Urban Green Space as a Reservoir of Predatory Syrphids (Diptera, Syrphidae) for Aphid Control in Cities
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Sites
2.2. Method of Sampling
2.2.1. Sampling of Syrphid Larvae
2.2.2. Sampling the Adults
2.2.3. Determination of Efficiency of Syrphid Larvae
2.3. Statistical Analysis
3. Results
3.1. The Species Compositions of Syrphidae Collected from the Aphid Colonies
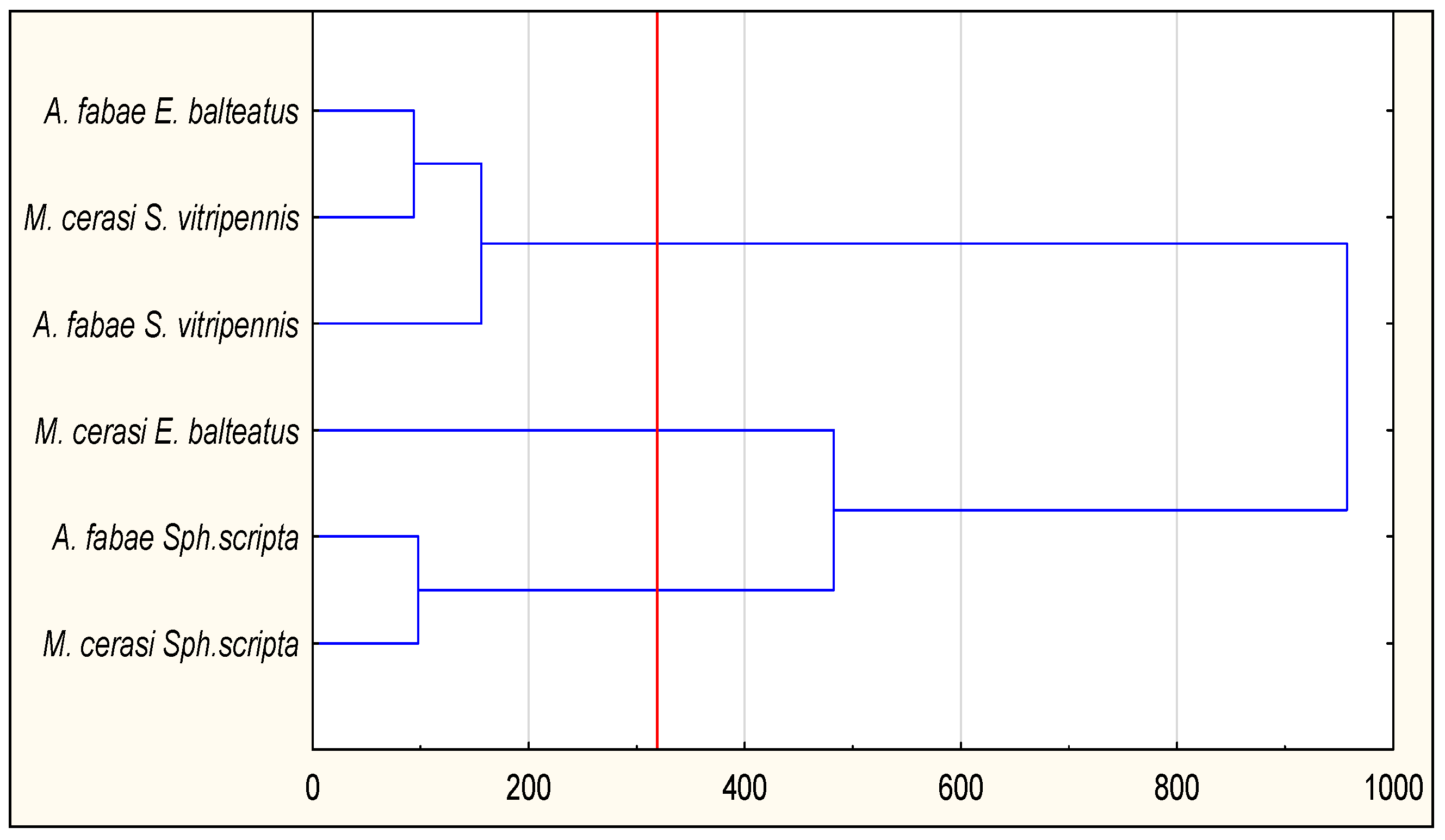
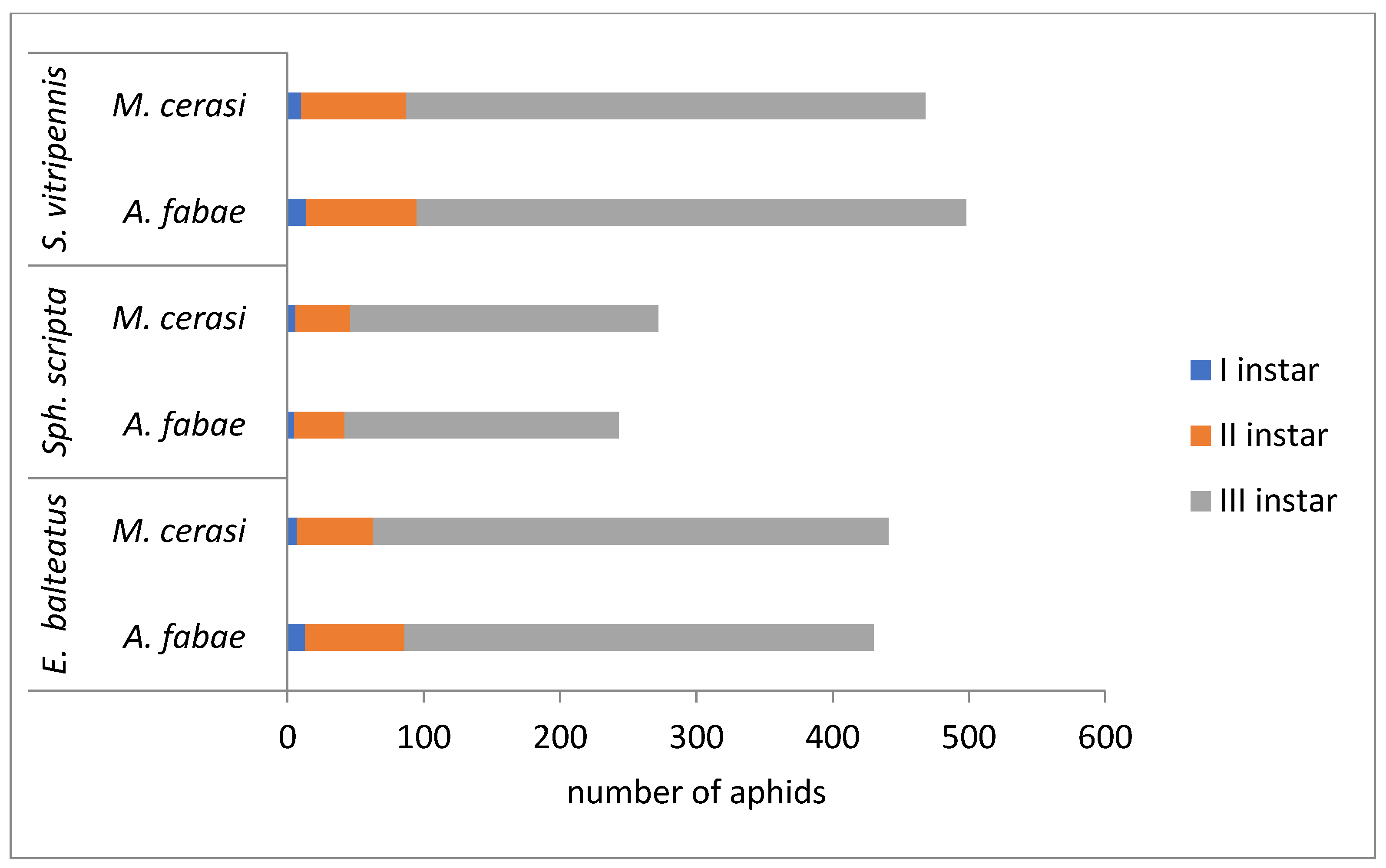
3.2. The Efficiency of Syrphid Larvae
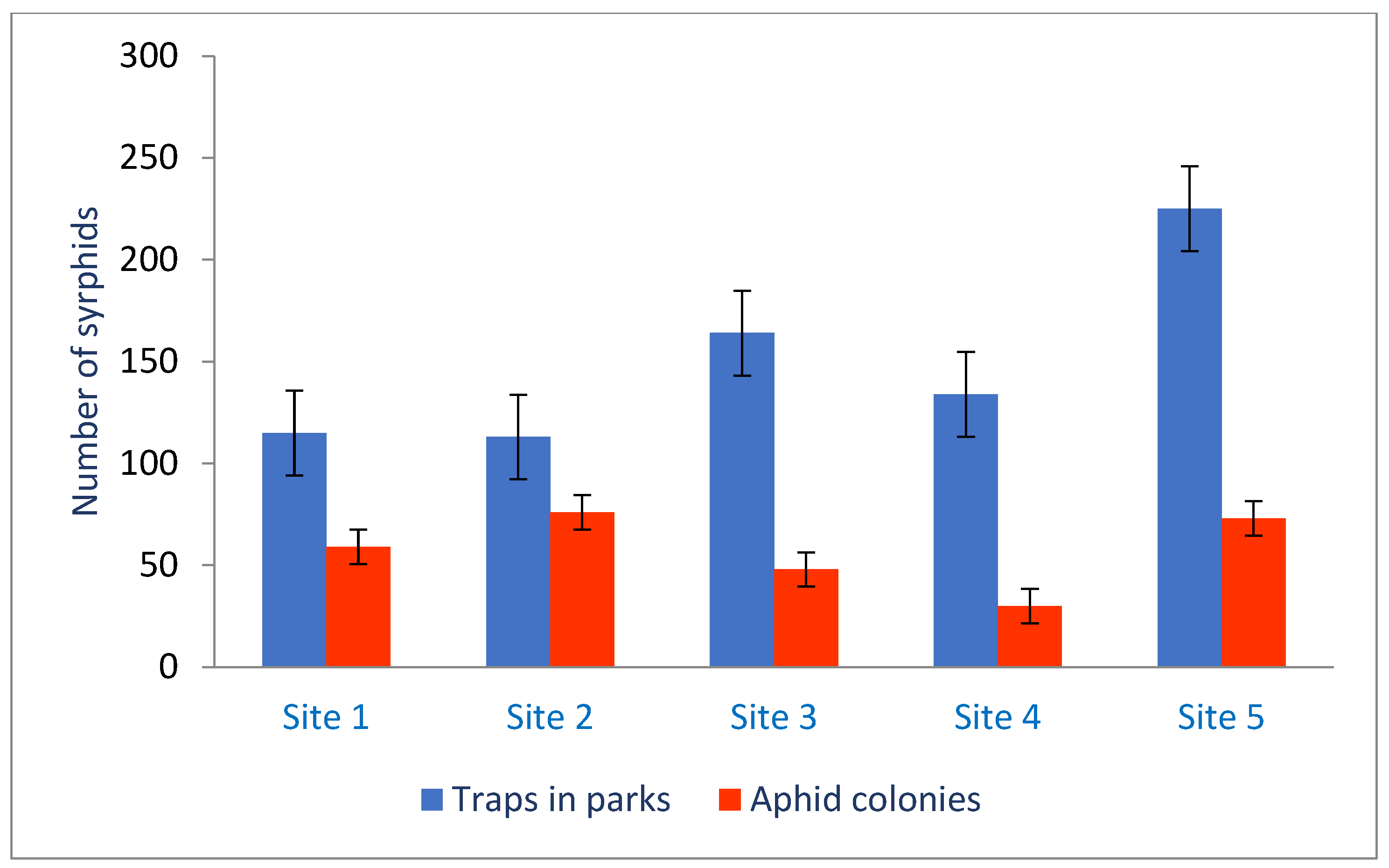
3.3. Syrphids Collected in Sweep Net and Moericke’s Traps
3.4. Significant Difference and Correlations Between the Syrphidae Collected from Aphid Colonies and from Sweep Net/Traps from Sampling Sites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llodrà-Llabrésa, J.; Cariñanos, P. Enhancing pollination ecosystem service in urban green areas: An opportunity for the conservation of pollinators. Urban For. Urban Green. 2022, 74, 127621. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Hahs, A.K.; Vesk, P.A. Urbanisation, plant traits and the composition of urban floras. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 78–86. [Google Scholar] [CrossRef]
- Lowenstein, D.M.; Minor, E.S. Diversity in flowering plants and their characteristics: Integrating humans as a driver of urban floral resources. Urban Ecosyst. 2016, 19, 1735–1748. [Google Scholar] [CrossRef]
- Aronson, M.F.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef]
- Lequerica Tamara, M.E.; Latty, T.; Threlfall, C.G.; Young, A.; Hochuli, D.F. Responses of hover fly diversity and abundance to urbanisation and local attributes of urban greenspaces. Basic. Appl. Ecol. 2023, 70, 12–26. [Google Scholar] [CrossRef]
- Smetana, S.M.; Crittenden, J.C. Sustainable plants in urban parks: A life cycle analysis of traditional and alternative lawns in Georgia, USA. Landsc. Urban Plan. 2014, 122, 140–151. [Google Scholar] [CrossRef]
- Md Meftaul, I.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Megharaj, M. Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar] [CrossRef]
- Alumai, A.; Salminen, S.O.; Richmond, D.S.; Cardina, J.; Grewal, P.S. Comparative evaluation of aesthetic, biological, and economic effectiveness of different lawn management programs. Urban Ecosyst. 2009, 12, 127–144. [Google Scholar] [CrossRef]
- Gaston, K.J.; Ávila-Jiménez, M.L.; Edmondson, J.L. Review: Managing urban ecosystems for goods and services. J. Appl. Ecol. 2013, 50, 830–840. [Google Scholar] [CrossRef]
- Graffigna, S.; González-Vaquero, R.A.; Torretta, J.P.; Marrero, H.J. Importance of urban green areas’ connectivity for the conservation of pollinators. Urban Ecosyst. 2024, 27, 417–426. [Google Scholar] [CrossRef]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Burkman, C.E.; Prajzner, S.P. The value of urban vacant land to support arthropod biodiversity and ecosystem services. Environ. Entomol. 2013, 42, 1123–1136. [Google Scholar] [CrossRef]
- Baldock, K.C.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; Stone, G.N.; et al. Where is the UKs pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. Royal Soc. B: Biol. Sci. 2015, 282, 20142849. [Google Scholar]
- Hall, D.M.; Camilo, G.R.; Tonietto, R.K.; Ollerton, J.; Ahrné, K.; Arduser, M.; Ascher, J.S.; Baldock, K.C.; Fowler, R.; Frankie, G.; et al. The city as a refuge for insect pollinators. Conserv. Biol. 2017, 31, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hennig, E.; Ghazoul, J. Pollinating Animals in the urban environment. Urban Ecosyst. 2012, 15, 149–166. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M.S. Habitat Management to Suppress Pest Populations: Progress and Prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]
- Lefebvre, V.; Fontaine, C.; Villemant, C.; Daugeron, C. Are empidine dance flies major flower visitors in alpine environments? A case study in the Alps, France. Biol. Lett. 2014, 10, 20140742. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.M.; Bianchi, F.J.J.A.; Entling, M.H.; Moonen, A.C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest. Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef]
- Wojciechowicz-Żytko, E.; Jankowska, B. Aphids and their predators occurring on some shrubs in the Botanical Garden of the Jagiellonian University in Kraków. Aphids Other Hemipter. Insects 2011, 17, 145–154. [Google Scholar]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Matteson, K.C.; Ascher, J.S.; Langellotto, G.A. Bee richness and abundance in New York City urban gardens. Ann. Entomol. Soc. Am. 2008, 101, 140–150. [Google Scholar] [CrossRef]
- Rousselin, A.; Bevacqua, D.; Sauge, M.H.; Lescourret, F.; Mody, K.; Jordan, M.O. Harnessing the aphid life cycle to reduce insecticide reliance in apple and peach orchards. A review. Agron. Sustain. Dev. 2017, 37, 38. [Google Scholar] [CrossRef]
- De França, S.M.; Breda, M.O.; Barbosa, D.R.; Araujo, A.M.; Guedes, C.A. The sublethal effects of insecticides in insects. In Biological Control of Pest and Vector Insects; Shields, V.D.C., Ed.; Intech Open: London, UK, 2017. [Google Scholar]
- Guzmán, G.; Ruiz, R.G. Side effects of insecticides on beneficial insects: A practical tool to identify organic agroecosystems. WJASS 2019, 4, 1–5. [Google Scholar]
- Trzciński, P.; Piekarska-Boniecka, H.; Rzańska-Wieczorek, M.; Kubasik, W. Changes in the fauna of zoophag-ous hoverflies (Syrphidae, Diptera) of green urban environments of Poznań in the light of multi-year observations. Nauka Przyr. Technol. 2016, 10, 3–39. [Google Scholar] [CrossRef]
- Wojciechowicz-Żytko, E. Attractiveness of some Apiaceae flowers for Syrphidae (Diptera)—Pollinators and biological control agents. In Proceedings of the ISHS Acta Horticulturae 1264: II International Symposium on Carrot and Other Apiaceae, Krakow, Poland, 19–22 September 2018. [Google Scholar] [CrossRef]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest. Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
- Passaseo, A.; Rochefort, S.; Pétremand, G.; Castella, E. Pollinators on Green Roofs: Diversity and Trait Analysis of Wild Bees (Hymenoptera: Anthophila) and Hoverflies (Diptera: Syrphidae) in an Urban Area (Geneva, Switzerland). Cities Environ. (CATE) 2021, 14, 1. [Google Scholar] [CrossRef]
- Schowalter, T.D.; Noriega, J.A.; Tscharntke, T. Insect effects on ecosystem services—Introduction. Basic. Appl. Ecol. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Kinzig, A.P. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Duffy, J.E. Why biodiversity is important to the functioning of real-world ecosystems. Front. Ecol. Environ. 2009, 7, 437–444. [Google Scholar] [CrossRef]
- Rodriguez-Gasol, N.; Alins, G.; Veronesi, E.; Wratten, S. The ecology of hoverflies as ecosystem-service providers in agricultural systems. Biol. Control 2020, 151, 104405. [Google Scholar] [CrossRef]
- Cichocka, E.; Goszczyński, W. The impact of urban pressure on species composition andnumber of Arthropoda on trees in a city on the example of Warsaw. Aphids Other Hemipter. Insects 2008, 14, 63–72. [Google Scholar]
- Trzciński, P.; Piekarska-Boniecka, H.; Rzańska, M. Hoverflies (Diptera, Syrphidae) of urban greenery as illustrated by the example of Adam Mickiewicz University Botanical Garden, Poznań. Prog. Plant Prot. 2014, 54, 326–333. [Google Scholar] [CrossRef][Green Version]
- Van Veen, M. Hoverflies of Northwest Europe: Identification Keys to the Syrphidae; KNNV. Publishing: Utrecht, The Netherlands, 2004. [Google Scholar]
- Moericke, V. Wie finden geflügelte Blattläuse ihre Wirtspflanze? In Angewandte Chemie; Mitteilungen aus der BiologischenReichsanstalt: Berlin, Germany, 1953; Volume 75, p. 90. [Google Scholar]
- Rotheray, G.E. Colour Guide to Hoverfly Larvae (Diptera, Syrphidae) in Britain and Europe. Diperists Dig. 1993, 9, 1–156. [Google Scholar]
- Soszyński, B. Syrphidae. In Checklist of Animals of Poland; Razowski, J., Ed.; Institute of Systematics and Evolution of Animals, Polish Academy of Sciences: Krakow, Poland, 1991. [Google Scholar]
- Kasprzak, K.; Niedbała, W. Biocenotic indicators used in ordering and analyzing data in quantitative research. In Methods Used in Soil Zoology; Górny, M., Grum, L., Eds.; Scientific PWN: Warsaw, Poland, 1981. [Google Scholar]
- Szujecki, A. Ecology of Forest Insects; PWN: Warszawa, Poland, 1980. [Google Scholar]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.-S. A new statistical approach for assessing 357 similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–149. [Google Scholar] [CrossRef]
- Verboven, H.; Uyttenbroeck, R.; Brys, R.; Hermy, M. Different responses of bees and hoverflies to land use in an urban–rural gradient show the importance of the nature on the rural land use. Landsc. Urban Plan. 2014, 126, 31–41. [Google Scholar] [CrossRef]
- Speight, M.C.D. Species accounts of European Syrphidae (Diptera), Glasgow 2011. Syrph Net Database Eur. Syrphidae 2011, 65, 285. [Google Scholar]
- Jacobs, J.; Beenaerts, N.; Artois, T. Green roofs and pollinators, useful green spots for some wild bee species (Hymenoptera: Anthophila), but not so much for hoverflies (Diptera: Syrphidae). Sci. Rep. 2023, 13, 1449. [Google Scholar] [CrossRef]
- Biesmeijer, J.; Roberts, S.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.; Potts, S.; Kleukers, R.; Thomas, C.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–353. [Google Scholar] [CrossRef]
- Kadas, G. Green Roofs and Biodiversity: Can Green Roofs Provide Habitat for Invertebrates in an Urban Environment? Lap Lambert Academic Publishing: Saarbrücken, Germany, 2010; Volume 312. [Google Scholar]
- Adams, T.H.L.; Chambers, R.J.; Dixon, A.F.G. Quantification of the impact of the hoverfly Metasyrphus corollae on the cereal aphid Sitobion avenae in winter wheat: Laboratory rates of kill. Entomol. Exp. Appl. 1987, 43, 153–157. [Google Scholar] [CrossRef]
- Natskova, V. The effect of basic ecological factors on the feeding capacities of some predators of aphids during their larval period. Ecol. Bulgaria 1985, 15, 35–42. [Google Scholar]
- Rotheray, G.E.; Martinat, P. Searching behaviour in relation to starvation of Syrphus ribesii. Entomol. Exp. Appl. 1984, 36, 17–21. [Google Scholar] [CrossRef]
- Tenhumberg, B. Estimating Predatory Efficiency of Episyrphus balteatus (Diptera: Syrphidae) in Cereal Fields. Environ. Entomol. 1995, 24, 687–691. [Google Scholar] [CrossRef]
- Wahbi, A.A. Untersuchungen Tiber den Einflu, 8 der Temperatur und der relativen Fuftfeuchtigkeit auf das Fra, Bvermogen von Syrphidenlarven (Diptera, Syrphidae). Ph.D. Thesis, University of Gottingen, Gottingen, Germany, 1967. [Google Scholar]
- Cornelius, M.; Barlow, C.A. Effect of aphid consumption by larvae on development and reproductive efficiency of a flower fly, Syrphus corollae (Diptera: Syrphidae). Can. Entomol. 1980, 112, 989–992. [Google Scholar] [CrossRef]
- Makhmoor, H.D.; Verma, A.K. Bionomics of major aphidophagous syrphids occurring in mid-hill regions of Himachal Pradesh. J. Bio Cont. 1987, 1, 23–31. [Google Scholar]
- Sharma, K.C.; Bhalla, O.P. Predatory potential of syrphid species on different aphids of cruciferous crops in the mid hill regions of Himachal Pradesh. Ind. J. Pl. Prot. 1991, 19, 73–75. [Google Scholar]
- Kumar, A.; Kapoor, V.C.; Mahal, M.S. Feeding behavior and efficacy of three aphidophagous syrphids. J. Ins. Sci. 1996, 9, 15–18. [Google Scholar]
- Agarwala, B.K.; Bhaumik, A.K.; Gilbert, F.S. Relative development and voracity of six species of aphidophagous syrphids in cruciferous crops. Proc. Ind. Acad. Sci. (Anim. Sci.) 1989, 98, 267–274. [Google Scholar] [CrossRef]
- Wojciechowicz-Żytko, E. The effectiveness of aphidophagous syrphid larvae (Diptera, Syrphidae) in the control of Aphis fabae Scop. (Homoptera, Aphidodea) on broad bean. J. Plant Prot. Res. 2000, 40, 152–157. [Google Scholar]
- Jiang, S.; Li, H.; Wu, K. Predation and Control Effect of Eupeodes corollae Fabricius (Diptera: Syrphidae) on Leguminous Plant Aphids. Agronomy 2023, 13, 1739. [Google Scholar] [CrossRef]
- Leir, V.; Barlow, C.A. effects of starvation and age on foraging efficiency and speed of consumption by larvae of a flower fly, Metasyrphus corollae (syrphidae). Can. Entomol. 1982, 114, 897–900. [Google Scholar] [CrossRef]
- Barahona-Segovia, R.M.; Chinga, J.; Durán-Sanzana, V.; Alfaro, E.; Murúa, M.; Pañinao-Monsalvéz, L. Syrphids in the City: A 10-Year Citizen Science Program Sheds Light on How the Greenness and Quality of Green Spaces Impact Flower Flies. J. Appl. Entom. 2025, 2025, 1–19. [Google Scholar] [CrossRef]
- Sharmin, M.; Tjoelker, M.G.; Rodriguez, M.E.; Katlav, A.; Gilpin, A.M.; Rymer, P.D.; Power, S.A. Urban greening with shrubs can supercharge invertebrate abundance and diversity. Sci. Rep. 2024, 14, 8735. [Google Scholar] [CrossRef] [PubMed]
- Bańkowska, R. Fly communities of the family Syrphidae in natural and anthropogenic habitats of Poland. Memorab. Zool. 1980, 33, 93. [Google Scholar]
- Persson, A.S.; Ekroosa, J.; Olsson, P.; Smith, H.G. Wild bees and hoverflies respond differently to urbanisation, human population density and urban form. Landsc. Urban Plan. 2020, 204, 103901. [Google Scholar] [CrossRef]
- Moquet, L.; Laurent, E.; Bacchetta, R.; Jacquemart, A.-L. Conservation of hoverflies (Diptera, Syrphidae) requires complementary resources at the landscape and local scales. Insect. Conserv. Divers. 2018, 11, 72–87. [Google Scholar] [CrossRef]
- De Groot, M.; Simončič, P.; Verlič, A.; Vilhar, U. Hoverflies (Diptera: Syrphidae) as biodiversity indicators for assessing urban forest habitats. Acta Silvae Ligni 2022. [Google Scholar] [CrossRef]
- Rocha, E.A.; Souza, E.N.F.; Bleakley, L.A.D.; Burley, C.; Mott, J.L.; Rue-Glutting, G.; Fellowes, M.D.E. Influence of urbanisation and garden plants on the diversity and abundance of aphids and their ladybird and hoverfly predators. Eur. J. Entomol. 2018, 115, 140–149. [Google Scholar] [CrossRef]
- Aleixo, K.P.; Biral de Faria, L.; Groppo, M.; Castro, M.M.d.N.; da Silva, C.I. Spatiotemporal distribution of floral resources in a Brazilian city: Implications for the maintenance of pollinators, especially bees. Urban For. Urban Green. 2014, 13, 689–696. [Google Scholar] [CrossRef]
- Laubertie, E.A.; Wratten, S.D.; Hemptinne, J.L. The contribution of potential beneficial insectary plant species to adult hoverfly (Diptera: Syrphidae) fitness. Biol. Control 2012, 61, 1–6. [Google Scholar] [CrossRef]



| Species | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | f | No. | % | f | No. | % | f | No. | % | f | No. | % | f | No. | % | |
| Epistrophe eligans (Harr.) | 6 | 10.2 Ed | 20 | 3 | 6.3 D | 15 | 14 | 18.4 Ed | 30 | 5 | 16.7 Ed | 15 | 10 | 13.7 Ed | 20 | 38 | 13.3 Ed |
| Episyrphus balteatus (Deg.) | 24 | 40.7 Ed | 85 | 14 | 29.2 Ed | 70 | 44 | 57.9 Ed | 90 | 12 | 40.0 Ed | 50 | 36 | 49.3 Ed | 90 | 130 | 45.5 Ed |
| Eupeodes corollae (Fabr.) | 4 | 6.8 D | 10 | 4 | 8.3 D | 10 | 7 | 9.2 D | 15 | 2 | 6.7 D | 5 | 4 | 5.5 D | 15 | 21 | 7.3 D |
| Melanostoma scalare (Fabr.) | 1 | 2.1 Sd | 5 | 1 | 1.4 | 5 | 2 | 0.7 Sr | |||||||||
| Meligramma triangulifera (Zett.) | 3 | 5.1 D | 10 | 3 | 3.9 Sd | 10 | 1 | 3.3 Sd | 5 | 2 | 2.7 Sd | 5 | 9 | 3.2 Sd | |||
| Platycheirus scutatus (Meig.) | 3 | 5.1 D | 10 | 2 | 2.7 Sd | 5 | 5 | 1.7 R | |||||||||
| Scaeva pyrastri (L.) | 3 | 5.1 D | 10 | 2 | 4.2 Sd | 5 | 2 | 6.7 D | 10 | 5 | 6.8 D | 10 | 12 | 4.2 Sd | |||
| Sphaerophoria scripta (L.) | 3 | 5.1 D | 10 | 4 | 8.3 D | 15 | 2 | 2.6 Sd | 10 | 3 | 10.0 D | 10 | 5 | 6.8 D | 15 | 17 | 5.9 D |
| Syrphus ribesii (L.) | 5 | 8.5 D | 15 | 4 | 8.3 D | 10 | 3 | 3.9 Sd | 15 | 2 | 6.7 D | 10 | 3 | 4.1 Sd | 10 | 17 | 5.9 D |
| Syrphus vitripennis (Meig.) | 8 | 13.6 Ed | 25 | 16 | 33.3 Ed | 50 | 3 | 3.9 Sd | 10 | 3 | 10.0 D | 10 | 5 | 6.8 D | 15 | 35 | 12.2 Ed |
| No. of specimens | 59 | 100 | 48 | 100 | 76 | 100 | 30 | 100 | 73 | 100 | 286 | 100 | |||||
| No. of species | 9 | 8 | 7 | 8 | 10 |
| Habitat | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 |
|---|---|---|---|---|---|
| specimens | |||||
| Aphid colonies | 5.9 ± 1.2 ab | 4.8 ± 0.9 ab | 7.6 ± 1.4 b | 3.0 ± 0.8 a | 7.3 ± 1.9 b |
| Traps/s.n. | 11.5 ± 2.0 a | 11.3 ± 2.2 a | 16.4 ± 2.3 ab | 13.4 ± 2.8 a | 22.5 ± 4.1 b |
| species | |||||
| Aphid colonies | 4.8 ± 1.0 a | 4.8 ± 1.1 a | 4.8 ± 1.0 a | 4.2 ± 1.3 a | 5.4 ± 1.5 a |
| Traps/s.n. | 6.8 ± 1.4 a | 6.4 ± 1.5 a | 8.4 ± 1.3 a | 5.8 ± 1.7 a | 10.0 ± 2.0 a |
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trap/ s.n. | a.c. | Trap/ s.n | a.c. | Trap/ s.n | a.c. | Trap/ s.n | a.c. | Trap/ s.n | a.c. | |
| No. of species | 11 | 9 | 11 | 8 | 13 | 7 | 12 | 8 | 19 | 10 |
| Species richness | 4.9 | 4.4 | 4.8 | 4.1 | 5.5 | 3.2 | 5.2 | 4.7 | 7.5 | 4.7 |
| E. balteatus | S. scripta | S. vitripennis | |
|---|---|---|---|
| A. fabae | 430.2 ± 6.2 b | 243.0 ± 19.4 a | 498.6 ± 12.3 c |
| M. cerasi | 441.2 ± 10.8 b | 272.4 ± 19.5 a | 468.0 ± 12.2 b |
| Gatunek | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | f | No. | % | f | No. | % | f | No. | % | f | No. | % | f | No. | % | |
| Baccha elongate (Fabr.) | 4 | 3.4 Sd | 10 | 7 | 5.2 D | 15 | 16 | 7.1 D | 25 | 27 | 3.6 Sd | ||||||
| Chrysotoxum cautum (Harr.) | 1 | 0.4 Sr | 5 | 1 | 0.1 Sr | ||||||||||||
| Chrysotoxum vernale (Loew) | 2 | 1.7 R | 10 | 2 | 0.3 Sr | ||||||||||||
| Dasysyrphus tricinctus (F.) | 1 | 0.9 Sr | 5 | 1 | 0.6 Sr | 5 | 2 | 0.9 Sr | 5 | 4 | 0.5 Sr | ||||||
| Didea fasciata (Macq.) | 3 | 2.7 Sd | 10 | 3 | 0.4 Sr | ||||||||||||
| Episyrphus balteatus (Deg.) | 24 | 20.9 Ed | 50 | 62 | 54.9 Ed | 90 | 96 | 58.5 Ed | 85 | 63 | 47.0 Ed | 80 | 59 | 26.2 Ed | 75 | 304 | 40.5 Ed |
| Epistrophe eligans (Harr.) | 3 | 1.8 Sr | 10 | 1 | 0.7 Sr | 5 | 10 | 4.4 Sd | 20 | 14 | 1.9 R | ||||||
| Eupeodes corollae (F.) | 3 | 2.6 Sd | 10 | 1 | 0.9 Sr | 5 | 2 | 1.2 R | 5 | 2 | 1.5 R | 10 | 15 | 6.7 D | 40 | 23 | 3.1 Sd |
| Eupeodes latifasciatus (Macq.) | 5 | 4.4 Sd | 15 | 2 | 0.9 Sr | 5 | 7 | 0.9 Sr | |||||||||
| Lapposyrphus (Eupeodes) lapponicus (Zett.) | 1 | 0.4 Sr | 5 | 1 | 0.1 Sr | ||||||||||||
| Melanostoma mellinum (L.) | 4 | 3.5 Sd | 10 | 6 | 3.7 Sd | 20 | 3 | 2.2 Sd | 10 | 4 | 1.8 R | 10 | 17 | 2.3 Sd | |||
| Melanostoma scalare (F.) | 8 | 7.0 D | 25 | 2 | 1.8 R | 5 | 2 | 1.2 Sr | 10 | 20 | 14.9 Ed | 30 | 7 | 3.1 Sd | 15 | 39 | 5.2 D |
| Meligramma triangulifera (Zett.) | 2 | 1.7 R | 5 | 4 | 2.4 Sd | 10 | 4 | 1.8 R | 10 | 10 | 1.3 R | ||||||
| Meliscaeva cinctella (Zett.) | 1 | 0.4 Sr | 1 | 0.1 Sr | |||||||||||||
| Platycheirus albimanus (F.) | 3 | 2.6 Sd | 15 | 4 | 2.4 Sd | 15 | 12 | 9.0 D | 25 | 3 | 1.3 R | 10 | 22 | 2.9 Sd | |||
| Platycheirus scutatus (Meig.) | 4 | 3.5 Sd | 10 | 6 | 5.3 D | 15 | 10 | 6.1 D | 30 | 4 | 3.0 Sd | 2 | 0.9 Sr | 5 | 26 | 3.5 Sd | |
| Sphaerophoria scripta (L.) | 59 | 51.3 Ed | 75 | 16 | 14.2 Ed | 50 | 19 | 11.6 Ed | 55 | 9 | 6.7 D | 25 | 21 | 9.3 D | 65 | 124 | 16.5 Ed |
| Syrphus ribesii (L.) | 4 | 3.5 Sd | 10 | 3 | 2.7 Sd | 10 | 3 | 2.2 Sd | 15 | 25 | 11.1 Ed | 50 | 35 | 4.7 Sd | |||
| Syrphus torvus (O.-S.) | 1 | 0.6 Sr | 5 | 13 | 5.8 D | 25 | 14 | 1.9 R | |||||||||
| Syrphus vitripennis (Meig.) | 5 | 4.3 Sd | 10 | 10 | 8.8 D | 30 | 12 | 7.3 D | 40 | 9 | 6.7 D | 25 | 24 | 10.7 Ed | 75 | 60 | 8.0 D |
| Xanthogramma pedissequum (Harris) | 1 | 0.9 Sr | 5 | 1 | 0.7 Sr | 5 | 15 | 6.7 D | 25 | 17 | 2.3 Sd | ||||||
| No. of specimens | 115 | 100 | 113 | 100 | 164 | 100 | 134 | 100 | 225 | 100 | 751 | 100 | |||||
| No. of species | 11 | 11 | 13 | 12 | 19 | ||||||||||||
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | |
|---|---|---|---|---|---|
| Site 1 | x | 0.5 | 0.6 | 0.4 | 0.5 |
| Site 2 | 0.8 | x | 0.7 | 0.6 | 0.5 |
| Site 3 | 0.7 | 0.7 | x | 0.7 | 0.7 |
| Site 4 | 0.9 | 0.9 | 0.8 | x | 0.6 |
| Site 5 | 0.9 | 0.7 | 0.8 | 0.8 | x |
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a.c. | Traps | a.c. | Traps | a.c. | Traps | a.c. | Traps | a.c. | Traps | |
| Mean ± SD (N = 5) | 5.9 ± 1.2 | 11.5 ± 2.0 | 4.8 ± 0.9 | 11.3 ± 2.2 | 7.6 ± 1.4 | 16.4 ± 2.3 | 3.0 ± 0.8 | 13.4 ± 2.8 | 7.3 ± 1.9 | 22.5 ± 4.1 |
| r2 | 0.247241 | 0.295475 | 0.379607 | 0.423426 | 0.405910 | |||||
| p | 0.025708 | 0.013241 | 0.003820 | 0.001891 | 0.002518 | |||||
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a.c. | Traps | a.c. | Traps | a.c. | Traps | a.c. | Traps | a.c. | Traps | |
| Mean ± SD (N = 5) | 4.8 ± 3.1 | 4.8 ± 2.9 | 2.8 ± 1.4 | 12.4 ± 5.8 | 8.8 ± 6.5 | 19.2 ± 7.7 | 2.4 ± 2.1 | 12.6 ± 9.0 | 7.2 ± 6.05 | 11.8 ± 5.5 |
| r2 | 0.745411 | 0.853970 | 0.964677 | 0.826913 | 0.828358 | |||||
| p | 0.059366 | 0.024811 | 0.002848 | 0.032315 | 0.031896 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowicz-Żytko, E.; Dobińska-Graczyk, M. Urban Green Space as a Reservoir of Predatory Syrphids (Diptera, Syrphidae) for Aphid Control in Cities. Agronomy 2025, 15, 953. https://doi.org/10.3390/agronomy15040953
Wojciechowicz-Żytko E, Dobińska-Graczyk M. Urban Green Space as a Reservoir of Predatory Syrphids (Diptera, Syrphidae) for Aphid Control in Cities. Agronomy. 2025; 15(4):953. https://doi.org/10.3390/agronomy15040953
Chicago/Turabian StyleWojciechowicz-Żytko, Elżbieta, and Maja Dobińska-Graczyk. 2025. "Urban Green Space as a Reservoir of Predatory Syrphids (Diptera, Syrphidae) for Aphid Control in Cities" Agronomy 15, no. 4: 953. https://doi.org/10.3390/agronomy15040953
APA StyleWojciechowicz-Żytko, E., & Dobińska-Graczyk, M. (2025). Urban Green Space as a Reservoir of Predatory Syrphids (Diptera, Syrphidae) for Aphid Control in Cities. Agronomy, 15(4), 953. https://doi.org/10.3390/agronomy15040953






