Research Progress on the Regulation of Plant Rhizosphere Oxygen Environment by Micro-Nano Bubbles and Their Application Prospects in Alleviating Hypoxic Stress
Abstract
1. Introduction
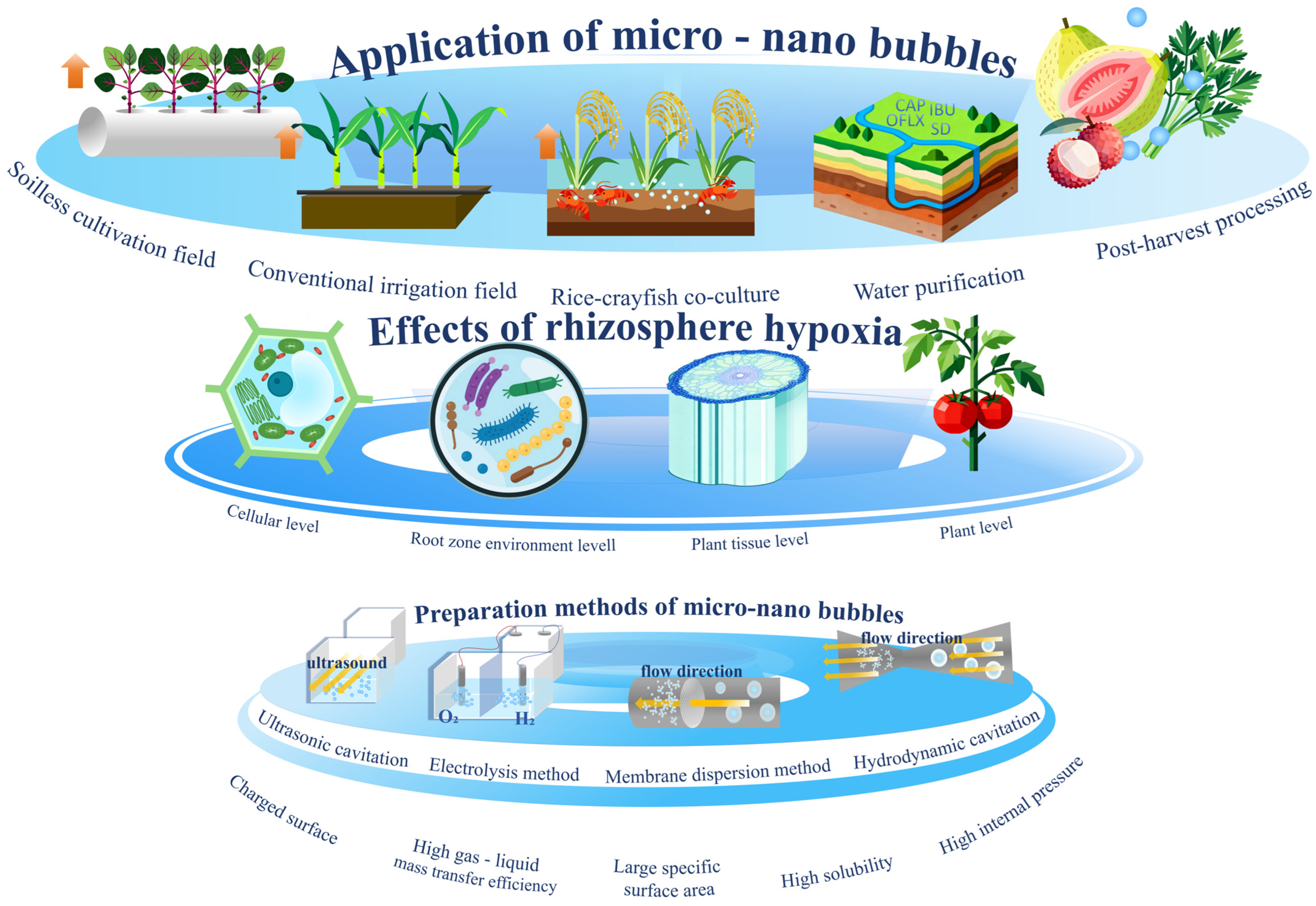
2. Physical Properties and Preparation Methods of MNBs
2.1. Physical Properties of MNBs
2.2. Preparation Methods of Micro-Nano Bubbles
2.2.1. Cavitation
- Ultrasonic cavitation
- 2.
- Hydrodynamic cavitation
2.2.2. Electrolysis Method
2.2.3. Membrane Dispersion Method
3. The Mechanism of Rhizosphere Hypoxia Affecting Plant Physiology, Plant Tolerance to Hypoxia, and the Alleviation of Hypoxia by MNBs
3.1. The Mechanism by Which Rhizosphere Hypoxia Influences Plant Physiological Processes
3.2. The Mechanism of Plant Tolerating Hypoxia
3.3. The Mechanism of MNBs Alleviating Hypoxia
3.4. Other Advantages of MNBs in Irrigation
3.5. The Potential Effects of ROS Generated After Their Collapse
4. The Application of MNBs in the Field of Agricultural Sciences
4.1. The Application of MNBs in the Field of Traditional Cultivation
4.2. The Application of MNBs in the Field of Soilless Cultivation
4.3. The Application of MNBs in Other Agricultural Science Fields
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peralta Ogorek, L.L.; Gao, Y.; Farrar, E.; Pandey, B.K. Soil compaction sensing mechanisms and root responses. Trends Plant Sci. 2025, 30, 565–575. [Google Scholar] [CrossRef]
- Keller, T.; Or, D. Farm vehicles approaching weights of sauropods exceed safe mechanical limits for soil functioning. Proc. Natl. Acad. Sci. USA 2022, 119, e2117699119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Peng, W.; Xie, Q.; Ran, E. Effects of Soil Compaction Stress Combined with Drought on Soil Pore Structure, Root System Development, and Maize Growth in Early Stage. Plants 2024, 13, 3185. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Jiang, F.; Yin, J.; Wang, Y.; Li, Y.; Yu, X.; Song, X.; Ottosen, C.O.; Rosenqvist, E.; Mittler, R.; et al. ROS-mediated waterlogging memory, induced by priming, mitigates photosynthesis inhibition in tomato under waterlogging stress. Front. Plant Sci. 2023, 14, 1238108. [Google Scholar] [CrossRef]
- Tan, X.; Xu, H.; Khan, S.; Equiza, M.A.; Lee, S.H.; Vaziriyeganeh, M.; Zwiazek, J.J. Plant water transport and aquaporins in oxygen-deprived environments. J. Plant Physiol. 2018, 227, 20–30. [Google Scholar] [CrossRef]
- Drew, M.; Armstrong, W. Root Growth and Metabolism Under Oxygen Deficiency. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2002; pp. 729–761. [Google Scholar]
- Liu, M.; Tan, X.; Sun, X.; Zwiazek, J.J. Properties of root water transport in canola (Brassica napus) subjected to waterlogging at the seedling, flowering and podding growth stages. Plant Soil 2020, 454, 431–445. [Google Scholar] [CrossRef]
- Liu, Z.; Hartman, S.; van Veen, H.; Zhang, H.; Leeggangers, H.; Martopawiro, S.; Bosman, F.; de Deugd, F.; Su, P.; Hummel, M.; et al. Ethylene augments root hypoxia tolerance via growth cessation and reactive oxygen species amelioration. Plant Physiol. 2022, 190, 1365–1383. [Google Scholar] [CrossRef]
- Greenway, H.; Gibbs, J. Review: Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Funct. Plant Biol. 2003, 30, 999–1036. [Google Scholar] [CrossRef]
- Nio, S.A.; Mantilen Ludong, D.P. Beneficial Root-Associated Microbiome during Drought and Flooding Stress in Plants. Pak. J. Biol. Sci. 2023, 26, 287–299. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and pathogenic plant-microbe interactions during flooding stress. Plant Cell Environ. 2022, 45, 2875–2897. [Google Scholar] [CrossRef]
- Moreno, S.R. The breathless niche: Unrevealing the metabolic landscape of root stem cells under hypoxia stress. Plant Physiol. 2023, 193, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhu, Z.; Chen, H.; Pan, H.; Jiang, L.; Su, W.-H.; Chen, Q.; Tang, Y.; Pan, J.; Yu, K. Full life circle of micro-nano bubbles: Generation, characterization and applications. Chem. Eng. J. 2023, 471, 144621. [Google Scholar] [CrossRef]
- Malahlela, H.K.; Belay, Z.A.; Mphahlele, R.R.; Caleb, O.J. Micro-nano bubble water technology: Sustainable solution for the postharvest quality and safety management of fresh fruits and vegetables—A review. Innov. Food Sci. Emerg. Technol. 2024, 94, 103665. [Google Scholar] [CrossRef]
- Xu, Q.; Nakajima, M.; Ichikawa, S.; Nakamura, N.; Shiina, T. A comparative study of microbubble generation by mechanical agitation and sonication. Innov. Food Sci. Emerg. Technol. 2008, 9, 489–494. [Google Scholar] [CrossRef]
- Li, H.; Hu, L.; Xia, Z. Impact of Groundwater Salinity on Bioremediation Enhanced by Micro-Nano Bubbles. Materials 2013, 6, 3676–3687. [Google Scholar] [CrossRef]
- Khaled Abdella Ahmed, A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Marhaba, T.; Zhang, W. Colloidal Properties of Air, Oxygen, and Nitrogen Nanobubbles in Water: Effects of Ionic Strength, Natural Organic Matters, and Surfactants. Environ. Eng. Sci. 2017, 35, 720–727. [Google Scholar] [CrossRef]
- Hu, L.; Xia, Z. Application of ozone micro-nano-bubbles to groundwater remediation. J. Hazard. Mater. 2018, 342, 446–453. [Google Scholar] [CrossRef]
- Li, H.; Hu, L.; Song, D.; Lin, F. Characteristics of micro-nano bubbles and potential application in groundwater bioremediation. Water Environ. Res. 2014, 86, 844–851. [Google Scholar] [CrossRef]
- Yao, G.J.; Ren, J.Q.; Zhou, F.; Liu, Y.D.; Li, W. Micro-nano aeration is a promising alternative for achieving high-rate partial nitrification. Sci. Total Environ. 2021, 795, 148899. [Google Scholar] [CrossRef]
- Khan, P.; Wang, H.; Gao, W.; Huang, F.; Khan, N.A.; Shakoor, N. Effects of micro-nano bubble with CO2 treated water on the growth of Amaranth green (Amaranthus viridis). Environ. Sci. Pollut. Res. 2022, 29, 72033–72044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Zhong, Y.; Zhou, Z.; Huang, Y.; Sun, C.Q. Nanobubble Skin Supersolidity. Langmuir 2016, 32, 11321–11327. [Google Scholar] [CrossRef] [PubMed]
- Gevari, M.T.; Abbasiasl, T.; Niazi, S.; Ghorbani, M.; Koşar, A. Direct and indirect thermal applications of hydrodynamic and acoustic cavitation: A review. Appl. Therm. Eng. 2020, 171, 115065. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, T.; Shang, J.; Tao, J.; Liu, Y.; Yang, T.; Ren, H.; Hu, G. Bubble dynamics model and its revelation of ultrasonic cavitation behavior in advanced oxidation processes: A review. J. Water Process Eng. 2024, 63, 105470. [Google Scholar] [CrossRef]
- Leong, T.; Ashokkumar, M.; Kentish, S.E. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 59–63. [Google Scholar]
- Cao, J.; Xue, H.H.; Zheng, Y.N.; Wang, L.; Sun, L.T. Enhanced microbubble-mediated cavitation by using acoustic droplet vaporization. Appl. Acoust. 2024, 219, 109919. [Google Scholar] [CrossRef]
- David Fernandez, R.; Prosperetti, A.; Zijlstra, A.G.; Lohse, D.; Han, J.G.E.G. Efficient Sonochemistry through Microbubbles Generated with Micromachined Surfaces. Angew. Chem. (Int. Ed. Engl.) 2012, 49, 9699–9701. [Google Scholar] [CrossRef]
- Panda, D.; Saharan, V.K.; Manickam, S. Controlled Hydrodynamic Cavitation: A Review of Recent Advances and Perspectives for Greener Processing. Processes 2020, 8, 220. [Google Scholar] [CrossRef]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Microbubble-aided water and wastewater purification: A review. Rev. Chem. Eng. 2012, 28, 191–221. [Google Scholar] [CrossRef]
- Ochoa, E.D.; García, M.C.; Padilla, N.D.; Remolina, A.M. Design and experimental evaluation of a Venturi and Venturi-Vortex microbubble aeration system. Heliyon 2022, 8, e10824. [Google Scholar] [CrossRef]
- Wu, M.; Yuan, S.; Song, H.; Li, X. Micro-nano bubbles production using a swirling-type venturi bubble generator. Chem. Eng. Process. 2022, 170, 108697. [Google Scholar] [CrossRef]
- Wu, M.; Song, H.; Liang, X.; Huang, N.; Li, X. Generation of micro-nano bubbles by self-developed swirl-type micro-nano bubble generator. Chem. Eng. Process. 2022, 181, 109136. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, B.; Wu, C.; Xu, X.; Xue, M.; Zheng, X. Numerical Simulation and Structural Optimization of Swirl Flow Micro-Nano Bubble Generator. Coatings 2023, 13, 1468. [Google Scholar] [CrossRef]
- Zhu, J.; An, H.; Alheshibri, M.; Liu, L.; Terpstra, P.M.; Liu, G.; Craig, V.S. Cleaning with Bulk Nanobubbles. Langmuir 2016, 32, 11203–11211. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.-T.; Yiacoumi, S.; Tsouris, C. Experiments on Electrostatic Dispersion of Air in Water. Ind. Eng. Chem. Res. 1997, 36, 3647–3655. [Google Scholar] [CrossRef]
- Kukizaki, M.; Goto, M. Size control of nanobubbles generated from Shirasu-porous-glass (SPG) membranes. J. Membr. Sci. 2006, 281, 386–396. [Google Scholar] [CrossRef]
- Hill, R.D.; Igamberdiev, A.U.; Stasolla, C. Preserving root stem cell functionality under low oxygen stress: The role of nitric oxide and phytoglobins. Planta 2023, 258, 89. [Google Scholar] [CrossRef]
- Dittert, K.; Wötzel, J.; Sattelmacher, B. Responses of Alnus glutinosa to anaerobic conditions—Mechanisms and rate of oxygen flux into the roots. Plant Biol. 2006, 8, 212–223. [Google Scholar] [CrossRef]
- Tong, S.; Kjær, J.E.; Peralta Ogorek, L.L.; Pellegrini, E.; Song, Z.; Pedersen, O.; Herzog, M. Responses of key root traits in the genus Oryza to soil flooding mimicked by stagnant, deoxygenated nutrient solution. J. Exp. Bot. 2023, 74, 2112–2126. [Google Scholar] [CrossRef]
- Mira, M.M.; Hill, R.D.; Hilo, A.; Langer, M.; Robertson, S.; Igamberdiev, A.U.; Wilkins, O.; Rolletschek, H.; Stasolla, C. Plant stem cells under low oxygen: Metabolic rewiring by phytoglobin underlies stem cell functionality. Plant Physiol. 2023, 193, 1416–1432. [Google Scholar] [CrossRef]
- Striker, G.G. An overview of oxygen transport in plants: Diffusion and convection. Plant Biol. 2023, 25, 842–847. [Google Scholar] [CrossRef]
- da-Silva, C.J.; do Amarante, L. Short-term nitrate supply decreases fermentation and oxidative stress caused by waterlogging in soybean plants. Environ. Exp. Bot. 2020, 176, 104078. [Google Scholar] [CrossRef]
- Rathnayaka Pathiranage, R.G.L.; Mira, M.M.; Hill, R.D.; Stasolla, C. The inhibition of maize (Zea mays L.) root stem cell regeneration by low oxygen is attenuated by Phytoglobin 1 (Pgb1) through changes in auxin and jasmonic acid. Planta 2023, 257, 120. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.S.; Da-Silva, C.J.; Shabala, S.; Striker, G.G.; Carvalho, I.R.; de Oliveira, A.C.B.; do Amarante, L. Understanding plant responses to saline waterlogging: Insights from halophytes and implications for crop tolerance. Planta 2023, 259, 24. [Google Scholar] [CrossRef] [PubMed]
- Howell, T.; Moriconi, J.I.; Zhao, X.; Hegarty, J.; Fahima, T.; Santa-Maria, G.E.; Dubcovsky, J. A wheat/rye polymorphism affects seminal root length and yield across different irrigation regimes. J. Exp. Bot. 2019, 70, 4027–4037. [Google Scholar] [CrossRef]
- Munir, R.; Konnerup, D.; Khan, H.A.; Siddique, K.H.M.; Colmer, T.D. Sensitivity of chickpea and faba bean to root-zone hypoxia, elevated ethylene, and carbon dioxide. Plant Cell Environ. 2019, 42, 85–97. [Google Scholar] [CrossRef]
- Huang, X.; Shabala, L.; Zhang, X.; Zhou, M.; Voesenek, L.; Hartman, S.; Yu, M.; Shabala, S. Cation transporters in cell fate determination and plant adaptive responses to a low-oxygen environment. J. Exp. Bot. 2022, 73, 636–645. [Google Scholar] [CrossRef]
- Liu, M.; Khan, S.; Zwiazek, J.J. Overexpression of Nicotiana tabacum PIP1;3 enhances root aeration and oxygen metabolism in canola (Brassica napus) plants exposed to root hypoxia. Plant Physiol. Biochem. 2024, 216, 109122. [Google Scholar] [CrossRef]
- Lehmann, J.; Jørgensen, M.E.; Fratz, S.; Müller, H.M.; Kusch, J.; Scherzer, S.; Navarro-Retamal, C.; Mayer, D.; Böhm, J.; Konrad, K.R.; et al. Acidosis-induced activation of anion channel SLAH3 in the flooding-related stress response of Arabidopsis. Curr. Biol. 2021, 31, 3575–3585.e3579. [Google Scholar] [CrossRef]
- Li, G.; Cheng, H.; Qiao, C.; Feng, J.; Yan, P.; Yang, R.; Song, J.; Sun, J.; Zhao, Y.; Zhang, Z. Root-zone oxygen supply mitigates waterlogging stress in tomato by enhancing root growth, photosynthetic performance, and antioxidant capacity. Plant Physiol. Biochem. 2025, 222, 109744. [Google Scholar] [CrossRef]
- Bai, T.; Li, C.; Li, C.; Liang, D.; Ma, F. Contrasting hypoxia tolerance and adaptation in Malus species is linked to differences in stomatal behavior and photosynthesis. Physiol. Plant. 2013, 147, 514–523. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Kawabata, S.; Thuyet, D.Q. Nanobubble Water’s Promotion Effect of Barley (Hordeum vulgare L.) Sprouts Supported by RNA-Seq Analysis. Langmuir 2017, 33, 12478–12486. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wang, Q.; Mu, W.; Ma, C.; Wei, K.; Sun, Y.; Zhao, X. Application of activated water irrigation technology: A sustainable way to improve soil fertility and crop adaptability in the sandy area of southern Xinjiang. Soil Tillage Res. 2025, 254, 106731. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, L.; Chen, L.; Zhang, H.; Li, G.; Wang, G.; Cui, J.; Filatova, I.; Liu, Y. Effect of micro-nano bubbles on the remediation of saline-alkali soil with microbial agent. Sci. Total Environ. 2024, 912, 168940. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; He, Y.; He, Y.; Zhan, J.; Shi, C.; Xu, Y.; Wang, X.; Wang, J.; Zhang, C. Micro-nano bubble water subsurface drip irrigation affects strawberry yield and quality by modulation of microbial communities. Agric. Water Manag. 2025, 307, 109228. [Google Scholar] [CrossRef]
- Zhou, Y.; Bastida, F.; Liu, Y.; He, J.; Chen, W.; Wang, X.; Xiao, Y.; Song, P.; Li, Y. Impacts and mechanisms of nanobubbles level in drip irrigation system on soil fertility, water use efficiency and crop production: The perspective of soil microbial community. J. Clean. Prod. 2022, 333, 130050. [Google Scholar] [CrossRef]
- Marcelino, K.R.; Wongkiew, S.; Shitanaka, T.; Surendra, K.C.; Song, B.; Khanal, S.K. Micronanobubble Aeration Enhances Plant Yield and Nitrification in Aquaponic Systems. ACS EST Eng. 2023, 3, 2081–2096. [Google Scholar] [CrossRef]
- Zhou, Y.; Bastida, F.; Zhou, B.; Sun, Y.; Gu, T.; Li, S.; Li, Y. Soil fertility and crop production are fostered by micro-nano bubble irrigation with associated changes in soil bacterial community. Soil Biol. Biochem. 2020, 141, 107663. [Google Scholar] [CrossRef]
- Li, H.; Li, P.; Li, J.; Jiang, Y.; Huang, X. Influence of micro/nano aeration on the diversity of the microbial community in drip irrigation to reduce emitter clogging. Biosys. Eng. 2023, 235, 116–130. [Google Scholar] [CrossRef]
- Wang, L.; Ali, J.; Wang, Z.; Oladoja, N.; Cheng, R.; Zhang, C.; Mailhot, G.; Pan Frsc, G. Oxygen nanobubbles enhanced photodegradation of oxytetracycline under visible light: Synergistic effect and mechanism. Chem. Eng. J. 2020, 388, 124227. [Google Scholar] [CrossRef]
- Bhattacharjee, S. ROS and Oxidative Stress: Origin and Implication. In Reactive Oxygen Species in Plant Biology; Bhattacharjee, S., Ed.; Springer: New Delhi, India, 2019; pp. 1–31. [Google Scholar]
- Marcelino, K.R.; Ling, L.; Wongkiew, S.; Nhan, H.T.; Surendra, K.C.; Shitanaka, T.; Lu, H.; Khanal, S.K. Nanobubble technology applications in environmental and agricultural systems: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1378–1403. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS Produced by Nanobubbles and Their Positive and Negative Effects on Vegetable Seed Germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. C. R. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative Capacity of Nanobubbles and Its Effect on Seed Germination. ACS Sustain. Chem. Eng. 2016, 4, 1347–1353. [Google Scholar] [CrossRef]
- Huchzermeyer, B.; Menghani, E.; Khardia, P.; Shilu, A. Metabolic Pathway of Natural Antioxidants, Antioxidant Enzymes and ROS Providence. Antioxidants 2022, 11, 761. [Google Scholar] [CrossRef]
- Bian, Q.; Dong, Z.; Zhao, Y.; Feng, Y.; Fu, Y.; Wang, Z.; Zhu, J. Phosphorus Supply Under Micro-Nano Bubble Water Drip Irrigation Enhances Maize Yield and Phosphorus Use Efficiency. Plants 2024, 13, 3046. [Google Scholar] [CrossRef]
- Guo, X.; Chen, R.; Li, H.; Li, G.; Zhao, Q.; Wang, D.; Wang, J. Micro-nano bubble hydrogen water irrigation improves the Cd tolerance of Ipomoea aquatica Forssk.: Rhizosphere micro-biotas modulation and oxidative stress alleviation. Environ. Pollut. 2025, 376, 126394. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, P.; Jin, Q.; Chen, J.; Chen, D.; Dai, X.; Ding, S.; Chu, L. Aeration Alleviated the Adverse Effects of Nitrogen Topdressing Reduction on Tomato Root Vigor, Photosynthetic Performance, and Fruit Development. Plants 2024, 13, 1378. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, R.; Wen, Y.; Zhang, J.; Yin, F.; Javed, T.; Zheng, J.; Wang, Z. Processing tomato (Lycopersicon esculentum Miller) yield and quality in arid regions through micro-nano aerated drip irrigation coupled with humic acid application. Agric. Water Manag. 2025, 308, 109317. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zhang, J.; Liang, X.; Wang, H.; Yang, Z.; Tang, R.; Yu, Q.; Zhang, Y. Micro-nano aerated subsurface drip irrigation and biochar promote photosynthesis, dry matter accumulation and yield of cucumbers in greenhouse. Agric. Water Manag. 2025, 308, 109295. [Google Scholar] [CrossRef]
- Qian, Y.; Guan, X.; Shao, C.; Qiu, C.; Chen, X.; Chen, J.; Peng, C. Effects of Different Concentrations of Micro-Nano Bubbles on Grain Yield and Nitrogen Absorption and Utilization of Double Cropping Rice in South China. Agronomy 2022, 12, 2196. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, J.; Li, S.; Lei, W.; Liu, A. Effects of micro/nano-ozone bubble nutrient solutions on growth promotion and rhizosphere microbial community diversity in soilless cultivated lettuces. Front. Plant Sci. 2024, 15, 1393905. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar] [CrossRef]
- Bian, Q.; Dong, Z.; Zhao, Y.; Feng, Y.; Fu, Y.; Wang, Z.; Zhu, J.; Ma, L. Micro-/nanobubble oxygenation irrigation enhances soil phosphorus availability and yield by altering soil bacterial community abundance and core microbial populations. Front. Plant Sci. 2024, 15, 1497952. [Google Scholar] [CrossRef]
- Ma, D.; Yin, R.; Liang, Z.; Liang, Q.; Xu, G.; Lian, Q.; Wong, P.K.; He, C.; Xia, D.; Lu, H. Photo-sterilization of groundwater by tellurium and enhancement by micro/nano bubbles. Water Res. 2023, 233, 119781. [Google Scholar] [CrossRef]
- Jing, M.; Zhang, J.; Li, G.; Zhang, D.; Liu, F.; Yang, S. Micro-nano bubbles enhanced immobilized Chlorella vulgaris to remove ofloxacin from groundwater. J. Contam. Hydrol. 2025, 268, 104458. [Google Scholar] [CrossRef]
- Zhu, T.; Jing, M.; Zhang, J.; Li, H.; Zhou, M.; Li, G. Efficacy of micro-nano bubble enhanced immobilized Chlorella vulgaris in the removal of typical antibiotics. RSC Adv. 2025, 15, 20268–20280. [Google Scholar] [CrossRef]
- Zhou, S.; Qiao, L.; Jia, Y.; Khanal, S.K.; Sun, L.; Lu, H. Micro-nano bubble ozonation for effective treatment of ibuprofen-laden wastewater and enhanced anaerobic digestion performance. Water Res. 2025, 273, 123006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Wang, M.; Lu, J.; Zhang, H.; Héroux, P.; Wang, G.; Tang, L.; Liu, Y. Evaluating micro-nano bubbles coupled with rice-crayfish co-culture systems: A field study promoting sustainable rice production intensification. Sci. Total Environ. 2024, 933, 173162. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Guo, L.; Wu, G.; Chen, Z.; Abbas, F.; Yin, Q. Preparation and evaluation of ozone micro-nano bubbles ice for Litchi precooling. Food Chem. 2025, 472, 142945. [Google Scholar] [CrossRef]
- Shi, J.; Cai, H.; Qin, Z.; Li, X.; Yuan, S.; Yue, X.; Sui, Y.; Sun, A.; Cui, J.; Zuo, J.; et al. Ozone micro-nano bubble water preserves the quality of postharvest parsley. Food Res. Int. 2023, 170, 113020. [Google Scholar] [CrossRef] [PubMed]
- Malahlela, H.K.; Belay, Z.A.; Mphahlele, R.R.; Caleb, O.J. Efficacy of Air and Oxygen Micro-nano Bubble Waters Against Colletotrichum gloeosporioides and Impacts on Postharvest Quality of ‘Fan Retief’ Guava Fruit. J. Food Prot. 2025, 88, 100437. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, W.; Xiong, J.; Huang, Z.; Gan, T.; Hu, H.; Qin, Y.; Zhang, Y. Polarized electric field induced by piezoelectric effect of ozone micro-nano bubbles/spontaneously polarized ceramic to boost ozonolysis for efficient fruit sterilization. Food Chem. 2025, 466, 142191. [Google Scholar] [CrossRef]
- Nakazawa, R.; Tanaka, A.; Hata, N.; Minagawa, H.; Harada, E. Nanobubbles in vase water inhibit transpiration and prolong the vase life of cut chrysanthemum flowers. Plant-Environ. Interact. 2023, 4, 309–316. [Google Scholar] [CrossRef]
- Li, L.; Yin, Q.; Zhang, T.; Cheng, P.; Xu, S.; Shen, W. Hydrogen Nanobubble Water Delays Petal Senescence and Prolongs the Vase Life of Cut Carnation (Dianthus caryophyllus L.) Flowers. Plants 2021, 10, 1662. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Tang, T.; Hu, J.; Zhou, X.; Zhang, L. Properties of CO2 Micro-Nanobubbles and Their Significant Applications in Sustainable Development. Nanomaterials 2025, 15, 1270. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Oshita, S.; Fan, W.; Liu, S. Mechanism for Enhancing the Ozonation Process of Micro-And Nanobubbles: Bubble Behavior and Interface Reaction. ACS EST Water 2023, 3, 3835–3847. [Google Scholar] [CrossRef]
- Pal, P.; Kioka, A. Micro and nanobubbles enhanced ozonation technology: A synergistic approach for pesticides removal. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70133. [Google Scholar] [CrossRef]


| System Type | Core Mechanism of Action | Practical Impacts |
|---|---|---|
| Conventional Irrigation System |
| |
| Soilless Cultivation System | ||
| Saline–Alkali Soil System | Soil CEC increased 1.3–1.7 times, pH reduced by 5–5.8% [55]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Zeng, H.; Liu, R.; Wu, L.; Pan, Y.; Li, J.; Shang, C. Research Progress on the Regulation of Plant Rhizosphere Oxygen Environment by Micro-Nano Bubbles and Their Application Prospects in Alleviating Hypoxic Stress. Agronomy 2025, 15, 2620. https://doi.org/10.3390/agronomy15112620
Zheng K, Zeng H, Liu R, Wu L, Pan Y, Li J, Shang C. Research Progress on the Regulation of Plant Rhizosphere Oxygen Environment by Micro-Nano Bubbles and Their Application Prospects in Alleviating Hypoxic Stress. Agronomy. 2025; 15(11):2620. https://doi.org/10.3390/agronomy15112620
Chicago/Turabian StyleZheng, Kexin, Honghao Zeng, Renyuan Liu, Lang Wu, Yu Pan, Jinhua Li, and Chunyu Shang. 2025. "Research Progress on the Regulation of Plant Rhizosphere Oxygen Environment by Micro-Nano Bubbles and Their Application Prospects in Alleviating Hypoxic Stress" Agronomy 15, no. 11: 2620. https://doi.org/10.3390/agronomy15112620
APA StyleZheng, K., Zeng, H., Liu, R., Wu, L., Pan, Y., Li, J., & Shang, C. (2025). Research Progress on the Regulation of Plant Rhizosphere Oxygen Environment by Micro-Nano Bubbles and Their Application Prospects in Alleviating Hypoxic Stress. Agronomy, 15(11), 2620. https://doi.org/10.3390/agronomy15112620







