parasitic nematodes remain largely unexplored [
17]. Given the economic importance of potato crops and the extensive damage caused by both insect and mollusk herbivores [
18], a deeper understanding of how these nematodes perceive and respond to plant-emitted VOCs is essential for optimizing their use in biological control strategies. Different nematode species exhibit distinct foraging strategies, with some actively seeking out hosts while others rely on ambush tactics [
7,
8]. Temperature is a key environmental factor influencing nematode activity, host-seeking behavior, and overall efficacy as biological control agents [
4,
5]. Investigating whether slug-parasitic nematodes exhibit varying chemotactic responses under different thermal conditions will help optimize their use across diverse agricultural environments. Additionally, potato plants release a variety of VOCs, some of which are known to attract beneficial nematodes [
12], yet it remains unclear whether slug-parasitic nematodes respond to the same chemical cues. Identifying the specific VOCs that influence their behavior will provide insights into their host-seeking mechanisms and improve their practical application in pest management. Furthermore, in many plant–herbivore–enemy systems, plants recruit natural enemies by releasing VOCs in response to herbivore damage [
9,
10]. If slug-parasitic nematodes are similarly attracted to VOCs emitted by herbivore-damaged potato tubers, this would suggest that plants indirectly recruit nematodes as part of their defense strategy. Clarifying this interaction will enhance our understanding of belowground multitrophic dynamics and contribute to the development of more sustainable pest management approaches.
This study aims to expand knowledge on the chemotactic behavior of slug-parasitic nematodes, specifically
P. papillosa,
O. myriophilus, and
O. onirici, in response to VOCs released by potato tubers. While previous research has extensively examined the behavioral responses of EPNs, the foraging strategies of nematodes that target mollusk pests remain largely unknown. To address this knowledge gap, this study is designed with the following aims: [
1]. Assess the variation in chemotactic responses among nematode species under different temperature conditions. Since temperature significantly influences nematode behavior and efficacy as biological control agents, understanding how different species respond to environmental fluctuations will help refine their application across diverse climatic regions [
2]. Investigate the behavioral effects of specific VOCs emitted by potato tubers on slug-parasitic nematodes. Identifying the key chemical compounds that attract or repel nematodes will provide insights into their host-seeking mechanisms and enhance the effectiveness of biological slug control strategies [
3]. Determine whether plant-emitted VOCs function as indirect defense signals by attracting slug-parasitic nematodes to herbivore-damaged sites. This will provide valuable insights into plant–nematode–herbivore interactions, offering a foundation for integrating slug-parasitic nematodes into sustainable pest management programs.
The scope of this study extends beyond fundamental nematode behavioral ecology, with direct applications for integrated pest management (IPM) strategies. By elucidating the mechanisms underlying nematode attraction to plant-emitted VOCs, this research contributes to improving biological control methods while reducing dependence on synthetic pesticides, thereby fostering environmentally sustainable agriculture.
2. Materials and Methods
2.1. Nematode Collection, Isolation and Preparation for Storage
For this study, native populations of
P. papillosa (GenBank accession number MT800511.1),
O. myriophilus (GenBank accession number OP684306.1), and
O. onirici (GenBank accession number PQ876382) were used, as their presence has been recently confirmed in Slovenia [
17].
The nematodes were cultured in vivo using freeze-killed Spanish slugs as a substrate. After 10 days of incubation, nematodes were extracted from the slug cadavers following a standardized protocol adapted for EPNs [
17]. The extraction process involved centrifugation in a 5% sodium hypochlorite solution to separate the nematodes from residual tissue and debris. This was followed by two successive washes with distilled water to ensure a clean suspension of infective juveniles (IJs).
The harvested infective juveniles (IJs) were suspended in M9 buffer—a commonly used isotonic solution containing KH
2PO
4, Na
2HPO
4, NaCl, and NH
4Cl, used to maintain nematode viability and osmotic balance [
1,
2]—and stored at 4 °C. The viability rate was assessed prior to each experiment by examining a random subsample of approximately 100 IJs under a stereomicroscope. The subsample was placed in a drop of M9 buffer on a glass slide and observed for motility. The nematodes were considered viable if they exhibited active movement, such as sinusoidal locomotion or twitching. Individuals showing no movement or displaying signs of degeneration were classified as non-viable. Only nematode batches less than two weeks old and exhibiting a viability rate exceeding 95% were selected for use in chemotaxis experiments to ensure optimal physiological activity and chemotactic responsiveness.
2.2. Tested Volatile Compounds
The selection of volatile organic compounds (VOCs) for this study was based on the findings of Weissteiner [
14], who identified key VOC emissions from potato tubers damaged by the northern cockchafer (
Melolontha hippocastani Fabricius). Using proton transfer reaction mass spectrometry (PTR-MS) and gas chromatography–mass spectrometry (GC-MS), Weissteiner detected seven major VOCs released in response to grub feeding: (1) decanal, (2) nonanal, (3) octanal, (4) undecane, (5) 1,2,4-trimethylbenzene, (6) 6-methyl-5-hepten-2-one, and (7) 2-ethyl-1-hexanol.
For our study, we used synthetically produced versions of these compounds (Sigma-Aldrich (now part of Merck), St. Louis, MO, USA), ensuring high purity levels for experimental precision. The following VOCs were purchased with their respective purities: decanal (≥97%), nonanal (97%), octanal (≥95%), undecane (≥99%), 1,2,4-trimethylbenzene (98%), 6-methyl-5-hepten-2-one (≥98%), and 2-ethyl-1-hexanol (≥99%). These compounds were tested at both their pure stock concentrations, as supplied by the manufacturer, and at a diluted concentration of 0.03 ppm, which corresponds to the average VOC levels detected in the soil 10 cm from the root system of potato tubers [
14].
To achieve the target 0.03 ppm concentration, pure VOCs were diluted in 96% ethanol. The dilution process involved precisely measuring the required volume of each compound and mixing it with ethanol to obtain the desired concentration. The prepared solutions were homogenized using a vortex mixer to ensure consistency before use in laboratory bioassays. This controlled approach allowed for standardized VOC exposure across experiments, ensuring the reliability and reproducibility of the results.
2.3. Chemotaxis Assay
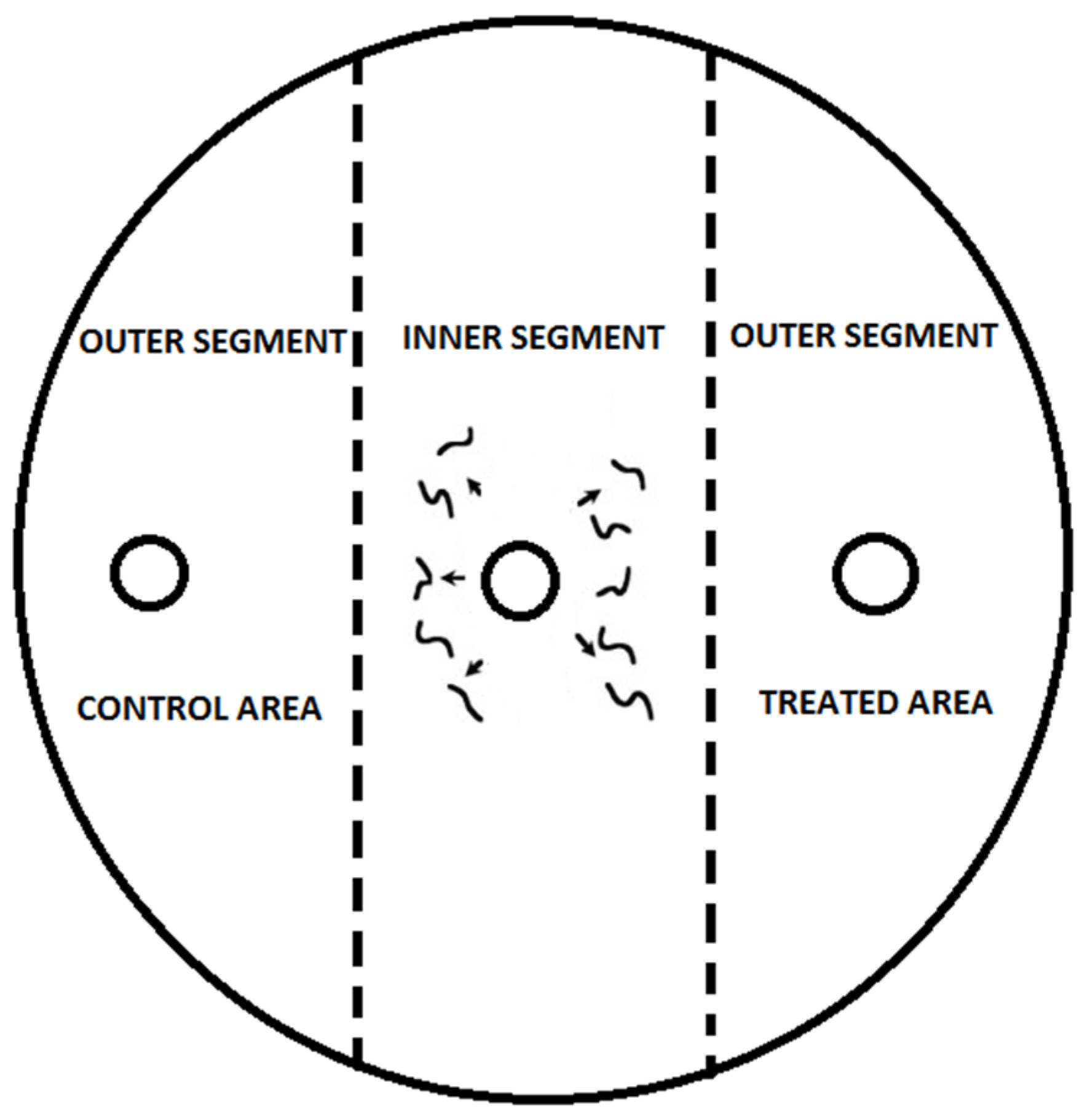
The chemotaxis assay (
Figure 1) was adapted from O’Halloran and Burnell [
19] and further refined by Laznik and Trdan [
12]. Petri dishes (ø = 9 cm) were filled with 25 mL of 1.6% technical agar (Biolife, Milan, Italy) supplemented with 5 mM potassium phosphate buffer (pH 6.0), 1 mM CaCl
2, and 1 mM MgSO
4. Each treatment was replicated 10 times, and the entire experiment was repeated three times to ensure consistency and reproducibility.
To prevent cross-contamination of VOCs between treatments, only one volatile compound was tested per experimental setup. The Petri dishes were securely sealed with laboratory film (Parafilm, Bemis Company, Inc., Neenah, WI, USA) to minimize evaporation and ensure that the VOCs remained localized within the designated treatment area. This setup maintained the integrity of the experiment by preventing unintended diffusion of VOCs between adjacent dishes.
The Petri dishes were placed in a rearing chamber (RK-900 CH, Kambič Laboratory Equipment, Semič, Slovenia) and incubated under dark conditions at 18 and 22 °C with 75% relative humidity. The nematodes were allowed to move freely within the assay plates for 24 h. After the incubation period, the dishes were rapidly frozen at −20 °C for 3 min to immobilize the nematodes, facilitating accurate counting. Nematode distribution in the treatment and control areas was assessed using a Nikon SMZ800N binocular microscope equipped with a 4K UHD Multi-output HDMI Camera (XCAM4K8MPB, Tucsen Photonics Co., Ltd., Fuzhou, China) at 25× magnification.
The chemotaxis index (CI) was calculated using the formula adapted from Bargmann and Horvitz [
20] and Laznik and Trdan [
12]:
CI values ranged from 1.0 (indicating complete attraction) to −1.0 (indicating complete repulsion). Based on the calculated CI, the compounds were classified as follows: attractants (CI ≥ 0.2), weak attractants (0.2 > CI ≥ 0.1), neutral (−0.1 ≤ CI < 0.1), weak repellents (−0.2 ≤ CI < −0.1), and repellents (CI ≤ −0.2) [
3].
2.4. Statistical Analysis
In the chemotaxis assay, directional movement of nematodes from the inner to the outer segments of the Petri dish—indicative of a preferential response—was analyzed using a paired Student’s t-test. Statistical significance was assessed at a threshold of p < 0.05. To evaluate differences in behavioral responses among species, the average percentage of infective juveniles (IJs) migrating to the outer segments or remaining in the inner segments was calculated for each replicate. These data were subjected to two-way analysis of variance (ANOVA), with significance determined at p < 0.05. The analysis tested the main effects of VOC identity, nematode species, temperature, and VOC concentration, as well as their interactions. Of all tested combinations, only the four-way interaction between VOC, nematode species, temperature, and concentration was found to be statistically significant and biologically interpretable.
Additionally, a separate two-way ANOVA was conducted on the chemotaxis index (CI) values to compare the overall responsiveness of different nematode species to the tested VOCs. Post hoc comparisons were performed using Duncan’s multiple-range test (p < 0.05) for mean separation. All data are presented as mean ± standard error (SE). Statistical analyses were carried out using Statgraphics Plus for Windows 4.0 (Statistical Graphics Corp., Manugistics, Inc., Rockville, MD, USA), and graphical visualizations were prepared using Microsoft Excel 2010.
3. Results
3.1. Nematode Motility
In this study, “motility” refers to the movement of nematode infective juveniles (IJs) from the inner segment to the outer segments of the assay dish (see
Figure 1). The proportion of nematodes reaching the outer zones was used as a measure of motility, which was significantly influenced by multiple factors (
Table 1). Among the primary factors, nematode species (F = 59.35,
p = 0.0001) and VOCs (F = 15.00,
p = 0.0001) had highly significant effects, confirming their central roles in directing nematode movement. In contrast, VOC concentration (F = 1.39,
p = 0.2388) and temperature (F = 1.25,
p = 0.2644) did not significantly influence motility, indicating that nematode responses remained stable across different concentrations and temperature conditions.
Several interactions between factors were also significant, revealing a more nuanced relationship between nematode motility and environmental variables. Nematode species × VOCs (S × V, F = 12.02, p = 0.0001) and nematode species × temperature (S × T, F = 46.70, p = 0.0001) demonstrated that different nematode species exhibit varying responses to VOCs and temperature. VOCs × concentration (V × C, F = 2.02, p = 0.0497) was marginally significant, suggesting that VOC effects on motility may be concentration-dependent, whereas VOCs × temperature (V × T, F = 0.83, p = 0.5653) was not significant, indicating that temperature does not substantially alter the effects of VOCs on nematode movement.
The analysis also highlighted complex three-way interactions, emphasizing the interplay between biological and environmental factors. Nematode species × VOCs × temperature (S × V × T, F = 4.72, p = 0.0001) was highly significant, suggesting that nematode responses to VOCs are temperature-dependent and species-specific. Likewise, nematode species × VOCs × concentration (S × V × C, F = 2.30, p = 0.0041) further supports the idea that VOC effects are influenced by both species identity and concentration levels.
These findings confirm that nematode species and VOCs are the primary drivers of IJ motility, while interactions provide a deeper insight into the environmental and chemical factors shaping their movement. Additionally, the significance of spatial replication suggests that positional effects within the assay setup should be taken into account in future studies to ensure accurate interpretations of nematode behavior.
Nematodes exposed to pure VOC compounds at 18 °C exhibited significantly higher motility compared to those treated with 0.03 ppm VOC solutions, indicating a concentration-dependent effect. Among the tested species,
O. myriophilus displayed the strongest motility response when exposed to octanal (pure compound), with over 30% of IJs migrating to the outer segments of the assay dish (
Figure 2). Species-specific differences in motility were evident, as
O. myriophilus consistently exhibited higher motility than
P. papillosa and
O. onirici, while
O. onirici displayed the lowest motility rates across all VOC exposures. These results suggest that nematode species respond differentially to VOC cues, with
O. myriophilus being the most sensitive to chemical stimuli. The increased motility observed under pure-compound treatments further supports the idea that higher VOC concentrations elicit stronger nematode responses. Additionally, the minimal movement in the control group reinforces the biological relevance of VOC-induced motility, confirming that nematode movement is primarily driven by chemical stimuli rather than random activity.
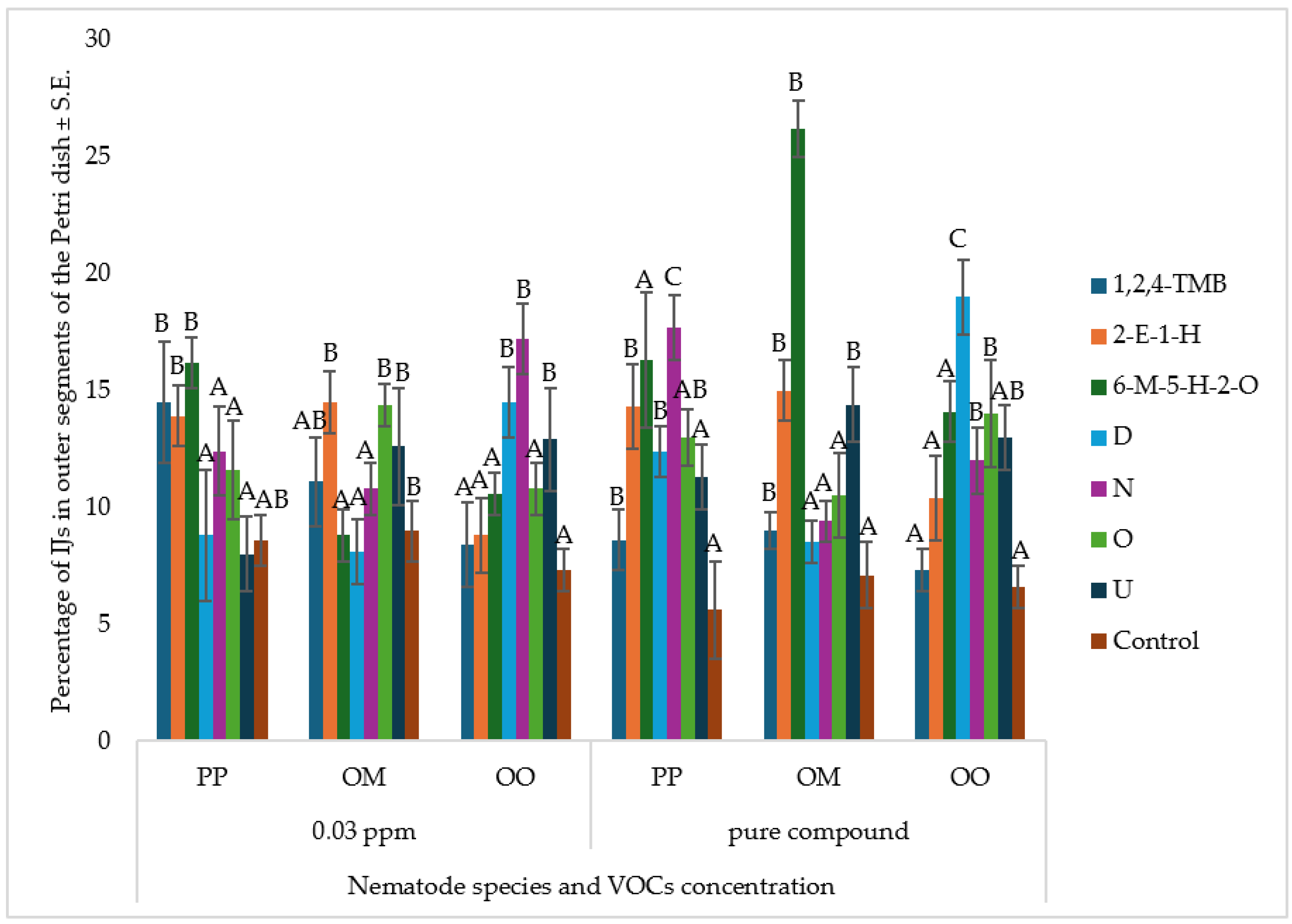
At 22 °C and a concentration of 0.03 ppm, the tested VOCs—1,2,4-trimethylbenzene, 2-ethyl-1-hexanol, and 6-methyl-5-hepten-2-one—elicited similar movement responses across nematode species (
Figure 3). The proportion of IJs reaching the outer segments of the Petri dish ranged between 5% and 15%, with no statistically significant differences among the VOC treatments. In contrast, the control treatment consistently exhibited the lowest IJ presence in the outer segments, which may suggest a potential behavioral response to VOCs, although the extremely low compound concentration warrants cautious interpretation. Under pure-compound exposure, more pronounced behavioral variations were observed (
Figure 3). Among the tested VOCs, 6-methyl-5-hepten-2-one induced the strongest IJ movement response, particularly in
O. myriophilus, where over 25 % of IJs were detected in the outer segments, indicating a strong behavioral attraction. Across all nematode species, the control group exhibited the lowest IJ distribution, further reinforcing the hypothesis that VOCs actively modulate nematode behavior.
These results demonstrate that nematode motility is primarily influenced by species identity and exposure to volatile organic compounds (VOCs), with higher VOC concentrations generally eliciting stronger chemotactic responses. Notably, O. myriophilus exhibited the highest sensitivity to VOC cues, indicating a more robust behavioral reaction to chemical stimuli. In contrast, O. onirici showed the weakest overall chemotactic response across all tested compounds, although certain individual VOCs—such as decanal—triggered lower responses in p. papillosa. These findings underscore the importance of both nematode species and VOC composition in shaping movement patterns, and they highlight the need for further research into the chemical ecology of nematode–host interactions, particularly in the context of sustainable pest management in agricultural systems.
3.2. Chemotaxis Index
The movement preference of nematode infective juveniles (IJs) was assessed using the chemotaxis index (CI).
Table 2 presents the ANOVA results for CI values, evaluating the effects of nematode species, VOCs, VOC concentration, temperature, and replication, along with their interactions. VOC type and temperature emerged as the most significant factors influencing chemotaxis, with strong effects observed for both (F = 19.94,
p = 0.0001 for VOCs; F = 23.93,
p = 0.0001 for temperature). In contrast, VOC concentration, temporal replication, and spatial replication did not significantly impact nematode chemotaxis (
p > 0.05). Among the interaction effects, the nematode species by VOC interaction (S × V, F = 9.63,
p = 0.0001) and the VOC by temperature interaction (V × T, F = 15.79,
p = 0.0001) were significant, indicating that nematode responses varied depending on specific VOCs and environmental conditions. Additionally, higher-order interactions, including species by VOC by concentration (S × V × C, F = 5.34,
p = 0.0001) and species by VOC by temperature (S × V × T, F = 25.59,
p = 0.0001), were also significant, underscoring the complex interplay between nematode species, chemical cues, and environmental factors. The residual variance accounted for a substantial proportion of the total variance, suggesting the presence of other unexplored variables influencing nematode chemotaxis.
The chemotaxis index (CI) values for
P. papillosa,
O. myriophilus, and
O. onirici under the influence of different VOCs at 18 °C are summarized in
Table 3. Among the pure VOCs, octanal acted as an attractant for
O. myriophilus, with a CI value of 0.21 ± 0.03, while it had a neutral effect on
P. papillosa (0.00 ± 0.01) and
O. onirici (0.05 ± 0.01). Conversely, nonanal was a weak attractant for
P. papillosa (0.17 ± 0.01), but a weak repellent for
O. myriophilus (−0.12 ± 0.02). The responses to other VOCs varied by species. For example, 6-methyl-5-hepten-2-one was a weak attractant for
P. papillosa (0.11 ± 0.01), but neutral for the other two species. Decanal had a neutral effect on
O. onirici (0.06 ± 0.02), while it was a weak repellent for
O. myriophilus (−0.12 ± 0.01). The control treatment (96% ethanol) did not induce significant chemotactic responses across all species, remaining in the neutral range.
At the lower VOC concentration (0.03 ppm), octanal remained a weak attractant for
O. myriophilus (0.18 ± 0.05) but had negligible (neutral) effects on
P. papillosa and
O. onirici. Notably, 1,2,4-trimethylbenzene exhibited a weak repellent effect for
P. papillosa at 0.03 ppm (−0.15 ± 0.02), whereas at the pure-compound level, it was neutral (−0.02 ± 0.01). Across all species, 2-ethyl-1-hexanol and 6-methyl-5-hepten-2-one showed neutral-to-weak chemotactic effects, depending on the species and concentration. These results highlight clear species-specific differences in chemotactic responses to VOCs and indicate that both compound identity and concentration significantly influence nematode movement patterns (
Table 3).
The chemotaxis index (CI) values for
P. papillosa,
O. myriophilus, and
O. onirici under the influence of different VOCs at 22 °C are summarized in
Table 4. Among the pure VOCs, octanal acted as a weak attractant for both
P. papillosa and
O. myriophilus, with CI values of 0.11 ± 0.01 and 0.11 ± 0.02, respectively, while the response in
O. onirici was neutral (CI = 0.01 ± 0.02). Similarly, 6-methyl-5-hepten-2-one functioned as a weak attractant for
O. myriophilus (CI = 0.16 ± 0.03), while
P. papillosa showed a neutral-to-weak attractant response (CI = 0.08 ± 0.02), and
O. onirici did not exhibit a significant response. Nonanal induced a weak repellent effect in
P. papillosa (CI = −0.11 ± 0.03), while responses in
O. myriophilus and
O. onirici remained neutral. Meanwhile, decanal and undecane showed neutral-to-weak chemotactic responses across all species. The control treatment (96% ethanol) did not elicit any significant chemotactic response, with CI values remaining within the neutral range.
At the lower VOC concentration (0.03 ppm), octanal remained a weak attractant for both
P. papillosa (CI = 0.10 ± 0.02) and
O. myriophilus (CI = 0.11 ± 0.01), while
O. onirici showed a neutral-to-weak attractant response (CI = 0.07 ± 0.01). In contrast, nonanal continued to act as a weak repellent for
P. papillosa (CI = −0.09 ± 0.02), but surprisingly elicited a weak attractant response in
O. onirici (CI = 0.10 ± 0.02). 6-methyl-5-hepten-2-one did not induce significant chemotactic activity at this concentration in any species, and 1,2,4-trimethylbenzene showed only minimal (neutral) influence across all nematodes. The control treatment (96% ethanol) remained inactive, with no statistically significant chemotactic response observed at this concentration (
Table 4).
4. Discussion
The chemotactic behavior of parasitic nematodes in response to volatile organic compounds (VOCs) offers valuable insights into belowground multitrophic interactions and their potential applications in sustainable pest management. This study examined the responses of P. papillosa, O. myriophilus, and O. onirici to VOCs emitted by potato (S. tuberosum) tubers, revealing significant species-specific, temperature-dependent, and concentration-driven behavioral patterns. These findings contribute to a broader understanding of plant–nematode–herbivore interactions and provide a foundation for optimizing VOC-based biocontrol strategies.
Distinct chemotactic responses among nematode species underscore the role of VOCs in mediating host-seeking behavior. Among the tested compounds, octanal consistently acted as a weak attractant, particularly for
O. myriophilus, whereas nonanal exhibited species-dependent effects, functioning as a weak attractant for
P. papillosa but a weak repellent for
O. myriophilus. These results align with previous studies indicating that aldehydes such as octanal and nonanal play key roles in nematode foraging behavior [
10,
12,
15]. In contrast, hydrocarbons such as undecane and 1,2,4-trimethylbenzene showed neutral-to-weak repellent effects, consistent with their lower volatility and reduced interaction with nematode sensory receptors [
15]. The weak attraction of nematodes to 6-methyl-5-hepten-2-one suggests a potential signaling role in plant–nematode interactions, reinforcing the idea that herbivore-induced VOCs contribute to belowground chemical communication networks. These findings emphasize the importance of further investigating the ecological relevance of these VOCs in natural soil environments.
Temperature significantly influenced nematode chemotaxis, with stronger weak attraction to octanal and 6-methyl-5-hepten-2-one observed at 18 °C than at 22 °C. This temperature-dependent variability suggests that nematode sensory perception and movement efficiency are influenced by environmental conditions, as previously reported [
10,
12,
17]. The observed species–VOC–temperature interactions further highlight the complexity of nematode foraging strategies, supporting the notion that different species occupy distinct ecological niches [
10,
12]. These findings have practical implications for biological control applications, as temperature fluctuations in the field may impact nematode recruitment and efficacy.
VOC concentration played a crucial role in shaping chemotactic responses. Higher concentrations of octanal and 6-methyl-5-hepten-2-one elicited more pronounced weak attraction, particularly in
O. myriophilus, whereas lower concentrations (0.03 ppm) resulted in neutral or weak responses. This threshold-dependent behavior is consistent with previous findings on entomopathogenic nematodes (EPNs), where higher VOC concentrations enhanced attraction while lower levels led to inconsistent responses [
12,
17]. Interestingly, 1,2,4-trimethylbenzene exhibited weak repellent activity at 0.03 ppm but was neutral at the pure-compound level, suggesting that certain VOCs may play dual roles depending on their concentration gradients [
17]. These findings underscore the need to fine-tune VOC concentrations to maximize attraction while avoiding potential repellency effects in field applications.
Our findings partly contrast with previous studies on EPNs. Laznik and Trdan [
12] reported weak chemotaxis to VOCs emitted by insect-damaged potato tubers, with the highest chemotaxis index (CI = 0.35 ± 0.08) observed in
H. bacteriophora when exposed to decanal—classifying it as an attractant in that case. Similarly, S. carpocapsae exhibited limited attraction to (E)-β-caryophyllene, linalool, and α-caryophyllene, suggesting that chemotactic responses are highly species- and strain-dependent. Our study further supports the hypothesis that responsiveness to VOCs varies among nematode taxa, with slug-parasitic species (
P. papillosa and
Oscheius spp.) showing stronger attraction to octanal than insect-parasitic EPNs [
12]. This highlights the need for species-specific optimization of VOC-based strategies for biological control. The observed differential responses indicate that VOC-based biocontrol approaches must be tailored to specific nematode–pest systems.
These findings have significant implications for biological control strategies targeting mollusk pests. The consistent weak attraction of O. myriophilus to octanal and 6-methyl-5-hepten-2-one suggests that these compounds could be leveraged to enhance nematode recruitment to pest-infested areas, improving their effectiveness as biocontrol agents. Integrating these VOCs into integrated pest management (IPM) programs could enhance slug control while reducing dependence on chemical molluscicides. However, several challenges must be addressed before field implementation. The success of VOC-based nematode attraction depends on multiple environmental factors, including soil composition, moisture, and microbial activity, which can alter VOC diffusion and persistence, thereby influencing nematode behavior. Field validation is necessary to determine whether synthetic VOCs can reliably enhance nematode recruitment under natural conditions, as real-world variability may impact efficacy. Additionally, temperature fluctuations could affect nematode activity, necessitating adaptive deployment strategies that account for seasonal and soil temperature dynamics.