3.3.1. Comparison of pH Measurements for Different Textiles
The pH values of substrates containing viscose fibers showed mild alkalinity throughout the experiment, with significant differences observed mainly in the composting systems (
Table 6). In outdoor composting, the pH ranged from 8.86 to 9.14, with the second sampling significantly higher than the first and third (
p = 0.00), suggesting increased microbial activity during the mid-phase of biodegradation. Controlled composting also revealed statistically significant differences (
p = 0.01), as pH increased from 8.87 to 9.12 and then slightly decreased to 8.96 in the final sampling. In contrast, outdoor (
p = 0.83) and home vermicomposting (
p = 0.914) did not show significant changes, maintaining relatively stable pH from 8.8 to 9.3, which indicates a well-buffered environment with stable biological processes.
For cotton-based substrates, pH values remained relatively stable in all composting systems, varying only slightly within the alkaline range of 8.75–9.27 (
Table 7). No statistically significant differences were detected across sampling times for any system (all
p > 0.05). Outdoor composting maintained an average pH of about 9.0, while controlled composting and both vermicomposting variants showed homogeneous pH (
p = 0.23–0.96). The stable pH profile indicates that cotton degradation proceeds under consistent biochemical conditions without acidification or strong alkalization phases.
Linen-containing substrates exhibited greater variability in pH among the systems (
Table 6). Significant differences were recorded during controlled composting (
p = 0.038), where the pH increased from 8.63 to 9.04 and then slightly decreased to 8.98. A similar trend was observed in outdoor composting (
p = 0.01), with the highest value (9.11) during the second sampling, followed by a minor decline to 8.96 in the third. In contrast, outdoor (
p = 0.11) and home vermicomposting (
p = 0.33) maintained stable values from 8.9 to 9.2 without statistically significant differences. This stability in vermicomposting systems suggests that the worm-assisted process buffered pH fluctuations more effectively than traditional composting.
3.3.2. Electrical Conductivity (EC)
EC reflects the dynamics of mineralization and nutrient release during biodegradation of the tested textiles (
Table 7). For viscose, the EC values showed a general downward trend during composting, while vermicomposting systems remained more stable. In outdoor composting, EC decreased from 1750 μS cm
−1 at the first sampling to 1551 μS cm
−1 and 1565 μS cm
−1 at the second and third samplings, respectively, with statistically significant differences among all samplings (letters c, a, b). In controlled composting, a continuous decrease was recorded (1730 → 1630 → 1332 μS cm
−1; a, ab, b), confirming a gradual reduction in soluble ion concentration as the compost matured. In outdoor vermicomposting, EC values (1536, 1500 and 1910 μS cm
−1; b, a, b) significantly differed, showing a temporary increase in the final sampling, possibly due to enhanced mineral release through worm activity. Home vermicomposting displayed only minor variations (1749, 1751 and 1711 μS cm
−1; b, a, b), yet with statistically detectable differences, indicating a mild but balanced ionic fluctuation.
Cotton-based substrates exhibited a similar pattern. In outdoor composting, EC decreased from 1836 to 1390 and 1530 μS cm−1 (a, b, b), revealing a significant reduction compared to the initial phase. Controlled composting (1644, 1680 and 1474 μS cm−1; all a) showed no significant differences, confirming a homogeneous decomposition process. Outdoor vermicomposting presented more pronounced variability (1604, 1580 and 1624 μS cm−1; c, a, b), whereas home vermicomposting (1835, 1640 and 1799 μS cm−1; a, b, b) demonstrated a decrease at the second sampling followed by partial recovery.
For linen, the EC in outdoor composting decreased markedly from 1908 to 1530 and 1481 μS cm−1 (b, a, ab), reflecting stabilization of soluble ions during organic matter decomposition. Controlled composting (1726, 1610 and 1434 μS cm−1; all a) revealed no significant changes, while outdoor vermicomposting (1682, 1560 and 1483 μS cm−1; c, a, b) exhibited a consistent decline. In home vermicomposting, EC values remained stable (1908, 1580 and 1913 μS cm−1; all a), indicating well-buffered conditions with no measurable accumulation of salts.
3.3.3. Volatile Solids (VS)
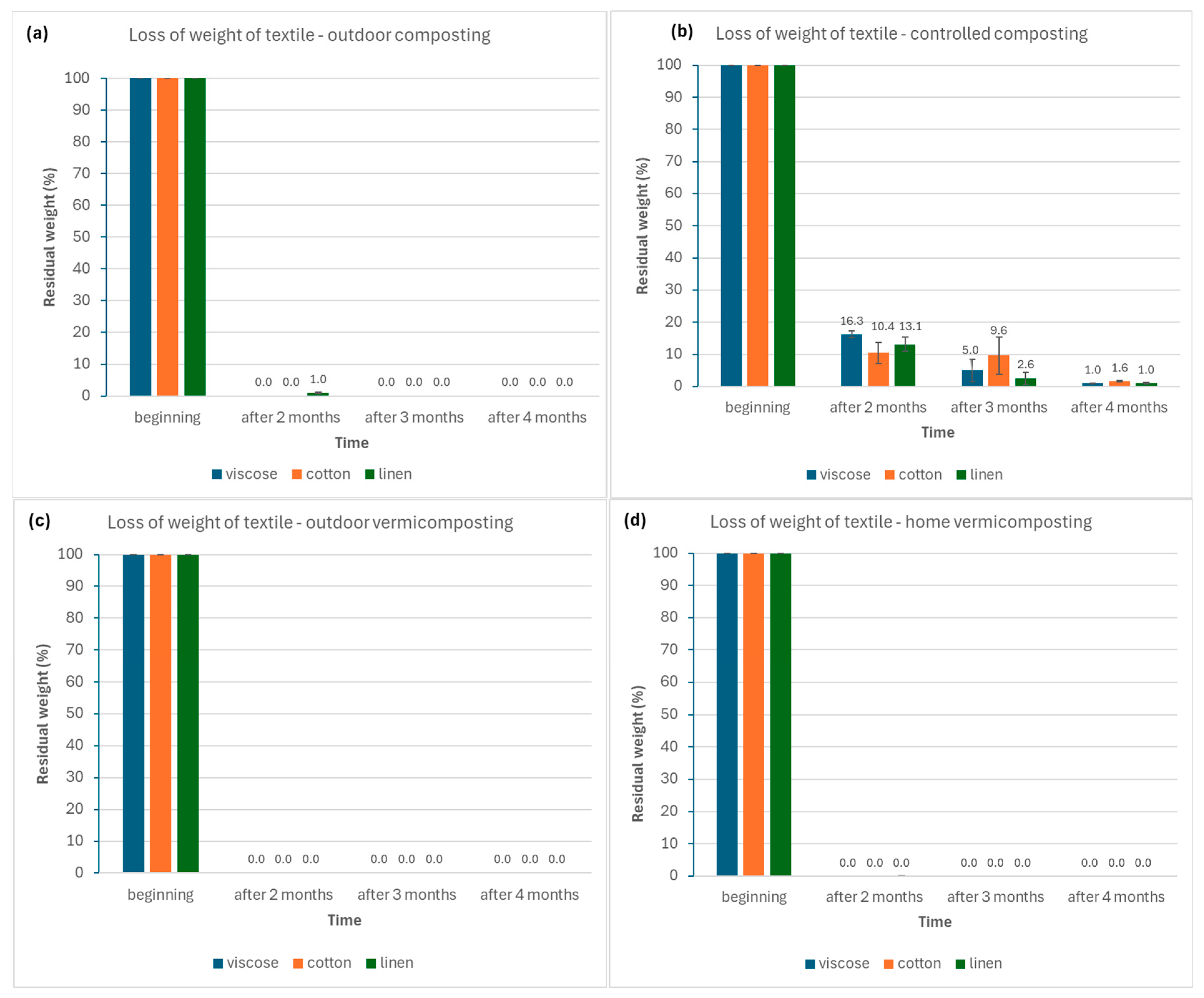
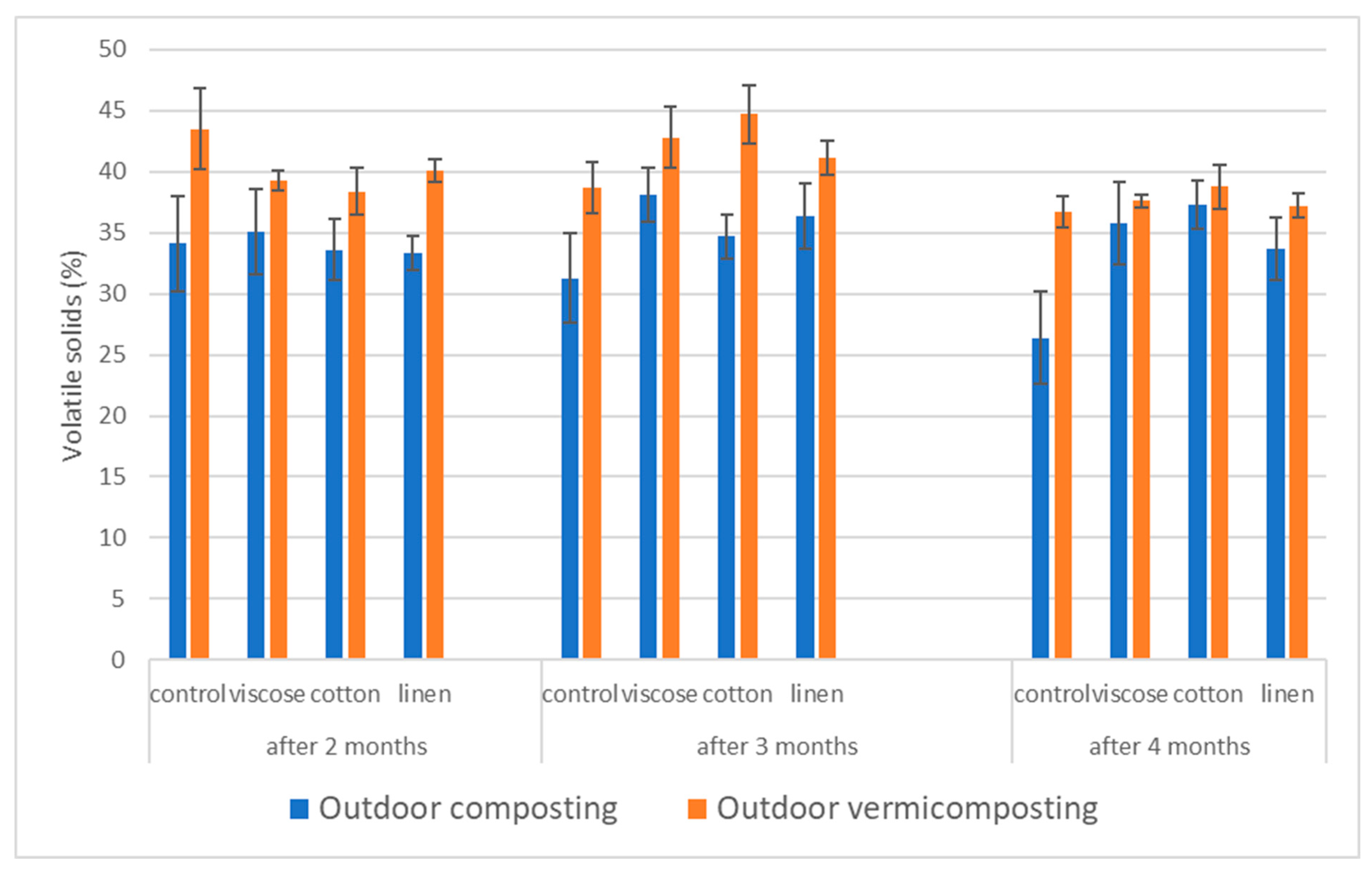
The proportion of vs. represents the percentage of organic matter subject to microbial degradation and serves as an indirect indicator of mineralization progress (
Figure 5).
For viscose, the outdoor composting system exhibited the highest vs. content at the second sampling (three months), followed by a gradual decline toward the third sampling, in line with organic matter stabilization. In outdoor vermicomposting, vs. values were initially higher than in composting systems but decreased steadily over time, reflecting enhanced decomposition mediated by earthworms.
In the case of cotton, outdoor composting initially showed values similar to those of other textiles (~34%), while at the final sampling the cotton variant maintained the highest vs. content among composts. Outdoor vermicomposting showed the highest vs. fraction at the second sampling, remaining elevated even after the third, suggesting slower degradation of cellulose-rich fibers under vermicomposting conditions.
Linen substrates displayed a decreasing trend in vs. during outdoor composting, consistent with continuing biodegradation. In outdoor vermicomposting, linen achieved the highest vs. proportion among all vermicomposts during the first sampling (~40%), which subsequently declined toward the final phase, reflecting progressive mineralization of fibrous material.
3.3.4. Carbon and Nitrogen Contents and the C/N Ratio
The balance of total carbon and nitrogen, expressed as the C/N ratio, provides insight into microbial activity and substrate stability. The biodegradation of viscose-containing substrates showed only minor fluctuations in total C and N contents across all composting and vermicomposting systems (
Table 8). Total C ranged from 15.1 to 21.7%, while N varied from 1.4 to 1.9%. The resulting C/N ratios remained stable, typically 11.0–11.6, suggesting balanced microbial activity and efficient organic matter stabilization. In outdoor composting, no significant differences were found between sampling times, whereas in controlled composting, a slight increase in C/N ratio was observed at the final sampling (10.7–11.6,
p ≤ 0.05), indicating mild carbon enrichment during the maturation phase. Outdoor vermicomposting displayed small but measurable variations (10.9–11.6), while home vermicomposting maintained a steady ratio near 11.3, with only marginal declines in both C and N at the last sampling.
In substrates containing cotton fibers, C and N dynamics remained stable throughout the composting and vermicomposting processes (
Table 9). Total C ranged from 16.8 to 20.3%, while total N varied between 1.47 and 1.8%. The C/N ratio stayed within a narrow range of 10.9–11.5, indicating balanced microbial decomposition and uniform organic matter stabilization across all systems. Neither outdoor nor controlled composting showed statistically significant differences between samplings (
p > 0.05). Similarly, both outdoor and home vermicomposting maintained consistent C and N levels, reflecting steady mineralization without accumulation or loss of nutrients. The slight fluctuations observed were within natural variability and did not affect substrate maturity.
In substrates containing linen fibers, C and N concentrations remained relatively stable across all composting and vermicomposting systems (
Table 10). Total C ranged between 16.1 and 20.9%, while N varied from 1.5 to 1.8%, indicating steady organic matter decomposition. The C/N ratio fluctuated only slightly, remaining between 10.7 and 11.8, which is characteristic of well-stabilized composted material. In outdoor composting, C and N contents showed no statistically significant changes between samplings (
p > 0.05), and the C/N ratio remained close to 11. Controlled composting exhibited similar stability, with minor fluctuations (C/N 10.7–11.6), confirming balanced microbial mineralization. In outdoor vermicomposting, the C/N ratio slightly decreased from 11.4 to 10.7 during the second sampling, then increased again to 11.3 in the final sampling, reflecting normal biological variability (
p ≤ 0.05). Home vermicomposting displayed the most consistent results, maintaining a nearly constant C/N ratio (11.6–11.8) throughout the process.
3.3.5. Macroelements
During composting, the content of macroelements, specifically phosphorus (P), potassium (K) and magnesium (Mg), were monitored in the samples containing the textile fibers (
Table 11). In the case of phosphorus, slightly significant differences were observed only in the substrate with flax (
p = 0.02761), where the P content in the first sampling was higher than in the following ones. The other groups (cotton, viscose, control) did not show significant differences between samplings (
p > 0.05). For K, no statistically significant differences were found between individual samplings or between individual textiles (
p > 0.05). The values ranged from 9375 to 12,536 mg/kg and the variability between groups was low, which indicates stable behavior during the process. For Mg, statistically significant differences were observed especially in the cotton and linen variants, while the viscose and control variants did not show significant variability (
p > 0.05). For the cotton substrate (
p = 0.01043), the highest magnesium content was recorded at the first sampling, after which the concentration decreased. For the flax substrate, the differences were also significant (
p = 0.02278), with the first sampling showing significantly higher values than the subsequent samplings.
In vermicomposting (
Table 12), different trends appeared between the samples and materials for all monitored macroelements. For P, statistically significant differences were recorded with viscose (
p = 0.01) and flax (
p = 0.03). For both viscose and linen variants, the value in the second sample was lower than in the first and third. For the variant with cotton and the control, the differences between samples were nonsignificant (
p = 0.06 and
p = 0.13). For K, the differences between samples were less pronounced. Statistically significant changes were found only with cotton (
p = 0.02), where the K concentration increased in the third sample (14,483 mg/kg). For other fibers and the control, the differences between the samples were nonsignificant (
p > 0.05).
The most significant differences were observed in Mg concentrations. With cotton, a significantly higher Mg content was found in the first and third compared to the second samples (p = 0.00). A similar trend was also observed with viscose (p = 0.01) and linen (p = 0.03), while in the control these changes were also significant (p = 0.01), with the lowest value in the second sample. Overall, it can be said that the content of the monitored elements was partially influenced by the sample timing, with Mg showing the greatest variability. However, differences between individual textiles were often comparable to the control, suggesting that these changes may be more related to the properties and dynamics of the vermicompost itself than to the type of fiber used.
When comparing classical composting and vermicomposting, certain differences in the dynamics of nutrient concentrations have been observed with the most significant differences being recorded for Mg. In general, vermicompost has a higher Mg content, while the differences between the samples were more often statistically significant than for traditional compost. This may be due to the activity of earthworms, which accelerate the decomposition and mineralization of organic matter and mobilization of Mg. As for P, in vermicompost, significant differences between samples were recorded more often, especially for viscose and linen, while in compost, the changes were less pronounced and mostly not statistically significant. This result may be related to differences in microbial composition and the presence of earthworms, which affect the P cycle and its release from organic residues. For K, the values were relatively stable in both compost types, and most of the changes in K between compost and vermicompost samples were not statistically significant, indicating the relative stability of this element during the biodegradation process.
These data suggest greater variability in vermicomposting in the content of some nutrients, particularly Mg and P, probably because of the biological activity of the earthworms. Composting shows more stability but slower mineralization.
3.3.6. Potentially Toxic Elements (PTEs)
In
Table 13, the concentrations of seven PTEs (Cd, Pb, As, Cr, Cu, Ni and Zn) were measured in the compost and vermicompost during three samplings for different types of fibers (cotton, viscose, flax) and the control. The PTE levels in all of the analyzed samples were well below the limits specified in Regulation (EU) 2019/1009 of the European Parliament and of the Council [
26], verifying the environmental safety of these composted materials. For Cd, significant differences were recorded only in the control (
p = 0.01), while for textiles the values were above the significance limit: cotton (
p = 0.19), viscose (
p = 0.05), and linen (
p = 0.13). The levels of Pb did not differ significantly between samplings in any of the samples (
p > 0.05). For Ni, linen did not show statistical significance (
p = 0.01). Arsenic showed very significant differences in all samples with lower As content observed during the sampling period. Chromium changed significantly in the cotton variant (
p = 0.02) and in the linen variant (
p = 0.03), while in the viscose variant and the control sample the differences were statistically nonsignificant (
p = 0.56 and
p = 0.31). Cu changed significantly only in the viscose variant (
p = 0.03), while for the other types of fibers the
p-values were >0.1. Zinc showed statistically significant differences in the cotton variant (
p = 0.03) and the linen variant (
p = 0.03). In the viscose variant and the control samples the differences were not significant (
p = 0.50 and
p = 0.65).
In terms of the effect of composting time on PTE concentrations, a slight decrease in the values of some hazardous elements was observed during the sampling, especially for As, where this trend was evident across all monitored textile types and the control sample. Similar decreasing trends were noted for Cd and Pb in some samples, although these changes were not always statistically significant. These results suggest that partial immobilization or transformation of some metals may occur during composting, thus reducing their bioavailability.
The content of PTEs in vermicompost (
Table 14) differed slightly between textiles, but none of the measured values exceeded the limits of toxic concentrations set by European Union legislation [
26]. In the case of Cd, similar concentrations were measured in most samples. Statistically significant differences were recorded for cotton (
p = 0.03) and viscose (
p = 0.01), which indicates a change in Cd concentration during sampling. In the control, the values were also statistically significant (
p = 0.03). In the case of Pb, the values were again lower than the limit. In the cotton variant, the differences between the samples were statistically significant (
p = 0.00), while in the other textiles the differences were not statistically significant. Interesting results were found for As, where it significantly decreased over time in all fiber types. The
p-values were very low for all textiles (
p <0.00), confirming a statistically significant decrease in the content of this element.
Chromium changed significantly in most textiles: cotton (p = 0.01), viscose (p = 0.00) and linen (p = 0.02). However, in the control, the differences between the samples were not significant (p = 0.08). In the case of Cu, the changes in values were statistically significant, but still lower than the limit with all textiles: cotton (p = 0.03), viscose (p = 0.01), linen (p = 0.01) and control (p = 0.05). This trend, namely that the value increased significantly from the second sample to the third, was seen for all three types of textiles and the control. In the case of Ni, the differences between the samples in most textiles were significant: cotton (p = 0.00), viscose (p = 0.04) and linen (p = 0.01). However, the values remained well below the toxicity limits. The control was nonsignificant (p = 0.08). Zinc was the most abundant element, but its content remained well below the toxicity limit (800 mg kg−1). Statistically significant differences were observed with viscose (p = 0.01) and linen (p = 0.01), but the variant with cotton was right at the limit of significance (p = 0.05) and the control was not significant (p = 0.08). Here again, a trend of increasing values from the second to the third sampling was evident.
The differences between compost and vermicompost in terms of PTE content were generally moderate, but in some cases statistically significant. For example, for As, both types of biodegradation showed significant decreases in concentrations during the sampling period, with p-values being highly significant in all cases. For Cu and Cr, statistically significant differences between sampling periods were more common in vermicompost than in compost, which may be related to the biological activity of earthworms and their influence on the mobilization or accumulation of these elements. For Cd and Pb, the contents were comparable and well below the toxicity limits, but in vermicompost, statistically significant fluctuations between sampling periods were recorded in several samples. In contrast, Ni and Zn did not show dramatic differences between biodegradation types, although their variability was somewhat higher in vermicompost. Overall, none of the monitored PTEs exceeded the legislative limits, and the differences between compost and vermicompost can be attributed to biological activity and process dynamics rather than the presence of the textile itself.