Influence of Temperature on the Autumn Population Structure of Rhopalosiphum padi (L.) in Western Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphid Sampling
2.2. Meteorological Data
2.3. Statistical Analysis
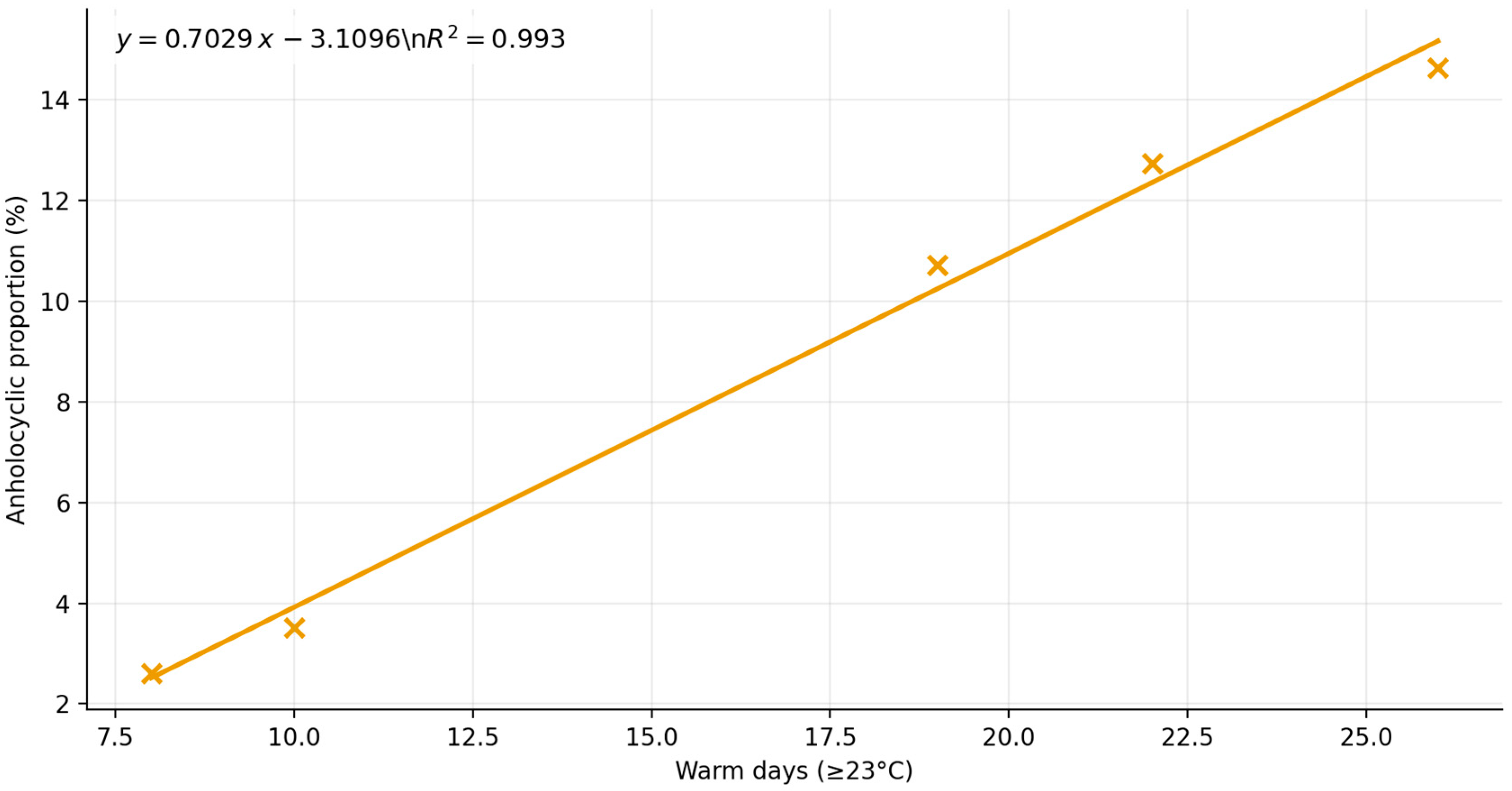
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops. In An Identification and Information Guide; The Natural History Museum: London, UK, 2000; p. 466. ISBN 978-0471851912. [Google Scholar]
- Blackman, R.L. Photoperiodic determination of the male and female sexual morphs of Myzus persicae. J. Insect Physiol. 1975, 21, 435–453. [Google Scholar] [CrossRef]
- Matsuka, M.; Mittler, T.E. Production of males and gynoparae by apterous viviparae of Myzus persicae continously expose to different scotoperiods. J. Insect Physiol. 1979, 25, 586–593. [Google Scholar] [CrossRef]
- Brodel, C.F.; Schaeters, G.A. The influence of temperature on the production of sexuals by Aphis rubicola under short-day conditions. Entomol. Exp. Appl. 1980, 37, 127–132. [Google Scholar] [CrossRef]
- Mittler, T.E.; Wilhoit, L. Sexual morph production by two regional biotypes of Myzus persicae (Homoptera: Aphididae) in relation to photoperiod. Environ. Entomol. 1990, 19, 111–126. [Google Scholar] [CrossRef]
- Harrington, R.; Bale, J.S.; Tatchell, G.M. Aphids in a changing climate. In Insects in a Changing Environment; Harrington, R., Stark, N.E., Eds.; Academic Press: London, UK, 1995; pp. 126–150, 535. [Google Scholar]
- Anderson, M.; Bromley, A.K. Sensory System. In World Crop Pests. Aphids. Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 2A, pp. 153–161. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Resource Tracking: Mechanism—Cyclical Parthenogenesis. In Aphid Ecology; Dixon, A.F.G., Ed.; Chapman & Hall: London, UK, 1998; pp. 83–84. [Google Scholar]
- Kawada, K. Polymorphism and Morph Determination. In World Crop Pests. Aphids. Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 2A, pp. 255–265. [Google Scholar]
- Bennetts, D.A. The Hadley Centre Transient Climate Experiment. In Insects in a Changing Environment; Harrington, R., Stork, N.E., Eds.; Academic Press: London, UK, 1995; pp. 50–58. [Google Scholar]
- Austin, A.B.M.; Tatchell, G.M.; Harrington, R.; Bale, J.S. Adaptative Significance of Changes in Morph Production during the Transition from the Parthenogenetic to Sexual Reproduction in the Aphid Rhopalosiphum padi L. (Homoptera: Aphididae). Bull. Entomol. Res. 1996, 86, 93–99. [Google Scholar] [CrossRef]
- Leszczyński, B.; Szynkarczyk, S.; Jóźwiak, B.; Laskowska, J. Bird-Cherry Oat Aphid Feeding Behaviour on Primary and Secondary Hosts. In Aphids and Other Homopterous Insects; Cichocka, E., Goszczyński, W., Wiech, K., Eds.; Warsaw Agricultural University: Warsaw, Poland, 1998; Volume 6, pp. 47–53. [Google Scholar]
- Leszczyński, B.; Urbańska, A.; Wereda, I. Some Factors Influencing Spring and Autumn Migrations of Bird Cherry-Oat Aphid in Eastern Poland. In Aphids and Other Homopterous Insects; Cichocka, E., Goszczyński, W., Leszczyński, B., Ruszkowska, M., Wojciechowski, W., Eds.; PAS: Siedlce, Poland, 2001; Volume 8, pp. 223–230. [Google Scholar]
- Pankanin-Franczyk, M.; Ceryngier, P. On Some Factors Affecting the Population Dynamics of Cereal Aphids. In Aphids and Other Homopterous Insects; PAS: Olsztyn, Poland, 2000; Volume 7, pp. 289–295. [Google Scholar]
- Yamamura, K.; Kiritari, K. A Simple Method to Estimate the Potential Increase in the Number of Generations under Global Warming in Temperate Zones. Appl. Entomol. Zool. 1998, 33, 289–298. [Google Scholar] [CrossRef]
- Ruszkowska, M. Aphids on Winter Cereals. In Aphids in Natural and Managed Ecosystems; Nieto Nafria, J.M., Dixon, A.G., Eds.; Universidad de León: León, Spain, 1998; pp. 609–613. [Google Scholar]
- Harrington, R. Insect Pest and Global Environmental Change. Causes and Consequences of Global Environmental Change. In Encyclopedia of Global Environmental Change; Munn, T., Ed.; J. Wiley & Sons Ltd.: Chichester, UK, 2002; Volume 3, pp. 381–386. [Google Scholar]
- Ruszkowska, M. Forecasting the Abundance of Rhopalosiphum padi (L.) by Means of Suction Trap Catch and Meteorological Data. Acta Phytopathol. Entomol. Hung. 1990, 25, 447–451. [Google Scholar] [CrossRef]
- Dean, J.G.W. Effect of Temperature on the Cereal Aphids Metopolophium dirhodum (Walk.), Rhopalosiphum padi (L.) and Macrosiphum avenae (F.) (Hem., Aphididae). Bull. Entomol. Res. 1974, 63, 401–409. [Google Scholar] [CrossRef]
- Ruszkowska, M. Across the Transformation Life Cycle of Rhopalosiphum padi (L.) (Homoptera: Aphidoidea): Coevolution with Temperature; Institute of Plant Protection: Poznań, Poland, 2007; p. 60. [Google Scholar]
- Hand, S.C.; Hand, L. Monitoring of the Winter Populations of Cereal Aphids near Wageningen, the Netherlands, in 1982/1983. Neth. J. Plant Pathol. 1986, 92, 137–146. [Google Scholar] [CrossRef]
- Slavíková, L.; Fryč, D.; Kundu, J.K. Analysis of Twenty Years of Suction Trap Data on the Flight Activity of Myzus persicae and Brevicoryne brassicae, Two Main Vectors of Oilseed Rape Infection Viruses. Agronomy 2024, 14, 1931. [Google Scholar] [CrossRef]
- Peng, X.; Qiao, X.; Chen, M. Responses of holocyclic and anholocyclic Rhopalosiphum padi populations to low-temperature and short-photoperiod induction. Ecol. Evol. 2017, 7, 1030–1042. [Google Scholar] [CrossRef]
- Mann, J.A.; Harrington, R. Key Factors for Modeling Secondary Spread of Barley Yellow Dwarf Virus. In Project Report No. 129 (Grant Project No. 0037/1/94); IACR Rothamsted: Harpenden, UK, 1996; p. 25. [Google Scholar]
- Ruszkowska, M. Biodiversity of Autumn Winged Forms of Rhopalosiphum padi (L.) (Homoptera: Aphidoidea) in Johnson Suction Trap in Poznań. In Aphids and Other Homopterous Insects; Cichocka, E., Goszczyński, W., Leszczyński, B., Ruszkowska, M., Wojciechowski, W., Eds.; PAS: Siedlce, Poland, 2001; Volume 8, pp. 65–70. [Google Scholar]
- Ruszkowska, M. Biodiversity of Rhopalosiphum padi (Linnaeus, 1758) (Hemiptera: Aphidoidea) and BYDV Expansion in Poland. In Aphids and Other Hemipterous Insects; Cichocka, E., Ed.; PAS: Warsaw, Poland, 2008; Volume 14, pp. 135–145. [Google Scholar]
- Rispe, C.; Hullé, M.; Gautier, J.P.; Pierre, J.S.; Harrington, R. Effect of Climate on the Population of Sexuals in the Autumn Flight of the Aphid Rhopalosiphum padi L. (Hom., Aphididae). J. Appl. Entomol. 1998, 122, 129–136. [Google Scholar] [CrossRef]
- Adler, L.S.; de Valpine, P.; Harte, J.; Call, J. Effect of Long-Term Experimental Warming on Aphid Density in the Field. J. Kans. Entomol. Soc. 2007, 89, 156–168. [Google Scholar] [CrossRef]
- Taylor, L.R. An Improved Suction Trap for Insects. Ann. Appl. Biol. 1951, 38, 582–591. [Google Scholar] [CrossRef]
- Remaudière, G.; Remaudière, M. Catalogue des Aphididae du Monde (World’s Aphididae Catalogue); INRA: Paris, France, 1997; p. 473. [Google Scholar]
- Lowles, A. A Quick Method for Distinguishing between the Two Autumn Winged Female Morphs of the Aphid Rhopalosiphum padi. Entomol. Exp. Appl. 1995, 74, 95–98. [Google Scholar] [CrossRef]
- Strażyński, P. Population Structure of Rhopalosiphum padi (Linnaeus, 1758) (Hemiptera, Aphidoidea) in Wielkopolska Region in 2003–2008 in the Context of Winter Cereals Threat of BYDV Expansion. In Aphids and Other Hemipterous Insects; Goszczyński, W., Herczek, A., Leszczyński, B., Łabanowski, G., Podsiadło, E., Rakauskas, R., Ruszkowska, M., Wilkaniec, B., Wojciechowski, W., Eds.; The John Paul II Catholic University of Lublin: Lublin, Poland, 2010; Volume 16, pp. 91–105. [Google Scholar]
- Strażyński, P.; Ruszkowska, M. The Life Cycle Functional Response of Rhopalosiphum padi (L.) to Higher Temperature: Territorial Expansion of Permanent Parthenogenetic Development as a Result of Warmer Weather Conditions. J. Plant Prot. Res. 2015, 55, 162–165. [Google Scholar] [CrossRef]
- Ruszkowska, M. Population Dynamics of Cereal Aphids in Poland from 1973 to 1984. In Population Structure, Genetics and Taxonomy of Aphids and Thysanoptera; Holman, J., Ed.; SPB Academic Publishing: The Hague, The Netherlands, 1987; pp. 209–218. [Google Scholar]
- Asin, L.; Pons, X. Effect of High Temperature on the Growth and Reproduction of Corn Aphids (Homoptera: Aphididae) and Implications for Their Population Dynamics on the Northeastern Iberian Peninsula. Environ. Entomol. 2001, 30, 1127–1134. [Google Scholar] [CrossRef]
- Maurice, N.G.; Ramteke, P.W.; Shukla, P.K.; John, S.A. Global Climate Change: A Threat to Aphids Population. In Dynamics of Crop Protection and Climate Change; Chattopadhyay, C., Prasad, D., Eds.; Studera Press: Delhi, India, 2016; pp. 61–91. [Google Scholar]
- Strażyński, P.; Trzmiel, K. Structure of the Autumn Populations of Bird Cherry-Oat Aphid (Rhopalosiphum padi L.)—Practical Importance in a Consideration of Real Threat of BYDV in Different Regions of Poland. Prog. Plant Prot. 2008, 48, 804–807. [Google Scholar]
- Pons, X.; Comas, J.; Albajes, R. Occurrence of Holocyclic and Anholocyclic Population of Rhopalosiphum padi and Sitobion avenae (Hom., Aphididae) in the Northeast of Spain. J. Appl. Entomol. 1995, 119, 1171–1175. [Google Scholar] [CrossRef]
- Schliephake, E.; Graichen, K.; Rabenstein, F. Investigations on the Vector Transmission of the Beet Mild Yellowing Virus (BMYV) and the Turnip Yellows Virus (TuYV). J. Plant Dis. Prot. 2000, 107, 81–87. [Google Scholar]
- Strażyński, P.; Ruszkowska, M.; Jeżewska, M.; Trzmiel, K. Evaluation of the Risk of the Autumn Infections of Winter Barley with Barley Yellow Dwarf Viruses in Poland in the Years 2006–2008. J. Plant Prot. Res. 2011, 51, 314–321. [Google Scholar] [CrossRef]

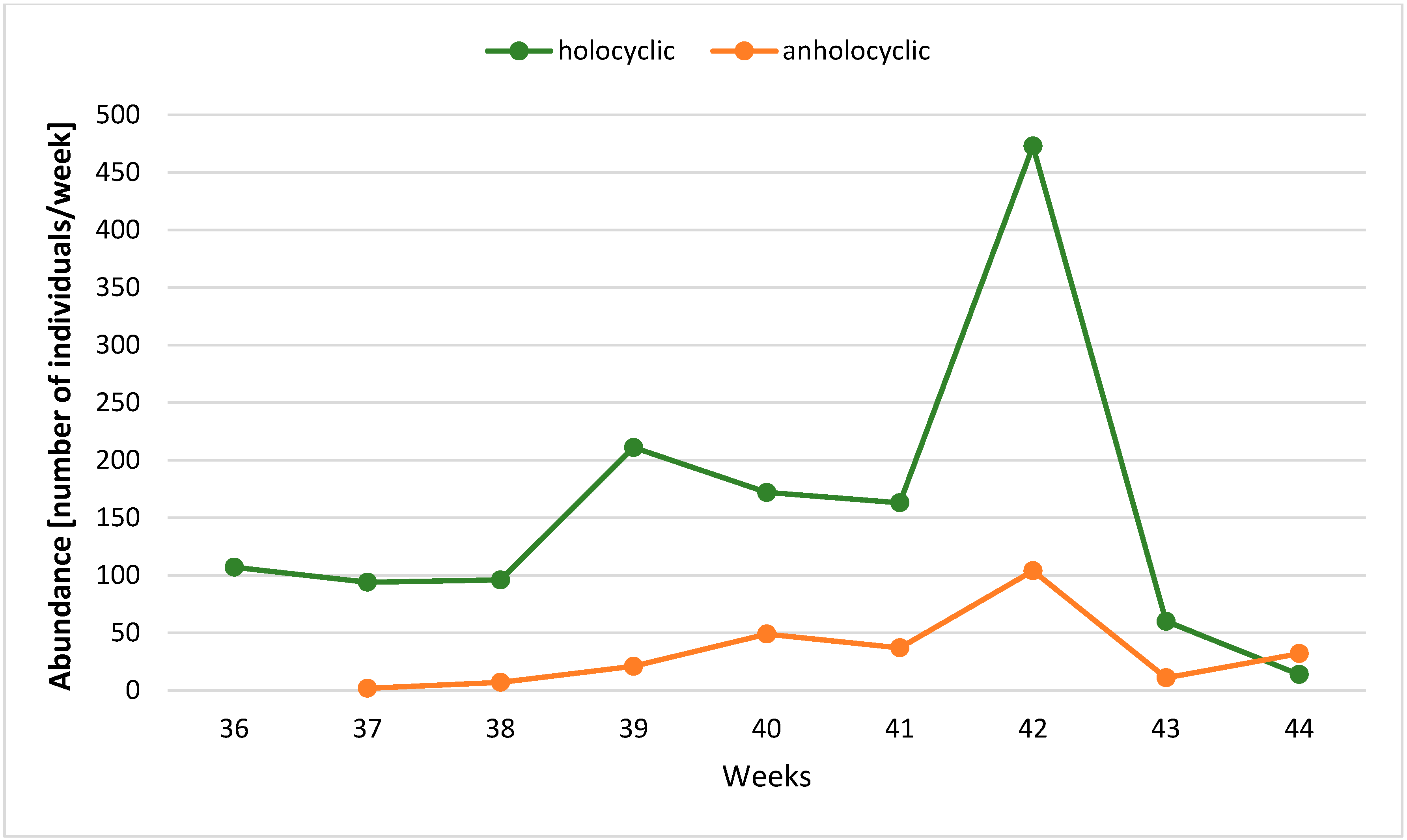
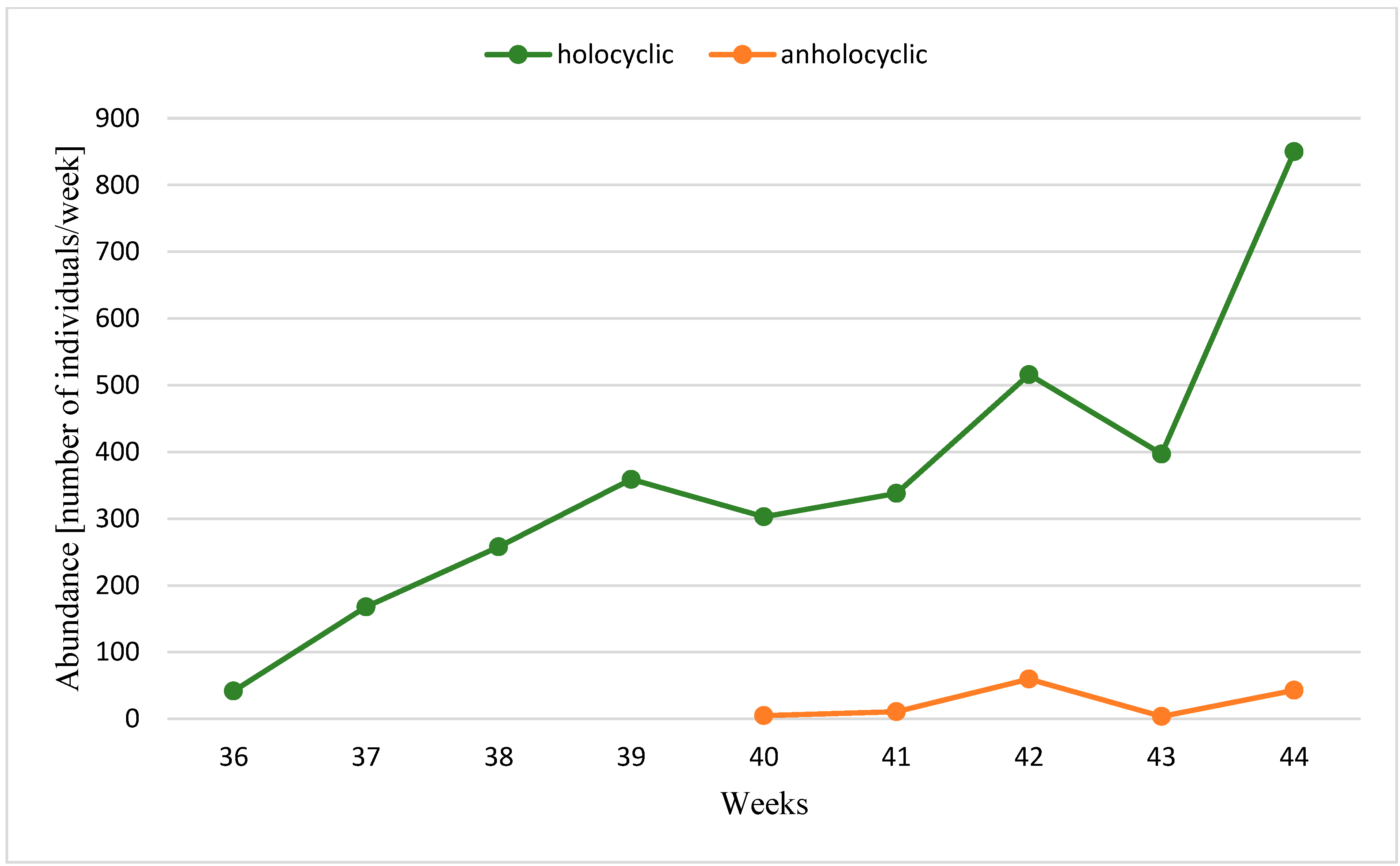
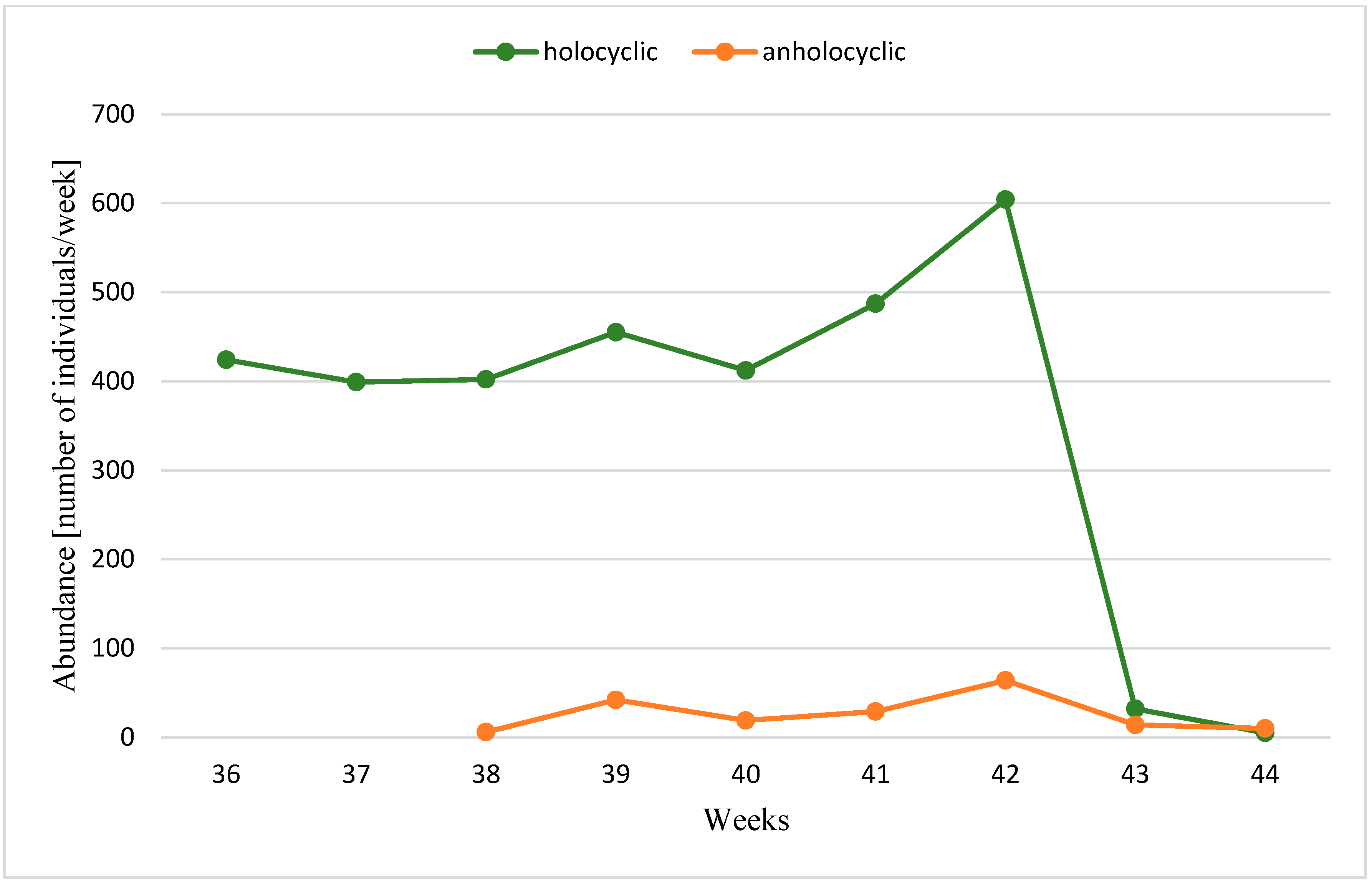
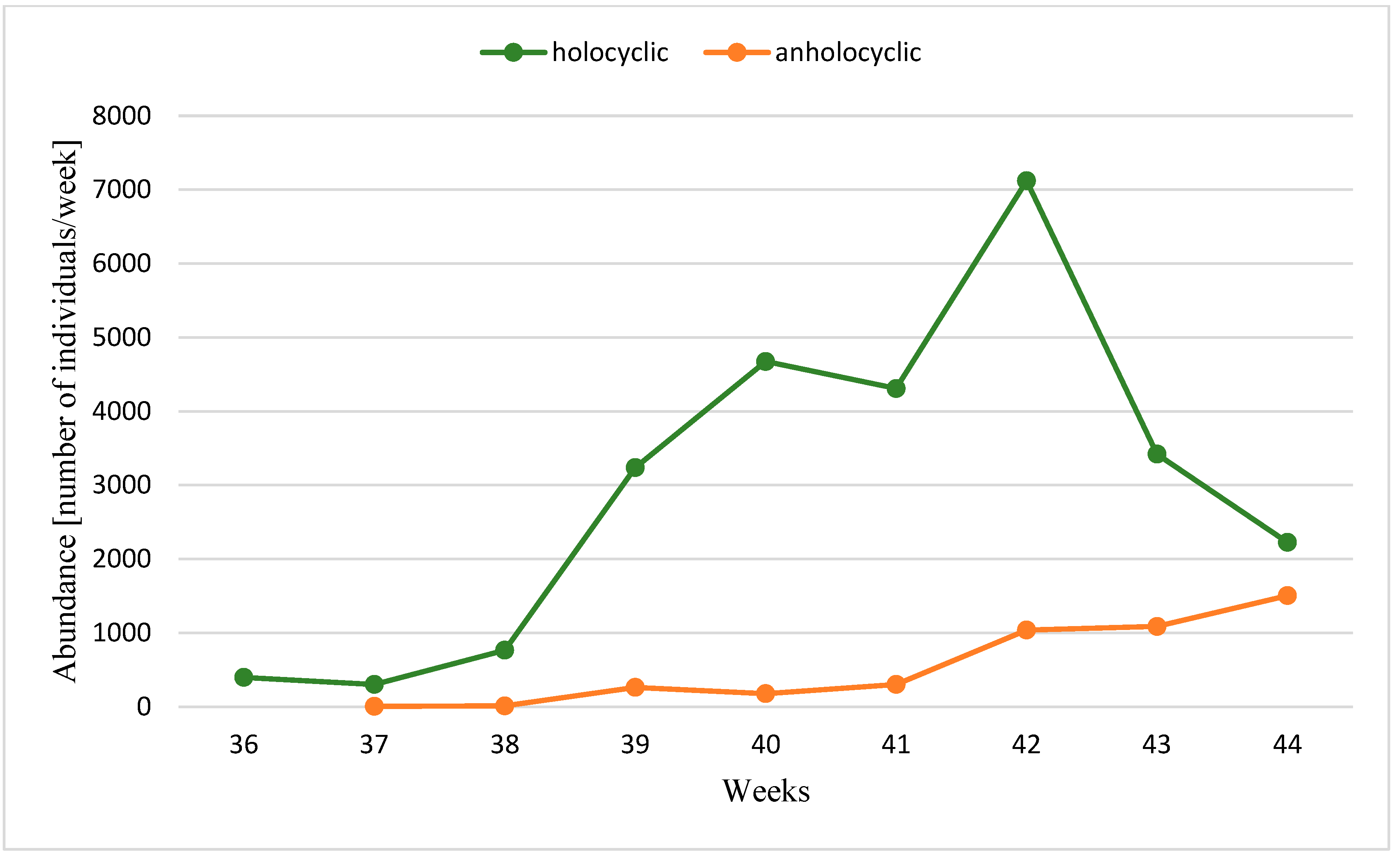
| Years | Temperature | Gynoparae | Males (% of the Population) | Anholocyclic Forms (% of Population) | Ratio of Holo- to Anholocyclic Forms | Total Autumn Catch | ||
|---|---|---|---|---|---|---|---|---|
| ≥25 °C | 23–24.9 °C | Total | ||||||
| 2018 | 7 | 12 | 19 | 819 | 123 (13.05%) | 101 (10.72%) | 8.10 | 942 |
| 2019 | 13 | 13 | 26 | 1663 | 203 (10.87%) | 273 (14.63%) | 6.10 | 1866 |
| 2020 | 4 | 4 | 8 | 3354 | 1357 (28.80%) | 123 (2.61%) | 27.26 | 4711 |
| 2021 | 4 | 6 | 10 | 3604 | 1626 (31.08%) | 184 (3.51%) | 19.58 | 5230 |
| 2022 | 10 | 11 | 22 | 30,929 | 4298 (12.20%) | 4485 (12.73%) | 6.90 | 35,227 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strażyński, P.; Kubasik, W.; Baran, M. Influence of Temperature on the Autumn Population Structure of Rhopalosiphum padi (L.) in Western Poland. Agronomy 2025, 15, 2664. https://doi.org/10.3390/agronomy15112664
Strażyński P, Kubasik W, Baran M. Influence of Temperature on the Autumn Population Structure of Rhopalosiphum padi (L.) in Western Poland. Agronomy. 2025; 15(11):2664. https://doi.org/10.3390/agronomy15112664
Chicago/Turabian StyleStrażyński, Przemysław, Wojciech Kubasik, and Marcin Baran. 2025. "Influence of Temperature on the Autumn Population Structure of Rhopalosiphum padi (L.) in Western Poland" Agronomy 15, no. 11: 2664. https://doi.org/10.3390/agronomy15112664
APA StyleStrażyński, P., Kubasik, W., & Baran, M. (2025). Influence of Temperature on the Autumn Population Structure of Rhopalosiphum padi (L.) in Western Poland. Agronomy, 15(11), 2664. https://doi.org/10.3390/agronomy15112664






