Changes of Catalase and Peroxidase Activity and Expression Under Cold Stress in Prunus persica Cultivars with Different Cold Tolerances
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. The Main Characteristics of the Cultivars
2.2.1. “Loadel”
2.2.2. “Springold”
2.2.3. “Podarok Like”
2.2.4. “Temisovskij”
2.3. Experimental Design
2.4. Antioxidant Enzyme Activity Assay
2.5. Total RNA Isolation and cDNA Synthesis
2.6. Expression of Some Peroxidases and Catalases Based on RT-PCR
2.7. Statistics
3. Results
3.1. Phenotypical Analyse
3.2. Biochemical Analyse
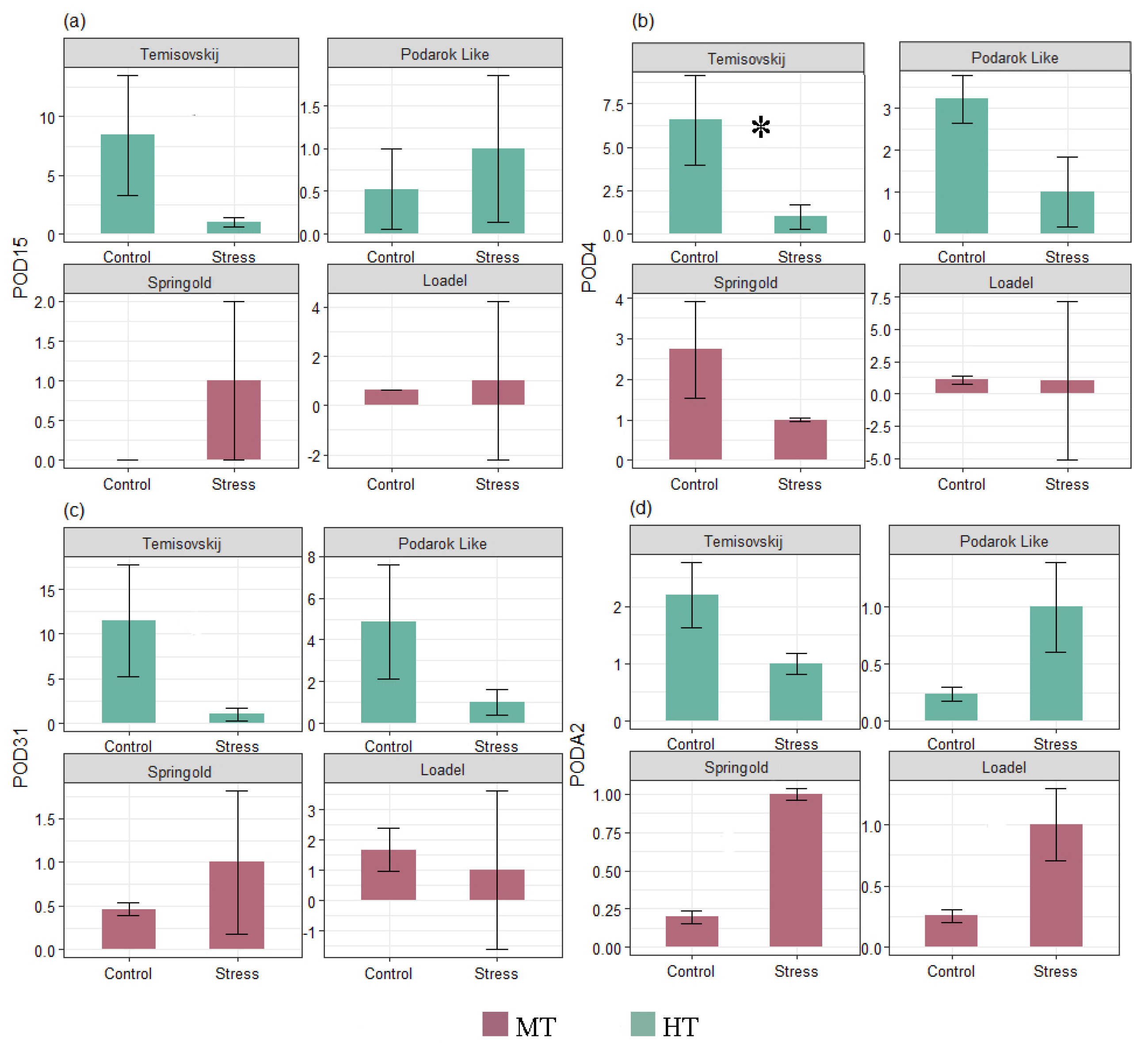
3.3. Gene Expression
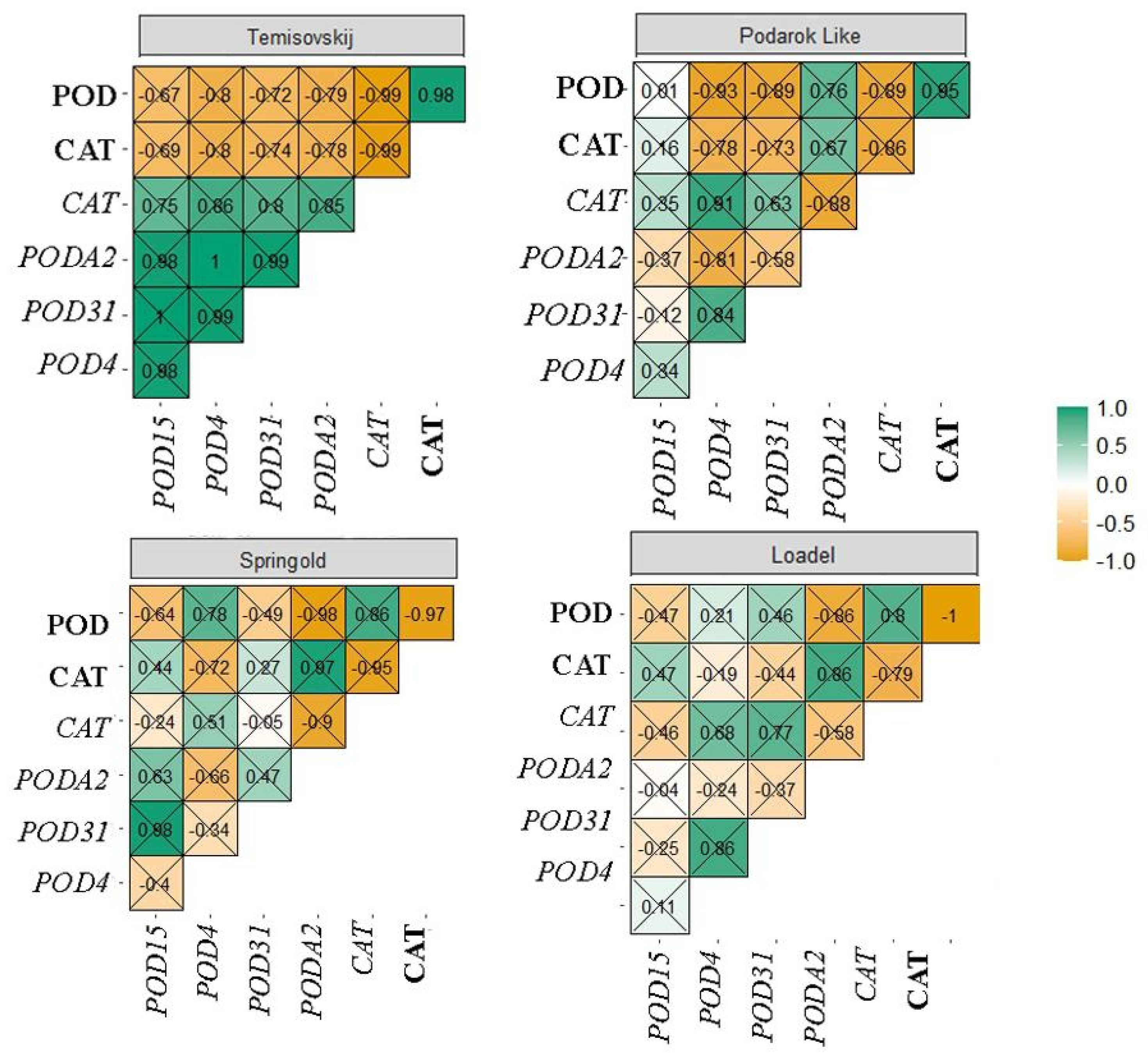
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mrázová, M.; Rampáčková, E.; Šnurkovič, P.; Ondrášek, I.; Nečas, T.; Ercisli, S. Determination of Selected Beneficial Substances in Peach Fruits. Sustainability 2021, 13, 14028. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverria, G. Current situation, trends and challenges for efficient and sustainable peach production. Sci. Hortic. 2022, 296, 110899. [Google Scholar] [CrossRef]
- Niu, R.; Zhao, X.; Wang, C.; Wang, F. Physiochemical responses and ecological adaptations of peach to low-temperature stress: Assessing the cold resistance of local peach varieties from Gansu, China. Plants 2023, 12, 4183. [Google Scholar] [CrossRef] [PubMed]
- Luna, V.; Reinoso, H.; Lorenzo, E.; Bottini, R.; Abdala, G. Dormancy in peach (Prunus persica L.) flower buds: II. Comparative morphology and phenology in floral and vegetative buds, and the effect of chilling and gibberellin A 3. Trees 1991, 5, 244–246. [Google Scholar] [CrossRef]
- Hillmann, L.; Elsysy, M.; Goeckeritz, C.; Hollender, C.; Rothwell, N.; Blanke, M.; Einhorn, T. Preanthesis changes in freeze resistance, relative water content, and ovary growth preempt bud phenology and signify dormancy release of sour cherry floral buds. Planta 2021, 254, 74. [Google Scholar] [CrossRef]
- Linden, L. Measuring Cold Hardiness in Woody Plants. Ph.D. Thesis, Department of Applied Biology, University of Helsinki, Helsinki, Finland, 2002. Publication No. 10. [Google Scholar]
- Chaar, J.E. Characterization of Peach [Prunus persica (L.) Batsch.] Cultivars for Frost Resistance. Acta Agronómica 2015, 64, 230–237. [Google Scholar] [CrossRef]
- Elmanova, T.S.; Opanasenko, N.E. Ecological and Physiological Characteristics of Peach; Agrarian Science: Moscow, Russia, 2010; pp. 17–54. (In Russian) [Google Scholar]
- Strimbeck, G.R.; Schaberg, P.G.; Fossdal, C.G.; Schröder, W.P.; Kjellsen, T.D. Extreme low temperature tolerance in woody plants. Front. Plant Sci. 2015, 6, 884. [Google Scholar] [CrossRef]
- Torres, E.; Miarnau, X. Frost Damage Mitigation in Flowers and Fruitlets of Peach and Almond from the Application of a Multi-Attribute Approach Biostimulant. Plants 2024, 13, 1603. [Google Scholar] [CrossRef]
- Drepper, B.; Bamps, B.; Gobin, A.; Van Orshoven, J. Strategies for managing spring frost risks in orchards: Effectiveness and conditionality—A systematic review protocol. Environ. Evid. 2021, 10, 32. [Google Scholar] [CrossRef]
- Ding, A.; Bao, F.; Ding, A.; Zhang, Q. Cold hardiness of prunus mume ‘Xiang ruibai’and its parents based on biological indexes and physical parameters. Forests 2022, 13, 2163. [Google Scholar] [CrossRef]
- Palonen, P.; Buszard, D. Current state of cold hardiness research on fruit crops. Can. J. Plant Sci. 1997, 77, 399–420. [Google Scholar] [CrossRef]
- Szalay, L.; Gyökös, I.G.; Békefi, Z. Cold Hardiness of Peach Flowers at Different Phenological Stages. Hort. Sci. 2018, 45, 119–124. [Google Scholar] [CrossRef]
- Layne, R.E.C. Cold hardiness of peaches and nectarines following a test winter. Fruit Var. J. 1982, 36, pp 90–98. [Google Scholar]
- Meng, Q.R.; Liang, Y.Q.; Wang, W.F.; Du, S.H.; Li, Y.H.; Yang, J.M. Study on Supercooling Point and Freeezing Point in Floral Organs of Apricot; Agricultural Sciences in China: Beijing, China, 2007; Volume 6, pp. 1330–1335. [Google Scholar]
- Gorina, V.M.; Richter, A.A.; Zaitsev, G.P. Response of generative buds of apricot plants to artificial freezing. Statistica 2012, 5, 6. (In Russian) [Google Scholar]
- Halász, J.; Hegedűs, A.; Karsai, I.; Tósaki, Á.; Szalay, L. Correspondence between SOC1 Genotypes and Time of Endodormancy Break in Peach (Prunus persica L. Batsch) Cultivars. Agronomy 2021, 11, 1298. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Ning, L.; Li, B.; Bao, M. Quantitative Proteomic Analysis Provides Novel Insights into Cold Stress Responses in Petunia Seedlings. Front. Plant Sci. 2016, 7, 136. [Google Scholar] [CrossRef]
- Bai, M.; Zeng, W.; Chen, F.; Ji, X.; Zhuang, Z.; Jin, B.; Wang, J.; Jia, L.; Peng, Y. Transcriptome expression profiles reveal response mechanisms to drought and drought-stress mitigation mechanisms by exogenous glycine betaine in maize. Biotechnol. Lett. 2022, 44, 367–386. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Sarwar, R.; Zhang, W.; Geng, R.; Zhu, K.-M.; Tan, X.-L. Research progress on the physiological response and molecular mechanism of cold response in plants. Front. Plant Sci. 2024, 15, 1334913. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 2016, 7, 570. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, A.; Dietz, K.J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Gould, K.S. Are betalain pigments the functional homologues of anthocyanins in plants? Environ. Exp. Bot. 2015, 119, 48–53. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Rong, J.D.; Li, S.P.; He, T.Y.; Chen, L.Y.; Zheng, Y.S. Effect of low temperature on biochemical characteristics of Prunus campanulata Maxim. J. Fujian Coll. For. 2013, 33, 326–329. [Google Scholar]
- Wang, Y.; Li, Y.; Wang, J.; Xiang, Z.; Xi, P.; Zhao, D. Physiological changes and differential gene expression of tea plants (Camellia sinensis (L.) Kuntze var. niaowangensis QH Chen) under cold stress. DNA Cell Biol. 2021, 40, 906–920. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- An, X.; Xu, Y.; Jiang, L.; Huan, C.; Yu, Z. Effects of postharvest temperature on apoptosis-related enzyme activity and gene expression in peach fruits (Prunus persica L. cv. Xiahui 8). Sci. Hortic. 2019, 245, 178–184. [Google Scholar] [CrossRef]
- Brizzolara, S.; Hertog, M.; Tosetti, R.; Nicolai, B.; Tonutti, P. Metabolic responses to low temperature of three peach fruit cultivars differently sensitive to cold storage. Front. Plant Sci. 2018, 9, 706. [Google Scholar] [CrossRef]
- Wang, K.; Yin, X.R.; Zhang, B.; Grierson, D.; Xu, C.J.; Chen, K.S. Transcriptomic and metabolic analyses provide new insights into chilling injury in peach fruit. Plant Cell Environ. 2017, 40, 1531–1551. [Google Scholar] [CrossRef]
- Nilo-Poyanco, R.; Vizoso, P.; Sanhueza, D.; Balic, I.; Meneses, C.; Orellana, A.; Campos-Vargas, R. A Prunus persica genome-wide RNA-seq approach uncovers major differences in the transcriptome among chilling injury sensitive and non-sensitive varieties. Physiol. Plant. 2019, 166, 772–793. [Google Scholar] [CrossRef] [PubMed]
- Szalay, L.; Hegedűs, A.; Stefanovits-Bányai, É. Presumable protective role of peroxidase and polyphenol oxidase enzymes against freezing stress in peach (Prunus persica/L./Batsch). Acta Biol. Szeged. 2005, 49, 121–122. [Google Scholar]
- Yu, D.J.; Jun, S.H.; Park, J.; Kwon, J.H.; Lee, H.J. Transcriptome analysis of genes involved in cold hardiness of peach tree (Prunus persica) shoots during cold acclimation and deacclimation. Genes 2020, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Tian, Q.; Zhou, Y.; Xu, J.; Chang, R.; Chen, H.; Liu, G. Quantitative Proteomic Analyses on the Mechanisms of Cold Tolerance in Two Peach Cultivars (Prunus persica L. Batsch) Based on ITRAQ. Eur. J. Hortic. Sci. 2021, 86, 308–319. [Google Scholar] [CrossRef]
- Jiao, Y.; Shen, Z.; Yan, J. Transcriptome analysis of peach [Prunus persica (L.) Batsch] stigma in response to low-temperature stress with digital gene expression profiling. J. Plant Biochem. Biotechnol. 2017, 26, 141–148. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Q.; Wang, Z.; Li, J.; Liu, S.; Chang, R.; Chen, H.; Liu, G. Integrated analysis of transcriptomics and metabolomics of peach under cold stress. Front. Plant Sci. 2023, 14, 1153902. [Google Scholar] [CrossRef]
- Plugatar, Y.V. (Ed.) Atlas of Cultivars of Fruit Crops From the Collection of the Nikitsky Botanical Gardens; PH “ARIAL”: Simferopol, Russia, 2018; 400p. (In Russian) [Google Scholar]
- Smykov, A.V.; Mesyats, N.V. Frost resistance and placement of peach cultivars in the Crimea. Plant Biol. Hortic. Theory Innov. 2024, 1, 67–80. (In Russian) [Google Scholar]
- Mitrofanova, I.; Lesnikova-Sedoshenko, N.; Tsiupka, V.; Smykov, A.; Mitrofanova, O. Use of Biotechnological Methods to Support the Production of New Peach Hybrids. Horticulturae 2021, 7, 533. [Google Scholar] [CrossRef]
- Fogle, H.W.K. Peach production. In Agriculture Handbook; USDA Agricultural Research Service: Washington, DC, USA, 1974; 90p. [Google Scholar]
- Korsakova, S.P.; Korsakov, P.B. Changes in climate norms on the southern coast of Crimea over the past 90 years. Plant Biol. Hortic. Theory Innov. 2023, 2, 84–95. (In Russian) [Google Scholar]
- De Melo-Abreu, J.P.; Mateos, L.; Villalobos, F.J. Frost protection. In Principles of Agronomy for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2024; pp. 497–511. [Google Scholar]
- Voskresenskaya, O.L. Bol’shoi Praktikum po Bioekologii (An Extended Practical Course in Biological Ecology); Mariisk. Univ.: Ioshkar Ola, Russia, 2006. [Google Scholar]
- Novikova, I.I.; Popova, E.V.; Kovalenko, N.M.; Krasnobaeva, I.L. The Effect of Bacillus subtilis in Combination with Chitosan Salicylate on Peroxidase and Catalase Activity in B. sorokiniana Infected Wheat. Appl. Biochem. Microbiol. 2024, 60, 241–250. [Google Scholar] [CrossRef]
- Vodiasova, E.; Meger, Y.; Uppe, V.; Tsiupka, V.; Chelebieva, E.; Smykov, A. Class III Peroxidases in the Peach (Prunus persica): Genome-Wide Identification and Functional Analysis. Plants 2024, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.S.; Kurjogi, M.M.; Khalil-ur-Rehman, M.; Pervez, T.; Songtao, J.; Fiaz, M.; Jogaiah, S.; Wang, C.; Fang, J. Drought stress revealed physiological, biochemical and gene-expressional variations in ‘Yoshihime’peach (Prunus persica L.) cultivar. J. Plant Interact. 2018, 13, 83–90. [Google Scholar] [CrossRef]
- Tong, Z.; Gao, Z.; Wang, F.; Zhou, J.; Zhang, Z. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol. Biol. 2009, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rezaie, R.; Abdollahi Mandoulakani, B.; Fattahi, M. Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Sci. Rep. 2020, 10, 5290. [Google Scholar] [CrossRef]
- Karami-Moalem, S.; Maali-Amiri, R.; Kazemi-Shahandashti, S.S. Effect of cold stress on oxidative damage and mitochondrial respiratory properties in chickpea. Plant Physiol. Biochem. 2018, 122, 31–39. [Google Scholar] [CrossRef]
- Su, T.; Wang, P.; Li, H.; Zhao, Y.; Lu, Y.; Dai, P.; Ren, T.; Wang, X.; Li, X.; Shao, Q.; et al. The Arabidopsis catalase triple mutant reveals important roles of catalases and peroxisome-derived signaling in plant development. J. Integr. Plant Biol. 2018, 60, 591–607. [Google Scholar] [CrossRef]
- Skadsen, R.W.; Schulze-Lefert, P.; Herbst, J.M. Molecular cloning, characterization and expression analysis of two catalase isozyme genes in barley. Plant Mol. Biol. 1995, 29, 1005–1014. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Y.; Chen, D.; Liu, D.; Hu, M.; Dong, J.; Zhang, X.; Song, L.; Shen, F. The catalase gene family in cotton: Genome-wide characterization and bioinformatics analysis. Cells 2019, 8, 86. [Google Scholar] [CrossRef]
- Lee, S.H.; An, C.S. Differential expression of three catalase genes in hot pepper (Capsicum annuum L.). Mol. Cells 2005, 20, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, F.; Danti, S.; Magherini, V.; Cozza, R.; Innocenti, A.M.; Racchi, M.L. Molecular cloning, characterisation and expression of two catalase genes from peach. Funct. Plant Biol. 2004, 31, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef]
- Oldonil, C.M.; Nienow, A.A.; Schons, J.; Mayer, A.N. Peroxidase activity and initial growth of ‘Barbosa’ peach on clonal rootstocks. Rev. Bras. Frutic. 2019, 41, e-086. [Google Scholar]
- Passardi, F.; Longet, D.; Penel, C.; Dunand, C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 2004, 65, 1879–1893. [Google Scholar] [CrossRef]
- Cai, K.W.; Liu, H.; Chen, S.; Zhao, X.Y.; Chen, S. Genome-wide identification and analysis of Class III peroxidases in Betula pendula. BMC Genom. 2021, 22, 314. [Google Scholar] [CrossRef]
- Su, P.S.; Yan, J.; Li, W.; Wang, L. A member of wheat class III peroxidase gene family, Ta PRX-2A, enhanced the tolerance of salt stress. BMC Plant Biol. 2020, 20, 15. [Google Scholar] [CrossRef]
- Aleem, M.; Riaz, A.; Raza, Q.; Aleem, M.; Aslam, M.; Kong, K.; Atif, R.M.; Kashif, M.; Bhat, J.A.; Zhao, T. Genome-wide characterization and functional analysis of class III peroxidase gene family in soybean reveal regulatory roles of GsPOD40 in drought tolerance. Genomics 2022, 114, 45–60. [Google Scholar] [CrossRef]
- Jiao, C.; Chai, Y.; Duan, Y. Inositol 1,4,5-trisphosphate mediates nitric-oxide-induced chilling tolerance and defense response in postharvest peach fruit. J. Agric. Food Chem. 2019, 67, 4764–4773. [Google Scholar] [CrossRef]
- Song, C.; Zhao, Y.; Li, A.; Qi, S.; Lin, Q.; Duan, Y. Postharvest nitric oxide treatment induced the alternative oxidase pathway to enhance antioxidant capacity and chilling tolerance in peach fruit. Plant Physiol. Biochem. 2021, 167, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Del-Saz, N.F.; Ribas-Carbo, M.; McDonald, A.E.; Lambers, H.; Fernie, A.R.; Florez-Sarasa, I. An in vivo perspective of the role (s) of the alternative oxidase pathway. Trends Plant Sci. 2018, 23, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rajakulendran, N.; Amirsadeghi, S.; Vanlerberghe, G.C. Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiol. Plant. 2011, 142, 339–351. [Google Scholar] [CrossRef] [PubMed]
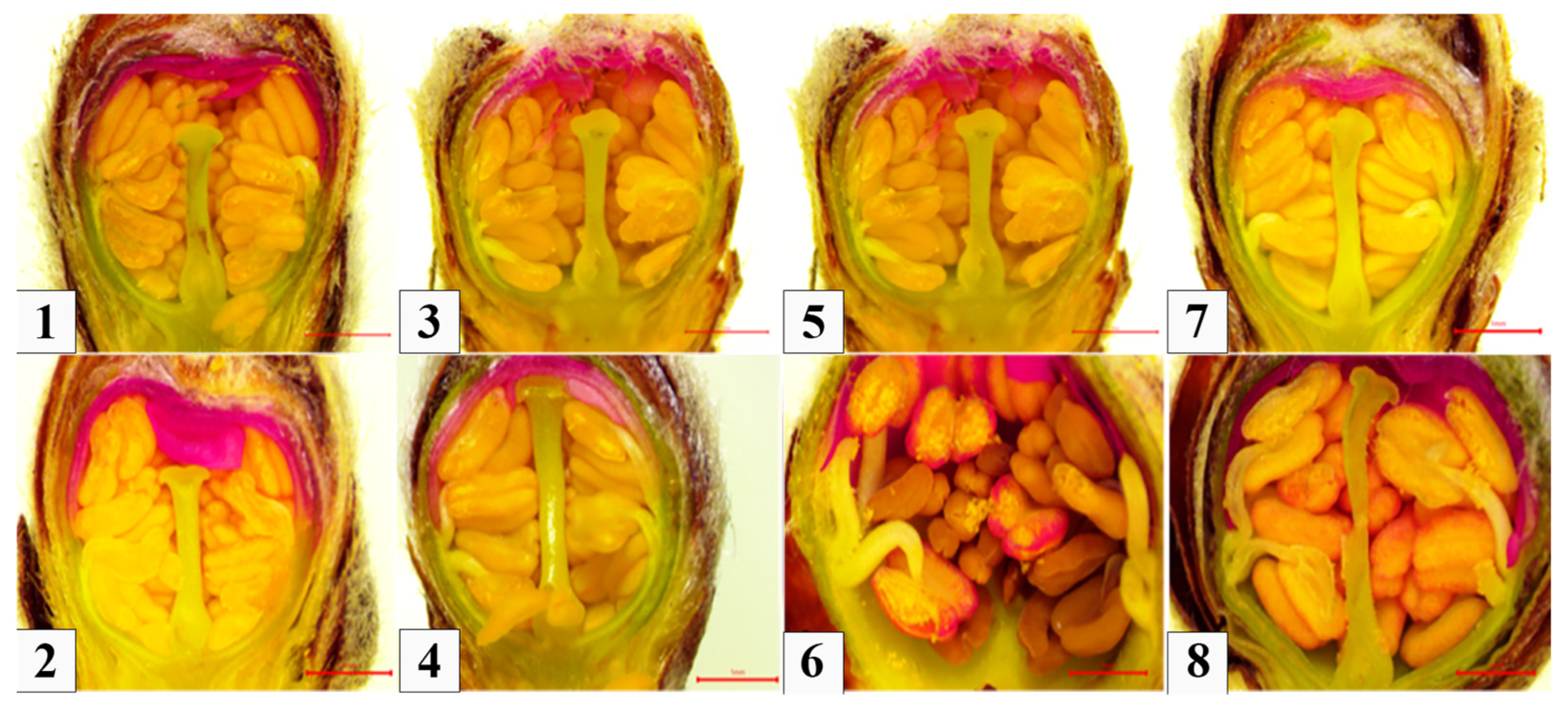
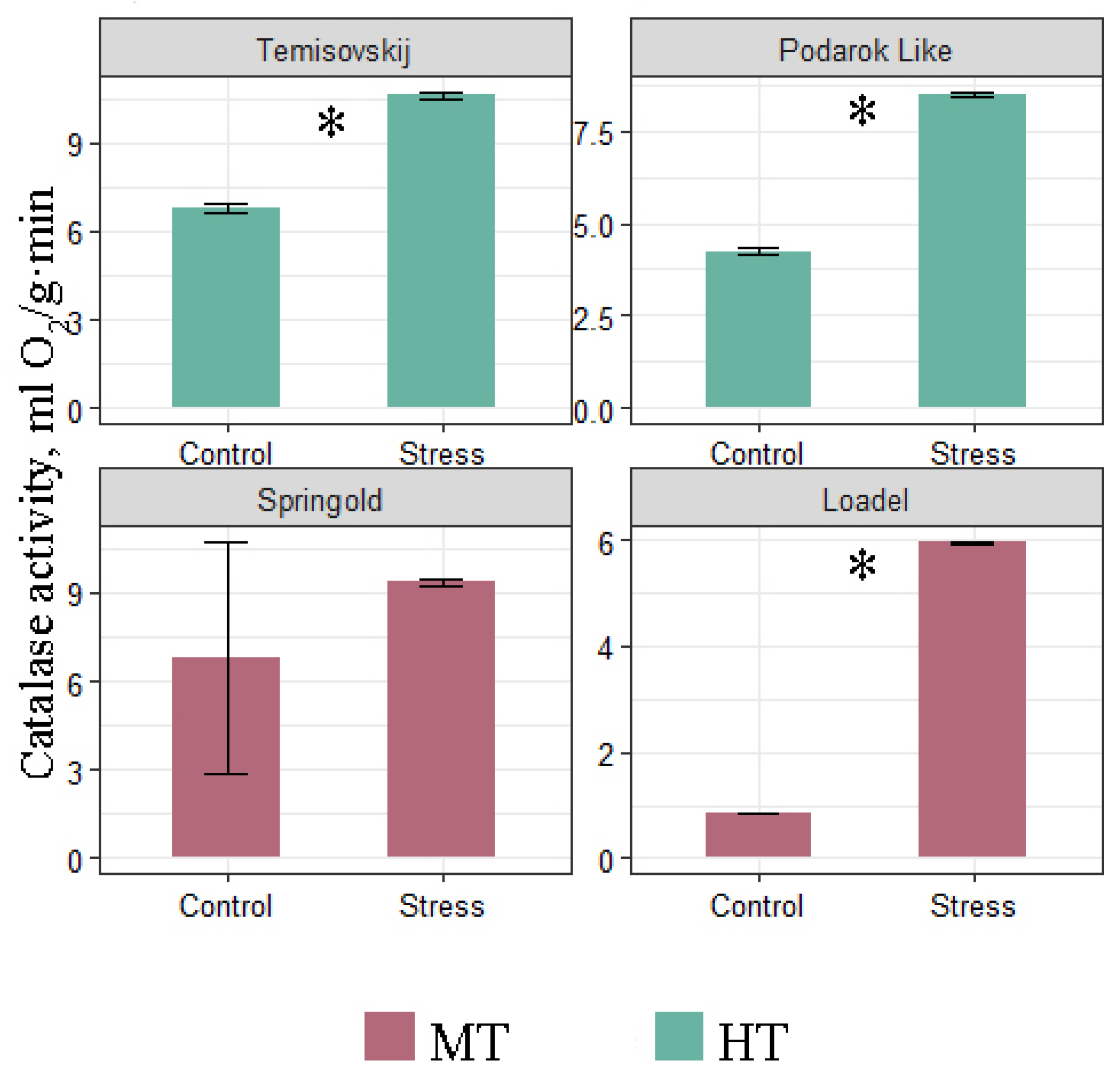
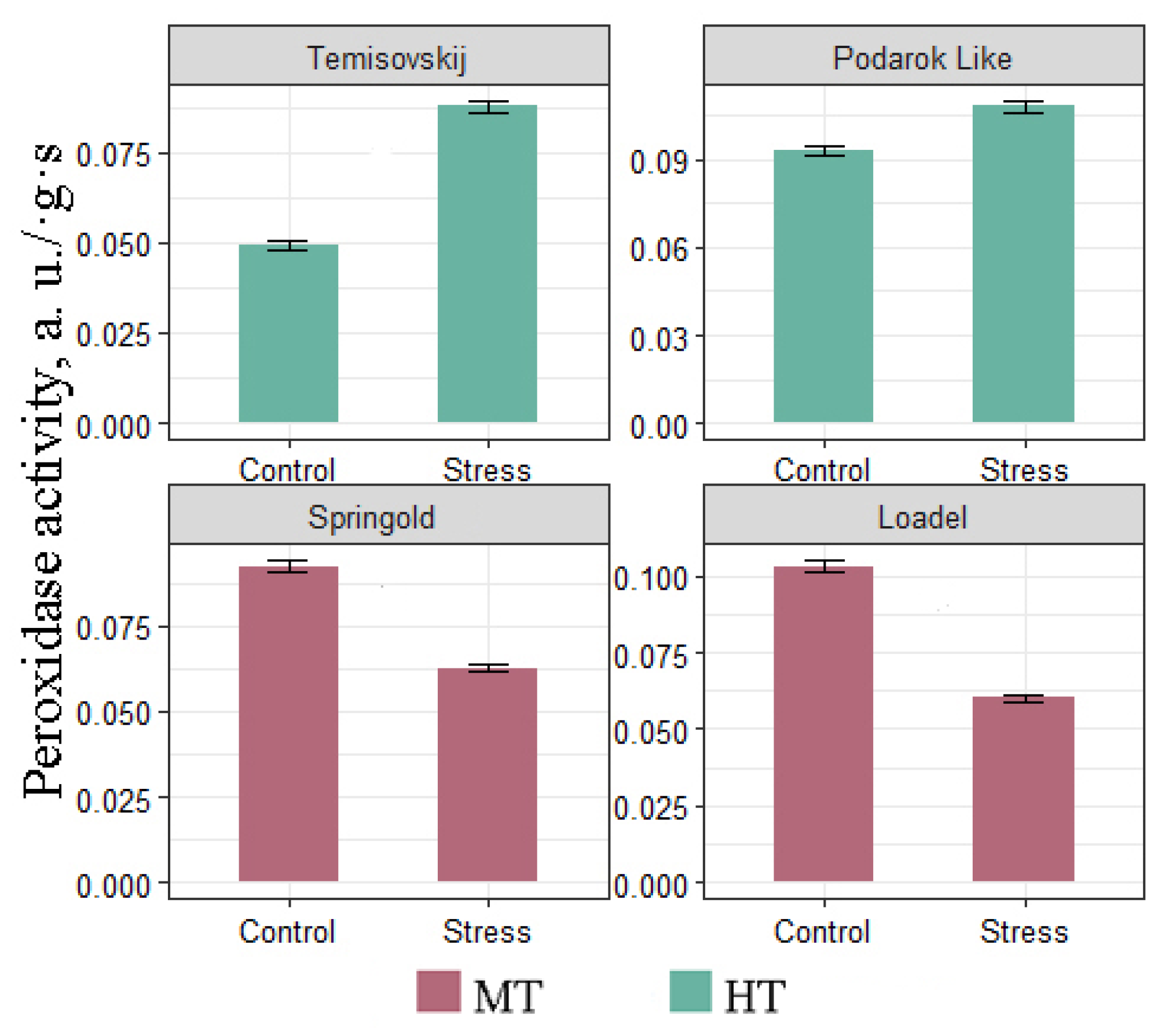
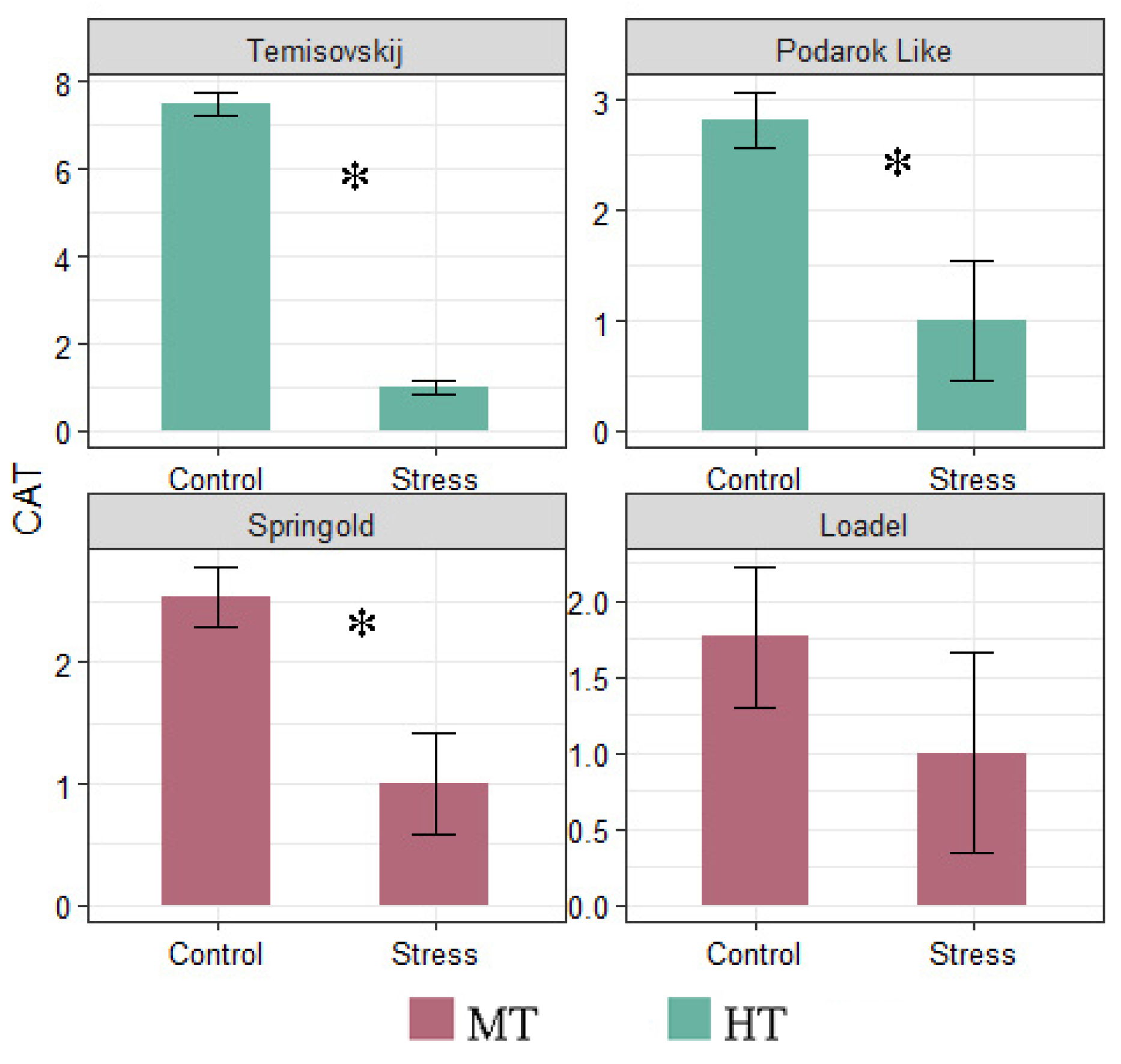

| Variety | Study Period, Freezing Mode | Reference | ||
|---|---|---|---|---|
| January −16 °C | February −16 °C | March −12 °C | ||
| Loadel | 25.9 | 25.0 | 15.9 | This study |
| Springold | 9.2 | 32.7 | 25.3 | This study |
| Podarok Like | 88.2 | 63.4 | 46.6 | [41] |
| Temisovskij | 97.5 | 56.1 | 77.3 | [41] |
| Gene Name | Sequence | Reference |
|---|---|---|
| CAT | TTCTGGAAAGCGTGAGAAGTGC | This study |
| TGTCTGGCGCCCAAGATCTGTA | ||
| PODA2 | ACTTAGACCCCACAACTCCG | [37] |
| CTCCCCATTACTTCCCACCA | ||
| POD4 | CATTGCTGCTCGAGACTCCGTT | This study |
| AGCTGGCTGAGGGTAGAAGTGG | ||
| POD15 | TCCAGGGTTGTGATGGTTCG | [49] |
| AGACAACACCAGGGCAAACA | ||
| POD31 | CCCTACTACAACGTACCGCT | [37] |
| ACCTGGATGAGCTGAGACAC | ||
| GAPDH | ATTTGGAATCGTTGAGGGTCTTATG | [50] |
| AATGATGTTGAAGGAAGCAGCAC | ||
| ACT | GTTATTCTTCATCGGCGTCTTCG | [50] |
| CTTCACCATTCCAGTTCCATTGTC |
| Df | Sum | Mean | F value | Pr (>F) | |
|---|---|---|---|---|---|
| POD15 | |||||
| Peach varieties | 3 | 49.14 | 16.38 | 1.93 | 0.175 |
| Temperature | 1 | 6.63 | 6.63 | 0.781 | 0.393 |
| Residuals | 13 | 110.35 | 8.489 | ||
| POD4 | |||||
| Peach varieties | 3 | 17.58 | 5.86 | 1.932 | 0.1743 |
| Temperature | 1 | 23.07 | 23.066 | 7.606 | 0.0163 * |
| Residuals | 13 | 39.43 | 3.033 | ||
| POD31 | |||||
| Peach varieties | 3 | 75.91 | 25.3 | 1.86 | 0.1862 |
| Temperature | 1 | 53.99 | 53.99 | 3.969 | 0.0678 |
| Residuals | 13 | 176.85 | 13.6 | ||
| PODA2 | |||||
| Peach varieties | 3 | 2.715 | 0.905 | 2.404 | 0.114 |
| Temperature | 1 | 0.453 | 0.453 | 1.203 | 0.293 |
| Residuals | 13 | 4.894 | 0.3765 | ||
| CAT | |||||
| Peach varieties | 3 | 22.58 | 7.53 | 4.072 | 0.03044 * |
| Temperature | 1 | 28.12 | 28.12 | 15.209 | 0.00183 ** |
| Residuals | 13 | 24.03 | 1.85 | ||
| CAT | |||||
| Peach varieties | 3 | 101.51 | 33.84 | 107.60 | 4.20 · 10−12 *** |
| Temperature | 1 | 92.71 | 92.71 | 294.80 | 4.99 · 10−13 *** |
| Residuals | 19 | 5.97 | 0.31 | ||
| POD | |||||
| Peach varieties | 3 | 0.003344 | 0.0011148 | 3.194 | 0.047 * |
| Temperature | 1 | 0.00014 | 0.0001402 | 0.402 | 0.534 |
| Residuals | 19 | 0.006631 | 0.000349 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vodiasova, E.; Chelebieva, E.; Kladchenko, E.; Grebennikova, O.; Uppe, V.; Tsiupka, V.; Dolgov, S.; Smykov, A. Changes of Catalase and Peroxidase Activity and Expression Under Cold Stress in Prunus persica Cultivars with Different Cold Tolerances. Agronomy 2025, 15, 556. https://doi.org/10.3390/agronomy15030556
Vodiasova E, Chelebieva E, Kladchenko E, Grebennikova O, Uppe V, Tsiupka V, Dolgov S, Smykov A. Changes of Catalase and Peroxidase Activity and Expression Under Cold Stress in Prunus persica Cultivars with Different Cold Tolerances. Agronomy. 2025; 15(3):556. https://doi.org/10.3390/agronomy15030556
Chicago/Turabian StyleVodiasova, Ekaterina, Elina Chelebieva, Ekaterina Kladchenko, Oksana Grebennikova, Victoria Uppe, Valentina Tsiupka, Sergey Dolgov, and Anatoly Smykov. 2025. "Changes of Catalase and Peroxidase Activity and Expression Under Cold Stress in Prunus persica Cultivars with Different Cold Tolerances" Agronomy 15, no. 3: 556. https://doi.org/10.3390/agronomy15030556
APA StyleVodiasova, E., Chelebieva, E., Kladchenko, E., Grebennikova, O., Uppe, V., Tsiupka, V., Dolgov, S., & Smykov, A. (2025). Changes of Catalase and Peroxidase Activity and Expression Under Cold Stress in Prunus persica Cultivars with Different Cold Tolerances. Agronomy, 15(3), 556. https://doi.org/10.3390/agronomy15030556






