Abstract
Investigating diverse techniques for promoting flowering in Xanthosoma is crucial for managing sexual reproduction, producing authentic botanical seeds, and increasing Xanthosoma’s genetic diversity. Therefore, the objective of this research was to investigate the impact of gibberellic acid (GA3) and foliar application of VIUSID® Agro on enhancing flower and seed production in Xanthosoma species. A controlled experiment was conducted in a completely randomized design using four treatments and three replicates. The treatments were a control and three GA3 concentrations of 500, 750, and 1000 mg L−1, all combined with 0.20 mg L−1 of VIUSID® Agro. The findings provide the first overview of the combination of GA3 and VIUSID® Agro on flowering and seed production in various Xanthosoma accessions. The results highlight that combining GA3 (1000 mg L−1) with VIUSID® Agro (0.20 mg L−1) resulted in significant flowering and seed production in all Xanthosoma accessions. This novel insight could be observed in the seed length (ranging from 1.0 to 1.5 mm), seed color (varying from light yellow to strong yellow), seed germination (92%), and seed mass (ranging from 0.60 to 3.10 g). Finally, we make suggestions for future research efforts in the use of GA3 and VIUSID® Agro to enhance the Xanthosoma breeding program.
1. Introduction
The white cocoyam, also known as ocumo, tannia, yautía, quequisque, tiquisque, or malanga (Xanthosoma sagittifolium), and the purple cocoyam (Xanthosoma violaceum) are plants that yield edible underground stems [1]. Cocoyam (Xanthosoma spp.), belonging to the Araceae family, originates from the Americas. This genus includes around 45 species, each containing various clones [2]. X. sagittifolium and X. violaceum are the main species more widely recognized [3]. Furthermore, the vegetative cycle is 8 to 12 months, and they are considered annual plant species [4].
Xanthosoma species is an important staple food in many tropical and subtropical regions, particularly in Africa, Asia, and the Pacific Islands [5]. It ranks as one of the six key root and tuber crops globally, feeding more than 400 million individuals [6]. The significance of cocoyam for food security in the region is extremely high [7]. The nutritional makeup of cocoyam differs based on its species, variety, and the conditions in which it is grown. It is a valuable source of complex carbohydrates, particularly starch, which offers dietary energy, especially for children and the elderly [8]. Additionally, cocoyam is rich in vitamins, including vitamin C, minerals, and phenolic compounds that can help shield the body from oxidative stress and may lower the risk of chronic diseases [9].
Malanga is critical to Cuba’s agricultural landscape and food security. As a starchy corm, it is an important staple crop in Cuba, contributing significantly to the caloric and protein intake of the population, especially in an environment where food self-sufficiency and reduced reliance on imports are strategic goals [10]. Malanga’s nutritional content, convenience of preparation, and digestive properties make it a widely requested commodity in the domestic market, as well as in institutional settings like hospitals and nursing homes [11]. The Research Institute of Tropical Roots and Tuber Crops (INIVIT) in Cuba actively maintains a varied germplasm collection of Xanthosoma species, concentrating on genetic improvement to maximize its productivity and nutritional benefits [10,11].
Both X. sagittifolium and X. violaceum are reproduced mainly through asexual reproduction, utilizing parts of corms or cormels [1]. The sexual seed propagation is not occurring due to the limited occurrence of flowering [2]. Xanthosoma species rarely or never flower under normal cultivation conditions due to a combination of the infrequent natural flowering, pollination challenges, and potential genetic factors, making their sexual propagation challenging [12,13]. Moreover, environmental factors, including temperature, humidity, and light, can greatly affect the process of flowering, which indicates that ideal conditions are not always achievable in cultivation conditions [14]. Some research shows that flowering induction in Xanthosoma species can be carried out through several methods, such as environmental control (i.e., temperature regulation, humidity control, and adequate light exposure), GA3 application, artificial pollination, and tissue culture [15].
GA3 stimulates flowering in many plant species, especially long-day plants and biennials, by orchestrating a complex molecular pathway [16]. The primary induced mechanism of flowering is through the regulation of genes involved in meristem identity and floral development [17]. One key mechanism is the degradation of DELLA proteins, which are GA3 signaling repressors, thereby releasing the inhibition on flowering-promoting genes such as FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) [18]. Additionally, during non-inductive photoperiods, GA3 can directly upregulate the expression of SOC1 and LEAFY (LFY), two MADS-box transcription factors essential for floral meristem identity and development, without the need for DELLA [19]. Furthermore, GA3 also interacts with the photoperiod pathway, where it can substitute for long-day signals to induce flowering, often by modulating the stability and activity of CONSTANS (CO) protein and the subsequent expression of FT [20].
Different methods of GA3 application are widely used to induce flowering in Xanthosoma plants, such as foliar application, direct injection, and pre-plant soaking [12,21,22]. The foliar spraying involves GA3 concentrations between 500 and 1500 mg L−1 [12,16,23,24]. Moreover, the injection of GA3 close to the apical meristem is a more precise approach, proving effective even in recalcitrant cultivars [15,21]. Additionally, pre-plant soaking at 1500 ppm of GA3 significantly enhanced flowering in Nigerian clones [14,22,25]. The flowering of cocoyam is influenced by both the genotype and the way GA3 is applied [10,26].
Biostimulants play an increasingly significant role in modern agriculture, offering a range of benefits that contribute to sustainable and efficient root and tuber crops [27,28,29]. These components can influence various physiological processes and may enhance flowering because they often contain microorganisms, minerals, vitamins, amino acids, oligosaccharides, natural plant hormones, and other components [30]. Plant growth and development can benefit significantly from amino acid-based biostimulants. In this scenario, VIUSID® Agro (a commercial amino acid growth promoter) contains a mixture of aspartic acid, arginine, glycine, and tryptophan, which could stimulate plant growth and productivity by activating several physiological and biochemical mechanisms [31,32,33]. Additionally, these amino acids act as a source of nitrogen reserves; promote phytohormones like auxin, cytokinins, and gibberellins; stimulate physiological and metabolic processes like increasing the uptake of nutrients; activate the biosynthesis of various metabolites; participate in the synthesis of other essential amino acids like lysine, threonine, isoleucine, and methionine; and are an essential component for the synthesis of nucleotides [30,31,34]. While specific studies on a mixture of these four amino acids for flowering and seed production have been reported in soybean and sunflower plants [26,33], in Xanthosoma, they might be limited.
In Cuba, Xanthosoma spp. seldom or never form flowers. For a long time, this genus has been propagated using rhizomes or their parts, leading to a decline in its ability to reproduce sexually [10]. Considering that the Genetic Improvement Program (GIP) at the Research Institute of Tropical Roots and Tuber Crops (INIVIT, by the Spanish abbreviation) has not successfully produced botanical seeds in this plant genus using traditional methods, we decided to modify our analysis. We will explore alternative methods to induce flowering and ultimately achieve botanical seed production through targeted crosses between selected parent plants. Therefore, the objective of this research was to investigate the impact of gibberellic acid (GA3) and foliar application of VIUSID® Agro on enhancing flower and seed production in Xanthosoma species.
2. Materials and Methods
2.1. Plant Materials
The preliminary study was carried out at the INIVIT (22°35′10″ N, 80°13′35″ W) as part of the Genetic Improvement Program (GIP) developed in the genus (Xanthosoma spp.). During this event, accessions were recognized that might indicate genetic diversity based on the corm mass color. Next, the “plus plants” from these accessions that are part of the cocoyam (Xanthosoma spp.) germplasm collection were separated, focusing on the edible species Xanthosoma sagittifolium (L.) Schott, a polymorphic species with a diversity of shapes and colors but bringing together many common characters—44 white-colored mass (2n = 26 chromosomes), 12 purple-colored mass (2n = 24 chromosomes), and 2 yellow-colored mass (2n = 26 chromosomes)—and Xanthosoma nigrum (Vell.) Mansf., a purple mass accession (2n = 24 chromosomes) [11]. The selected accessions, such as ‘Morada 1726’, ‘INIVIT-84’, ‘Cuarentena’, ‘México 3’, ‘Jibara’, and ‘Morada Cabaiguán’, were selected on the basis of their potential value to drought tolerance.
2.2. Growth Conditions
Cormels from each accession were chosen for their maximum weight, consistent size, and lack of damage from pests or mechanical issues. They were then placed into sealed, labeled plastic bags for transport from the germplasm bank to the Agamic Seed Reproduction Center (CRAS) area. Afterward, N24P12K36 fertilization was applied, and the cormels were planted in sterile substrate with good drainage. The cormels were planted in February of 2024, with a distance of 0.90 m between lines and 0.30 m between plants, obtaining 18 plants per plot. At CRAS, a sprinkler irrigation system was set up to operate every 3–4 days during the early stages, with adjustments made later based on rainfall.
We conducted a controlled experiment in the CRAS area, where the total rainfall recorded was 1362.5 mm. The average maximum temperature was 31.3 °C; the mean temperature was 24.7 °C; and the minimum temperature was 19.7 °C. Relative humidity was at 81%, with a wind speed of 5.3 km/h and evaporation measured at 5.1 mm. The photoperiod consisted of 12 h of daylight and 12 h of darkness, while light intensity was adjusted to 70% using a mesh net to create ideal conditions for plant growth.
2.3. Experimental Design
Each Xanthosoma accession was arranged in a completely randomized design with four treatments and three replications (plots). The treatments consisted of a control (without GA3 and VIUSID® Agro) and three GA3 concentrations—500 (GV1), 750 (GV2), and 1000 mg L−1 (GV3)—all combined with VIUSID® Agro through foliar spraying at a concentration of 0.20 mg L−1. The plot size was 5 m2, separated by a distance of 1.0 cm between each plot, and all experimental areas were divided into 48 experimental plots. The two central rows formed the plot’s useful area, with a border margin of 0.5 m. Furthermore, in each Xanthosoma accession treated with GV3, one plant per replication was manually self-fertilized. Additionally, in the GV3 treatment, one plant of the ‘Morada 1726’ accession per replication was manually crossed with ‘Jibara’ (X. sagittifolium), and another plant was manually crossed with ‘Morada Cabaiguán’ due to the importance of these accessions to drought resistance.
2.4. GA3 and VIUSID® Agro Application
The GA3 injection started when the bases of the plants’ stems measured 5 cm in diameter, which was about 90 days after planting, following the method outlined by Katsura [21]. Each concentration was applied at the base of the plants by injecting 5 mg using a disposable plastic syringe (Luer Lock, Kindly (KDL) Group, Shanghai, China), applied at three different times with weekly intervals (see Figure 1a). A week after completing the GA3 treatments, a foliar application of the plant growth promoter VIUSID® Agro was applied at a concentration of 0.20 mg L−1, as recommended by the Spanish company Catalysis S.L. Enterprise.
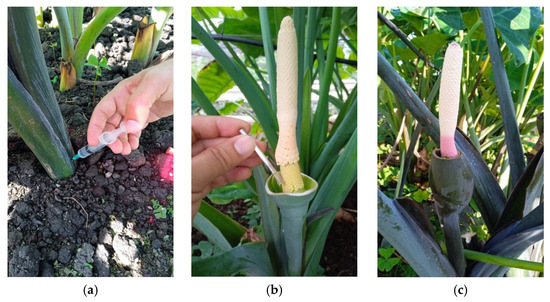
Figure 1.
(a) Method of GA3 injections near the meristem to Xanthosoma spp. accessions. (b) Hand-pollination with spathe removal. (c) Pollinated flower covered with the lower tubular portion of the freshly removed spathe. Photos were taken by the author.
2.5. GA3 and VIUSID® Agro Compositions
Berelex Tablets Gibberellic acid (GA3; Zhengzhou Delong Chemical Co., Ltd., Zhengzhou, China), containing 0.92 as GA3, was used for preparing the different concentrations of GA3. The declared composition of the amino acid-based growth promoter (commercialized as VIUSID® Agro by Catalysis S.L., Madrid, Spain) is as follows: aspartic acid (1.6% m/m), arginine (2.5% m/m), glycine (2.4% m/m), and tryptophan (0.5% m/m), with a pH of 6.80 and a net mass of 1.14 kg [33].
2.6. Hand-Pollination Methods
We applied the hand-pollination techniques previously outlined by Goenaga and Hepperly [12]. Briefly, the initial sign was the presence of a sweet scent, occasionally quite strong, emitted by the flowers. This occurred when the spathe was fully removed, revealing the reproductive organs before they opened (Figure 1b). The visible stigmas displayed a yellow, jelly-like substance, signaling that the ovaries were ready to receive pollen. A brush was used to apply mature pollen from different flowers onto them. To gather the emitted pollen for new crosses, the stigmas were covered with the lower tubular part of the spathe without cutting the spadix (Figure 1c).
2.7. Observations of Flowers, Pollen, and Seed Mass Parameters
The average number of flowers per inflorescence was directly counted from each replicate/plant of all treatments (~230 days after cormels were planted). Furthermore, we took a demonstrative photo to show the characteristics of the inflorescences and flowers of this species (see details in Figure 2b). A typical observation of Spadix with an abundant presence of anthers was also observed (see Figure 2c). The fertility of the pollen grains was examined using the acetocarmine staining technique [35]. Also, a demonstrative picture of pollen-fertilized grain was taken under a Leica MS5 stereomicroscope (Leica Microsystems (Schweiz) AG Verkaufsgesellschaft, Heerbrugg, Switzerland) at ×4 magnification (see Figure 2d). Furthermore, observation of the fruits close to maturation was performed, and the image is shown in Figure 2f.
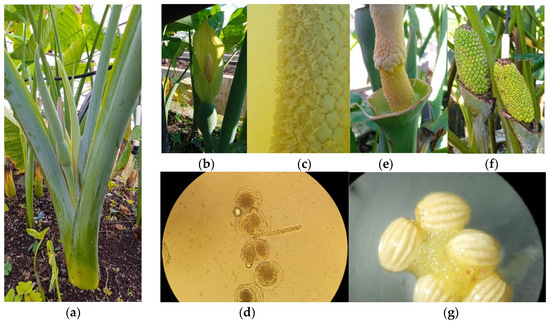
Figure 2.
Reproductive cycle of Xanthosoma cocoyam species. (a) Plant with the first signs of flowering: flag leaf. (b) Inflorescence typical of the genus Xanthosoma with open spathe. (c) Spadix with abundant presence of anthers (magnified image). (d) Germinated pollen grains in their last stage of meiotic division (magnified image). (e) Pollinated female flower. (f) Fruits close to maturation. (g) Botanical seeds of Xanthosoma cocoyam with the presence of mucilage (enlarged image).
After botanical seed harvest (280 days, Figure 2e), in all Xanthosoma accessions, seeds of each treatment were rinsed to eliminate the mucilage, and they were separated and collocated above paper towels to dry in laboratory conditions and ambient temperature rooms for approximately 72 h. Then, a digital precision weighing scale (0.1 mg; AR224CN, Ohaus Corporation, Shanghai, China) was used to record the seed mass (g) of each treatment for all Xanthosoma accessions. After the seeds’ mass determination, three replicates of 10 seeds of each accession were collocated into Petri dishes and measured under a Leica MS5 stereomicroscope (Heerbrugg, Switzerland) at ×4 magnification.
2.8. Seed Germination Bioassay
In this trial, we only used seeds of the crosses ‘Jibara’ (X. sagittifolium) with ‘Morada 1726’ (X. nigrum) and ‘Morada Cabaiguán’ with ‘Morada 1726’. In both crosses, the ‘Morada 1726’ accession was the female parent. After around nine months, seeds from both previously mentioned crosses were used for the seed germination test. Thirty random seeds of each cross were selected, and then three replicates of ten seeds each were placed in Petri dishes (9 mm) using three different substrates. The first medium tested was moist cotton. The second substrate consisted of a magenta container filled with moist black soil. The third substrate was a magenta container filled with red soil. The seeds were then cultured for germination under laboratory conditions as part of the soaking process (water was added periodically to maintain moisture levels) under laboratory conditions and at an ambient temperature (25 ± 2 °C). The total germination time for this experiment was 20 days. The germination of seeds was observed and recorded daily at 9 a.m. The standard used for germination was that the shoot length was more than one-half of the seed length, and the quantification was performed using Equation (1):
Germination (%) = (number of seeds germinated per treatment/total number of seeds per treatment) × 100
2.9. Statistical Analyses
Experimental data were examined through an analysis of variance (ANOVA) following tests for normality and homoscedasticity, which were conducted using the Shapiro–Wilk and Levene tests, respectively. Then, the data were analyzed using one-way ANOVA. Furthermore, all levels of statistical significance among the means of the treatments were determined using the Tukey test at a 95% significance limit. All statistical analyses were performed in the software R version 4.4 (2024, Vienna, Austria).
3. Results
The results indicated that flowering in Xanthosoma is rare, as evidenced by the control treatment. Ongoing factors, such as GA3 application, are known to encourage this process (Table 1). Flowering induction varied significantly among treatments in all Xanthosoma accessions. Applying GV3 induced a higher (100%) number of flowers per inflorescence in all Xanthosoma accessions than in the control treatment. However, in the ‘Morada 1726’ accession, all plants flowered (100%) under GV1 and GV2 treatments in comparison with the control treatment (Table 1). Also, in the ‘Morada 1726’ accession, the GV3 treatment significantly increased the number of flowers per inflorescence by ~50% compared to the GV1 and GV2 treatments (Table 1). For the remaining accessions, the application of GV1 and GV2 treatments did not produce any flowers and were similar to the control treatment (Table 1).

Table 1.
Average number of flowers per inflorescence obtained with the different injected GA3 concentrations (500, 750, and 1000 mg L−1) combined with foliar spraying of VIUSID® Agro at 0.20 mg L−1 and a control treatment.
The initial indication of flowering induction appears at 19 weeks after applying 500 and 750 mg L−1 of GA3 with 0.20 mg L−1 of VIUSID® Agro treatments in the Morada 1726 accession, or 1000 mg L−1 of GA3 and 0.20 mg L−1 of VIUSID® Agro treatment in all accessions was the appearance of flag leaves in the leaf axils (Figure 2a). Overall, the majority of the inflorescences produced appeared to be free of deformities (Figure 2b), aside from two inflorescences in the ‘Morada 1726’ accession treated with 500 and 750 mg L−1 of GA3 along with 0.2 mg L−1 of VIUSID® Agro, which produced two spathes, the first spathe surrounding the second.
A significant number of anthers on the staminodes in the treatments that stimulated flowering in all Xanthosoma accessions was observed (Figure 2c). This indicates a possible large quantity of pollen grains, leading to the pollen grains exhibiting a high rate of fertility and germination (Figure 2d). Also, covering the stigmas completely with the collected pollen (Figure 2e) led to the growth of consistent fruit in all Xanthosoma accessions (Figure 2f).
The seed length showed a significant difference between treatments; especially the GV3 treatment, which showed a significant increase in seed length as compared to the rest of the treatments in all Xanthosoma accessions (Table 2). In addition, the seed length in the GV3 treatment was significantly higher by 100 and 43% compared to the control and GV1 and GV2 treatments in the ‘Morada 1726’ accession. Although GV1 and GV2 revealed similar effects on the seed size, both exhibited higher seed sizes than in the control treatment (Table 2). Furthermore, the seed length ranged from 1.0 to 1.5 mm and displayed a color range from light to dark yellow (Table 2, Figure 2g).

Table 2.
Average seed length (mm) obtained with the different injected GA3 concentrations (500, 750, and 1000 mg L−1) combined with foliar spraying of VIUSID® Agro at 0.20 mg L−1 and a control treatment.
Overall, the seed mass collected in all accessions was 10.6 g. All accessions produced a higher seed mass under GV3 treatment, especially in the ‘Morada 1726’ accession; the increases were 100%, 42%, and 18% compared to the control, GV1, and GV2, respectively (Table 3). Moreover, the GV3 treatment contributed 84% of the total seed mass, whereas the ‘Morada 1726’ accession contributed approximately 45% of the overall seed production (Table 3).

Table 3.
Average seed mass per plant obtained with the different injected GA3 concentrations (500, 750, and 1000 mg L−1) combined with foliar spraying of VIUSID® Agro at 0.20 mg L−1 and a control treatment.
The seed germination in response to the crossbreeding pair and substrate showed no significant differences (Table 4, Figure 3). The average seed germination was 92%, independently of the crossbreeding pair and substrate used (Table 4, Figure 3). The testing on a random sample of seeds revealed that germination began 9 days after sowing, and the final emergence was 16 days at the three substrates, such as cotton (Figure 3a,b), black soil (Figure 3c), and red soil (Figure 3d). This pattern of seed germination was consistent across all three substrates and both crossbreeding pairs (Table 4, Figure 3).

Table 4.
Average seed germination in response to the crossbreeding and the three substrates employed using seed collected from the GV3 (1000 mg L−1 of GA3 combined with foliar spraying of VIUSID® Agro at 0.2 mg L−1) treatment.

Figure 3.
Percentage of Xanthomonas spp. seed emergence and germination in the substrates employed after different crossbreeding approaches. (a,b) Germinated seeds in cotton substrate (enlarged image). (c) Germinated seeds in red soil substrate (enlarged image). (d) Germinated seeds in black soil substrate (enlarged image). Photos were taken by the author.
4. Discussion
Currently, including both commercial and non-commercial genotypes in a genetic enhancement program for Xanthosoma (cocoyam or tannia) is crucial for a comprehensive and sustainable breeding strategy [26]. The commercial genotypes are often selected for specific desirable traits like high yield, disease resistance to prevalent issues, and market-preferred characteristics [36]. However, non-commercial (i.e., landraces and wild relatives) genotypes are essential for infusing genetic diversity, enhancing resilience, ensuring long-term sustainability, and addressing future challenges and opportunities in the Xanthosoma genetic improvement program (GIP) [26,36]. Often Xanthosoma breeding programs present propagation challenges that limit the GIP for Xanthosoma cocoyam in Cuba, which is organized into multiple phases, beginning with Phase 1, which involves selecting parent plants. During this phase, specific accessions are chosen from the germplasm bank based on their desirable traits that can be passed on to the offspring [37]. This stage is essential for gathering a diverse range of cocoyam germplasm. It encompasses local landraces, varieties chosen by farmers, and possibly wild relatives. The goal of this collection is to obtain the widest genetic diversity possible for the crop [10,38]. In essence, Phase 1 provides the essential information and resources needed to make strategic decisions in the subsequent phases of a cocoyam genetic improvement program.
The flower induction is a critical and indispensable step in Phase 1 of a Xanthosoma GIP. It overcomes the limitations of asexual reproduction, enables the creation of novel genetic combinations through hybridization, and lays the foundation for the development of improved Xanthosoma varieties [12,39]. Natural flowering and seed sets are often rare or inconsistent in many Xanthosoma cultivars, hindering the development of hybrid offspring with desirable traits [39]. Induced flowering enables controlled pollination and the production of genuine botanical seeds, which is an essential initial step in a traditional breeding program [40]. Furthermore, by obtaining these seeds, breeders can develop new genetic combinations by crossing various Xanthosoma accessions that have complementary traits, resulting in greater genetic diversity within the breeding population [39].
The main method used for flowering in Xanthosoma species (like tannia or cocoyam) is GA3, which is crucial in enabling controlled pollination and the production of botanical seeds in Xanthosoma, essentially resulting in genetic improvement and breeding programs [12]. GA3 can be applied through foliar application, injection, or pre-plant soak [21,41,42]. The concentration of GA3 needed for floral induction varies based on factors such as the plant variety, plant density, climate conditions (especially light), soil characteristics, and more [15,16,23]. Scientific studies indicate the application of GA3 concentrations ranging from 250 ppm to 1500 ppm [16,22,23,25]. The flowering of cocoyam is influenced by both the genotype and the technique used for applying GA3 [22]. A lower concentration of 250 ppm to 500 ppm is suggested because different cultivars respond differently to GA3 treatment [24,25]. Clones that tend to flower naturally do well with 500 ppm GA3, while those that seldom flower need 1500 ppm GA3 [14]. Moreover, the floral induction in tannia is influenced by the concentration of GA3 and the age of the plant, with a concentration of 500 mg L−1 yielding the most favorable outcome [16].
Correspondingly, the greatest number of inflorescences and the highest pollen quantity in Tannia were achieved with GA3 concentrations of 750 and 1000 ppm [3]. Similarly, plants that were soaked in 500 ppm of GA3 for 30 min right before planting showed early flower emergence [23]. Furthermore, applying GA3 at 1500 ppm, either as a foliar spray or through pre-plant soaking, successfully enhanced flowering in Nigerian clones [22]. As discussed above, the application of GA3 is a well-documented method for floral induction in Xanthosoma; other approaches have also been explored. In some studies, 6-Benzyladenine, a type of cytokinin, has been used in conjunction with GA3 to promote flowering in Xanthosoma species due to the fact that cytokinins play a role in cell division and differentiation, which can influence the transition to reproductive growth [43]. This is the first study showing that using GA3 in combination with VIUSID® Agro could be an alternative approach that requires additional evaluations and may be developed into new strategies or utilized as a floral inductor in future studies. Therefore, while GA3 is the most commonly reported method, other hormonal treatments, environmental manipulations, and tissue culture techniques hold potential for floral induction in Xanthosoma. However, further research is needed to optimize these methods and understand the underlying physiological mechanisms.
Applying exogenous amino acids can indeed be a powerful tool for boosting flowering induction and seed production in plants. A complementary application of VIUSID Agro can significantly influence flowering induction through several interconnected mechanisms, such as regulating the synthesis and signaling of hormones like auxins, gibberellins, cytokinins, and ethylene, all of which play critical roles in floral initiation and development [42,44]. Another possible mechanism is by the regulation of the flowering pathways, including photoperiodic, vernalization, and autonomous pathways, and also by influencing the expression of key flowering genes like FLOWERING LOCUS T (FT), which both play a crucial role in promoting flowering [45]. Furthermore, they can contribute to the development and function of floral structures essential for pollination and fertilization because they are involved in the synthesis of proteins that guide pollen tube growth, a critical step in fertilization [46]. The synergistic effect of applying these amino acids together could be more pronounced (e.g., earlier flowering and an increased number of flowers) than applying them individually [47].
Pollination and fertilization are crucial steps that directly lead to subsequent seed production [13]. Natural pollination is often limited, and hand pollination is usually required to obtain seeds after flowering induction [48]. Moreover, the pollination mechanism in Xanthosoma is an interesting case of a unique relationship between plants and insects, mainly featuring scarab beetles from the genus Cyclocephala [48,49]. Several species of Xanthosoma show protogyny, which means that the female flowers are ready to receive pollen before the male flowers release it. The inflorescences are typically open for two nights [48]. On the first night, when the female flowers are ready to receive pollen, the inflorescence blooms, usually around dusk. They generate heat (thermogenesis), increasing their temperature just above the surrounding air, and emit a strong, sweet fragrance. These signals draw in nocturnal Cyclocephala beetles [13,41,50]. The intricate relationship between Xanthosoma species and their Cyclocephala beetle pollinators showcases the importance of scent, thermogenesis, and floral structure in ensuring successful pollination. In this context, amino acid-based growth promoters play a multifaceted role in enhancing the pollination process in plants, including Xanthosoma, although direct research specifically on Xanthosoma might be limited. It is well known that pollen grains contain a high level of amino acids, which are essential for their growth, development, and survival [51]. Moreover, amino acids can provide energy for developing pollen grains and the growing pollen tube, aiding the energy-demanding processes needed for fertilization [52]. Research indicates a positive link between certain amino acids, such as glycine and arginine, and the length of pollen tubes, which is crucial for successful fertilization [53]. These methods, particularly the use of gibberellic acid, have been crucial in enabling controlled pollination and the production of botanical seeds in Xanthosoma, which are essential for genetic improvement and breeding programs.
For effective fertilization and fruit maintenance, great attention is given to fruit sets with frequent application of irrigation and care of mechanical damage by insects. However, successful fertilization, which involves the merging of male and female gametes to create seeds, is due to their infrequent production of inflorescences or the sterility of the flowers. When breeding aims to enhance genetic diversity through fertilization, several methods can be valuable, based on research findings such as GA3 application, hand pollination, and the insect genus Cyclocephala [53,54]. Also, another factor that influences successful fertilization is plant maturity and health, timing of pollination, pollen viability, and controlled conditions, such as appropriate light, temperature, humidity, and plant density, and fertilization [48,55,56].
The capacity to generate seeds is essential for enhancing genetics, managing diseases, and gaining insights into the biology of the crop. Ongoing research focused on promoting flowering and producing viable seeds is important for the future growth and sustainability of Xanthosoma farming [12,57]. Studies indicate that Xanthosoma seeds can exhibit high germination rates of 85% [12]. In the current study, using GA3 in combination with VIUSID® Agro showed a high germination rate (92%), which was superior to that observed in this plant species (85%) with solely GA3 [12]. A possible explanation for this fact is due to various factors like photoperiod, fertilization, genotype, temperature, humidity, plant density, etc. Another probable cause of increased seed germination rate is the complementary application of VIUSID® Agro, which can activate the synthesis of storage compounds in seeds, which are crucial for germination and seedling establishment [58]. Also, it can regulate the nitrogen metabolism and nutrient uptake, which are essential for proper seed development [59]. Furthermore, it can enhance a plant’s tolerance to environmental stresses, which can negatively impact seed production, maintaining optimal reproductive development [60].
The findings of this research suggest that the combination of GA3 and VIUSID® Agro effectively promotes flowering and seed production in Xanthosoma species, maintaining a higher viability for three months after the seeds were stored at room temperature (8 °C and 80% relative humidity), leading to revitalizing the genetic diversity of these plants. Meanwhile, these findings are new for Cuba; there are still limitations in this research field. Further detailed studies are needed to explore more sustainable and cost-effective options, such as GA3 and VIUSID® Agro concentrations, organic fertilizers, biostimulants, and plant density, among others. These alternatives could enhance flowering and seed production, leading to the development of new lines that can better cope with climate change impacts. The introduction of new varieties from highly viable botanical seeds is a significant achievement, as few researchers and breeding programs for cocoyam Xanthosoma spp. have reached similar results. This advancement not only promotes the creation of new genetic combinations and the inheritance of traits but also enhances the overall adaptability of the crop. This situation aligns with the Sustainable Development Goals (established by the United Nations), like no poverty, zero hunger, good health and well-being, responsible consumption and production, climate action, and life on land, highlighting the importance of biodiversity in addressing food insecurity.
Breeding programs for Xanthosoma frequently arise due to the sporadic and erratic flowering of numerous cultivated varieties, which obstructs conventional cross-pollination methods. Even when flowering takes place, the low viability of pollen and seed production can complicate hybridization efforts. Moreover, the reliance on vegetative propagation via cormels restricts the genetic diversity available for selection, posing challenges in the introduction of new traits and enhancing resistance to pests and diseases. The limited availability of comprehensive genetic and genomic resources, in comparison to major crops, also constrains the use of advanced breeding techniques such as marker-assisted selection. In addition, a financially supported Xanthosoma breeding initiative may encounter expenses associated with land procurement, labor for cultivation and upkeep, specialized analytical equipment, and research staff, which could lead to a considerable financial commitment over multiple years without a promise of immediate returns. Furthermore, assessing the characteristics of new Xanthosoma cultivars in various field conditions is essential for identifying the best genotypes suited to the various challenges that are dependent on the regions.
5. Conclusions
This study provides the first comprehensive overview of how a combination of GA3 and VIUSID® Agro impacts flowering and seed production in various Xanthosoma accessions. Our findings demonstrate that applying GA3 (1000 mg L−1) combined with VIUSID® Agro (0.20 mg L−1) significantly boosts both flowering and subsequent seed production across all tested Xanthosoma accessions. This discovery is evident in key seed characteristics, including the seed length (1.0 to 1.5 mm), seed color (light yellow to strong yellow), a remarkable 92% seed germination rate, and seed mass (0.60 to 3.10 g). These novel insights highlight the potential of this combined phytohormonal and biostimulant approach to improve seed yield and quality in Xanthosoma species, offering valuable implications for future breeding and cultivation strategies. Further research could explore the broader applicability of this method in other understudied crops and could also investigate different dosages and intervals of application of VIUSID® Agro in promoting flowering with and without the presence of gibberellic acid (GA3), as well as how it interacts with environmental factors to enhance seed production. Additionally, the future of Xanthosoma improvement heavily relies on overcoming the limitations of its reproductive biology. The progress made in flower induction and seed production techniques, coupled with advancements in molecular breeding tools, offers a bright perspective for developing improved Xanthosoma varieties that can contribute to food security and economic development in the tropics.
Author Contributions
Conceptualization, A.J.-M. and A.M.; methodology, A.J.-M. and A.C.-H.; software, A.J.-M.; validation, A.J.-M., A.M., D.G.-G., A.C.-H. and K.P.-C.; formal analysis, A.M., A.M. and D.G.-G.; investigation, A.J.-M., A.M., D.G.-G., A.C.-H., K.P.-C. and B.K.; resources, A.M., A.M. and D.G.-G.; data curation, A.J.-M., A.M. and D.G.-G.; writing—original draft preparation, A.J.-M.; writing—review and editing, A.J.-M., A.M., D.G.-G., A.C.-H., K.P.-C. and B.K.; visualization, A.J.-M.; supervision, A.J.-M.; project administration, A.J.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
This manuscript includes all data generated or analyzed during this study. Other necessary data pertaining to this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank all the professionals and workers of the Research Institute of Tropical Roots and Tuber Crops (INIVIT) who collaborated directly or otherwise in obtaining the results. We also thank Bulent’s work team for providing commercial product VIUSID® Agro in its different presentations to carry out research. And special thanks go to the Vincent Lebot and Ivan Javier Pastrana Vargas for their support and contributions to the knowledge in the results.
Conflicts of Interest
Author Bulent Kukurtcu was employed by the company Catalysis, S.L. Macarena. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GA3 | Gibberellic acid |
| GIP | Genetic Improvement Program |
| INIVIT | Research Institute of Tropical Roots and Tuber Crops |
| CRAS | Agamic Seed Reproduction Center |
| GV1 | 500 mg L−1 of GA3 combined with foliar spraying of VIUSID® Agro at 0.2 mg L−1 |
| GV2 | 750 mg L−1 of GA3 combined with foliar spraying of VIUSID® Agro at 0.2 mg L−1 |
| GV3 | 1000 mg L−1 of GA3 combined with foliar spraying of VIUSID® Agro at 0.2 mg L−1 |
References
- Owusu-Darko, P.G.; Paterson, A.; Omenyo, E.L. Cocoyam (Corms and Cormels)—An Underexploited Food and Feed Resource. J. Agric. Chem. Environ. 2014, 3, 22–29. [Google Scholar] [CrossRef]
- O’Hair, S.K.; Asokan, M.P. Edible Aroids: Botany and Horticulture. In Horticultural Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1986; pp. 43–99. ISBN 978-1-118-06081-0. [Google Scholar]
- Soborío, F.; Gómez, L.; Torres, S.; Valverde, R. Introducción de Floración En Tiquisque (Xanthosoma ssp) En Cinco Regiones de Costa Rica. Agron. Costarric. 2000, 24, 37–45. [Google Scholar]
- Joseph, A.M.; Roland, A.G.K.G.; Georges, Y.K.A.; Bakari, K.A.; Simon-Pierre, N.A. Comparative Study of Dormant Bud Development in Taro [Xanthosoma sagittifolium, Xanthosoma sp. and Colocasia esculenta (L.) Schott] in Relation to Their Size and Localization on the Principal Tuber. J. Exp. Agric. Int. 2023, 45, 212–223. [Google Scholar] [CrossRef]
- Boakye, A.A.; Wireko-Manu, F.D.; Oduro, I.; Ellis, W.O.; Gudjónsdóttir, M.; Chronakis, I.S. Utilizing Cocoyam (Xanthosoma sagittifolium) for Food and Nutrition Security: A Review. Food Sci. Nutr. 2018, 6, 703–713. [Google Scholar] [CrossRef]
- Vaneker, K.; Slaats, E. Mapping Edible Aroids. Iridescent 2012, 2, 34–45. [Google Scholar] [CrossRef]
- Winara, A.; Fauziyah, E.; Suhartono; Widiyanto, A.; Sanudin; Sudomo, A.; Siarudin, M.; Hani, A.; Indrajaya, Y.; Achmad, B.; et al. Assessing the Productivity and Socioeconomic Feasibility of Cocoyam and Teak Agroforestry for Food Security. Sustainability 2022, 14, 11981. [Google Scholar] [CrossRef]
- Matikiti, A.; Allemann, J.; Kujeke, G.; Gasura, G.; Masekesa, T.; Chabata, I. Nutritional Composition of Cocoyam (Colocasia Esculenta), Grown in Manicaland Province in Zimbabwe. Asian J. Agric. Rural Dev. 2017, 7, 48–55. [Google Scholar] [CrossRef]
- Eleazu, C.O. Characterization of the Natural Products in Cocoyam (Colocasia Esculenta) Using GC–MS. Pharm. Biol. 2016, 54, 2880–2885. [Google Scholar] [CrossRef]
- Milián-Jiménez, M.D. Recursos genéticos de la malanga del género Xanthosoma Schott en Cuba. Cultiv. Trop. 2018, 39, 112–126. [Google Scholar]
- Jiménez, M.D.M.; Concepción, O.M.; Aguila, Y.F. Integrated Characterization of Cuban Germplasm of Cocoyam (Xanthosoma sagittifolium (L.) Schott). J. Plant Genet. Crop Res. 2018, 1, 1–18. [Google Scholar] [CrossRef]
- Goenaga, R.; Hepperly, P. Flowering Induction, Pollen and Seed Viability and Artificial Hybridization of Taniers (Xanthosoma Spp.). J. Agric. Univ. Puerto Rico 1990, 74, 253–260. [Google Scholar] [CrossRef]
- García-Robledo, C.; Kattan, G.; Murcia, C.; Quintero-Marín, P. Beetle Pollination and Fruit Predation of Xanthosoma daguense (Araceae) in an Andean Cloud Forest in Colombia. J. Trop. Ecol. 2004, 20, 459–469. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Kendabie, P.; Obidiegwu, O.; Amadi, C. Towards an Enhanced Breeding in Cocoyam: A Review of Past and Future Research Perspectives. Res. Rev. J. Bot. Sci. 2016, 5, 22–33. [Google Scholar]
- McDavid, C.R.; Alamu, S. Promotion of Flowering in Tannia (Xanthosoma sagittifolium) by Gibberellic Acid. Trop. Agric. 1976, 53, 373–374. [Google Scholar]
- McDavid, C.R.; Alamu, S. Effect of Daylength and Gibberellic Acid on the Growth and Promotion of Flowering in Tannia (Xanthosoma sagittifolium). Trop. Agric. 1979, 56, 17–23. [Google Scholar]
- Wu, K.; Xu, H.; Gao, X.; Fu, X. New Insights into Gibberellin Signaling in Regulating Plant Growth–Metabolic Coordination. Curr. Opin. Plant Biol. 2021, 63, 102074. [Google Scholar] [CrossRef] [PubMed]
- Galvão, V.C.; Horrer, D.; Küttner, F.; Schmid, M. Spatial Control of Flowering by DELLA Proteins in Arabidopsis Thaliana. Development 2012, 139, 4072–4082. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a Factor in Floral Regulatory Networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef]
- Sun, T. Gibberellin-GID1-DELLA: A Pivotal Regulatory Module for Plant Growth and Development. Plant Physiol. 2010, 154, 567–570. [Google Scholar] [CrossRef]
- Katsura, N.; Takayanagi, K.; Sato, T. Gibberellic Acid Induced Flowering in Cultivars of Japanese. Taro. J. Jpn. Soc. Hortic. Sci. 1986, 55, 69–74. [Google Scholar] [CrossRef]
- Wilson, J.E. Effects of Formulation and Method of Applying Gibberellic Acid on Flower Promotion in Cocoyam. Exp. Agric. 1981, 17, 317–322. [Google Scholar] [CrossRef]
- Alamu, S.; McDavid, C.R. Effect of Time and Method of Application of Gibberellic Acid on the Growth and Promotion of Flowering in Tannia (Xanthosoma sagittifolium). Trop. Agric. 1978, 55, 235–241. [Google Scholar]
- Onokpise, O.; Tambong, J.; Nyochembeng, L.; Wutoh, J. Acclimatization and Flower Induction of Tissue Culture Derived Cocoyam (Xanthosoma sagittifolium Schott) Plants. Agronomie 1992, 12, 193–199. [Google Scholar] [CrossRef]
- Alamu, S.; McDavid, C.R. Promotion of Flowering in Edible Aroids by Gibberellic Acid. Trop. Agric. 1978, 55, 81–86. [Google Scholar]
- Lebot, V.; Ivančič, A.; Lawac, F. Cocoyam (Xanthosoma sagittifolium (L.) Schott) Genetic Resources and Breeding: A Review of 50 Years of Research Efforts. Genet. Resour. Crop Evol. 2025, 72, 2593–2612. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Ginter, A.; Mystkowska, I.; Sikorska, A. The Positive Effects of Mechanical and Chemical Treatments with the Application of Biostimulants in the Cultivation of Solanum tuberosum L. Agriculture 2023, 13, 45. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.A.; da Silva, R.M.; Spaccini, R.; Mota, G.P.; Olivares, F.L. Biostimulants Using Humic Substances and Plant-Growth-Promoting Bacteria: Effects on Cassava (Manihot esculentus) and Okra (Abelmoschus esculentus) Yield. Agronomy 2023, 13, 80. [Google Scholar] [CrossRef]
- ALHadidi, N.; Pap, Z.; Ladányi, M.; Szentpéteri, V.; Kappel, N. Mycorrhizal Inoculation Effect on Sweet Potato (Ipomoea batatas (L.) Lam) Seedlings. Agronomy 2021, 11, 2019. [Google Scholar] [CrossRef]
- Calero Hurtado, A.; Meléndrez Rodríguez, J.F.; Peña Calzada, K.; Pérez Díaz, Y.; Jiménez Medina, A. Foliar Application of a Mixture of Amino Acid-Based Growth Promoters Enhances Tomato Seedling Production. Horticulturae 2025, 11, 582. [Google Scholar] [CrossRef]
- Calero Hurtado, A.; Aparecida Chiconato, D.; Sousa Junior, G.d.S.; Prado, R.d.M.; Peña Calzada, K.; Olivera Viciedo, D. Silicon Induces Salt Stress Amelioration in Sunflower Plants by Improving Photosynthetic Pigments and Mineral Status. Stresses 2024, 4, 860–869. [Google Scholar] [CrossRef]
- Peña-Calzada, K.; Calero-Hurtado, A.; Meléndrez-Rodríguez, J.F.; Rodríguez-Fernández, J.C.; Gutiérrez-Cádenas, O.G.; García-González, M.T.; Madrigal-Carmona, L.; Jiménez-Medina, A. Impacts of the Biostimulant VIUSID® Agro on Growth, Productivity, and Tolerance to Salt Stress in Crops: A Systematic Review. Horticulturae 2025, 11, 407. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Olivera Viciedo, D.; Habermann, E.; Calero Hurtado, A.; Lupino Gratão, P.; De Mello Prado, R.; Lata-Tenesaca, L.F.; Martinez, C.A.; Ajila Celi, G.E.; Rodríguez, J.C. Exogenous Application of Amino Acids Mitigates the Deleterious Effects of Salt Stress on Soybean Plants. Agronomy 2022, 12, 2014. [Google Scholar] [CrossRef]
- Calero Hurtado, A.; Peña Calzada, K.; Fasoli, J.V.B.; Jiménez, J.; Sánchez López, L. Synergic Effects of the Microbial Consortium and Amino Acid-Based Growth Promoter in Sunflower Productivity Under Water-Deficit Conditions. Water 2025, 17, 1365. [Google Scholar] [CrossRef]
- Marks, G.E. An Aceto-Carmine Glycerol Jelly for Use in Pollen-Fertility Counts. Stain Technol. 1954, 29, 277. [Google Scholar] [CrossRef] [PubMed]
- Cathebras, C.; Traore, R.; Malapa, R.; Risterucci, A.-M.; Chaïr, H. Characterization of Microsatellites in Xanthosoma sagittifolium (Araceae) and Cross-Amplification in Related Species. Appl. Plant Sci. 2014, 2, 1400027. [Google Scholar] [CrossRef]
- Pádua, J.G. Conservation of Crop Genetic Resources in Brazil in the Context of the Target 9 of the Global Strategy for Plant Conservation. Rodriguésia 2018, 69, 1557–1565. [Google Scholar] [CrossRef]
- Lebot, V. Tropical Root and Tuber Crops. Cassava, Sweet Potato, Yams and Aroids. Exp. Agric. 2009, 45, 382. [Google Scholar] [CrossRef]
- Beale, A.J.; Green, J.V.E.; Parrado, J.L. Inducement of Flowering in Taniers (Xanthosoma spp). J. Agric. Univ. Puerto Rico 1982, 66, 115–122. [Google Scholar] [CrossRef]
- Wilson, J.E. Cocoyam Breeding by Flower Induction, Pollination, and Germination, 4th ed.; Manual Ser; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 1979. [Google Scholar]
- Milet-Pinheiro, P.; Gomes Gonçalves, E.; do Amaral Ferraz Navarro, D.M.; Nuñez-Avellaneda, L.A.; Maia, A.C.D. Floral Scent Chemistry and Pollination in the Neotropical Aroid Genus Xanthosoma (Araceae). Flora 2017, 231, 1–10. [Google Scholar] [CrossRef]
- Khurana, J.P.; Tamot, B.K.; Maheshwari, S.C. Floral Induction in a Photoperiodically Insensitive Duckweed, Lemna Paucicostata LP6 1: Role of Glutamate, Aspartate, and Other Amino Acids and Amides. Plant Physiol. 1988, 86, 904–907. [Google Scholar] [CrossRef]
- Wu, W.; Du, K.; Kang, X.; Wei, H. The Diverse Roles of Cytokinins in Regulating Leaf Development. Hortic. Res. 2021, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Koshiyama, T.; Higashiyama, Y.; Mochizuki, I.; Yamada, T.; Kanekatsu, M. Ergothioneine Improves Seed Yield and Flower Number through FLOWERING LOCUS T Gene Expression in Arabidopsis Thaliana. Plants 2024, 13, 2487. [Google Scholar] [CrossRef]
- Navarro-León, E.; Borda, E.; Marín, C.; Sierras, N.; Blasco, B.; Ruiz, J.M. Application of an Enzymatic Hydrolysed L-α-Amino Acid Based Biostimulant to Improve Sunflower Tolerance to Imazamox. Plants 2022, 11, 2761. [Google Scholar] [CrossRef] [PubMed]
- Díaz, Y.P.; Hurtado, A.C.; Calzada, K.P.; Díaz, J.L.G.; González, V.R. Plant densities and foliar application of amino acids increasing sesame yield. Temas Agrar. 2024, 29, 100–112. [Google Scholar] [CrossRef]
- Gottsberger, G.; Silberbauer-Gottsberger, I.; Dötterl, S. Distant Populations of a Xanthosoma (Araceae) Species Have Different Floral Scents but the Same Cyclocephaline Beetle Pollinators. Acta Bot. Bras. 2020, 34, 580–588. [Google Scholar] [CrossRef]
- Amancio, G.; Hernández-Ortiz, V.; Aguirre-Jaimes, A.; Guevara, R.; Quesada, M. Feeding Specialization of Flies (Diptera: Richardiidae) in Aroid Infructescences (Araceae) of the Neotropics. J. Insect Sci. 2019, 19, 28. [Google Scholar] [CrossRef]
- Valerio, C. Notes on Phenology and Pollination of Xanthosoma wendlandii (Araceae) in Costa Rica. Rev. Biol. Trop. 1988, 36, 55–61. [Google Scholar]
- Jeannerod, L.; Carlier, A.; Schatz, B.; Daise, C.; Richel, A.; Agnan, Y.; Baude, M.; Jacquemart, A.-L. Some Bee-Pollinated Plants Provide Nutritionally Incomplete Pollen Amino Acid Resources to Their Pollinators. PLoS ONE 2022, 17, e0269992. [Google Scholar] [CrossRef]
- Kawade, K.; Tabeta, H.; Ferjani, A.; Hirai, M.Y. The Roles of Functional Amino Acids in Plant Growth and Development. Plant Cell Physiol. 2023, 64, 1482–1493. [Google Scholar] [CrossRef]
- Patel, T.; Singh, B.; Solanki, H. Effect of Abiotic Factors and Nutrition Elements on Pollen Germination and Pollen Viability. World J. Adv. Res. Rev. 2025, 25, 836–851. [Google Scholar] [CrossRef]
- Sama, A.E.; Hughes, H.G.; Abbas, M.S.; Shahba, M.A. An Efficient In Vitro Propagation Protocol of Cocoyam [Xanthosoma sagittifolium (L) Schott]. Sci. World J. 2012, 2012, 346595. [Google Scholar] [CrossRef]
- Asante, M.O.O.; Ahiakpa, J.K.; Amoatey, C.; Adjei-Nsiah, S. Effect of Shade and Level of Fertilizer Application on Nutrient Uptake and Dry Matter Partitioning in Cocoyam (Xanthosoma sagittifolium L.). J. Plant Nutr. 2017, 40, 2312–2325. [Google Scholar] [CrossRef]
- Suminarti, N.E.; Chriswanto, D.A.; Fajrin, A.N. Improvement of Taro Mbothe Yield (Xanthosoma sagittifolium L.) Schott on Dry Land Indonesia by Potassium Application and Tuber Size Selection. Asian J. Plant Sci. 2022, 21, 460–468. [Google Scholar] [CrossRef]
- Carole, D.A.; Benjamin, A.A.; Désiré, M.H.; Kévin, T.A.M.; Brice, A.S.; Maguy, N.B.A.; Motassy, M.D.; Jones, N.; Nicolas, N. Application of the PIF Method in Seed Multiplication in Xanthosoma sagittifolium L. Schott: Effect of the Mass of the Corm Fragment and Realization of the Field Transfer Test. Am. J. Agric. For. 2023, 11, 203–211. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wójtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling Biometric Traits, Yield and Nutritional and Antioxidant Properties of Seeds of Three Soybean Cultivars Through the Application of Biostimulant Containing Seaweed and Amino Acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Jeong, S.J.; Masabni, J.; Niu, G. Biostimulants Applied in Seedling Stage Can Improve Onion Early Bulb Growth: Cultivar- and Fertilizer-Type-Specific Positive Effects. Horticulturae 2025, 11, 402. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Liu, W.; Ji, J.; Zhang, K.; Li, H.; Feng, Y.; Xue, J.; Ji, C.; Zhang, L.; et al. Mechanisms of Branched Chain Amino Acids Promoting Growth and Lipid Accumulation in Camelina Sativa Seedlings under Drought and Salt Stress. Sustain. Energy Technol. Assess. 2025, 75, 104201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
