Abilities of the Newly Introduced Apple Cultivars (Malus × domestica Borkh.) ‘Eden’ and ‘Fryd’ to Promote Pollen Tube Growth and Fruit Set with Different Combinations of Pollinations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
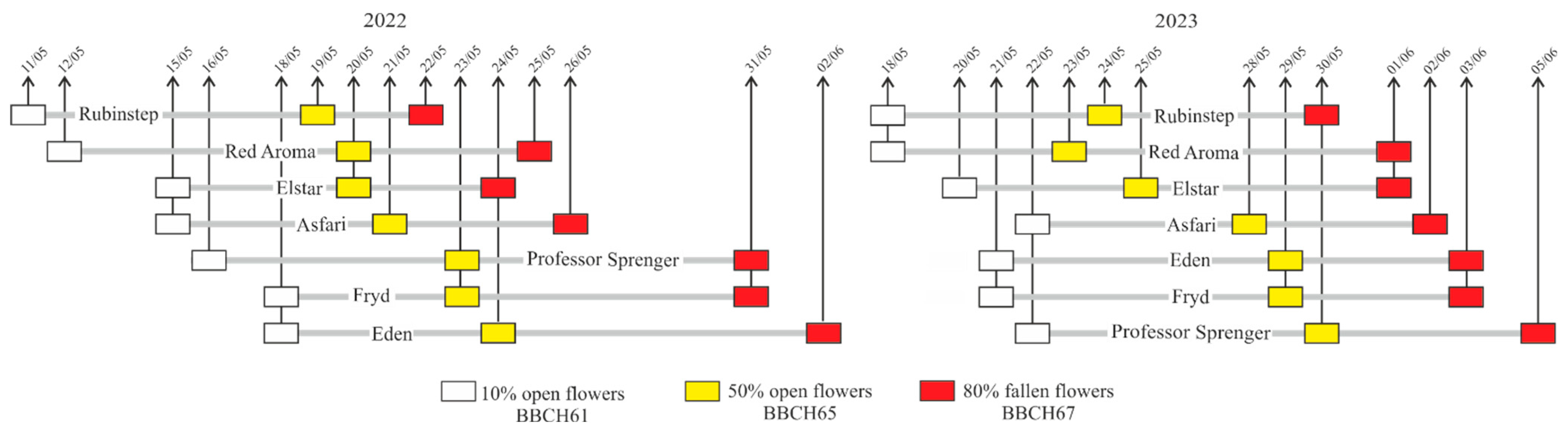
2.2. Climate Conditions and Flowering Time
2.3. Pollen Germination In Vitro
2.4. Pollination Procedure
2.5. Pollen Tube Growth In Vivo
2.6. Fruit Set
2.7. Statistical Analysis
3. Results
3.1. Time of Flowering and Climatic Conditions
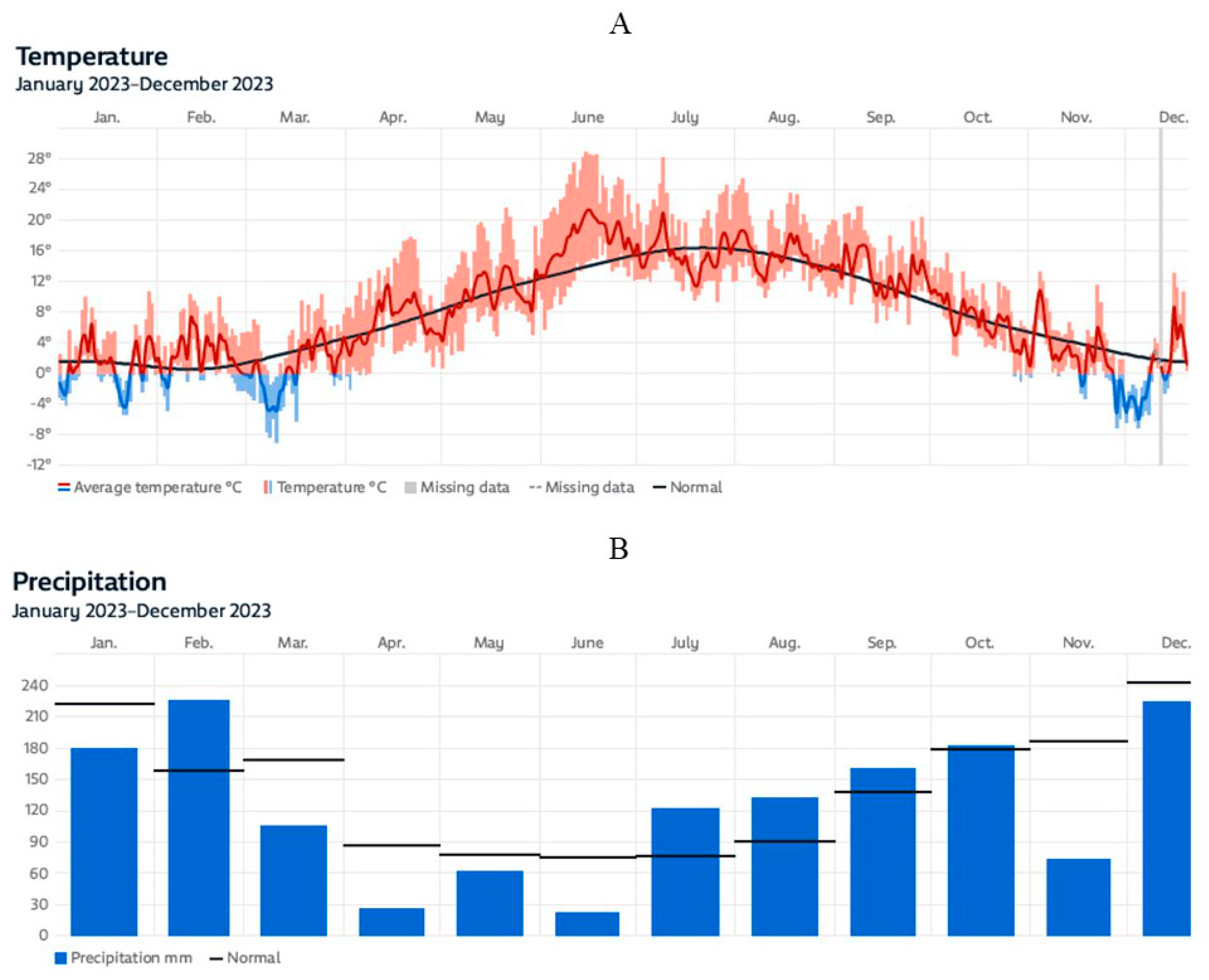
3.2. Air Temperature and Precipitation
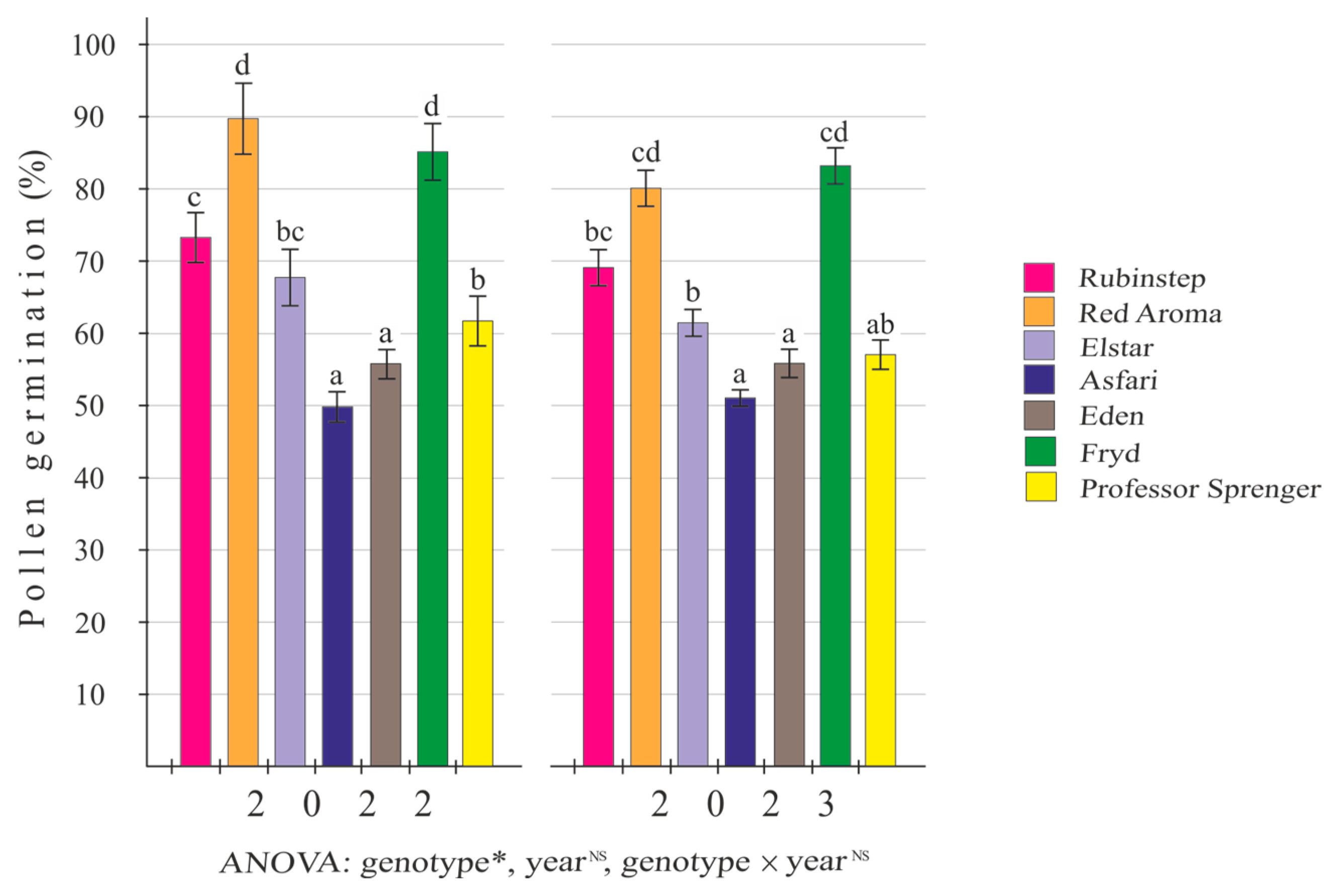
3.3. In Vitro Pollen Germination
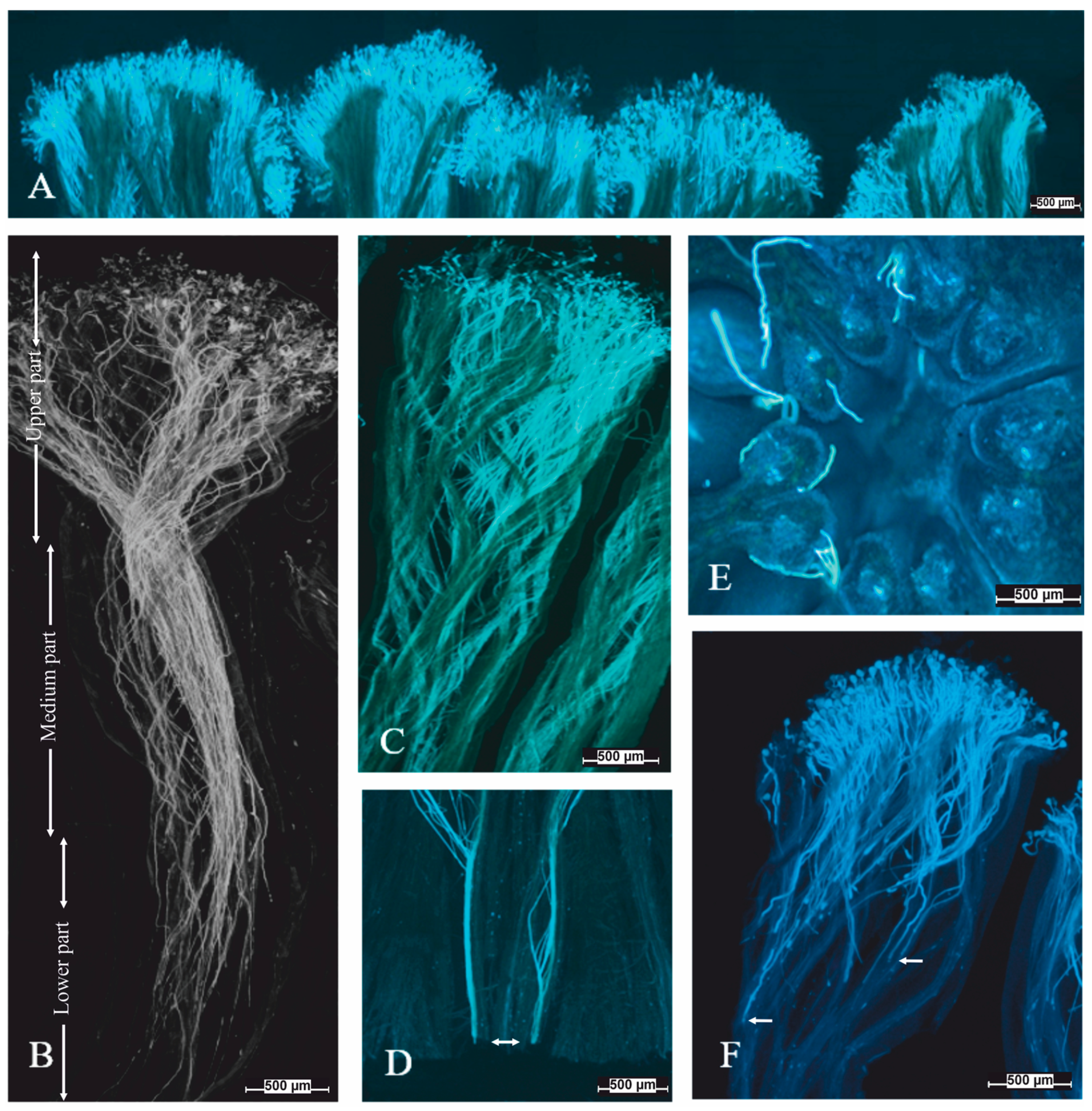
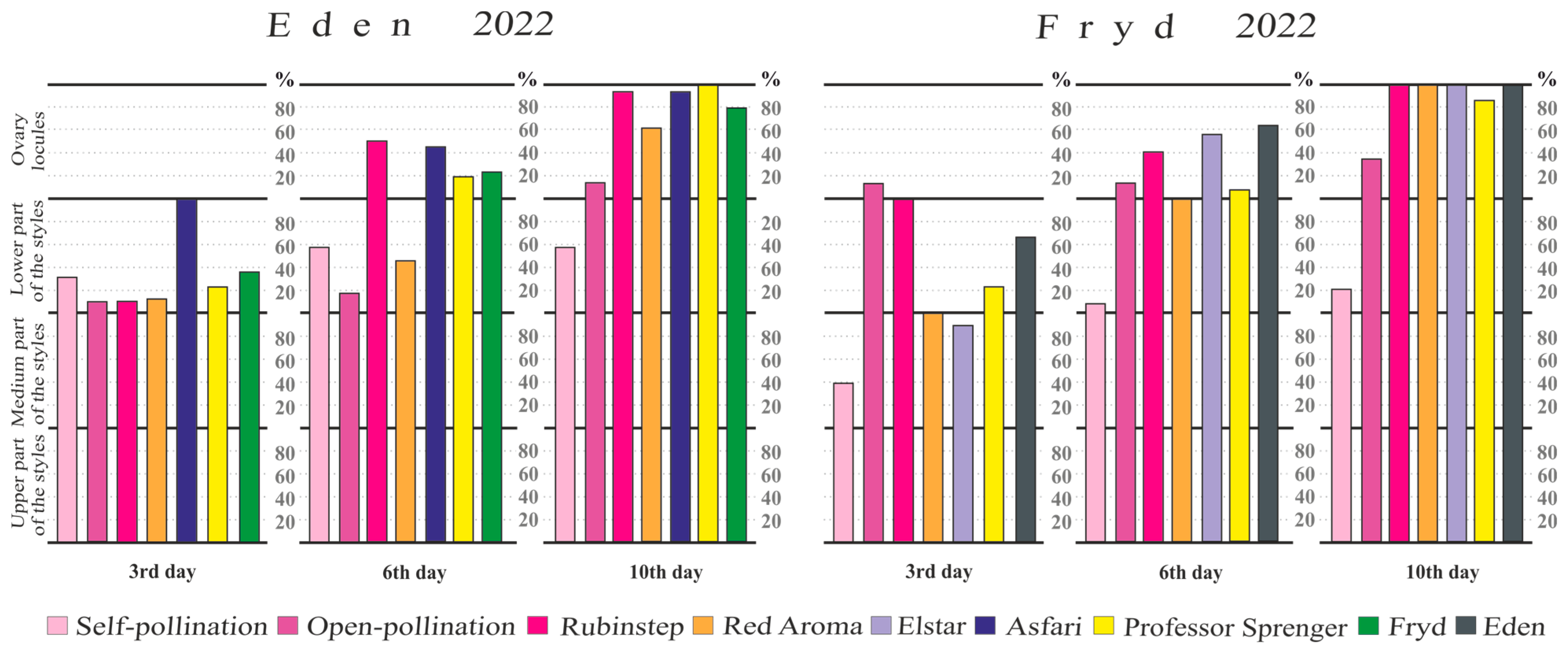
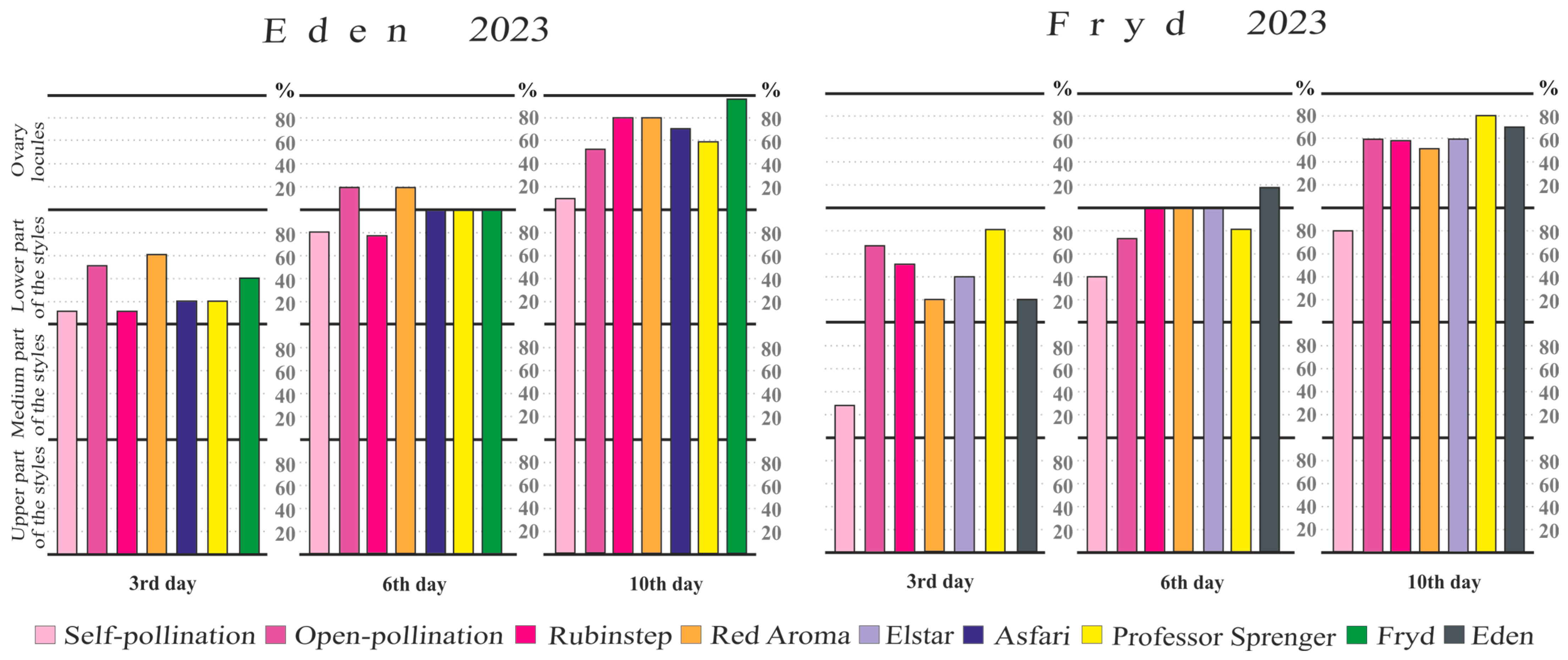
3.4. Pollen Tube Growth in the Pistil
3.5. Fruit Set
4. Discussion
4.1. In Vitro Pollen Germination
4.2. Pollen Tube Growth In Vivo
4.3. Fertilization and Fruit Set
4.4. Overlapping in Flowering Time
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Norway. 2023. Available online: https://www.ssb.no/en/statbank/table/10507/tableViewLayout1/ (accessed on 12 December 2024).
- Natić, M.; Dabić Zagorac, D.; Jakanovski, M.; Smailagić, A.; Čolić, S.; Meland, M.; Fotirić Akšić, M. Fruit Quality Attributes of Organically Grown Norwegian Apples Are Affected by Cultivar and Location. Plants 2024, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Ličina, V.; Krogstad, T.; Fotirić Akšić, M.; Meland, M. Apple Growing in Norway-Ecologic Factors, Current Fertilization Practices and Fruit Quality: A Case Study. Horticulturae 2024, 10, 233. [Google Scholar] [CrossRef]
- Vujadinović Mandić, M.; Vuković Vimić, A.; Fotirić Akšić, M.; Meland, M. Climate Potential for Apple Growing in Norway—Part 2: Assessment of Suitability of Heat Conditions under Future Climate Change. Atmosphere 2023, 14, 937. [Google Scholar] [CrossRef]
- Poldervaart, G. Fresh Forward introduces Wursixo and Wuranda. European. Fruit Mag. 2024, 10, 14–15. [Google Scholar]
- Sassa, H.; Mase, N.; Hirano, H.; Ikehashi, H. Identification of self-incompatibility-related glycoproteins in styles of apple (Malus × domestica). Theor. Appl. Genet. 1994, 89, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, F.; Davenport, T.L. Apple pollination: A review. Sci. Hortic. 2013, 162, 188–203. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Good-Avila, S.V.; Montaña, C.; Domínguez, C.A.; Eguiarte, L.E. Effect of variation in self-incompatibility on pollen limitation and inbreeding depression in Flourensia cernua (Asteraceae) scrubs of contrasting density. Ann. Bot. 2009, 103, 1077–1089. [Google Scholar] [CrossRef]
- Adachi, Y.; Komori, S.; Hoshikawa, Y.; Tanaka, N.; Abe, K.; Bessho, H.; Watanabe, M.; Suzuki, A. Characteristics of fruiting and pollen tube growth of apple autotetraploid cultivars showing self-compatibility. J. Jpn. Soc. Hortic. Sci. 2009, 78, 402–409. [Google Scholar] [CrossRef][Green Version]
- Liu, D.-D.; Dong, Q.-L.; Sun, C.; Wang, Q.-L.; You, C.-X.; Yao, Y.-X.; Hao, Y.-J. Functional characterization of an apple apomixis-related MhFIE gene in reproduction development. Plant Sci. 2012, 185, 105–111. [Google Scholar] [CrossRef]
- Liu, D.-D.; Fang, M.-J.; Dong, Q.-L.; Hu, D.-G.; Zhou, L.-J.; Sha, G.-L.; Jiang, Z.-W.; Liu, Z.; Hao, Y.-J. Unreduced embryo sacs escape fertilization via a ‘‘female-late-on-date’’ strategy to produce clonal seeds in apomictic crabapples. Sci. Hortic. 2014, 167, 76–83. [Google Scholar] [CrossRef]
- Nunes-Silva, P.; Costa, L.; Campbell, A.J. Radiofrequency identification (RFID) reveals long-distance flight and homing abilities of the stingless bee Melipona fasciculata. Apidologie 2020, 51, 240–253. [Google Scholar] [CrossRef]
- Imani, A.; Barzegar, K.; Piripireivatlou, S.; Hassan Masomi, S.H. Storage of apple pollen and in vitro germination. Afr. J. Agric. Res. 2011, 6, 624–629. [Google Scholar]
- Roeder, S.; Serra, S.; Musacchi, S. Novel Metrics to Characterize In Vitro Pollen Tube Growth Performance of Apple Cultivars. Plants 2021, 16, 1460. [Google Scholar] [CrossRef]
- DeLong, C.N.; Keith, S.; Yoder, S.K.; Combs, L.; Peck, M.G. Apple Pollen Tube Growth Rates Are Regulated by Parentage and Environment. J. Amer. Soc. Hort. Sci. 2016, 141, 548–554. [Google Scholar] [CrossRef]
- Yang, X.; Ma, H.; Liu, G.; Lü, D.; Qin, S.; Du, G. Anatomical Study on the Multi-Ovule Development and Abortion of Hanfu Apple. J. Integr. Agric. 2014, 13, 770–777. [Google Scholar] [CrossRef]
- Wei, H.; Wang, B.; Xu, Y.; Fan, W.; Zhang, M.; Huang, F.; Shi, C.; Li, T.; Wang, S.; Wang, S. The Mechanism of Ovule Abortion in Self-Pollinated ‘Hanfu’ Apple Fruits and Related Gene Screening. Plants 2024, 13, 996. [Google Scholar] [CrossRef]
- Jahed, R.K.; Hirst, M.P. Pollen Tube Growth and Fruit Set in Apple. HortScience 2017, 52, 1054–1059. [Google Scholar] [CrossRef]
- Kang, I.K.; Lee, G.J.; Kim, M.J.; Kwon, S.I.; Paek, P.Y.; Choi, D.G. Selection of crab apples as pollinizers for major apple cultivars in apple orchard. Kor. J. Hort. Sci. Technol. 2002, 20, 330–334. [Google Scholar]
- Kwon, S.-I.; Yoo, J.; Lee, J.; Moon, Y.-S.; Choi, C.; Jung, H.Y.; Lee, D.H.; Kim, C.K.; Kang, I.-K. Evaluation of crab apples for apple production in high-density apple orchards. J. Plant Biotechnol. 2015, 42, 271–276. [Google Scholar] [CrossRef]
- Aizen, A.M.; Harder, D.L. Expanding the limits of the pollen-limitation concept: Effect of pollen quantity and quality. Ecology 2007, 88, 271–281. [Google Scholar] [CrossRef]
- Knight, M.T.; Steets, A.J.; Vamosi, C.J.; Mazer, J.S.; Burd, M.; Diane, R.; Campbell, R.D.; Dudash, R.M.; Johnston, O.M.; Mitchell, J.R.; et al. Pollen Limitation of Plant Reproduction: Pattern and Process. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 467–497. [Google Scholar] [CrossRef]
- Bartomeus, I.; Potts, S.G.; Steffan-Dewenter, I.; Vaissière, B.E.; Woyciechowski, M.; Krewenka, K.M.; Tscheulin, T.; Roberts, S.P.M.; Szentgyörgyi, H.Ž.; Westphal, C.; et al. Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. PeerJ. 2014, 2, e328. [Google Scholar] [CrossRef]
- Samnegård, U.; Hambäck, P.A.; Smith, H.G. Pollination treatment affects fruit set and modifies marketable and storable fruit quality of commercial apples. R. Soc. Open Sci. 2019, 6, 190326. [Google Scholar] [CrossRef] [PubMed]
- Garratt MP, D.; Breeze, T.D.; Boreux, V.; Fountain, M.T.; McKerchar, M.; Webber, S.M.; Coston, D.J.; Jenner, N.; Dean, R.; Westbury, D.B.; et al. Apple pollination: Demand depends on variety and supply depends on pollinator identity. PLoS ONE 2016, 11, e0153889. [Google Scholar]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar]
- Radičević, S.; Cerović, R.; Đorđević, M. Ovule senescence and unusual pollen tube growth in the ovary of sweet cherry as affected by pistilar genotype and temperature. Span. J. Agri. Res. 2018, 16, e0704. [Google Scholar] [CrossRef]
- Cerović, R.; Fotirić Akšić, M.; Meland, M. Success Rate of Individual Pollinizers for the Pear Cultivars “Ingeborg” and “Celina” in a Nordic Climate. Agronomy 2020, 10, 970. [Google Scholar] [CrossRef]
- Cerović, R.; Fotirić Akšić, M.; Đorđević, M.; Meland, M. The effects of pollinizers on pollen tube growth and fruit set of European plum (Prunus domestica L.) in a Nordic climate. Sci. Hortic. 2021, 288, 110390. [Google Scholar]
- Naor, A.; Naschitz, S.; Peres, M.; Gal, Y. Responses of apple fruit size to tree water status and crop load. Tree Physiol. 2008, 28, 1255–1261. [Google Scholar]
- Meland, M.; Kaiser, C. Ethephon as a blossom and fruitlet thinner affects crop load, fruit weight, fruit quality, and return bloom of ‘Summerred’ apple (Malus domestica) Borkh. HortScience 2011, 46, 432–438. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Martin Heß, M.; Peter, D.; Lancashire, D.P.; Schnock, U.; Stauß, R.; et al. The BBCH system to coding the phenological growth stages of plants—History and publications. J. Für Kult. 2009, 61, 41–52. [Google Scholar]
- Galleta, G.J. Pollen and seed management. In Methods in Fruit Breeding; Moore, J.N., Janick, J., Eds.; Purdue University Press: West Lafayette, IN, USA, 1983; pp. 23–47. [Google Scholar]
- Preil, W. Observing of pollen tube in pistil and ovarian tissue by means of fluorescence microscopy. Zeiss Inf. 1970, 75, 24–25. [Google Scholar]
- Kho, Y.O.; Baër, J. Fluorescence microscopy in botanical research. Zeiss Inf. 1971, 76, 54–57. [Google Scholar]
- NIBIO LandbruksMeteorologisk Tjeneste. 2025. Available online: https://lmt.nibio.no/stationinfo/54/ (accessed on 15 December 2024).
- Gasi, G.; Pojskić, N.; Kalamujić Stroil, B.; Frøynes, O.; Fotirić Akšić, M.; Meland, M. Determining Pollinizer Success Rates among Several Apple (Malus domestica L.) Cultivars Using Microsatellite Markers. Agronomy 2023, 13, 1106. [Google Scholar] [CrossRef]
- Ćalić, D.; Milojević, J.; Belić, M.; Miletić, R.; Zdravković-Korać, S. Impact of Storage Temperature on Pollen Viability and Germinability of Four Serbian Autochthon Apple Cultivars. Front. Plant Sci. 2021, 12, 709231. [Google Scholar]
- Albuquerque Junior, C.L.; Denardi, F.; Dantas, A.C.; Nodari, R.O. Número de anteras por flor, grãos de pólen por antera ecapacidade germinativa do pólen de diferentes cultivares de macieiras. Rev. Bras. Frutic. 2010, 32, 1255–1260. [Google Scholar] [CrossRef]
- Javid, R.; Rather, G. Functional pollen ability of different crab apples used as pollinizers for apple. J. Pharmacogn. Phytochem. 2019, 8, 617–620. [Google Scholar]
- Hebbar, K.B.; Rose, H.M.; Nair, A.R.; Kannan, S.; Niral, V.; Arivalagan, M.; Gupta, A.; Samsudeen, K.; Chandran, K.P.; Chowdappa, P.; et al. Differences in in vitro pollen germination and pollen tube growth of coconut (Cocos nucifera L.) cultivars in response to high-temperature stress. Environ. Exp. Bot. 2018, 153, 35–44. [Google Scholar]
- Vuletin Selak, G.; Perica, S.; Goreta Ban, S.; Poljak, M. The effect of temperature and genotype on pollen performance in olive (Olea europaea L.). Sci. Hortic. 2013, 156, 38–46. [Google Scholar]
- Sharafi, Y.; Talebi, S.F.; Talei, D. Effects of heavy metals on male gametes of sweet cherry. Caryologia 2017, 70, 166–173. [Google Scholar] [CrossRef]
- Sharafi, Y. Study of pollen germination in pome fruit tree of Rosaceae family in vitro. Afr. J. Plant Sci. 2011, 5, 483–488. [Google Scholar]
- Heslop-Harrison, Y. Control gates and micro-ecology: The pollen–stigma interaction in perspective. Ann. Bot. 2000, 85, 5–13. [Google Scholar]
- Losada, J.M.; Herrero, M. Flower strategy and stigma performance in the apple inflorescence. Sci. Hortic. 2013, 150, 283–289. [Google Scholar]
- McClure, B.A.; Franklin-Tong, V. Gametophytic self-incompatibility: Understanding the cellular mechanisms involved in ‘self’ pollen tube inhibition. Planta 2006, 224, 233–245. [Google Scholar] [CrossRef]
- Pusey, P.L.; Rudell, D.R.; Curry, E.A.; Mattheis, J.P. Characterization of Stigma Exudates in Aqueous Extracts from Apple and Pear Flowers. Hortscience 2008, 43, 1471–1478. [Google Scholar] [CrossRef]
- Hiscock, S.J.; Allen, A.M. Diverse cell signaling pathways regulate pollen-stigma interaction: The search for consensus. New Phytol. 2008, 179, 286–317. [Google Scholar] [CrossRef]
- Zheng, R.H.; Su, S.H.; Xiao, H.; Tian, H.Q. Calcium: A Critical Factor in Pollen Germination and Tube Elongation. Int. J. Mol. Sci. 2019, 20, 420. [Google Scholar] [CrossRef]
- Zhao, W.; Hou, Q.; Qi, Y.; Wu, S.; Wan, X. Structural and molecular basis of pollen germination. Plant Physiol. Bioch. 2023, 203, 108042. [Google Scholar]
- Williams, H.J. Pollen Tube Growth Rates and the Diversification of Flowering Plant Reproductive Cycles. Int. J. Sci. 2012, 173, 649–661. [Google Scholar]
- Herrero, M. Ovary signals for directional pollen tube growth. Sex. Plant Reprod. 2001, 14, 3–7. [Google Scholar]
- Bayer, I.; Stösser, R. Veränderungen der Narbenstruktur während der Blüte bei Pflaumen und Zwetschen (Prunus × domestica L.). Angewandte Botanik 2022, 76, 99–106. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Franklin-Tong, N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol. Plant 2013, 6, 1018–1036. [Google Scholar]
- Kron, P.; Husband, C.B. The effects of pollen diversity on plant reproduction: Insights from apple. Sex. Plant Reprod. 2006, 19, 125–131. [Google Scholar]
- Mert, C. Temperature responses of pollen germination in walnut (Juglans regia L.). J. Biol. Environ. Sci. 2009, 3, 37–43. [Google Scholar]
- Pirlak, L. The effects of temperature on pollen germination and pol-len tube growth of apricot and sweet cherry. Gartenbauwissenschaft 2002, 67, 61–64. [Google Scholar]
- Sorkheh, K.; Shiran, B.; Rouhi, V.; Khodambashi, M.; Wolukau, J.N.; Ercisli, S. Response of in vitro pollen germination and pollen tube growth of almond (Prunus dulcis Mill.) to temperature, polyamines and polyamine synthesis inhibitor. Biochem. Syst. Ecol. 2011, 39, 749–757. [Google Scholar]
- Deng, L.; Wang, T.; Hu, J.; Yang, X.; Yao, Y.; Jin, Z.; Huang, Z.; Sun, G.; Xiong, B.; Liao, L.; et al. Effects of Pollen Sources on Fruit Set and Fruit Characteristics of ‘Fengtangli’ Plum (Prunus salicina Lindl.) Based on Microscopic and Transcriptomic Analysis. Int. J. Mol. Sci. 2022, 23, 12959. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Cerović, R.; Hjeltnes, S.H.; Meland, M. The Effective Pollination Period of European Plum (Prunus domestica L.) Cultivars in Western Norway. Horticulturae 2022, 8, 55. [Google Scholar] [CrossRef]
- Campbell, T.; Kalcsits, L. Strategies to overcome biennial bearing in apple—A review. Eur. J. Agron. 2024, 158, 127213. [Google Scholar]
- Fioravanço, J.C.; Costa Czermainski, A.B. Biennial bearing in apple cultivars . Rev. Ceres, Viçosa 2018, 65, 144–149. [Google Scholar]
- Cerović, R.; Ružić, Đ.; Mićić, N. 2000. Viability of plum ovules at different temperatures. Ann. Appl. Biol. 2000, 137, 53–59. [Google Scholar] [CrossRef]
- Crespo-Martínez, S.; Oneka, O.; Laquidáin, M.J.; Urrestarazu, J.; Santesteban, L.G.; Miranda, C. Pollen viability, self-incompatibility, and a very singular S-allele structure between the reasons for the limited potential productivity of traditional Basque cider apple varieties. Sci. Hortic. 2023, 322, 112395. [Google Scholar] [CrossRef]
- Racsko, J.; Leite, G.B.; Petri, J.L.; Zhongfu, S.; Wang, Y.; Szabó, Z.; Soltész, M.; Nyéki, J. Fruit drop: The role of inner agents and environmental factors in the drop of flowers and fruits. Int. J. Hortic. Sci. 2007, 13, 13–23. [Google Scholar] [CrossRef]
- Costa, G.; Dal Cin, V.; Ramina, A. Physiological, molecular and practical aspects of fruit abscission. Acta Hortic. 2006, 727, 301–310. [Google Scholar]
- Baena-Gonzalez, E.; Sheen, J. Convergent energy and stress signaling. Trends Plant Sci. 2008, 13, 474–482. [Google Scholar]
- Arseneault, M.H.; Cline, J.A. A review of apple preharvest fruit drop and practices for horticultural management. Sci. Hortic. 2016, 211, 40–52. [Google Scholar] [CrossRef]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–339. [Google Scholar] [CrossRef]
- Bessho, H.; Wada, M.; Kudo, K.; Inomata, Y.; Iwanami, H.; Abe, K.; Suzuki, A. Selection of crabapple pollinizers for ‘Fuji’ and ‘Tsugaru’ apple. J. Amer. Pomol. Soc. 2009, 63, 2–13. [Google Scholar]
- Das, B.; Ahmad, N.; Srivastava, K.K.; Ranjan, P. Top working method and bloom density of pollinizers as productive determinant for spur type apple (Malus·domestica Borkh.) cultivars. Sci. Hort. 2011, 129, 642–648. [Google Scholar]
- Jackson, J.E. Flowers and fruits. In The Biology of Apples and Pears; Cambridge University Press: Cambridge, MA, USA, 2003; pp. 268–340. [Google Scholar]
- Dennis, F.J. Flowering, pollination and fruit set and development. In Apples Botany Production and Uses; Ferree, D.C., Ed.; CAB International: Cambridge, MA, USA, 2003; pp. 153–166. [Google Scholar]
- Kron, P.; Husband, B.C.; Kevan, P.G.; Belaoussoff, S. Factors Affecting Pollen Dispersal in High-density Apple Orchards. Hortscience 2001, 36, 1039–1046. [Google Scholar]
- Delaplane, K.S.; Mayer, D.F. Crop Pollination by Bees; CABI: New York, NY, USA, 2000; pp. 143–153. [Google Scholar]
- Delgado, A.; Quinet, M.; Dapena, E. Analysis of the Variability of Floral and Pollen Traits in Apple Cultivars—Selecting Suitable Pollen Donors for Cider Apple Orchards. Agronomy 2021, 11, 1717. [Google Scholar] [CrossRef]
- Petri, J.L.; Hawerroth, F.J.; Leite, G.B. Fenologia de espécies silvestres demacieira como polinizadora das cultivares gala e fuji. Rev. Brasil. Fruticult. 2008, 30, 868–874. [Google Scholar]
- Fotirić Akšić, M.M.; Pešić, M.B.; Pećinar, I.; Dramićanin, A.; Milinčić, D.D.; Kostić, A.Ž.; Gašić, U.; Jakanovski, M.; Kitanović, M.; Meland, M. Diversity and Chemical Characterization of Apple (Malus sp.) Pollen: High Antioxidant and Nutritional Values for Both Humans and Insects. Antioxidants 2024, 13, 1374. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerović, R.; Fotirić Akšić, M.; Kitanović, M.; Meland, M. Abilities of the Newly Introduced Apple Cultivars (Malus × domestica Borkh.) ‘Eden’ and ‘Fryd’ to Promote Pollen Tube Growth and Fruit Set with Different Combinations of Pollinations. Agronomy 2025, 15, 909. https://doi.org/10.3390/agronomy15040909
Cerović R, Fotirić Akšić M, Kitanović M, Meland M. Abilities of the Newly Introduced Apple Cultivars (Malus × domestica Borkh.) ‘Eden’ and ‘Fryd’ to Promote Pollen Tube Growth and Fruit Set with Different Combinations of Pollinations. Agronomy. 2025; 15(4):909. https://doi.org/10.3390/agronomy15040909
Chicago/Turabian StyleCerović, Radosav, Milica Fotirić Akšić, Marko Kitanović, and Mekjell Meland. 2025. "Abilities of the Newly Introduced Apple Cultivars (Malus × domestica Borkh.) ‘Eden’ and ‘Fryd’ to Promote Pollen Tube Growth and Fruit Set with Different Combinations of Pollinations" Agronomy 15, no. 4: 909. https://doi.org/10.3390/agronomy15040909
APA StyleCerović, R., Fotirić Akšić, M., Kitanović, M., & Meland, M. (2025). Abilities of the Newly Introduced Apple Cultivars (Malus × domestica Borkh.) ‘Eden’ and ‘Fryd’ to Promote Pollen Tube Growth and Fruit Set with Different Combinations of Pollinations. Agronomy, 15(4), 909. https://doi.org/10.3390/agronomy15040909





