Influence of Fruit Ripeness on Physiological Seed Quality of Maax Pepper (Capsicum annuum L. var. glabriusculum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
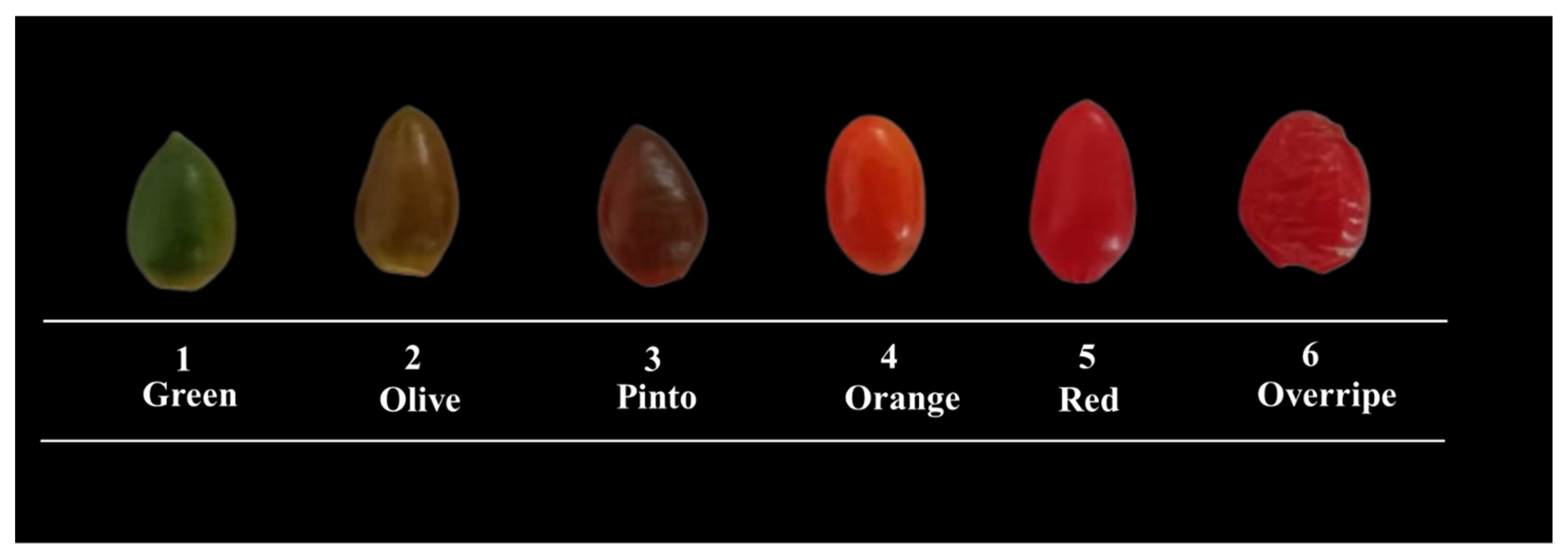
2.2. Plant Material
2.3. Variables Evaluated
2.4. Germination Test and Germination Rate Index
2.5. Emergency Test and Emergency Rate Index
2.6. Evaluation of the Physical Traits of the Seed
2.7. Experimental Design and Statistical Analysis
3. Results
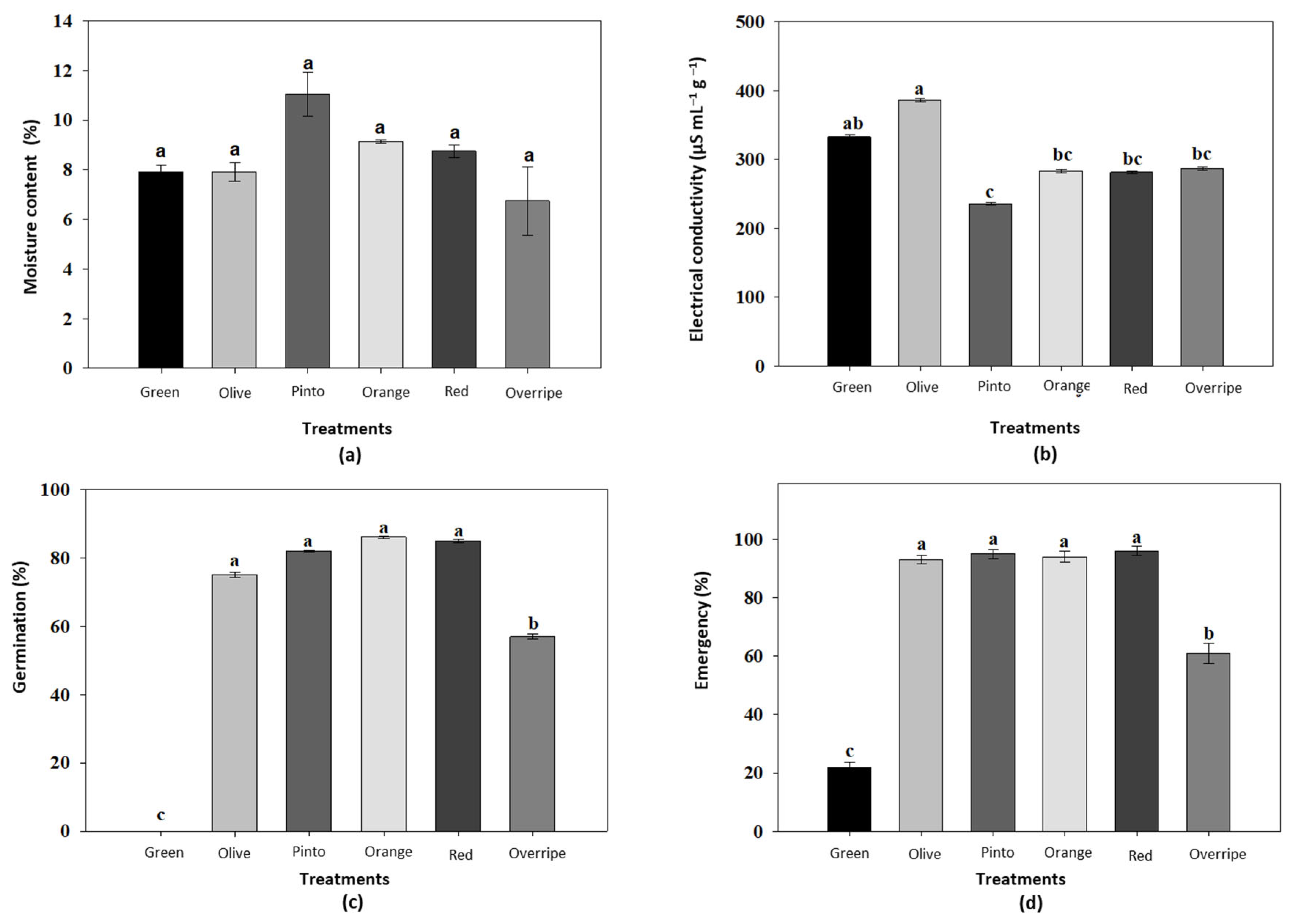
3.1. Physiological Parameters of Maax Pepper Seeds (Capsicum annuum L. var. glabriusculum)
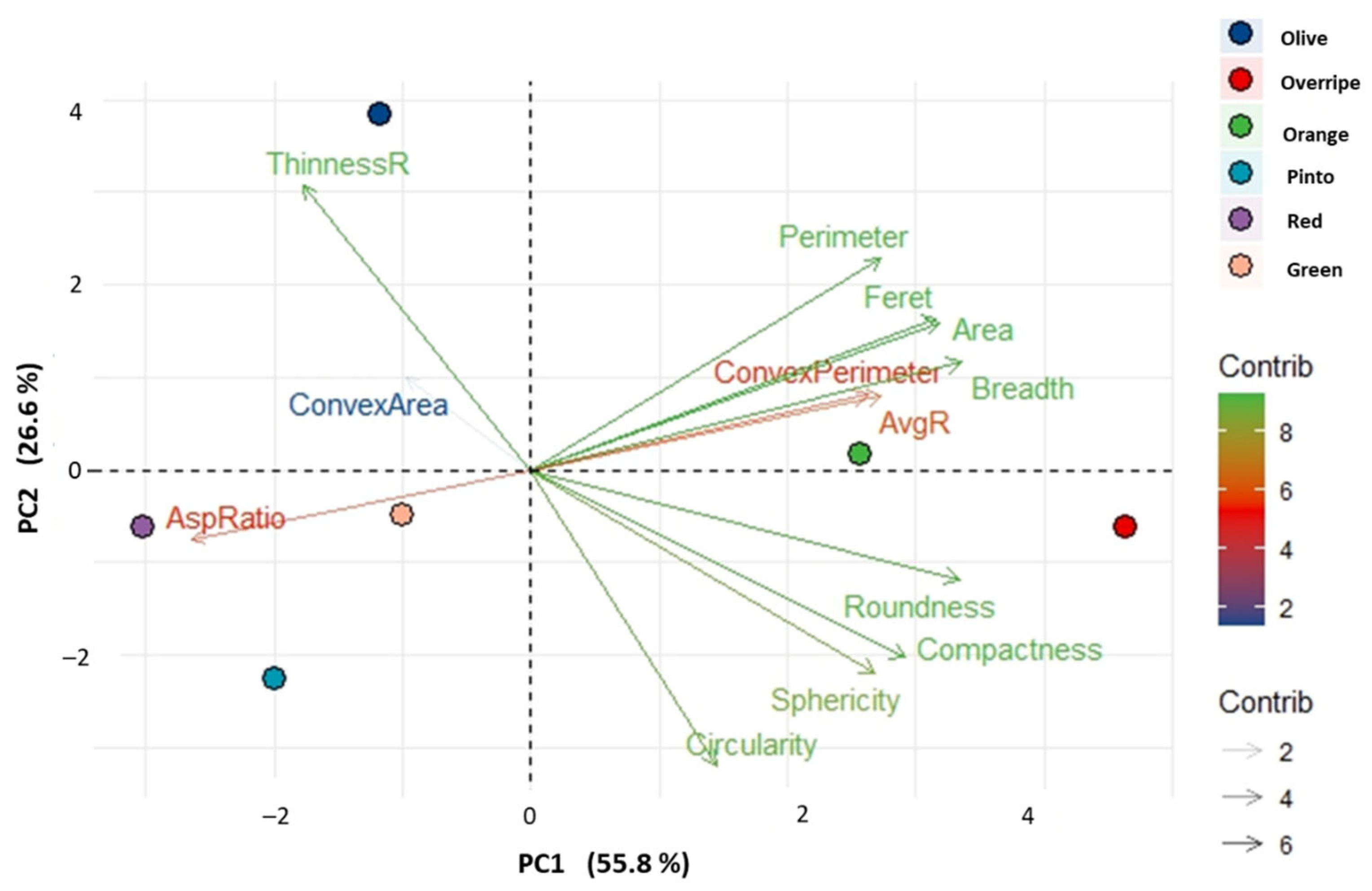
3.2. Morphological Traits of Maax Pepper Seeds (Capsicum annuum L. var. glabriusculum)
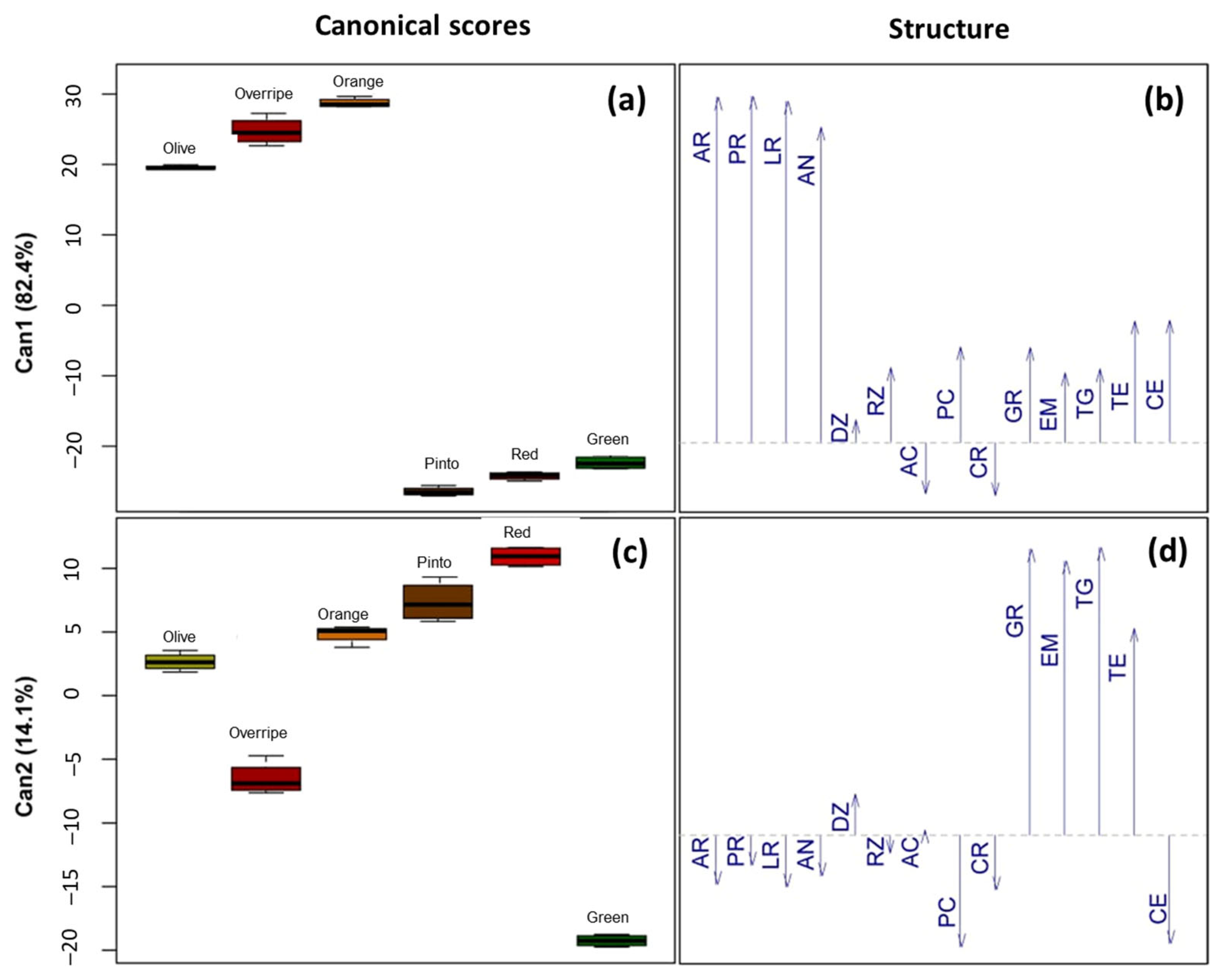
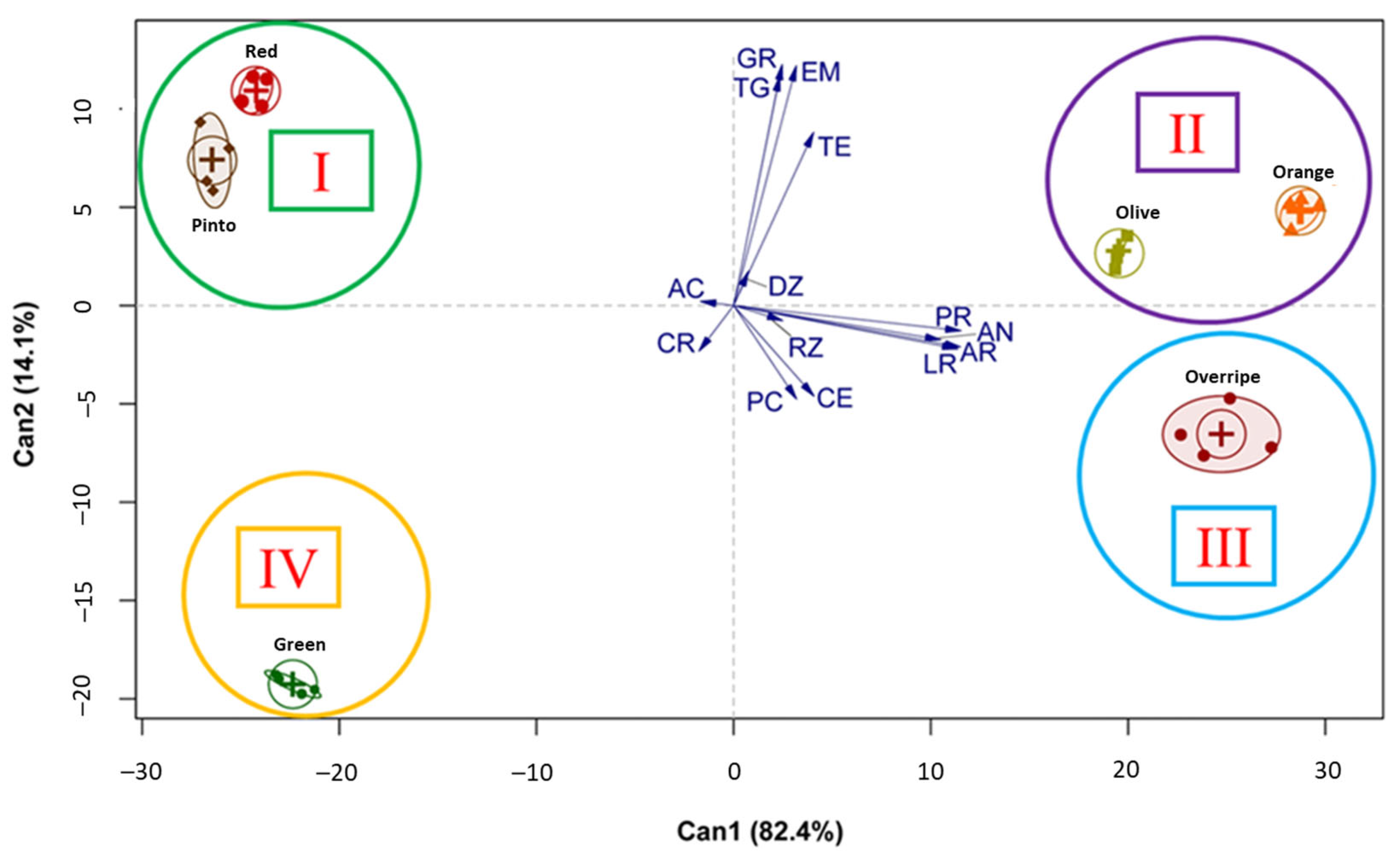
3.3. Relationship Between Physical and Physiological Traits of Seed and Ripening Stages of Maax Pepper Fruit (Capsicum annuum L. var. glabriusculum)
4. Discussion
4.1. Moisture Content and Electrical Conductivity of Maax Pepper (Capsicum annuum L. var. glabriusculum)
4.2. Seed Germination and Seedling Emergence of Maax Pepper (Capsicum annuum L. var. glabriusculum)
4.3. Seed Germination Rate and Seedling Emergence Rate of Maax Pepper (Capsicum annuum L. var. glabriusculum)
4.4. Physical Traits of Maax Pepper Seeds (Capsicum annuum L. var. glabriusculum)
4.5. Relationship Between the Stage of Fruit Ripening, Physical, and Physiological Traits of the Seed of Maax Pepper (Capsicum annuum L. var. glabriusculum)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, B.; Hu, F.; Cai, X.; Cheng, J.; Zhang, Y.; Lin, H.; Hu, K.; Wu, Z. Integrative analysis of the metabolome and transcriptome of a cultivated pepper and its wild progenitor chiltepin (Capsicum annuum L. var. glabriusculum) revealed the loss of pungency during capsicum domestication. Front. Plant Sci. 2022, 12, 783496. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Ventura, J.C.; Márquez-Quiroz, C.; De la Cruz-Lázaro, E.; Osorio-Osorio, R.; Preciado-Rangel, P. Morphological variation of wild peppers (Capsicum spp.) from the state of Tabasco, Mexico. Emir. J. Food. Agri. 2018, 30, 115–121. [Google Scholar]
- Gutiérrez-Burón, R.; Latournerie-Moreno, L.; Garruña-Hernández, R.; Ruiz-Sánchez, E.; Lara-Martín, A.R.; Castañón-Nájera, G. Phenotypic diversity of chilli Amashito from Tabasco and Chiapas, Mexico. Rev. Mex. Cienc. Agríc. 2020, 11, 649–662. [Google Scholar]
- Cano-Vázquez, A.; López-Peralta, M.C.; Zavaleta-Mancera, H.A.; Cruz-Huerta, N.; Ramírez-Ramírez, I.; Gardea-Béjar, A.; González-Hernández, V.A. Variación en grados de latencia en semillas entre colectas de chile piquín (Capsicum annuum var. glabriusculum). Bot. Sci. 2015, 93, 175–184. [Google Scholar] [CrossRef]
- Alcalá-Rico, J.S.G.J.; López-Benítez, A.; Vázquez-Badillo, M.E.; Sánchez-Aspeytia, D.; Rodríguez-Herrera, S.A.; Pérez-Rodríguez, M.Á.; Ramírez-Godina, F. Seed Physiological Potential of Capsicum annuum var. glabriusculum Genotypes and Their Answers to Pre-Germination Treatments. Agronomy 2019, 9, 325. [Google Scholar] [CrossRef]
- Domínguez Orta, J.C.; Herrera Martínez, G. El papá de todos los chiles. Desde Herb. CICY 2019, 11, 98–101. [Google Scholar]
- Díaz-Sánchez, D.D.; López-Sánchez, H.; Silva-Rojas, H.V.; Gardea-Béjar, A.A.; Cruz-Huerta, N.; Ramírez-Ramírez, I.; González-Hernández, V.A. Pungency and fruit quality in Mexican landraces of piquín pepper (Capsicum annuum var. glabriusculum) as affected by plant growth environment and postharvest handling. Chil. J. Agric. Res. 2021, 81, 546–556. [Google Scholar] [CrossRef]
- Hayano Kanashiro, C.; Gámez Meza, N.; Medina Juárez, L.Á. Wild pepper Capsicum annuum L. var. glabriusculum: Taxonomy, plant morphology, distribution, genetic diversity, genome sequencing, and phytochemical compounds. Crop Sci. 2016, 56, 1–11. [Google Scholar] [CrossRef]
- González-Cortés, N.; Jiménez, V.R.; Guerra, B.E.C.; Silos, E.H.; de la Payro, C.E. Germination of amashito Chili (Capsicum annuum L. var. Glabriusculum) in southeastern Mexico. Rev. Mex. Cienc. Agríc. 2015, 6, 2211–2218. [Google Scholar]
- Prado-Urbina, G.; Lagunes-Espinoza, L.; García-López, E.; Bautista-Muñoz, C.; Camacho-Chiu, W.; Mirafuentes, G.F.; Aguilar-Rincón, V.H. Seed germination of wild chili peppers in response to pre-germination treatments. Ecosistemas Recur. Agropecu. 2015, 2, 139–149. [Google Scholar]
- Votava, E.J.; Nabhan, G.P.; Bosland, P.W. Genetic diversity and similarity revealed via molecular analysis among and within an in situ population and ex situ accessions of chiltepin (Capsicum annuum var. glabriusculum). Conserv. Genet. 2002, 3, 123–129. [Google Scholar] [CrossRef]
- Sandoval-Rangel, A.; Tapia González, A.; De la Fuente, M.C.; González Fuentes, J.A.; Benavides-Mendoza, A. Age, yield and gibberellic acid affect the germination of piquín chili pepper plants. Rev. Mex. Cienc. Agríc. 2018, 9, 4199–4209. [Google Scholar]
- Mares-Quiñones, M.D.; Valiente-Banuet, J.I. Horticultural aspects for the cultivated production of piquin peppers (Capsicum annuum L. var. glabriusculum) a review. Hortic. Sci. 2019, 54, 70–75. [Google Scholar] [CrossRef]
- Beltrán-Burboa, J.N.; López Peralta, M.C.; Hernández Meneses, E.; Cruz-Huerta, N. In vitro seed germination of chiltepin pepper (Capsicum annuum L. var. glabriusculum) and plant regeneration via organogenesis. Agrociencia 2020, 54, 195–208. [Google Scholar]
- Brondo-Ricárdez, R.; Domínguez-Angulo, S.; Pérez-Hernández, I.; D’Artola-Barceló, L.A. Tratamientos pregerminativos a semillas y desarrollo inicial de plántulas de chile amashito (Capsicum annuum L. var. glabriusculum). Agroproductividad 2020, 13, 53–60. [Google Scholar]
- García-Tierrablanca, E.A.; Raya-Pérez, J.C.; Covarrubias-Prieto, J.; Dorantes-González, J.A.R.; Chablé-Moreno, F.; Ramírez-Pimentel, J.G.; Aguirre-Mancilla, C. Assessment of emasculation techniques and maturation of fruit for seed production of pepper (Capsicum annuum L.). Rev. Mex. Cienc. Agríc. 2015, 6, 2129–2137. [Google Scholar]
- Santamaria, B.F.; Zavala, L.M. Maturity stages of Xcat ik pepper (Capsicum annuum L) and its relationship with the storage period in seeds germination. BJAER 2021, 4, 4674–4683. [Google Scholar]
- Hernández-Pinto, C.; Garruña, R.; Andueza-Noh, R.; Hernández-Núñez, E.; Zavala-León, M.J.; Pérez-Gutiérrez, A. Post-harvest storage of fruits: An alternative to improve physiological quality in habanero pepper seeds. BioScience 2020, 7, e796. [Google Scholar]
- Vidigal, D.; Dias, D.; Von-Pinho, E.R.V.; Dias, L.A.S. Sweet pepper seed quality and lea-protein activity in relation to fruit maturation and post-harvest storage. Seed Sci. Technol. 2009, 37, 192–201. [Google Scholar] [CrossRef]
- International Seed Testing Association. Available online: https://www.seedtest.org/ (accessed on 24 June 2024).
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Loddo, A.; Di Ruberto, C.; Vale, A.M.P.G.; Ucchesu, M.; Soares, J.M.; Bacchetta, G. An effective and friendly tool for seed image analysis. Vis. Comput. 2022, 39, 335–352. [Google Scholar] [CrossRef]
- The National Seed Inspection and Certification Service. Available online: https://www.gob.mx/snics (accessed on 10 February 2024).
- Ayala-Villegas, M.J.; Ayala-Garay, Ó.J.; Aguilar-Rincón, V.H.; Corona-Torres, T. Evolución de la calidad de semilla de Capsicum annuum L. durante su desarrollo en el fruto. Rev. Fitotec. Mex. 2014, 37, 79–87. [Google Scholar] [CrossRef]
- Neto, A.F.; Cruz, A.F.; Almeida, F.A.C.; Vieira, J.F.; Rodrigue, M.B. Physiological Seed Quality of Melon as Affected by Fruit Ripeness. Int. J. Veg. Sci. 2016, 22, 219–226. [Google Scholar] [CrossRef]
- Popović, V.; Lekić, S.; Kiprovski, B.; Takač, A. The effect of ripeness phases on seed and fruit quality of eggplant (Solanum melongena L.). Emir. J. Food. Agric. 2022, 34, 144–150. [Google Scholar] [CrossRef]
- Dos Santos, H.O.; Dutra, S.M.; Pereira, R.W.; Pires, R.D.O.; Von Pinho, E.D.R.; Da Rosa, S.D.V.F.; De Carvalho, L.M. Physiological quality of habanero pepper (Capisicum chinense) seeds based on development and drying process. Afr. J. Agric. Res. 2016, 11, 1102–1109. [Google Scholar]
- Sripathy, K.V.; Groot, S.P. Seed Development and Maturation. In Seed Science and Technology Biology, Production, Quality; Springer: New Delhi, India, 2023; pp. 17–38. [Google Scholar]
- Valdez-Eleuterio, G.; Uscanga-Mortera, E.; Kohashi-Shibata, J.; García-Nava, R.; Martínez-Moreno, D.; Torres-García, J.; García-Esteva, A. Seed size, substrate granulometry and sowing depth in seed and seedling vigor of two weeds. Agrociencia 2015, 49, 899–915. [Google Scholar]
- Criollo, H.; Upegui, P.A. Determinación de la madurez fisiológica de semillas de uvilla (Physalis peruviana L.). Rev. Mex. Cienc. Agric. 2005, 22, 56–69. [Google Scholar]
- Sanches, L.A.; Ramalho, A.B.; Camili, E.C.; Guimarães, R.A.P. Fruit biometrics and maturity on the quality of Diospyros inconstans Jacq. Seeds. J. Seed Sci. 2023, 45, e202345023. [Google Scholar] [CrossRef]
- Silva, L.D.S.; Gentil, D.F.D.O.; Ferreira, S.A.D.N. Maturation and germination of Trichosanthes cucumerina L. seeds. J. Seed Sci. 2022, 44, e202244027. [Google Scholar] [CrossRef]
- Santos, T.P.; Sá, M.E.; Malagutti, E.S.; Pinto, M.S.; Ferreira, A.F.A.; Monteiro, L.N.H.; Rodrigues, M.G.F. Effects of gibberellic acid concentration and fruit maturation stage on seed germination and vigor of pitahaya seedlings. Braz. J. Biol. 2022, 84, e260650. [Google Scholar] [CrossRef]
- Martínez, J.S.J.; Aquino Bolaños, T.; Ortiz Hernández, Y.D.; Cruz Izquierdo, S. Características de fruto y semilla de chile huacle (Capsicum annuum L.) producido en hidroponia. Idesia 2019, 37, 87–94. [Google Scholar] [CrossRef]
- Quevedo, M.; Laurentin, H. Caracterización fenotípica de tres cultivares de ají dulce (Capsicum chinense Jacq.) venezolano. Agron. Mesoam. 2020, 31, 729–741. [Google Scholar]
- Pinheiro, D.T.; Medeiros, A.D.; Zavala-León, M.J.; Dias, D.C.F.S.; da Silva, L.J. Physical and physiological quality of Jatropha curcas L. Seeds at different maturity stages using image análisis. Span. J. Agric. Res. 2020, 18, e0206. [Google Scholar] [CrossRef]
- Ellis, R.H. Temporal patterns of seed quality development, decline, and timing of máximum quality during seed development and maturation. Seed. Sci. Res. 2019, 29, 135–142. [Google Scholar] [CrossRef]
- Medeiros, A.D.; de Araújo, J.O.; Zavala-León, M.J.; Silva, L.J.; Dias, D.C.F.S. Parameters based on X-ray images to assess the physical and physiological quality of Leucaena leucocephala seeds. Cienc. Agrotec. 2018, 42, 643–652. [Google Scholar] [CrossRef]
| Treatments | Germination Rate (Seeds Germinated/Day) | Emergency Rate (Seedlings Emerged/Day) |
|---|---|---|
| Green | 0 ± 0.00 c | 0.28 ± 0.01 d |
| Olive | 4.68 ± 0.44 ab | 10.87 ± 0.65 a |
| Pinto | 5.07 ± 0.09 a | 8.08 ± 0.80 ab |
| Orange | 5.28 ± 0.26 a | 9.36 ± 0.093 ab |
| Red | 5.67 ± 0.22 a | 6.45 ± 0.66 bc |
| Overripe | 3.40 ± 0.22 b | 2.91 ± 0.40 cd |
| Canonical Variable | Canonical Correlation | Self-Value | Eigenvalue Ratio | Cumulative Proportion | Probability Value |
|---|---|---|---|---|---|
| Can 1 | 0.998 | 801.296 | 82.389 | 82.389 | 6 × 10−11 *** |
| Can 2 | 0.992 | 137.234 | 14.110 | 96.500 | 2 × 10−06 *** |
| Can 3 | 0.967 | 30.109 | 3.095 | 99.596 | 0.005183 ** |
| Can 4 | 0.781 | 3.574 | 0.367 | 99.963 | 0.441521 |
| Can 5 | 0.262 | 0.356 | 0.036 | 100.000 | 0.954468 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzib-Ek, M.G.; Andueza-Noh, R.H.; Garruña, R.; Zavala-León, M.J.; Villanueva-Couoh, E.; Rivera-Hernández, B.; Torres-Cab, W.J.; Alvarado-López, C.J.; Ruíz-Santiago, R.R. Influence of Fruit Ripeness on Physiological Seed Quality of Maax Pepper (Capsicum annuum L. var. glabriusculum). Agronomy 2025, 15, 747. https://doi.org/10.3390/agronomy15030747
Dzib-Ek MG, Andueza-Noh RH, Garruña R, Zavala-León MJ, Villanueva-Couoh E, Rivera-Hernández B, Torres-Cab WJ, Alvarado-López CJ, Ruíz-Santiago RR. Influence of Fruit Ripeness on Physiological Seed Quality of Maax Pepper (Capsicum annuum L. var. glabriusculum). Agronomy. 2025; 15(3):747. https://doi.org/10.3390/agronomy15030747
Chicago/Turabian StyleDzib-Ek, María Gabriela, Rubén Humberto Andueza-Noh, René Garruña, Manuel Jesús Zavala-León, Eduardo Villanueva-Couoh, Benigno Rivera-Hernández, Walther Jesús Torres-Cab, Carlos Juan Alvarado-López, and Roberto Rafael Ruíz-Santiago. 2025. "Influence of Fruit Ripeness on Physiological Seed Quality of Maax Pepper (Capsicum annuum L. var. glabriusculum)" Agronomy 15, no. 3: 747. https://doi.org/10.3390/agronomy15030747
APA StyleDzib-Ek, M. G., Andueza-Noh, R. H., Garruña, R., Zavala-León, M. J., Villanueva-Couoh, E., Rivera-Hernández, B., Torres-Cab, W. J., Alvarado-López, C. J., & Ruíz-Santiago, R. R. (2025). Influence of Fruit Ripeness on Physiological Seed Quality of Maax Pepper (Capsicum annuum L. var. glabriusculum). Agronomy, 15(3), 747. https://doi.org/10.3390/agronomy15030747







