Enhancing the Valorization of Spent Pleurotus Substrate Through Anaerobic Digestion by Extracted Enzymes
Abstract
1. Introduction
2. Materials and Methods
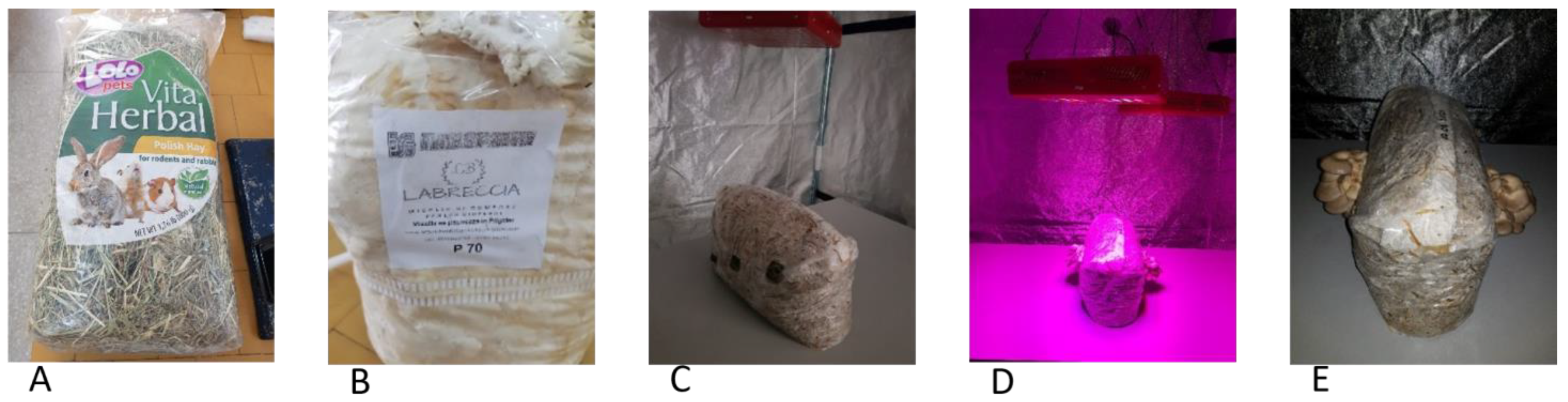
2.1. Materials
2.2. Cultivation of P. ostreatus
2.3. Hay and SPS Characterization
2.4. Enzyme Extraction
2.5. Cellulase Activity
2.6. Laccase Activity
2.7. Mechanical and Enzymatic Pretreatment of SPS
2.8. Anaerobic Digestion
2.9. Dry Weight and Moisture
2.10. Chemical Oxygen Demand
2.11. Elemental Analysis
2.12. GC-MS Analysis of Volatile Compounds from Liquid Digestate
2.13. Inductively Coupled Plasma Optical Emission Spectroscopy
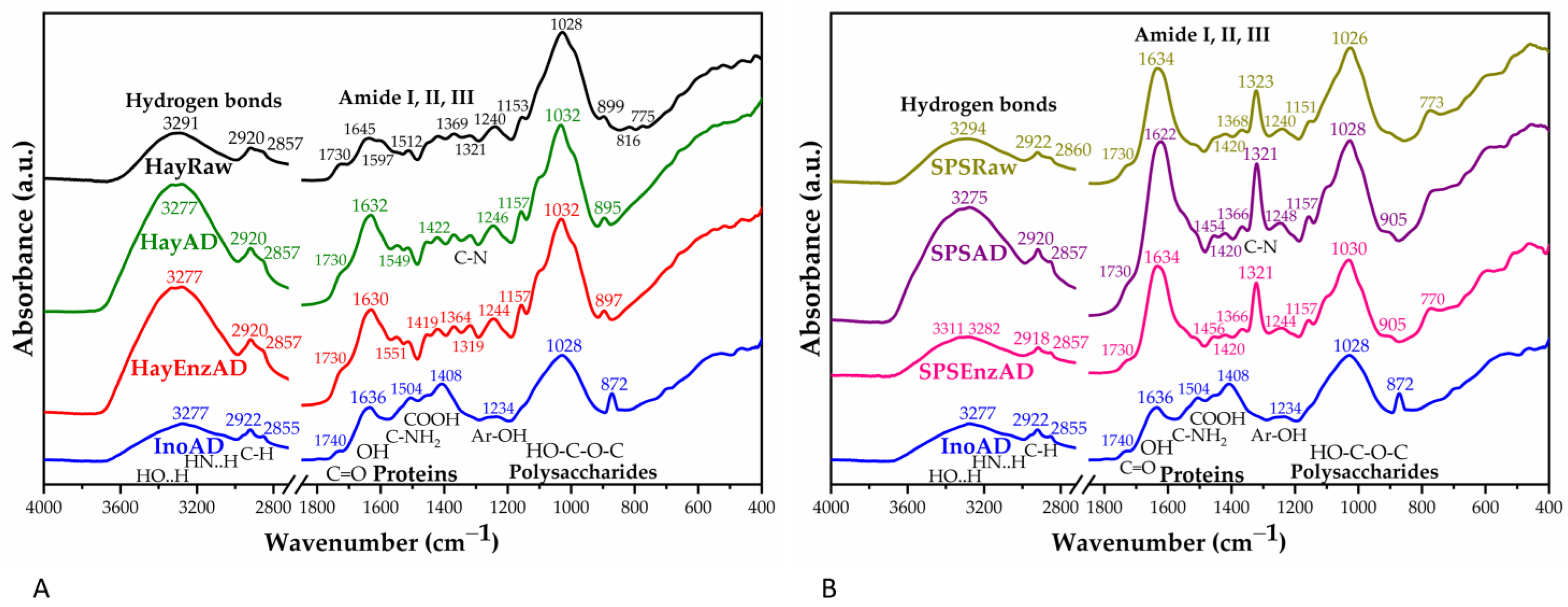
2.14. Fourier Transform-Infrared Spectroscopy
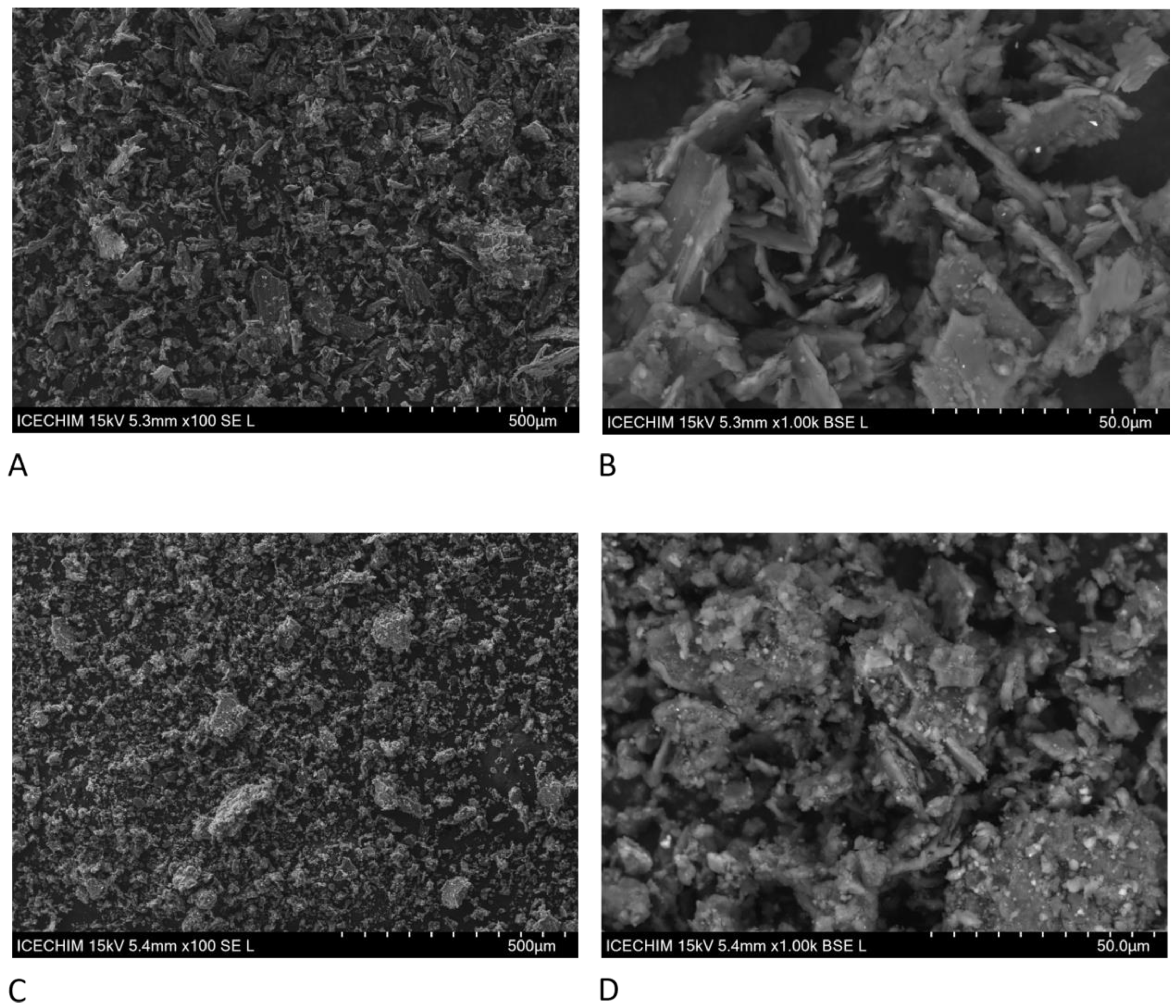
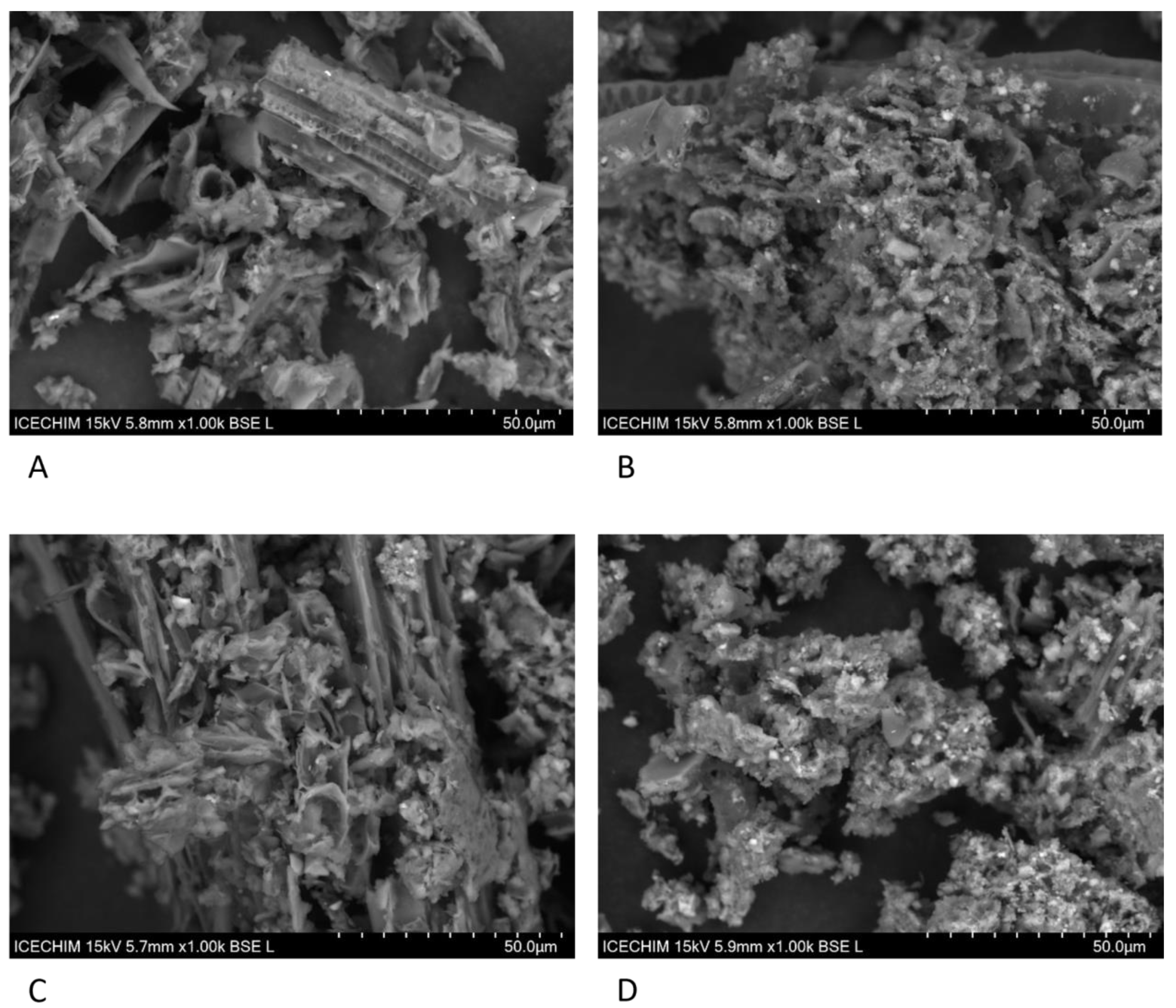
2.15. Scanning Electron Microscopy
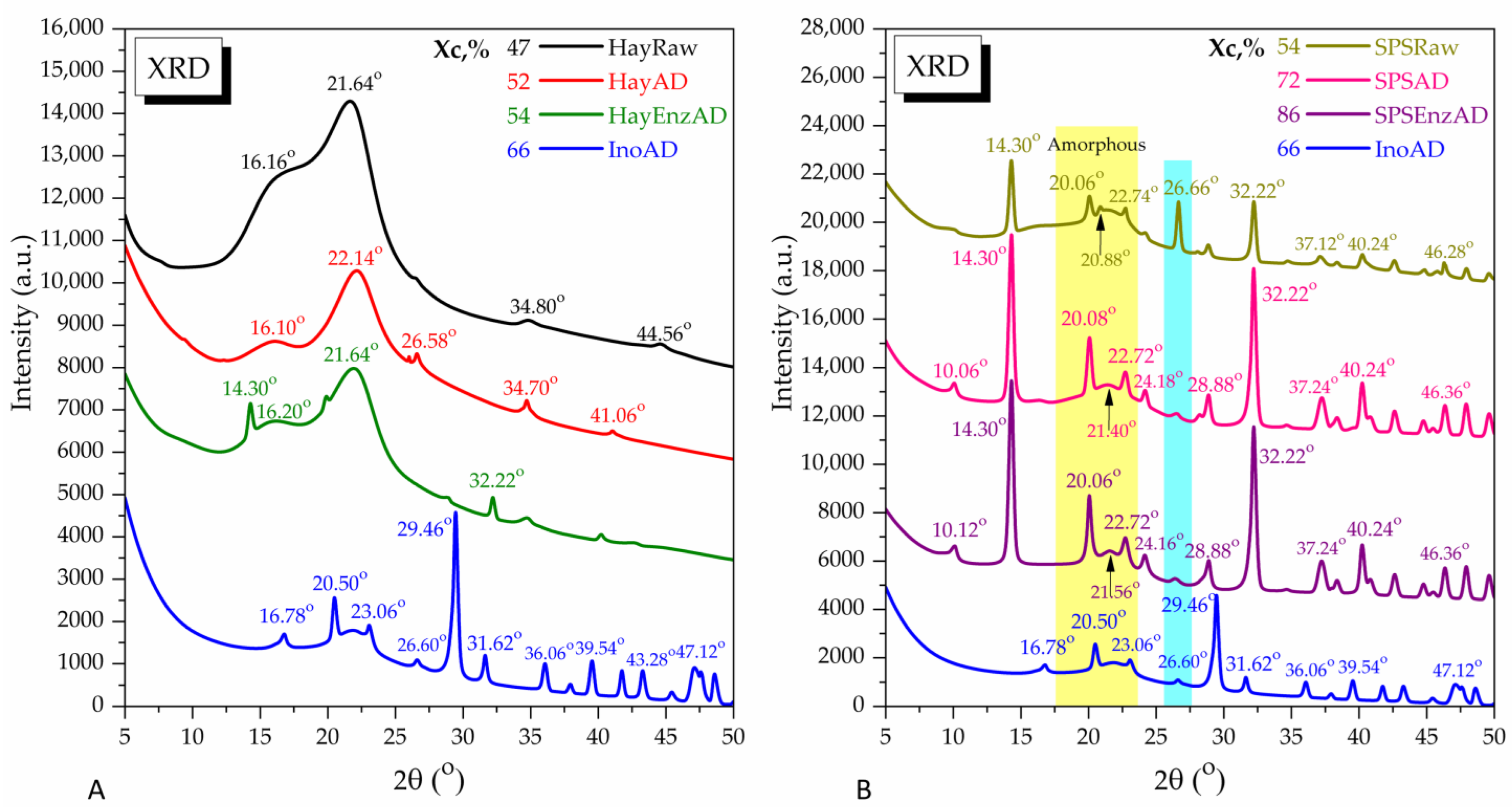
2.16. X-Ray Diffraction Analysis
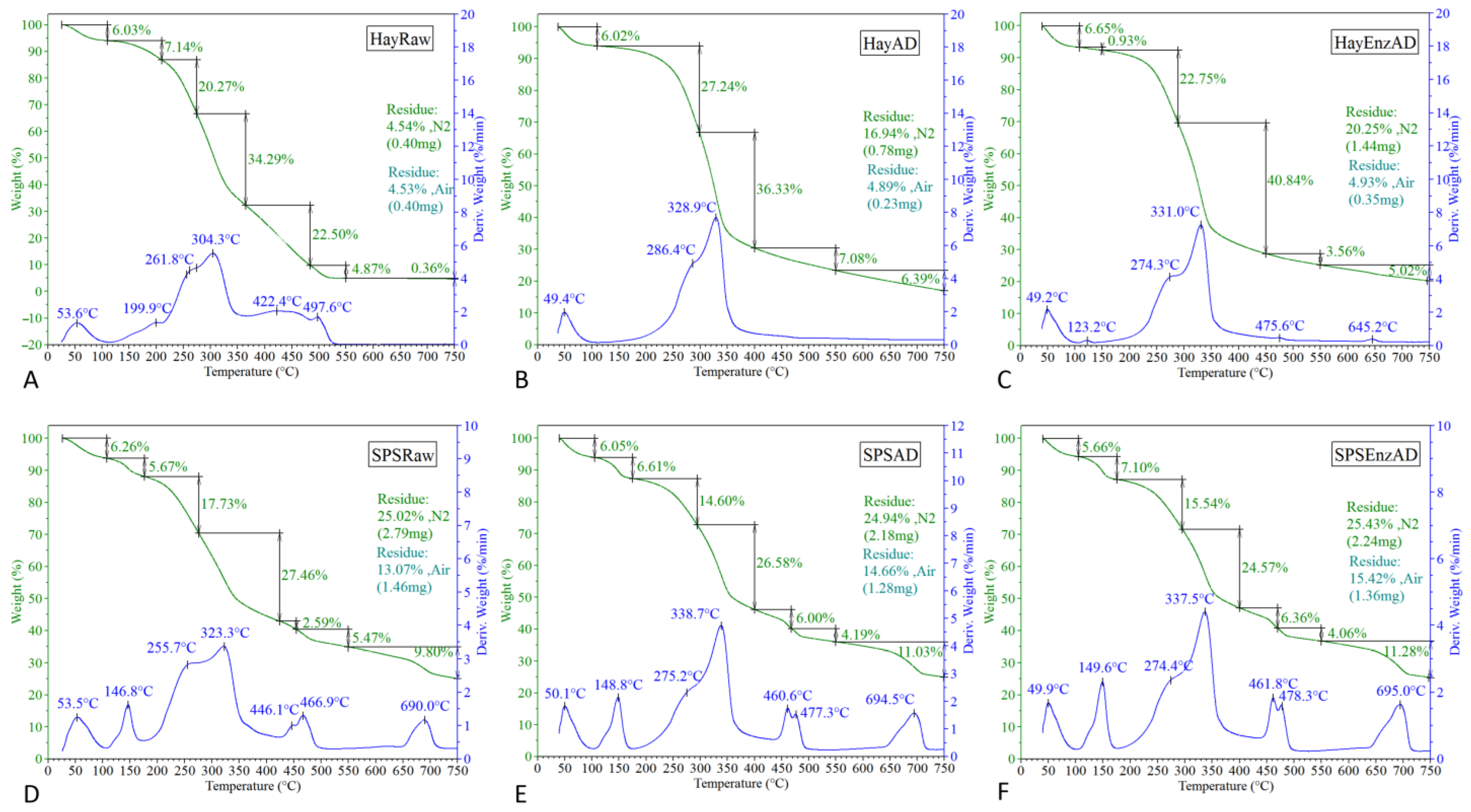
2.17. Thermogravimetric Analysis
2.18. Statistical Analysis
3. Results and Discussion
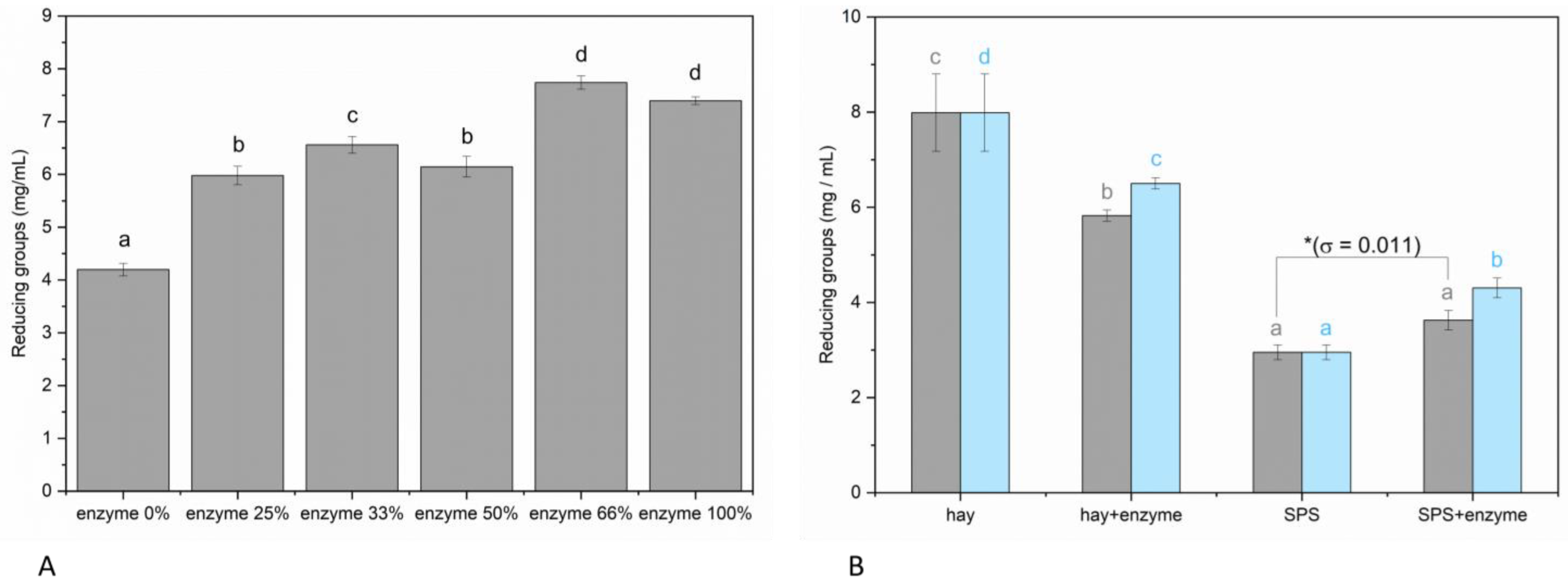
3.1. Effect of Pleurotus Enzymes on Lignocellulosic Substrate
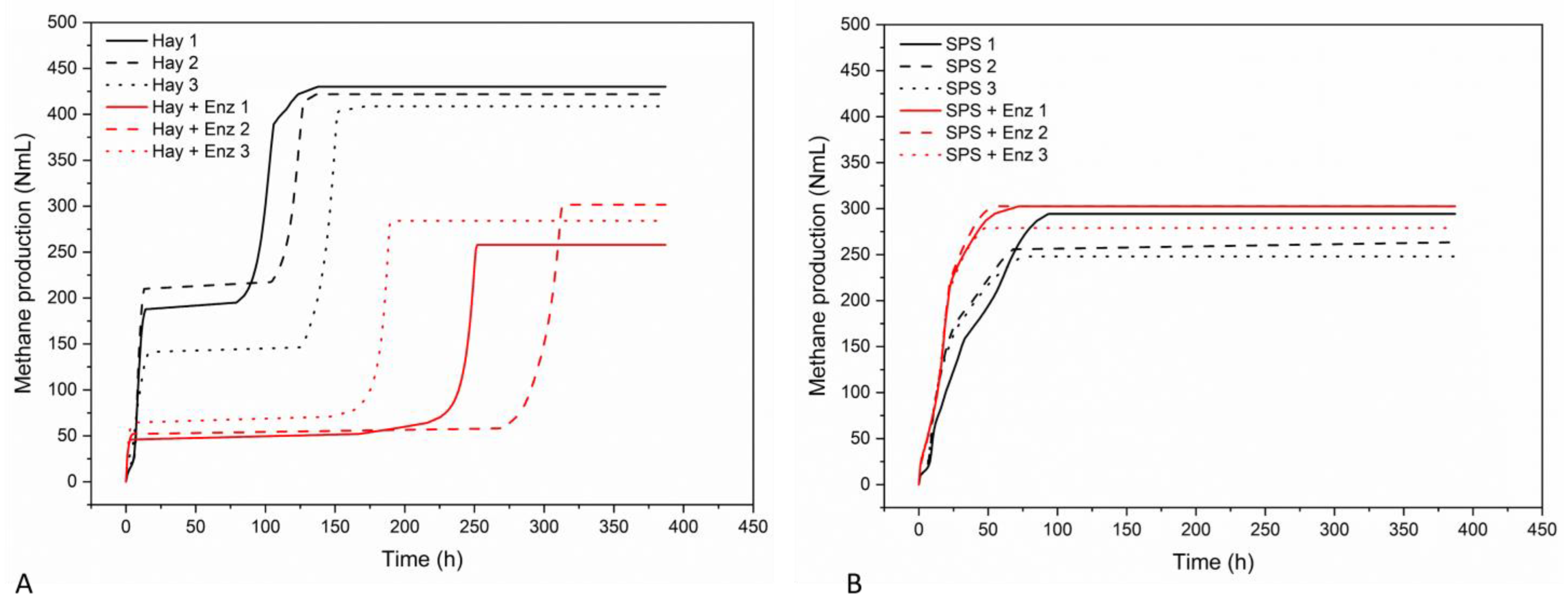
3.2. Effect of Enzyme Extract from SPS on Methane Production
3.3. Characterization of Solid and Liquid Digestate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| AD | Anaerobic Digestion |
| BHT | Butylated Hydroxytoluene |
| CMC | Carboxymethylcellulose |
| COD | Chemical Oxygen Demand |
| DNS | 3,5-Dinitrosalicylic acid |
| DTG | Derivative Thermogram |
| FAE | Feruloyl Esterase |
| FT-IR | Fourier Transform-Infrared |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HayAD | Hay After Anaerobic Digestion |
| HayEnzAD | Enzymatically Treated Hay After Anaerobic Digestion |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectroscopy |
| PES | Polyethersulfone |
| SEM | Scanning Electron Microscopy |
| SPS | Spent Pleurotus Substrate |
| SPSAD | SPS After Anaerobic Digestion |
| SPSEnzAD | Enzymatically Treated SPS After Anaerobic Digestion |
| TGA | Thermogravimetric Analysis |
| VFA | Volatile Fatty Acids |
| XRD | X-Ray Diffraction |
References
- Leong, Y.K.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Valorization of spent mushroom substrate for low-carbon biofuel production: Recent advances and developments. Bioresour. Technol. 2022, 363, 128012. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.-W.; Chang, J.-S.; Yang, F.-C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2022, 344, 126157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wu, M.; Chen, X.; Li, C.; Zhang, A.; Lu, W. Growth performance and hematological changes in growing sika deers fed with spent mushroom substrate of Pleurotus ostreatus. Animals 2022, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.Y.; Liu, C.L.; Tsai, C.F.; Lin, W.C.; Chang, S.C.; Shih, H.D.; Shy, Y.M.; Lee, T.-T. Evaluation of Waste Mushroom Compost as a Feed Supplement and Its Effects on the Fat Metabolism and Antioxidant Capacity of Broilers. Animals 2020, 10, 445. [Google Scholar] [CrossRef]
- Wang, S.; Xu, F.; Li, Z.; Zhao, S.; Song, S.; Rong, C.; Geng, X.; Liu, Y. The spent mushroom substrates of Hypsizigus marmoreus can be an effective component for growing the oyster mushroom Pleurotus ostreatus. Sci. Hortic. 2015, 186, 217–222. [Google Scholar] [CrossRef]
- De Bonis, M.; Sambo, P.; Zanin, G.; Cardarelli, M.; Nicoletto, C. Spent Pleurotus substrate as organic fertilizer to improve yield and soil fertility: The case of baby leaf lettuce production. J. Sci. Food Agric. 2025, 105, 5874–5886. [Google Scholar] [CrossRef]
- Tuhy, Ł.; Samoraj, M.; Witkowska, Z.; Wilk, R.; Chojnacka, K. Using spent mushroom substrate as the base for organic-mineral micronutrient fertilizer–field tests on maize. BioResources 2015, 10, 5709–5719. [Google Scholar] [CrossRef]
- Ma, X.; Han, F.; Yang, G.; Wu, J.; Ma, Y. Enhanced soil ecosystem multifunctionality and microbial community shifts following spent mushroom substrate application in vineyards. Appl. Soil Ecol. 2025, 213, 106230. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.; Wang, G.; Kong, L.; Guan, Q.; Yang, R.; Jin, Y.; Liu, X.; Qu, J. Remediation of cadmium contaminated soil by composite spent mushroom substrate organic amendment under high nitrogen level. J. Hazard. Mater. 2022, 430, 128345. [Google Scholar] [CrossRef]
- Oancea, F.; Raut, I.; Sesan, T.E.; Doni, M.; Popescu, M.; Jecu, M.L. Enhancement of biostimulant activity of spent Pleurotus substrate for seedling production. Acta Hortic. 2017, 1164, 55–62. [Google Scholar] [CrossRef]
- Kousar, A.; Khan, H.A.; Farid, S.; Zhao, Q.; Zeb, I. Recent advances on environmentally sustainable valorization of spent mushroom substrate: A review. Biofuels Bioprod. Biorefining 2024, 18, 639–651. [Google Scholar] [CrossRef]
- Subbarao, P.M.V.; D’ Silva, T.C.; Adlak, K.; Kumar, S.; Chandra, R.; Vijay, V.K. Anaerobic digestion as a sustainable technology for efficiently utilizing biomass in the context of carbon neutrality and circular economy. Environ. Res. 2023, 234, 116286. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Dincă, M.-N.; Ferdeș, M.; Zăbavă, B.-Ș.; Ionescu, M.; Moiceanu, G.; Paraschiv, G. Effective Valorization of Anaerobic Digestate—A Sustainable Approach to Circular Economy. Appl. Sci. 2025, 15, 8939. [Google Scholar] [CrossRef]
- Pérez-Chávez, A.M.; Mayer, L.; Albertó, E. Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy Sustain. Dev. 2019, 50, 50–60. [Google Scholar] [CrossRef]
- Bisaria, R.; Vasudevan, P.; Bisaria, V.S. Utilization of spent agro-residues from mushroom cultivation for biogas production. Appl. Microbiol. Biotechnol. 1990, 33, 607–609. [Google Scholar] [CrossRef]
- Bernat, K.; Le, T.C.T.; Kulikowska, D.; Thapa, R. Anaerobic Digestion as a Possible Method of Managing Waste from Mushroom Production with Sewage Sludge as Co-Substrate. Energies 2024, 17, 1938. [Google Scholar] [CrossRef]
- Xiao, Z.; Lin, M.; Fan, J.; Chen, Y.; Zhao, C.; Liu, B. Anaerobic digestion of spent mushroom substrate under thermophilic conditions: Performance and microbial community analysis. Appl. Microbiol. Biotechnol. 2018, 102, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yuan, X.; Wang, S.; Sun, F.; Hou, Z.; Hu, Q.; Zhai, L.; Cui, Z.; Zou, Y. Methane production and characteristics of the microbial community in the co-digestion of spent mushroom substrate with dairy manure. Bioresour. Technol. 2018, 250, 611–620. [Google Scholar] [CrossRef]
- Vasilakis, G.; Rigos, E.-M.; Giannakis, N.; Diamantopoulou, P.; Papanikolaou, S. Spent Mushroom Substrate Hydrolysis and Utilization as Potential Alternative Feedstock for Anaerobic Co-Digestion. Microorganisms 2023, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tang, X.; Zhao, K.; Balan, V.; Zhu, Q. Biogas Production from Anaerobic Co-Digestion of Spent Mushroom Substrate with Different Livestock Manure. Energies 2021, 14, 570. [Google Scholar] [CrossRef]
- Lee, J.; Ryu, D.-y.; Jang, K.H.; Lee, J.W.; Kim, D. Influence of Different Pretreatment Methods and Conditions on the Anaerobic Digestion Efficiency of Spent Mushroom Substrate. Sustainability 2022, 14, 15854. [Google Scholar] [CrossRef]
- Zhu, W.; Lai, X.; Liu, C.; Wu, X.; Bai, X.; Cai, Y.; Zhao, X.; Li, Z.; Hao, Y.; Huang, Y.; et al. Enhancement of Biomethane Yield from Spent Mushroom Substrate: Biological Pretreatment with the Chlamydospores of Trichoderma viride. Fermentation 2025, 11, 152. [Google Scholar] [CrossRef]
- López-Balladares, O.H.; De la Lama-Calvente, D.; Flores-Flor, F.J.; Borja, R. Valorization of Lignocellulosic Biomass Through Anaerobic Digestion after the Cultivation of the Edible Mushroom Lentinula Edodes and Enzymatic Pretreatment. Waste Biomass Valorization 2025, 1–13. [Google Scholar] [CrossRef]
- Ravlikovsky, A.; Pinheiro, M.N.C.; Kucheruk, P.; Symochko, L. Spent Mushroom Substrate as a Renewable Energy Resource: Evaluating Its Biogas Production Potential. Sustainability 2025, 17, 1800. [Google Scholar] [CrossRef]
- Bala, I.-A.; Tritean, N.; Enache, A.A.; Trică, B.; Constantinescu-Aruxandei, D.; Oancea, F. Effects of Blue-Light Laser Irradiation on the Enzymatic Activities and Sporulation of Trichoderma atroviride Grown on Rice Husks. Appl. Sci. 2023, 13, 9191. [Google Scholar] [CrossRef]
- Shyaula, M.; Regmi, S.; Khadka, D.; Poudel, R.C.; Dhakal, A.; Koirala, D.; Sijapati, J.; Singh, A.; Maharjan, J. Characterization of Thermostable Cellulase from Bacillus licheniformis PANG L Isolated from the Himalayan Soil. Int. J. Microbiol. 2023, 2023, 3615757. [Google Scholar] [CrossRef]
- Yuliana, T.; Maharddhika, A.; Rialita, T.; Lembong, E.; Anastassya, F.; Krama, A.; Ratu, S. Optimization of Laccase Production from Marasmius sp. in a Submerged Fermentation System. Pak. J. Biol. Sci. 2024, 27, 283–288. [Google Scholar] [CrossRef]
- Ben Khedher, N.; Lattieff, F.A.; Mahdi, J.M.; Ghanim, M.S.; Majdi, H.S.; Jweeg, M.J.; Baazaoui, N. Modeling of biogas production and biodegradability of date palm fruit wastes with different moisture contents. J. Clean. Prod. 2022, 375, 134103. [Google Scholar] [CrossRef]
- Ivan, G.R.; Ion, I.; Capra, L.; Oprea, O.; Ion, A.C. The Influence of the Chemical Composition of Natural Waters about the Triclocarban Sorption on Pristine and Irradiated MWCNTs. Separations 2023, 10, 46. [Google Scholar] [CrossRef]
- Restrepo Londoño, C.; Alvarado Torres, P.; Moreno, A.; Giraldo Gil, A. Characterization of Spent Mushroom Compost and Evaluation of Its Potential for Thermochemical Valorization through Ash Reduction Treatments. Biomass 2024, 4, 978–989. [Google Scholar] [CrossRef]
- Sheng, T.; Zhao, L.; Gao, L.; Liu, W.; Wu, G.; Wu, J.; Wang, A. Enhanced biohydrogen production from nutrient-free anaerobic fermentation medium with edible fungal pretreated rice straw. RSC Adv. 2018, 8, 22924–22930. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.M.; Mota, T.R.; Salatta, F.V.; de Almeida, G.H.G.; Olher, V.G.A.; Oliveira, M.A.S.; Marchiosi, R.; Ferrarese-Filho, O.; dos Santos, W.D. Feruloyl esterase activity and its role in regulating the feruloylation of maize cell walls. Plant Physiol. Biochem. 2020, 156, 49–54. [Google Scholar] [CrossRef]
- Kamusoko, R.; Mukumba, P. Potential of Wheat Straw for Biogas Production by Anaerobic Digestion in South Africa: A Review. Energies 2024, 17, 4662. [Google Scholar] [CrossRef]
- Schroyen, M.; Vervaeren, H.; Vandepitte, H.; Van Hulle, S.W.H.; Raes, K. Effect of enzymatic pretreatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour. Technol. 2015, 192, 696–702. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.; Wen, J.; Zhao, C.; Dong, L.; Liu, H. Underestimated humic acids release and influence on anaerobic digestion during sludge thermal hydrolysis. Water Res. 2021, 201, 117310. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; van Loosdrecht, M.C.M.; Luo, Y.; Cao, D. Effect of humic acids on batch anaerobic digestion of excess sludge. Water Res. 2019, 155, 431–443. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Yang, X.; Feng, Y.; Dong, L.; Zhang, Y.; Feng, L.; Mazarji, M.; Pan, J. From feedstock to digestion: Unraveling the impact of humic acid composition on anaerobic digestion. Sci. Total Environ. 2023, 902, 166495. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, H.; Wen, J.; Huang, S.; Zheng, Z.; Zhang, X.; Fu, B.; Li, Y.; Wang, A.; Liu, H. Influences of humic acids released during sludge thermal hydrolysis on anaerobic digestion: New insights from enzymatic perspectives. Chem. Eng. J. 2023, 474, 145849. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, H.; Zhang, X.; Li, Y.; Shi, M.; Huang, F.; Dong, L.; Wen, J.; Liu, H. Humic acids promotion or inhibition of sludge anaerobic digestion depends on their redox potentials. Chem. Eng. J. 2023, 464, 142653. [Google Scholar] [CrossRef]
- Li, S.; Sun, K.; Latif, A.; Si, Y.; Gao, Y.; Huang, Q. Insights into the Applications of Extracellular Laccase-Aided Humification in Livestock Manure Composting. Environ. Sci. Technol. 2022, 56, 7412–7425. [Google Scholar] [CrossRef] [PubMed]
- Schroyen, M.; Van Hulle, S.W.H.; Holemans, S.; Vervaeren, H.; Raes, K. Laccase enzyme detoxifies hydrolysates and improves biogas production from hemp straw and miscanthus. Bioresour. Technol. 2017, 244, 597–604. [Google Scholar] [CrossRef]
- Lin, Y.; Ge, X.; Li, Y. Solid-state anaerobic co-digestion of spent mushroom substrate with yard trimmings and wheat straw for biogas production. Bioresour. Technol. 2014, 169, 468–474. [Google Scholar] [CrossRef]
- Córdoba, V.; Colavolpe, M.B.; Fernández, M.; Santalla, E.; Albertó, E. Potential methane production of spent sawdust used in the cultivation of Gymnopilus pampeanus. J. Environ. Chem. Eng. 2016, 4, 4418–4425. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Chen, J.; Xie, W.; Kuo, J.; Lu, X.; Chang, K.; Wen, S.; Sun, G.; Cai, H.; et al. Combustion behaviors of spent mushroom substrate using TG-MS and TG-FTIR: Thermal conversion, kinetic, thermodynamic and emission analyses. Bioresour. Technol. 2018, 266, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Czerwinska, J.; Szufa, S.; Unyay, H.; Wielgosinski, G. Emission of Total Volatile Organic Compounds from the Torrefaction Process: Meadow Hay, Rye, and Oat Straw as Renewable Fuels. Energies 2025, 18, 4154. [Google Scholar] [CrossRef]
- Paredes, C.; Medina, E.; Moral, R.; Pérez-Murcia, M.; Moreno-Caselles, J.; Bustamante, M.; Cecilia, J. Characterization of the Different Organic Matter Fractions of Spent Mushroom Substrate. Commun. Soil Sci. Plant Anal. 2009, 40, 150–161. [Google Scholar] [CrossRef]
- Dima, S.O.; Constantinescu-Aruxandei, D.; Tritean, N.; Ghiurea, M.; Capra, L.; Nicolae, C.A.; Faraon, V.; Neamtu, C.; Oancea, F. Spectroscopic Analyses Highlight Plant Biostimulant Effects of Baker’s Yeast Vinasse and Selenium on Cabbage through Foliar Fertilization. Plants 2023, 12, 3016. [Google Scholar] [CrossRef]
- Ma, Y.H.; Wang, Q.; Sun, X.; Wang, X.; Su, W.; Song, N. A Study on Recycling of Spent Mushroom Substrate to Prepare Chars and Activated Carbon. BioResources 2014, 9, 3939–3954. [Google Scholar] [CrossRef]
- Popa-Tudor, I.; Tritean, N.; Dima, S.; Trica, B.; Ghiurea, M.; Cimpean, A.; Oancea, F.; Constantinescu-Aruxandei, D. Kombucha Versus Vegetal Cellulose for Affordable Mucoadhesive (nano)Formulations. Gels 2025, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Kapoor, S.; Thakur, R.; Sharma, E.; Tiwari, R.K.; Joshi, S.J. Lignocellulolytic enzymes and bioethanol production from spent biomass of edible mushrooms using Saccharomyces cerevisiae and Pachysolen tannophilus. Biomass Convers. Biorefinery 2025, 15, 28445–28459. [Google Scholar] [CrossRef]
- Chen, F.; Martín, C.; Lestander, T.A.; Grimm, A.; Xiong, S. Shiitake cultivation as biological preprocessing of lignocellulosic feedstocks—Substrate changes in crystallinity, syringyl/guaiacyl lignin and degradation-derived by-products. Bioresour. Technol. 2022, 344, 126256. [Google Scholar] [CrossRef]
- Long, J.; Wang, X.; Qiu, S.; Zhou, W.; Zhou, S.; Shen, K.; Xie, L.; Ma, X.; Zhang, X. Construction of cellulose-degrading microbial consortium and evaluation of their ability to degrade spent mushroom substrate. Front. Microbiol. 2024, 15, 1356903. [Google Scholar] [CrossRef] [PubMed]
- Facchinatto, W.M.; Santos, D.M.d.; Fiamingo, A.; Bernardes-Filho, R.; Campana-Filho, S.P.; Azevedo, E.R.d.; Colnago, L.A. Evaluation of chitosan crystallinity: A high-resolution solid-state NMR spectroscopy approach. Carbohydr. Polym. 2020, 250, 116891. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Wolcott, M.P.; Liu, H.; Glandon, N.; Wang, J. The influence of pre-fibrillation via planetary ball milling on the extraction and properties of chitin nanofibers. Cellulose 2020, 27, 6205–6216. [Google Scholar] [CrossRef]
- Guo, H.; Bian, K.; Ding, S.; Cai, H.; Zhang, H.; Chen, X.; Wang, C.; Yao, S.; Chen, X. Efficient Utilization of Biomass Hydrolysis Residues in Preparing a Metal/Acid Bifunctional Catalyst for Butyl Levulinate Hydrogenation to γ-Valerolactone. Ind. Eng. Chem. Res. 2023, 62, 5502–5514. [Google Scholar] [CrossRef]
- Poerio, A.; Girardet, T.; Petit, C.; Fleutot, S.; Jehl, J.; Arab-Tehrany, E.; Mano, J.; Cleymand, F. Comparison of the Physicochemical Properties of Chitin Extracted from Cicada orni Sloughs Harvested in Three Different Years and Characterization of the Resulting Chitosan. Appl. Sci. 2021, 11, 1278. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Bansal, V.; Ahmad, A.; Sastry, M. Fungus-Mediated Biotransformation of Amorphous Silica in Rice Husk to Nanocrystalline Silica. J. Am. Chem. Soc. 2006, 128, 14059–14066. [Google Scholar] [CrossRef]
- Rajavat, A.S.; Rai, S.; Pandiyan, K.; Kushwaha, P.; Choudhary, P.; Kumar, M.; Chakdar, H.; Singh, A.; Karthikeyan, N.; Bagul, S.Y.; et al. Sustainable use of the spent mushroom substrate of Pleurotus florida for production of lignocellulolytic enzymes. J. Basic Microbiol. 2020, 60, 173–184. [Google Scholar] [CrossRef]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hong, S.; Shen, X.-J.; Xue, Z.; Sun, Z.; Yuan, T.-Q. Structure–function relationships of deep eutectic solvents for lignin extraction and chemical transformation. Green Chem. 2020, 22, 7219–7232. [Google Scholar] [CrossRef]
- Zuffi, V.; Puliga, F.; Zambonelli, A.; Trincone, L.; Sanchez-Cortes, S.; Francioso, O. Sustainable Management of Anaerobic Digestate: From Biogas Plant to Full-Scale Cultivation of Pleurotus ostreatus. Agronomy 2023, 13, 950. [Google Scholar] [CrossRef]
- Banerjee, A.; Sahu, S.; Bhaskar, T.; Ghosh, D. Enhanced Glucan–Chitin Complex Extraction from Deoiled Yeast Biomass for Sustainable Biorefinery Applications. ACS Sustain. Chem. Eng. 2024, 12, 17450–17459. [Google Scholar] [CrossRef]
- Bekiaris, G.; Koutrotsios, G.; Tarantilis, P.A.; Pappas, C.S.; Zervakis, G.I. FTIR assessment of compositional changes in lignocellulosic wastes during cultivation of Cyclocybe cylindracea mushrooms and use of chemometric models to predict production performance. J. Mater. Cycles Waste Manag. 2020, 22, 1027–1035. [Google Scholar] [CrossRef]
- Gaur, R.; Agrawal, R.; Kumar, R.; Ramu, E.; Bansal, V.R.; Gupta, R.P.; Kumar, R.; Tuli, D.K.; Das, B. Evaluation of recalcitrant features impacting enzymatic saccharification of diverse agricultural residues treated by steam explosion and dilute acid. RSC Adv. 2015, 5, 60754–60762. [Google Scholar] [CrossRef]
- Battista, F.; Bolzonella, D. Beyond Anaerobic Digestion: New Perspectives for the Development of a Biorefinery Platform for the Simultaneous Production of Medium-Chain Fatty Acids by Chain Elongation and Biogas from Food Wastes. ACS Sustain. Chem. Eng. 2024, 12, 15294–15306. [Google Scholar] [CrossRef]
- Anderson, A.J.; Kim, Y.C. The Plant-Stress Metabolites, Hexanoic Aacid and Melatonin, Are Potential “Vaccines” for Plant Health Promotion. Plant Pathol. J. 2021, 37, 415–427. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Li, J.; Dong, Z.; Zhao, J.; Shao, T.; Yuan, X. Effects of hexanoic acid on microbial communities, fermentation, and hygienic quality of corn silages infested with toxigenic fungi. J. Sci. Food Agric. 2022, 102, 3522–3534. [Google Scholar] [CrossRef]
- D’Ambrosio, V.; Angelini, A.; Pastore, C. Hexanoic acid upgrading into hexyl hexanoate: An efficient way to obtain a new sustainable biofuel. Fuel 2024, 368, 131631. [Google Scholar] [CrossRef]
- Hong, D.H.; Gebresillase, M.N.; Seo, J.G. Upscaled Catalytic Production of Renewable Biofuels from Hexanoic Acid. Korean J. Chem. Eng. 2025, 42, 1033–1043. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, X.; Weng, L.; Zhu, X.; Liu, L. Study on low dielectric laminate modified by hyperbranched polyester of caprylic acid and hexanoic acid co-blocking. J. Mater. Sci. Mater. Electron. 2020, 31, 5068–5076. [Google Scholar] [CrossRef]
- Shi, J.; Weng, L.; Wang, X.; Sun, X.; Du, S.; Gao, F.; Zhang, X. Synthesis and evaluation of epoxy resin modified by hyperbranched polyester of caprylic acid and hexanoic acid. Pigment Resin Technol. 2021, 51, 33–41. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, L.; Ji, X.; Chen, R.; Zhu, J. Hexanoic Acid Production from Chinese Cabbage Waste Driven by In Situ Lactic Acid Pre-Fermentation: Effect of pH. Appl. Biochem. Biotechnol. 2025, 197, 6154–6168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, Y.; Zhou, X.; Wang, X.; Zhu, J. Effect of different vegetable wastes on the performance of volatile fatty acids production by anaerobic fermentation. Sci. Total Environ. 2020, 748, 142390. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Dávila, C.A.; Carrión, V.J.; Vonk, V.R.; Buisman, C.N.J.; Strik, D.P.B.T.B. Consecutive lactate formation and chain elongation to reduce exogenous chemicals input in repeated-batch food waste fermentation. Water Res. 2020, 169, 115215. [Google Scholar] [CrossRef] [PubMed]


| Variant | Composition |
|---|---|
| hay | 21 g hay, 180 mL water, 20 mL inoculum |
| hay + enzyme | 21 g hay, 100 mL 66% enzyme, 80 mL water, 20 mL inoculum |
| SPS 1 | 21 g SPS, 180 mL water, 20 mL inoculum |
| SPS + enzyme | 21 g SPS, 100 mL 66% enzyme, 80 mL water, 20 mL inoculum |
| Control | 200 mL water, 20 mL inoculum |
| Sample Name | Elemental Analysis % (w/w) | ICP-OES ISO 11885:2009, % (w/w) | Gravimetric Analysis % (w/w) | |||
|---|---|---|---|---|---|---|
| Total Nitrogen | Total Carbon | P (λ = 213.617 nm) | K (λ = 766.490 nm) | VS (550 °C) | Ash (550 °C) | |
| Hay | 1.52 | 43.00 | 0.20 | 0.31 | 94.89 ± 0.29 | 5.11 ± 0.29 |
| SPS | 2.48 | 40.00 | 0.21 | 0.69 | 80.28 ± 0.30 | 19.72 ± 0.30 |
| Sample | B1 (NmL/g VS) | Rm1 (NmL/g VS/h | λ1 (h) |
| Hay | 9.917 ± 1.899 b# | 1.503 ± 0.789 b | 3.989 ± 2.472 b |
| Hay + Enzyme | 3.008 ± 0.456 a | 1.077 ± 0.042 ab | −0.160 ± 0.012 a |
| SPS | 16.975 ± 1.581 c | 0.356 ± 0.058 a | −0.769 ± 0.735 a |
| SPS + Enzyme | 17.504 ± 0.800 c | 0.638 ± 0.037 ab | 2.503 ± 0.487 ab |
| Sample | B2 (NmL/g VS) | Rm2 (NmL/g VS) | λ2 (NmL/g VS) |
| Hay | 12.642 ± 1.515 | 0.798 ± 0.124 | 113.092 ± 22.690 |
| Hay + Enzyme | 11.271 ± 1.153 (p = 0.280) | 0.710 ± 0.183 (p = 0.530) | 234.181 ± 56.129 * (p = 0.026) |
| Sample Name | Liquid Digestate | Solid Digestate (Freeze-Dried) | |
|---|---|---|---|
| Dry Weight (%) | Dry Weight (g) | Moisture (%) | |
| Hay | 2.40 ± 0.10 b,# | 16.75 ± 1.94 bc | 79.40 ± 1.76 b |
| Hay + Enzyme | 3.42 ± 0.01 d | 18.72 ± 2.20 c | 77.50 ± 0.62 b |
| SPS | 3.18 ± 0.06 c | 14.53 ± 0.20 b | 71.60 ± 0.54 a |
| SPS + Enzyme | 4.28 ± 0.02 e | 14.02 ± 0.56 b | 69.90 ± 1.00 a |
| Control | 0.81 ± 0.02 a | 0.55 ± 0.03 a | 92.00 ± 2.25 c |
| Sample | T (°C); WL (%) | ResN2 | ResAir | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H2O & VOBs | Small Organics | Hemicellulose | Cellulose |
Lignin
Chitin |
Lignin
FC | CaCO3 | w% | w% | |
| 25–105 °C | 105–200 °C | 200–290 °C | 290–350 °C | 350–460 °C | 460–550 °C | 550–750 °C | 750 °C | 750 °C | |
| HayRaw | 53; 6.03 | 199.9; 7.14 | 261.8; 20.27 | 304.3; 34.29 | 422.4; 22.50 | 497.6; 4.87 | 650.0; 0.36 | 4.54 | 4.53 |
| SPSRaw | 53.5; 6.26 | 146.8; 5.67 | 255.7; 17.73 | 323.3; 27.46 | 446.1; 2.59 | 466.9; 5.47 | 690.0; 9.80 | 25.02 | 13.07 |
| HayAD | 49.4; 6.02 | - | 286.4; 27.24 | 328.9; 36.33 | - | 470.0; 7.08 | 650.0; 6.39 | 16.94 | 4.89 |
| SPSAD | 50.1; 6.05 | 148.8; 6.61 | 275.2; 14.60 | 338.7; 26.58 | 460.6; 6.00 | 477.3; 4.19 | 694.5; 11.03 | 24.94 | 14.66 |
| HayEnzAD | 49.2; 6.65 | 123.2; 0.93 | 274.3; 22.75 | 331.0; 40.84 | - | 475.6; 3.56 | 645.2; 5.02 | 20.25 | 4.93 |
| SPSEnzAD | 49.9; 5.66 | 149.6; 7.10 | 274.; 15.54 | 337.5; 24.57 | 461.8; 6.36 | 478.3; 4.06 | 695.0; 11.28 | 25.43 | 15.42 |
| Sample | Liquid Digestate | Solid Digestate (Freeze-Dried) |
|---|---|---|
| COD (mg/L) | COD (mg/g) | |
| Hay | 23,508.3 ± 471.6 b# | 1535.6 ± 263.2 b |
| Hay + Enzyme | 23,133.3 ± 358.5 b | 1211.4 ± 298.0 ab |
| SPS | 21,731.33 ± 3727.3 b | 1308.8 ± 65.9 ab |
| SPS + Enzyme | 29,391.7 ± 312.6 c | 1019.1 ± 216.0 ab |
| Control | 613.3 ± 270.2 a | 898.7 ± 237.7 a |
| Compound | A | B | C | D |
|---|---|---|---|---|
| Hay | Hay + Enzyme | SPS | SPS + Enzyme | |
| Area, % | Area, % | Area, % | Area, % | |
| Ethyl acetate | 0.39 ± 0.05 a,# | 0.73 ± 0.09 b A ** (p = 0.005) | 0.98 ± 0.14 c | 0.81 ± 0.08 bc |
| 2-Propanol | 0.00 ± 0.00 a | 0.74 ± 0.78 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Lactic acid | 0.00 ± 0.00 a | 0.62 ± 0.15 b A ** (p = 0.002) | 0.63 ± 0.04 b | 0.43 ± 0.06 b C ** (p = 0.007) |
| 2-Butanol | 0.52 ± 0.13 b | 1.91 ± 0.19 c A *** (p < 0.001) | 0.00 ± 0.00 a | 0.07 ± 0.08 a |
| Acetic acid | 0.00 ± 0.00 a | 0.29 ± 0.26 a | 0.73 ± 0.73 a | 0.95 ± 0.13 a A *** (p < 0.001); B * (p = 0.016) |
| Propanoic acid | 0.13 ± 0.13 a | 1.05 ± 0.08 c A ** (p = 0.001) | 0.45 ± 0.08 b | 0.48 ± 0.13 b |
| 2-methyl propanoic acid | 1.76 ± 0.05 a | 1.94 ± 1.58 a | 0.88 ± 0.13 a A *** (p < 0.001) | 1.01 ± 0.08 a A *** (p < 0.001) |
| Butanoic acid | 15.35 ± 2.28 a | 20.24 ± 9.73 a | 24.32 ± 1.73 a A ** (p = 0.006) | 23.09 ± 0.96 a A ** (p = 0.006) |
| 2-methyl butanoic acid | 1.32 ± 0.05 a | 2.72 ± 0.18 b A *** (p < 0.001) | 1.03 ± 0.11 a A * | 3.96 ± 0.24 c C *** (p < 0.001) |
| Pentanoic acid | 4.75 ± 0.11 ab | 6.74 ± 2.12 b | 2.31 ± 0.10 a | 4.51 ± 0.68 ab C ** (p = 0.005) |
| 4-methyl pentanoic acid | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.70 ± 0.23 b | 0.64 ± 0.23 b |
| Hexanoic acid | 64.51 ± 1.50 c | 53.11 ± 5.07 a A * (p = 0.02) | 58.75 ± 2.67 ab | 53.45 ± 0.64 a C * (p = 0.029) |
| Butylated hydroxytoluene | 1.42 ± 0.08 a | 2.81 ± 0.40 b A ** (p = 0.004) | 3.89 ± 0.32 c | 3.01 ± 0.49 bc C (ms) (p = 0.06) |
| Heptanoic acid | 2.38 ± 0.51 a | 3.15 ± 2.29 a | 1.04 ± 0.20 a | 2.75 ± 0.71 a C * (p = 0.016) |
| Octanoic acid | 3.61 ± 0.40 a | 2.62 ± 1.96 a | 2.33 ± 0.68 a | 3.39 ± 0.38 a C (ms) (p = 0.077) |
| Cyclohexanecarboxylic acid | 3.88 ± 0.14 b | 0.00 ± 0.00 a A *** (p < 0.001) | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Sample Name | Total Nitrogen | Total Carbon |
|---|---|---|
| Mean * (%) | Mean * (%) | |
| Solid digestate | ||
| Hay | 2.23 ± 0.34 a,# | 47.45 ± 0.07 c |
| Hay + Enzyme | 2.11 ± 0.21 a | 47.60 ± 0.57 c |
| SPS | 2.12 ± 0.26 a | 37.10 ± 0.14 b |
| SPS + Enzyme | 1.97 ± 0.30 a | 37.00 ± 0.14 b |
| Control | 3.40 ± 0.46 b | 31.30 ± 0.28 a |
| Liquid digestate | ||
| Hay | <0.19 ** | 0.87 ± 0.08 a |
| Hay + Enzyme | <0.19 ** | 1.19 ± 0.10 bc |
| SPS | <0.19 ** | 1.17 ± 0.09 b |
| SPS + Enzyme | <0.19 ** | 1.39 ± 0.02 c |
| Control | <0.19 ** | <0.52 ** |
| Sample Name | Element | |
|---|---|---|
| P % (w/w) | K % (w/w) | |
| Solid digestate | ||
| Hay | 0.200 ± 0.000 bc,# | 0.250 ± 0.010 a |
| Hay + Enzyme | 0.233 ± 0.015 c | 0.333 ± 0.012 b |
| SPS | 0.157 ± 0.015 a | 0.280 ± 0.017 ab |
| SPS + Enzyme | 0.187 ± 0.015 ab | 0.333 ± 0.012 b |
| Control | 2.127 ± 0.150 | 0.577 ± 0.051 c |
| Liquid digestate | ||
| Hay | 0.026 ± 0.006 a | 0.055 ± 0.008 b |
| Hay + Enzyme | 0.052 ± 0.027 a | 0.084 ± 0.002 c |
| SPS | 0.020 ± 0.003 a | 0.086 ± 0.011 c |
| SPS + Enzyme | 0.028 ± 0.003 a | 0.113 ± 0.006 d |
| Control | 0.017 ± 0.011 a | 0.027 ± 0.002 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinescu-Aruxandei, D.; Vlaicu, A.; Popa, D.G.; Dima, Ș.-O.; Deșliu-Avram, M.; Vintilă, A.C.N.; Ghiurea, M.; Cilțea-Udrescu, M.; Popa-Tudor, I.; Tritean, N.; et al. Enhancing the Valorization of Spent Pleurotus Substrate Through Anaerobic Digestion by Extracted Enzymes. Agronomy 2025, 15, 2663. https://doi.org/10.3390/agronomy15112663
Constantinescu-Aruxandei D, Vlaicu A, Popa DG, Dima Ș-O, Deșliu-Avram M, Vintilă ACN, Ghiurea M, Cilțea-Udrescu M, Popa-Tudor I, Tritean N, et al. Enhancing the Valorization of Spent Pleurotus Substrate Through Anaerobic Digestion by Extracted Enzymes. Agronomy. 2025; 15(11):2663. https://doi.org/10.3390/agronomy15112663
Chicago/Turabian StyleConstantinescu-Aruxandei, Diana, Alexandru Vlaicu, Daria Gabriela Popa, Ștefan-Ovidiu Dima, Mălina Deșliu-Avram, Alin Cristian Nicolae Vintilă, Marius Ghiurea, Mihaela Cilțea-Udrescu, Ioana Popa-Tudor, Naomi Tritean, and et al. 2025. "Enhancing the Valorization of Spent Pleurotus Substrate Through Anaerobic Digestion by Extracted Enzymes" Agronomy 15, no. 11: 2663. https://doi.org/10.3390/agronomy15112663
APA StyleConstantinescu-Aruxandei, D., Vlaicu, A., Popa, D. G., Dima, Ș.-O., Deșliu-Avram, M., Vintilă, A. C. N., Ghiurea, M., Cilțea-Udrescu, M., Popa-Tudor, I., Tritean, N., Ivan, G. R., Nicolae, C.-A., Ganciarov, M., Vasilievici, G., & Oancea, F. (2025). Enhancing the Valorization of Spent Pleurotus Substrate Through Anaerobic Digestion by Extracted Enzymes. Agronomy, 15(11), 2663. https://doi.org/10.3390/agronomy15112663









