Harnessing Genomics and Transcriptomics to Combat PVY Resistance in Potato: From Gene Discovery to Breeding Applications
Abstract
1. Introduction
2. Potato Virus Y: Biology, Structure and Impact
3. Genetic Basis of PVY Resistance and Breeding Aspects
| Locus | Chromosome | Species of Origin | Position on DM1-3 v6.1 Genome | Associated Markers | Distance to the PVY Resistance Gene (cM) | Marker Type | Ploidy-Specific Applicability | Reference |
|---|---|---|---|---|---|---|---|---|
| Ny-1 | IX | S. tuberosum | 66,252,800–66,254,515 | SC8951139 | 0.5 | STS | Effective in tetraploid breeding programs | [33] |
| GP41443 | 1.0 | SCAR | [32] | |||||
| C2_At3g168401100 | 6.0 | COSII | ||||||
| S1d11 | 2 | CAPS | ||||||
| GP129 | 9 | |||||||
| TG591 | - | |||||||
| U276927 | 16 | |||||||
| TG591 | 5 | |||||||
| S1d11 | - | |||||||
| U38666 | 14 | |||||||
| TG186 | 19 | |||||||
| Ny-Smira | IX | S. tuberosum | - | Ry186 | 1.4 | STS | Tetraploid | [92,105] |
| Rchc | IX | S. chacoense | 65,488,223–65,491,765 | MG64-17 | - | Both diploid and tetraploid | [34] | |
| Ry_4099 | - | KASP | [106] | |||||
| Ry_3331 | - | |||||||
| Ry186 | 1.4 | STS | [92] | |||||
| Ny-2 | XI | Sarpo Mira | 1,655,790–1,661,555 | N1271164 | - | SCAR | Tetraploid | [32] |
| ShkB | 4.0 | CAPS | ||||||
| B11.6 | - | |||||||
| Ryadg | XI | S. tuberosum sp. Andigenum | 39,331,965–39,333,799 | RYSc3 | - | SCAR | Primarily Effective for tetraploid potato breeding efforts | [107,108] |
| RYSC4 | - | [24,107] | ||||||
| TG508 | 2.1 | RFLP | [89] | |||||
| GP125 | 4.2 | |||||||
| CD17 | 2.1 | |||||||
| CT168 | ~13.2 | |||||||
| M45 | ~0.2 | SCAR | [109,110] | |||||
| ADG1 | - | RFLP | [89] | |||||
| ADG2 | 1.3 | [89,111] | ||||||
| Rysto | XII | S. stoloniferum | 2,457,137–2,459,737 | STM0003 | - | SSR | Both diploid and tetraploid | [112,113,114] |
| Cat-in2 | 12.5 | SCAR | [112,114] | |||||
| YES3-3A | 0.53 | [90,115] | ||||||
| ST1 | 1.3 | [113,114] | ||||||
| STM0003-111 | 2.95 | SSR | ||||||
| M1 | 0.53 | RAPD | [113] | |||||
| M2 | 5.84 | |||||||
| M3 | 9.13 | |||||||
| SCARysto4 | 9.1 | SCAR | [116] | |||||
| GP122 | - | CAPS | [88,112] | |||||
| Ry-fsto | XII | S. stolonifer-um | 1,734,940–1,736,028 | GP122718 | 1.2′ | CAPS | ‘’ | [88] |
| GP122564 | - | [91] |
4. Transcriptomics in Understanding Potato-PVY Interactions
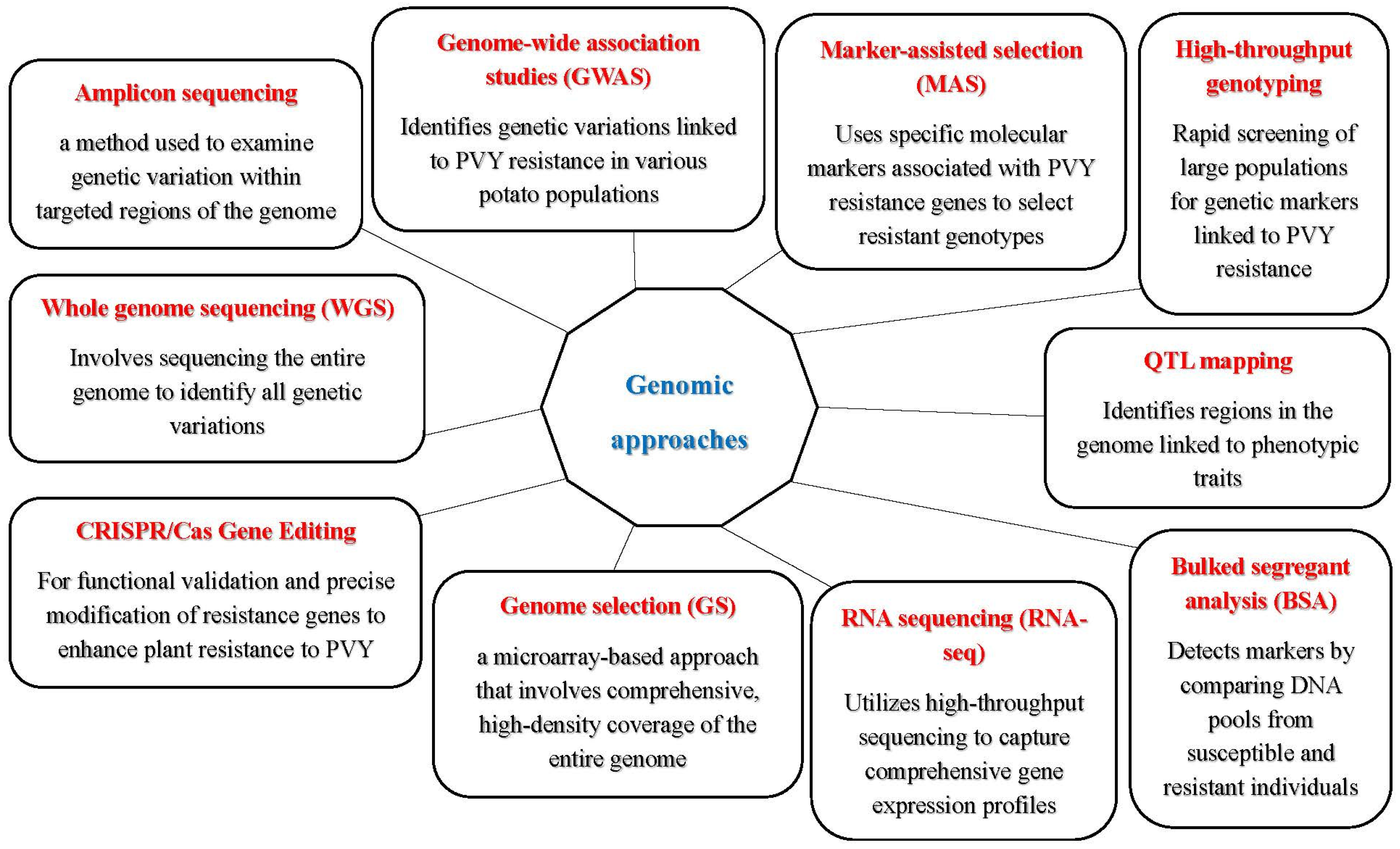
5. Genomic Approaches for Breeding PVY-Resistant Varieties
6. Limitations of MAS for PVY Resistance
7. Future Perspectives and Challenges
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crandall, S.G.; Gold, K.M.; del Mar Jiménez-Gasco, M.; Filgueiras, C.C.; Willett, D.S. A Multi-Omics Approach to Solving Problems in Plant Disease Ecology. PLoS ONE 2020, 15, e0237975. [Google Scholar] [CrossRef]
- Qi, W.; Chen, J.; Han, Y.; Li, Z.; Su, X.; Yeo, F.K.S. Editorial: Omics-Driven Crop Improvement for Stress Tolerance. Front. Plant Sci. 2023, 14, 1172228. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F. Applications of Multi-Omics Technologies for Crop Improvement. Front. Plant Sci. 2021, 12, 563953. [Google Scholar] [CrossRef] [PubMed]
- Manasseh, R.; Sathuvalli, V.; Pappu, H.R. Transcriptional and Functional Predictors of Potato Virus Y-Induced Tuber Necrosis in Potato (Solanum tuberosum). Front. Plant Sci. 2024, 15, 1369846. [Google Scholar] [CrossRef] [PubMed]
- Stare, T.; Ramšak, Ž.; Križnik, M.; Gruden, K. Multiomics Analysis of Tolerant Interaction of Potato with Potato Virus Y. Sci. Data 2019, 6, 250. [Google Scholar] [CrossRef]
- Vleeshouwers, V.G.A.A.; Rietman, H.; Krenek, P.; Champouret, N.; Young, C.; Oh, S.K.; Wang, M.; Bouwmeester, K.; Vosman, B.; Visser, R.G.F.; et al. Effector Genomics Accelerates Discovery and Functional Profiling of Potato Disease Resistance and Phytophthora infestans Avirulence Genes. PLoS ONE 2008, 3, e2875. [Google Scholar] [CrossRef]
- Chung, B.Y.W.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An Overlapping Essential Gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Bradley, R.H.E. Infectivity of Aphids after Several Hours on Tobacco Infected with Potato Virus Y. Nature 1953, 171, 755–756. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, W.; Ding, W.; Chen, W.; Zhao, D. Effects of PVY-Infected Tobacco Plants on the Adaptation of Myzus persicae (Hemiptera: Aphididae). Insects 2022, 13, 1120. [Google Scholar] [CrossRef]
- Aramburu, J.; Galipienso, L.; Matas, M. Characterization of Potato Virus Y Isolates from Tomato Crops in Northeast Spain. Eur. J. Plant Pathol. 2006, 115, 247–258. [Google Scholar] [CrossRef]
- Grbin, D.; Pecman, A.; Musić, M.Š.; Kutnjak, D.; Škorić, D. First Report of Potato Virus S and Potato Virus Y in Tomatoes from Croatia. Plant Dis. 2023, 107, 975. [Google Scholar] [CrossRef]
- Fereres, A.; Perez, P.; Gemeno, C.; Ponz, F. Transmission of Spanish Pepper- and Potato-PVY Isolates by Aphid (Homoptera: Aphididae) Vectors: Epidemiological Implications. Environ. Entomol. 1993, 22, 1260–1265. [Google Scholar] [CrossRef]
- Vinodhini, J.; Rajendran, L.; Karthikeyan, G. Molecular Evidence of ‘N’ Strain of Potato Virus Y Causing Mosaic Disease on Hot Pepper (Capsicum annuum) in India. Indian Phytopathol. 2023, 76, 647–650. [Google Scholar] [CrossRef]
- Murphy, A.F.; Rondon, S.I.; Moreno, A.; Fereres, A. Effect of Potato Virus y Presence in Solanum tuberosum (Solanales: Solanaceae) and Chenopodium album on Aphid (Hemiptera: Aphididae) Behavior. Environ. Entomol. 2018, 47, 654–659. [Google Scholar] [CrossRef]
- Byarugaba, A.A.; Mukasa, S.B.; Barekye, A.; Rubaihayo, P.R. Interactive Effects of Potato Virus Y and Potato Leafroll Virus Infection on Potato Yields in Uganda. Open Agric. 2020, 5, 726–739. [Google Scholar] [CrossRef]
- Beczner, L.; Horváth, J.; Romhányi, I.; Förster, H. Studies on the Etiology of Tuber Necrotic Ringspot Disease in Potato. Potato Res. 1984, 27, 339–352. [Google Scholar] [CrossRef]
- Bouhachem, S.B.; Khamassy, N.; Glais, L.; Kerlan, C. Occurrence in Tunisia of Potato Tuber Necrotic Ringspot Disease (PTNRD) Caused by Variant PVYNTN of Potato Virus Y. Plant Pathol. 2008, 57, 388. [Google Scholar] [CrossRef]
- Nolte, P.; Whitworth, J.L.; Thornton, M.K.; McIntosh, C.S. Effect of Seedborne Potato Virus Y on Performance of Russet Burbank, Russet Norkotah, and Shepody Potato. Plant Dis. 2004, 88, 248–252. [Google Scholar] [CrossRef]
- Whitworth, J.L.; Hamm, P.B.; McIntosh, C.S. Effect of Potato Virus Y on Yield of a Clonal Selection of Russet Norkotah. Am. J. Potato Res. 2010, 87, 310–314. [Google Scholar] [CrossRef]
- Boquel, S.; Zhang, J.; Goyer, C.; Giguère, M.A.; Clark, C.; Pelletier, Y. Effect of Insecticide-Treated Potato Plants on Aphid Behavior and Potato Virus Y Acquisition. Pest Manag. Sci. 2015, 71, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, M.; Achal, V. A Comprehensive Review on Environmental and Human Health Impacts of Chemical Pesticide Usage. Emerg. Contam. 2025, 11, 100410. [Google Scholar] [CrossRef]
- Goyer, A.; Hamlin, L.; Crosslin, J.M.; Buchanan, A.; Chang, J.H. RNA-Seq Analysis of Resistant and Susceptible Potato Varieties during the Early Stages of Potato Virus Y Infection. BMC Genom. 2015, 16, 472. [Google Scholar] [CrossRef]
- Del Rosario Herrera, M.; Vidalon, L.J.; Montenegro, J.D.; Riccio, C.; Guzman, F.; Bartolini, I.; Ghislain, M. Molecular and Genetic Characterization of the Ryadg Locus on Chromosome XI from Andigena Potatoes Conferring Extreme Resistance to Potato Virus Y. Theor. Appl. Genet. 2018, 131, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.K.; Chakrabarti, S.K.; Kumar, V.; Gopal, J.; Singh, B.P.; Pandey, S.K.; Pattanayak, D. Identification of Host Gene Conferring Resistance to Potato Virus Y Using Ry Gene-Based Molecular Markers. Indian J. Hortic. 2013, 70, 373–377. [Google Scholar]
- Palloix, A.; Ayme, V.; Moury, B. Durability of Plant Major Resistance Genes to Pathogens Depends on the Genetic Background, Experimental Evidence and Consequences for Breeding Strategies. New Phytol. 2009, 183, 190–199. [Google Scholar] [CrossRef]
- Xu, C.; Guo, H.; Li, R.; Lan, X.; Zhang, Y.; Xie, Q.; Zhu, D.; Mu, Q.; Wang, Z.; An, M. Transcriptomic and Functional Analyses Reveal the Molecular Mechanisms Underlying Fe-Mediated Tobacco Resistance to Potato Virus Y Infection. Front. Plant Sci. 2023, 14, 1163679. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Witek, K.; Szajko, K.; Witek, A.I.; Morgiewicz, K.; Wasilewicz-Flis, I.; Jakuczun, H.; Marczewski, W.; Jones, J.D.G.; Hennig, J. Extreme Resistance to Potato Virus Y in Potato Carrying the Rysto Gene Is Mediated by a TIR-NLR Immune Receptor. Plant Biotechnol. J. 2020, 18, 655–667. [Google Scholar] [CrossRef]
- Ross, B.T.; Zidack, N.; McDonald, R.; Flenniken, M.L. Transcriptome and Small RNA Profiling of Potato Virus Y Infected Potato Cultivars, Including Systemically Infected Russet Burbank. Viruses 2022, 14, 523. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, X.; Bai, J.; Lv, W.; Chen, Q.; Hu, J.; Liu, G.; Zhao, Y.; Zhou, H.; Zhao, M.; et al. Transcriptome Analysis of Genes Involved in the Pathogenesis Mechanism of Potato Virus Y in Potato Cultivar YouJin. Front. Microbiol. 2024, 15, 1353814. [Google Scholar] [CrossRef]
- Paluchowska, P.; Lim Rossmann, S.; Lysøe, E.; Janiszewska, M.; Michalak, K.; Heydarnajad Giglou, R.; Torabi Giglou, M.; Brurberg, M.B.; Śliwka, J.; Yin, Z. Diversity of the Rysto Gene Conferring Resistance to Potato Virus Y in Wild Relatives of Potato. BMC Plant Biol. 2024, 24, 375. [Google Scholar] [CrossRef] [PubMed]
- Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W. Ny-1 and Ny-2 Genes Conferring Hypersensitive Response to Potato Virus Y (PVY) in Cultivated Potatoes: Mapping and Marker-Assisted Selection Validation for PVY Resistance in Potato Breeding. Mol. Breed. 2014, 34, 267–271. [Google Scholar] [CrossRef]
- Szajko, K.; Chrzanowska, M.; Witek, K.; Strzelczyk-Zyta, D.; Zagórska, H.; Gebhardt, C.; Hennig, J.; Marczewski, W. The Novel Gene Ny-1 on Potato Chromosome IX Confers Hypersensitive Resistance to Potato Virus Y and Is an Alternative to Ry Genes in Potato Breeding for PVY Resistance. Theor. Appl. Genet. 2008, 116, 297–303. [Google Scholar] [CrossRef]
- Li, G.; Shao, J.; Wang, Y.; Liu, T.; Tong, Y.; Jansky, S.; Xie, C.; Song, B.; Cai, X. Rychc Confers Extreme Resistance to Potato Virus Y in Potato. Cells 2022, 11, 2577. [Google Scholar] [CrossRef] [PubMed]
- Torrance, L.; Talianksy, M.E. Potato Virus y Emergence and Evolution from the Andes of South America to Become a Major Destructive Pathogen of Potato and Other Solanaceous Crops Worldwide. Viruses 2020, 12, 1430. [Google Scholar] [CrossRef] [PubMed]
- Syller, J.; Grupa, A. The Effects of Co-Infection by Different Otato Virus Y (PVY) Isolates on Virus Concentration in Solanaceous Hosts and Efficiency of Transmission. Plant Pathol. 2014, 63, 466–475. [Google Scholar] [CrossRef]
- Noureen, A.; Khan, M.Z.; Amin, I.; Zainab, T.; Mansoor, S. CRISPR/Cas9-Mediated Targeting of Susceptibility Factor EIF4E-Enhanced Resistance Against Potato Virus Y. Front. Genet. 2022, 13, 922019. [Google Scholar] [CrossRef]
- Zhan, X.; Liu, W.; Nie, B.; Zhang, F.; Zhang, J. Cas13d-Mediated Multiplex RNA Targeting Confers a Broad-Spectrum Resistance against RNA Viruses in Potato. Commun. Biol. 2023, 6, 855. [Google Scholar] [CrossRef]
- Moury, B.; Desbiez, C. Host Range Evolution of Potyviruses: A Global Phylogenetic Analysis. Viruses 2020, 12, 111. [Google Scholar] [CrossRef]
- Slater, A.T.; Schultz, L.; Lombardi, M.; Rodoni, B.C.; Bottcher, C.; Cogan, N.O.I.; Forster, J.W. Screening for Resistance to PVY in Australian Potato Germplasm. Genes 2020, 11, 429. [Google Scholar] [CrossRef]
- Bellardi, M.G.; Rubies-Autonell, C.; Vicchi, V. Virus Infections of Surfinia in Italy. Acta Hortic. 1996, 432, 306–311. [Google Scholar] [CrossRef]
- Kubaa, R.A.; Choueiri, E.; De Stradis, A.; Jreijiri, F.; Saponari, M.; Cillo, F. Occurrence and Distribution of Major Viruses Infecting Eggplant in Lebanon and Molecular Characterization of a Local Potato Virus X Isolate. Agriculture 2021, 11, 126. [Google Scholar] [CrossRef]
- Whitworth, J.L.; Nolte, P.; McIntosh, C.; Davidson, R. Effect of Potato Virus Y on Yield of Three Potato Cultivars Grown under Different Nitrogen Levels. Plant Dis. 2006, 90, 73–76. [Google Scholar] [CrossRef]
- Dupuis, B.; Nkuriyingoma, P.; Ballmer, T. Economic Impact of Potato Virus Y (PVY) in Europe. Potato Res. 2024, 67, 55–72. [Google Scholar] [CrossRef]
- Okeyo, G.O.; Sharma, K.; Atieno, E.; Narla, R.D.; Miano, D.W.; Schulte-Geldermann, E. Effectiveness of Positive Selection in Managing Seed-Borne Potato Viruses. J. Agric. Sci. 2018, 10, 71. [Google Scholar] [CrossRef]
- Priegnitz, U.; Lommen, W.J.M.; van der Vlugt, R.A.A.; Struik, P.C. Impact of Positive Selection on Incidence of Different Viruses During Multiple Generations of Potato Seed Tubers in Uganda. Potato Res. 2019, 62, 1–30. [Google Scholar] [CrossRef]
- Gildemacher, P.R.; Kaguongo, W.; Ortiz, O.; Tesfaye, A.; Woldegiorgis, G.; Wagoire, W.W.; Kakuhenzire, R.; Kinyae, P.M.; Nyongesa, M.; Struik, P.C.; et al. Improving Potato Production in Kenya, Uganda and Ethiopia: A System Diagnosis. Potato Res. 2009, 52, 173–205. [Google Scholar] [CrossRef]
- Beriso, K.; Mohammed, W.; Yusuf, A.; Kumar, A. Single and Mixed Infections of Six Major Potato Viruses in Four Major Potato-Growing Districts of Eastern Ethiopia. Crop Prot. 2024, 184, 106860. [Google Scholar] [CrossRef]
- Nyakio, M.; Were, M.; Wekesa, C.; Lungayia, H.; Okoth, P.; Were, H. Molecular Footprints of Potato Virus Y Isolate Infecting Potatoes (Solanum tuberosum) in Kenya. Adv. Virol. 2024, 2024, 2197725. [Google Scholar] [CrossRef] [PubMed]
- Onditi, J.; Nyongesa, M.; van der Vlugt, R. Prevalence, Distribution and Control of Potato Virus Y (PVY) Strains in Kenyan Potato Cultivars. Trop. Plant Pathol. 2022, 47, 659–671. [Google Scholar] [CrossRef]
- Pirone, T.P.; Perry, K.L. Aphids: Non-Persistent Transmission. Adv. Bot. Res. 2002, 36, 1–19. [Google Scholar] [CrossRef]
- Woodford, J.A.T. Virus Transmission by Aphids in Potato Crops. Neth. J. Plant Pathol. 1992, 98, 47–54. [Google Scholar] [CrossRef]
- Collar, J.L.; Avilla, C.; Duque, M.; Fereres, A. Behavioral Response and Virus Vector Ability of Myzus persicae (Homoptera: Aphididae) Probing on Pepper Plants Treated with Aphicides. J. Econ. Entomol. 1997, 90, 1628–1634. [Google Scholar] [CrossRef]
- da Silva, W.; Kutnjak, D.; Xu, Y.; Xu, Y.; Giovannoni, J.; Elena, S.F.; Gray, S. Transmission Modes Affect the Population Structure of Potato Virus Y in Potato. PLoS Pathog. 2020, 16, e1008608. [Google Scholar] [CrossRef]
- Inoue-Nagata, A.K.; Jordan, R.; Kreuze, J.; Li, F.; López-Moya, J.J.; Mäkinen, K.; Ohshima, K.; Wylie, S.J.; Siddell, S.G.; Lefkowitz, E.J.; et al. ICTV Virus Taxonomy Profile: Potyviridae 2022. J. Gen. Virol. 2022, 103, 001738. [Google Scholar] [CrossRef]
- Smith, K.M. On the Composite Nature of Certain Potato Virus Diseases of the Mosaic Group as Revealed by the Use of Plant Indicators and Selective Methods of Transmission. Proc. R. Soc. London Ser. B Contain. Pap. Biol. Character 1931, 109, 251–267. [Google Scholar]
- Ogawa, T.; Nakagawa, A.; Hataya, T.; Ohshima, K. The Genetic Structure of Populations of Potato Virus Y in Japan; Based on the Analysis of 20 Full Genomic Sequences. J. Phytopathol. 2012, 160, 661–673. [Google Scholar] [CrossRef]
- Robaglia, C.; Durand-Tardif, M.; Tronchet, M.; Boudazin, G.; Astier-Manifacier, S.; Casse-Delbart, F. Nucleotide Sequence of Potato Virus Y (N Strain) Genomic RNA. J. Gen. Virol. 1989, 70 Pt 4, 935–947. [Google Scholar] [CrossRef]
- Moyo, L.; Ramesh, S.V.; Kappagantu, M.; Mitter, N.; Sathuvalli, V.; Pappu, H.R. The Effects of Potato Virus Y-Derived Virus Small Interfering RNAs of Three Biologically Distinct Strains on Potato (Solanum tuberosum) Transcriptome. Virol. J. 2017, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Pompe-Novak, M.; Gruden, K.; Baebler, Š.; Krečič-Stres, H.; Kovač, M.; Jongsma, M.; Ravnikar, M. Potato Virus Y Induced Changes in the Gene Expression of Potato (Solanum tuberosum L.). Physiol. Mol. Plant Pathol. 2006, 67, 237–247. [Google Scholar] [CrossRef]
- Quenouille, J.; Vassilakos, N.; Moury, B. Potato Virus Y: A Major Crop Pathogen That Has Provided Major Insights into the Evolution of Viral Pathogenicity. Mol. Plant Pathol. 2013, 14, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Crosslin, J.M.; Hamm, P.B.; Eastwell, K.C.; Thornton, R.E.; Brown, C.R.; Corsini, D.; Shiel, P.J.; Berger, P.H. First Report of the Necrotic Strain of Potato Virus Y (PVYN) on Potatoes in the Northwestern United States. Plant Dis. 2002, 86, 1177. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. Occurrence of Potato Tuber Necrotic Isolates of Potato Virus Y in a Commercial Tobacco Field in Southern Ontario, Canada. Plant Dis. 2008, 92, 1586. [Google Scholar] [CrossRef]
- Funke, C.N.; Nikolaeva, O.V.; Green, K.J.; Tran, L.T.; Chikh-Ali, M.; Quintero-Ferrer, A.; Cating, R.A.; Frost, K.E.; Hamm, P.B.; Olsen, N. Strain-Specific Resistance to Potato Virus Y (PVY) in Potato and Its Effect on the Relative Abundance of PVY Strains in Commercial Potato Fields. Plant Dis. 2017, 101, 20–28. [Google Scholar] [CrossRef]
- Srinivasan, R.; Alvarez, J.M. Effect of Mixed Viral Infections (Potato Virus Y-Potato Leafroll Virus) on Biology and Preference of Vectors Myzus persicae and Macrosiphum euphorbiae (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 1047–1052. [Google Scholar] [CrossRef]
- Kerlan, C.; Nikolaeva, O.V.; Hu, X.; Meacham, T.; Gray, S.M.; Karasev, A. V Identification of the Molecular Make-Up of the Potato Virus Y Strain PVYZ: Genetic Typing of PVYZ-NTN. Phytopathology 2011, 101, 1052–1060. [Google Scholar] [CrossRef]
- Glais, L.; Tribodet, M.; Kerlan, C. Genomic Variability in Potato Potyvirus Y (PVY): Evidence That PVY NW and PVY NTN Variants Are Single to Multiple Recombinants between PVY O and PVY N Isolates. Arch. Virol. 2002, 147, 363–378. [Google Scholar] [CrossRef]
- Chare, E.R.; Holmes, E.C. A Phylogenetic Survey of Recombination Frequency in Plant RNA Viruses. Arch. Virol. 2006, 151, 933–946. [Google Scholar] [CrossRef]
- Schubert, J.; Fomitcheva, V.; Sztangret-Wiśniewska, J. Differentiation of Potato Virus Y Strains Using Improved Sets of Diagnostic PCR-Primers. J. Virol. Methods 2007, 140, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sarawaneeyaruk, S.; Iwakawa, H.O.; Mizumoto, H.; Murakami, H.; Kaido, M.; Mise, K.; Okuno, T. Host-Dependent Roles of the Viral 5′ Untranslated Region (UTR) in RNA Stabilization and Cap-Independent Translational Enhancement Mediated by the 3′ UTR of Red Clover Necrotic Mosaic Virus RNA1. Virology 2009, 391, 107–118. [Google Scholar] [CrossRef]
- Sriskanda, V.S.; Pruss, G.; Ge, X.; Vance, V.B. An Eight-Nucleotide Sequence in the Potato Virus X 3′ Untranslated Region Is Required for Both Host Protein Binding and Viral Multiplication. J. Virol. 1996, 70, 5266–5271. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, P. Nucleotide Sequence and Genome Organization of a Canadian Isolate of the Common Strain of Potato Virus Y (PVY°). Can. J. Plant Pathol. 1996, 18, 209–224. [Google Scholar] [CrossRef]
- Galvino-Costa, S.B.F.; dos Reis Figueira, A.; de Assis Câmara Rabelo-Filho, F.; Moraes, F.H.R.; Nikolaeva, O.V.; Karasev, A.V. Molecular and Serological Typing of Potato Virus Y Isolates from Brazil Reveals a Diverse Set of Recombinant Strains. Plant Dis. 2012, 96, 1451–1458. [Google Scholar] [CrossRef]
- Nie, X.; Singh, R.P. Evolution of North American PVY(NTN) Strain Tu 660 from Local PVY(N) by Mutation Rather than Recombination. Virus Genes 2003, 26, 39–47. [Google Scholar] [CrossRef]
- Yin, Z.; Xie, F.; Michalak, K.; Pawełkowicz, M.; Zhang, B.; Murawska, Z.; Lebecka, R.; Zimnoch-Guzowska, E. Potato Cultivar Etola Exhibits Hypersensitive Resistance to PVYNTN and Partial Resistance to PVYZ-NTN and PVYN-Wi Strains and Strain-Specific Alterations of Certain Host MiRNAs Might Correlate with Symptom Severity. Plant Pathol. 2017, 66, 539–550. [Google Scholar] [CrossRef]
- Jakab, G.; Droz, E.; Brigneti, G.; Baulcombe, D.; Malnoë, P. Infectious in Vivo and in Vitro Transcripts from a Full-Length CDNA Clone of PVY-N605, a Swiss Necrotic Isolate of Potato Virus Y. J. Gen. Virol. 1997, 78, 3141–3145. [Google Scholar] [CrossRef] [PubMed]
- Dullemans, A.M.; Cuperus, C.; Verbeek, M.; van der Vlugt, R.A.A. Complete Nucleotide Sequence of a Potato Isolate of Strain Group C of Potato Virus Y from 1938. Arch. Virol. 2011, 156, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Kosakowski, J.; Palucha, A.; Chrzanowska, M. Whole Genome Sequencing of Polish Reference PVY(N)W Isolate. “Old” Wilga After 30 Years of Passaging from Plant to Plant; Department of Protein Biosynthesis, Institute of Biochemistry and Biophysics PAS: Warsaw, Poland, 2007; submitted.
- Barker, H.; McGeachy, K.D.; Toplak, N.; Gruden, K.; Žel, J.; Browning, I. Comparison of Genome Sequence of PVY Isolates with Biological Properties. Am. J. Potato Res. 2009, 86, 227–238. [Google Scholar] [CrossRef]
- Fanigliulo, A.; Comes, S.; Pacella, R.; Harrach, B.; Martin, D.P.; Crescenzi, A. Characterisation of Potato Virus Y Nnp Strain Inducing Veinal Necrosis in Pepper: A Naturally Occurring Recombinant Strain of PVY. Arch. Virol. 2005, 150, 709–720. [Google Scholar] [CrossRef]
- Hühnlein, A.; Drechsler, N.; Steinbach, P.; Thieme, T.; Schubert, J. Comparison of Three Methods for the Detection of Potato Virus Y in Seed Potato Certification. J. Plant Dis. Prot. 2013, 120, 57–69. [Google Scholar] [CrossRef]
- Goh, C.J.; Hahn, Y. Analysis of Proteolytic Processing Sites in Potyvirus Polyproteins Revealed Differential Amino Acid Preferences of NIa-pro Protease in Each of Seven Cleavage Sites. PLoS ONE 2021, 16, e0245853. [Google Scholar] [CrossRef]
- Choi, S.H.; Hagiwara-Komoda, Y.; Nakahara, K.S.; Atsumi, G.; Shimada, R.; Hisa, Y.; Naito, S.; Uyeda, I. Quantitative and Qualitative Involvement of P3N-PIPO in Overcoming Recessive Resistance against Clover Yellow Vein Virus in Pea Carrying the Cyv1 Gene. J. Virol. 2013, 87, 7326–7337. [Google Scholar] [CrossRef]
- Baebler, Š.; Coll, A.; Gruden, K. Plant Molecular Responses to Potato Virus Y: A Continuum of Outcomes from Sensitivity and Tolerance to Resistance. Viruses 2020, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, J.P.T. Elucidation of Virus-Host Interactions to Enhance Resistance Breeding for Control of Virus Diseases in Potato. Breed. Sci. 2015, 65, 69–76. [Google Scholar] [CrossRef]
- Elison, G.L.; Novy, R.G.; Whitworth, J.L.; Yilma, S. Correction to: Russet Potato Breeding Clones with Extreme Resistance to Potato Virus Y Conferred by Rychc as Well as Resistance to Late Blight and Cold-Induced Sweetening. Am. J. Potato Res. 2021, 98, 411–419. [Google Scholar] [CrossRef]
- Torrance, L.; Cowan, G.H.; McLean, K.; MacFarlane, S.; Al-Abedy, A.N.; Armstrong, M.; Lim, T.Y.; Hein, I.; Bryan, G.J. Natural Resistance to Potato Virus Y in Solanum tuberosum Group Phureja. Theor. Appl. Genet. 2020, 133, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Flis, B.; Hennig, J.; Strzelczyk-Żyta, D.; Gebhardt, C.; Marczewski, W. The Ry-Fsto Gene from Solanum Stoloniferum for Extreme Resistant to Potato Virus Y Maps to Potato Chromosome XII and Is Diagnosed by PCR Marker GP122718 in PVY Resistant Potato Cultivars. Mol. Breed. 2005, 15, 95–101. [Google Scholar] [CrossRef]
- Hämäläinen, J.H.; Watanabe, K.N.; Valkonen, J.P.T.; Arihara, A.; Plaisted, R.L.; Pehu, E.; Miller, L.; Slack, S.A. Mapping and Marker-Assisted Selection for a Gene for Extreme Resistance to Potato Virus Y. Theor. Appl. Genet. 1997, 94, 192–197. [Google Scholar] [CrossRef]
- Kondrák, M.; Kopp, A.; Uri, C.; Sós-Hegedűs, A.; Csákvári, E.; Schiller, M.; Barta, E.; Cernák, I.; Polgár, Z.; Taller, J.; et al. Mapping and DNA Sequence Characterisation of the Rysto Locus Conferring Extreme Virus Resistance to Potato Cultivar “White Lady. ” PLoS ONE 2020, 15, e0224534. [Google Scholar] [CrossRef]
- Witek, K.; Strzelczyk-Żyta, D.; Hennig, J.; Marczewski, W. A Multiplex PCR Approach to Simultaneously Genotype Potato towards the Resistance Alleles Ry-Fstoand Ns. Mol. Breed. 2006, 18, 273–275. [Google Scholar] [CrossRef]
- Tomczyńska, I.; Jupe, F.; Hein, I.; Marczewski, W.; Śliwka, J. Hypersensitive Response to Potato Virus Y in Potato Cultivar Sárpo Mira Is Conferred by the Ny-Smira Gene Located on the Long Arm of Chromosome IX. Mol. Breed. 2014, 34, 471–480. [Google Scholar] [CrossRef]
- Quenouille, J.; Saint-Felix, L.; Moury, B.; Palloix, A. Diversity of Genetic Backgrounds Modulating the Durability of a Major Resistance Gene. Analysis of a Core Collection of Pepper Landraces Resistant to P Otato Virus Y. Mol. Plant Pathol. 2016, 17, 296–302. [Google Scholar] [CrossRef]
- Janzac, B.; Tribodet, M.; Lacroix, C.; Moury, B.; Verrier, J.L.; Jacquot, E. Evolutionary Pathways to Break down the Resistance of Allelic Versions of the PVY Resistance Gene Va. Plant Dis. 2014, 98, 1521–1529. [Google Scholar] [CrossRef]
- Acosta-Leal, R.; Xiong, Z. Intrahost Mechanisms Governing Emergence of Resistance-Breaking Variants of Potato Virus Y. Virology 2013, 437, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Caruana, B.M.; Rodoni, B.C.; Constable, F.; Slater, A.T.; Cogan, N.O.I. Genome Enhanced Marker Improvement for Potato Virus y Disease Resistance in Potato. Agronomy 2021, 11, 832. [Google Scholar] [CrossRef]
- Rowley, J.S.; Gray, S.M.; Karasev, A. V Screening Potato Cultivars for New Sources of Resistance to Potato Virus Y. Am. J. Potato Res. 2015, 92, 38–48. [Google Scholar] [CrossRef]
- Jones, R.A.C. Strain Group Specific and Virus Specific Hypersensitive Reactions to Infection with Potyviruses in Potato Cultivars. Ann. Appl. Biol. 1990, 117, 93–105. [Google Scholar] [CrossRef]
- Visser, J.C.; Bellstedt, D.U.; Pirie, M.D. The Recent Recombinant Evolution of a Major Crop Pathogen, Potato Virus Y. PLoS ONE 2012, 7, e50631. [Google Scholar] [CrossRef]
- Tian, D.; Traw, M.B.; Chen, J.Q.; Kreitman, M.; Bergelson, J. Fitness Costs of R-Gene-Mediated Resistance in Arabidopsis thaliana. Nature 2003, 423, 74–77. [Google Scholar] [CrossRef]
- Ungerer, M.C.; Halldorsdottir, S.S.; Purugganan, M.D.; Mackay, T.F.C. Genotype-Environment Interactions at Quantitative Trait Loci Affecting Inflorescence Development in Arabidopsis Thaliana. Genetics 2003, 165, 353–365. [Google Scholar] [CrossRef]
- Massa, A.N.; Manrique-Carpintero, N.C.; Coombs, J.; Haynes, K.G.; Bethke, P.C.; Brandt, T.L.; Gupta, S.K.; Yencho, G.C.; Novy, R.G.; Douches, D.S. Linkage Analysis and QTL Mapping in a Tetraploid Russet Mapping Population of Potato. BMC Genet. 2018, 19, 87. [Google Scholar] [CrossRef]
- Leuenberger, J.; Sharma, S.K.; McLean, K.; Pelle, R.; Berard, A.; Lesage, M.-L.; Porhel, D.; Dantec, M.-A.; Chauvin, J.-E.; Bryan, G.J. A Genomic Dataset Integrating Genotyping-by-Sequencing, SolCAP Array and PCR Marker Data on Tetraploid Potato Advanced Breeding Lines. Front. Plant Sci. 2024, 15, 1384401. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Vincent, S.J. Strain-Specific Hypersensitive and Extreme Resistance Phenotypes Elicited by Potato Virus y among 39 Potato Cultivars Released in Three World Regions over a 117-Year Period. Plant Dis. 2018, 102, 185–196. [Google Scholar] [CrossRef]
- Mori, K.; Sakamoto, Y.; Mukojima, N.; Tamiya, S.; Nakao, T.; Ishii, T.; Hosaka, K. Development of a Multiplex PCR Method for Simultaneous Detection of Diagnostic DNA Markers of Five Disease and Pest Resistance Genes in Potato. Euphytica 2011, 180, 347–355. [Google Scholar] [CrossRef]
- Asano, K.; Endelman, J.B. Development of KASP Markers for the Potato Virus Y Resistance Gene Rychc Using Whole-Genome Resequencing Data. Am. J. Potato Res. 2024, 101, 114–121. [Google Scholar] [CrossRef]
- Kasai, K.; Morikawa, Y.; Sorri, V.A.; Valkonen, J.P.T.; Gebhardt, C.; Watanabe, K.N. Development of SCAR Markers to the PVY Resistance Gene RY(Adg) Based on a Common Feature of Plant Disease Resistance Genes. Genome 2000, 43, 1–8. [Google Scholar] [CrossRef]
- Biryukova, V.A.; Shmiglya, I.V.; Zharova, V.A.; Beketova, M.P.; Rogozina, E.V.; Mityushkin, A.V.; Meleshin, A.A. Molecular Markers of Genes for Extreme Resistance to Potato Virus Y in Solanum tuberosum L. Cultivars and Hybrids. Russ. Agric. Sci. 2019, 45, 517–523. [Google Scholar] [CrossRef]
- Brigneti, G.; Garcia-Mas, J.; Baulcombe, D.C. Molecular Mapping of the Potato Virus Y Resistance Gene Ry(Sto) in Potato. Theor. Appl. Genet. 1997, 94, 198–203. [Google Scholar] [CrossRef]
- Dalla Rizza, M.; Vilaró, F.L.; Torres, D.G.; Maeso, D. Detection of PVY Extreme Resistance Genes in Potato Germplasm from the Uruguayan Breeding Program. Am. J. Potato Res. 2006, 83, 297–304. [Google Scholar] [CrossRef]
- Sorri, V.A.; Watanabe, K.N.; Valkonen, J.P.T. Predicted Kinase-3a Motif of a Resistance Gene Analogue as a Unique Marker for Virus Resistance. Theor. Appl. Genet. 1999, 99, 164–170. [Google Scholar] [CrossRef]
- Song, Y.-S.; Schwarzfischer, A.A. Development of STS Markers for Selection of Extreme Resistance (Rysto) to PVY and Maternal Pedigree Analysis of Extremely Resistant Cultivars. Am. J. Potato Res. 2008, 85, 159–170. [Google Scholar] [CrossRef]
- Cernák, I.; Taller, J.; Wolf, I.; Fehér, E.; Babinszky, G.; Alföldi, Z.; Csanádi, G.; Polgár, Z. Analysis of the Applicability of Molecular Markers Linked to the PVY Extreme Resistance Gene Rysto, and the Identification of New Markers. Acta Biol. Hung. 2008, 59, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Decsi, K.; Cernák, I.; Bánfalvi, Z.; Korom, E.; Wolf, I.; Vaszily, Z.; Taller, J.; Polgár, Z. Marker Assisted Selection of the Solanum stoloniferum Based PVY Resistance in the Breeding Material of Keszthely. ScienceMED 2012, 3, 215–219. [Google Scholar]
- Fulladolsa, A.C.; Navarro, F.M.; Kota, R.; Severson, K.; Palta, J.P.; Charkowski, A.O. Application of Marker Assisted Selection for Potato Virus Y Resistance in the University of Wisconsin Potato Breeding Program. Am. J. Potato Res. 2015, 92, 444–450. [Google Scholar] [CrossRef]
- Cernák, I.; Decsi, K.; Nagy, S.; Wolf, I.; Polgár, Z.; Gulyás, G.; Hirata, Y.; Taller, J. Development of a Locus-Specific Marker and Localization of the Ry Sto Gene Based on Linkage to a Catalase Gene on Chromosome XII in the Tetraploid Potato Genome. Breed. Sci. 2008, 58, 309–314. [Google Scholar] [CrossRef]
- Venkatesh, J.; An, J.; Kang, W.H.; Jahn, M.; Kang, B.C. Fine Mapping of the Dominant Potyvirus Resistance Gene Pvr7 Reveals a Relationship with Pvr4 in Capsicum Annuum. Phytopathology 2018, 108, 142–148. [Google Scholar] [CrossRef]
- Liu, J.; Yue, J.; Wang, H.; Xie, L.; Zhao, Y.; Zhao, M.; Zhou, H. Strategies for Engineering Virus Resistance in Potato. Plants 2023, 12, 1736. [Google Scholar] [CrossRef]
- Kogovšek, P.; Pompe-Novak, M.; Baebler, Š.; Rotter, A.; Gow, L.; Gruden, K.; Foster, G.D.; Boonham, N.; Ravnikar, M. Aggressive and Mild Potato Virus Y Isolates Trigger Different Specific Responses in Susceptible Potato Plants. Plant Pathol. 2010, 59, 1121–1132. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Hafeez, M.N.; Wattoo, J.I.; Ali, A.; Sharif, M.N.; Rashid, B.; Tabassum, B.; Nasir, I.A. Prediction of Host-Derived MiRNAs with the Potential to Target PVY in Potato Plants. Front. Genet. 2016, 7, 159. [Google Scholar] [CrossRef]
- Nie, X.; Sutherland, D.; Dickison, V.; Singh, M.; Murphy, A.M.; De Koeyer, D. Development and Validation of High-Resolution Melting Markers Derived from Rysto STS Markers for High-Throughput Marker-Assisted Selection of Potato Carrying Rysto. Phytopathology 2016, 106, 1366–1375. [Google Scholar] [CrossRef]
- Kante, M.; Lindqvist-Kreuze, H.; Portal, L.; David, M.; Gastelo, M. Kompetitive Allele Specific Pcr (Kasp) Markers for Potato: An Effective Tool for Increased Genetic Gains. Agronomy 2021, 11, 2315. [Google Scholar] [CrossRef]
- Saidi, A.; Hajibarat, Z. Approaches for Developing Molecular Markers Associated with Virus Resistances in Potato (Solanum tuberosum). J. Plant Dis. Prot. 2021, 128, 649–662. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, C.; Du, Y.; Zhao, Y.; Jiang, R.; Zhang, K.; Lv, D. Genetic Profiling and PVY Resistance Identification of Potato Germplasm Resources. Front. Plant Sci. 2024, 15, 1444281. [Google Scholar] [CrossRef]
- Hameed, A.; Shan-e-Ali Zaidi, S.; Sattar, M.N.; Iqbal, Z.; Tahir, M.N. CRISPR Technology to Combat Plant RNA Viruses: A Theoretical Model for Potato Virus Y (PVY)Resistance. Microb. Pathog. 2019, 133, 103551. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, W.; Hua, J. Temperature Modulates Plant Defense Responses through NB-LRR Proteins. PLoS Pathog. 2010, 6, e1000844. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Sood, S.; Kumar, A. Efficiency and Reliability of Marker Assisted Selection for Resistance to Major Biotic Stresses in Potato. Potato J. 2019, 46, 56–66. [Google Scholar]
- Kneib, R.B.; Kneib, R.B.; da Silva Pereira, A.; Castro, C.M. Allele Dosage of PVY Resistance Genes in Potato Clones Using Molecular Markers. Crop Breed. Appl. Biotechnol. 2017, 17, 306–312. [Google Scholar] [CrossRef]
- Njuguna, J.N.; Clark, L.V.; Lipka, A.E.; Anzoua, K.G.; Bagmet, L.; Chebukin, P.; Dwiyanti, M.S.; Dzyubenko, E.; Dzyubenko, N.; Ghimire, B.K. Impact of Genotype-calling Methodologies on Genome-wide Association and Genomic Prediction in Polyploids. Plant Genome 2023, 16, e20401. [Google Scholar] [CrossRef]
- Meirmans, P.G.; Liu, S.; van Tienderen, P.H. The Analysis of Polyploid Genetic Data. J. Hered. 2018, 109, 283–296. [Google Scholar] [CrossRef]
- Bourke, P.M.; Voorrips, R.E.; Kranenburg, T.; Jansen, J.; Visser, R.G.F.; Maliepaard, C. Integrating Haplotype-Specific Linkage Maps in Tetraploid Species Using SNP Markers. Theor. Appl. Genet. 2016, 129, 2211–2226. [Google Scholar] [CrossRef]
- Endelman, J.B.; Carley, C.A.S.; Bethke, P.C.; Coombs, J.J.; Clough, M.E.; da Silva, W.L.; De Jong, W.S.; Douches, D.S.; Frederick, C.M.; Haynes, K.G. Genetic Variance Partitioning and Genome-Wide Prediction with Allele Dosage Information in Autotetraploid Potato. Genetics 2018, 209, 77–87. [Google Scholar] [CrossRef]
- Gebhardt, C.; Valkonen, J.P.T. Organization of Genes Controlling Disease Resistance in the Potato Genome. Annu. Rev. Phytopathol. 2001, 39, 79–102. [Google Scholar] [CrossRef]
- Barone, A. Molecular Marker-Assisted Selection for Potato Breeding. Am. J. Potato Res. 2004, 81, 111–117. [Google Scholar] [CrossRef]
- Lyzenga, W.J.; Pozniak, C.J.; Kagale, S. Advanced Domestication: Harnessing the Precision of Gene Editing in Crop Breeding. Plant Biotechnol. J. 2021, 19, 660–670. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.; Cheng, S.; Zhang, B.; Mu, D.; Ni, P.; Zhang, G.; Yang, S.; Li, R.; Wang, J.; et al. Genome Sequence and Analysis of the Tuber Crop Potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef]
- Park, T.-H. Complete Chloroplast Genome Sequence of the Wild Diploid Potato Relative, Solanum acaule. Mitochondrial DNA Part B 2021, 6, 1189–1191. [Google Scholar] [CrossRef]
- van Lieshout, N.; van der Burgt, A.; de Vries, M.E.; ter Maat, M.; Eickholt, D.; Esselink, D.; van Kaauwen, M.P.W.; Kodde, L.P.; Visser, R.G.F.; Lindhout, P.; et al. Solyntus, the New Highly Contiguous Reference Genome for Potato (Solanum tuberosum). G3 Genes Genomes Genet. 2020, 10, 3489–3495. [Google Scholar] [CrossRef]
- Achakkagari, S.R.; Kyriakidou, M.; Gardner, K.M.; De Koeyer, D.; De Jong, H.; Strömvik, M.V.; Tai, H.H. Genome Sequencing of Adapted Diploid Potato Clones. Front. Plant Sci. 2022, 13, 954933. [Google Scholar] [CrossRef]
- Hajibarat, Z.; Saidi, A.; Zeinalabedini, M.; Mousapour Gorji, A.; Ghaffari, M.R.; Shariati, V.; Ahmadvand, R. Genotyping-by-Sequencing and Weighted Gene Co-Expression Network Analysis of Genes Responsive against Potato Virus Y in Commercial Potato Cultivars. PLoS ONE 2024, 19, e0303783. [Google Scholar] [CrossRef]
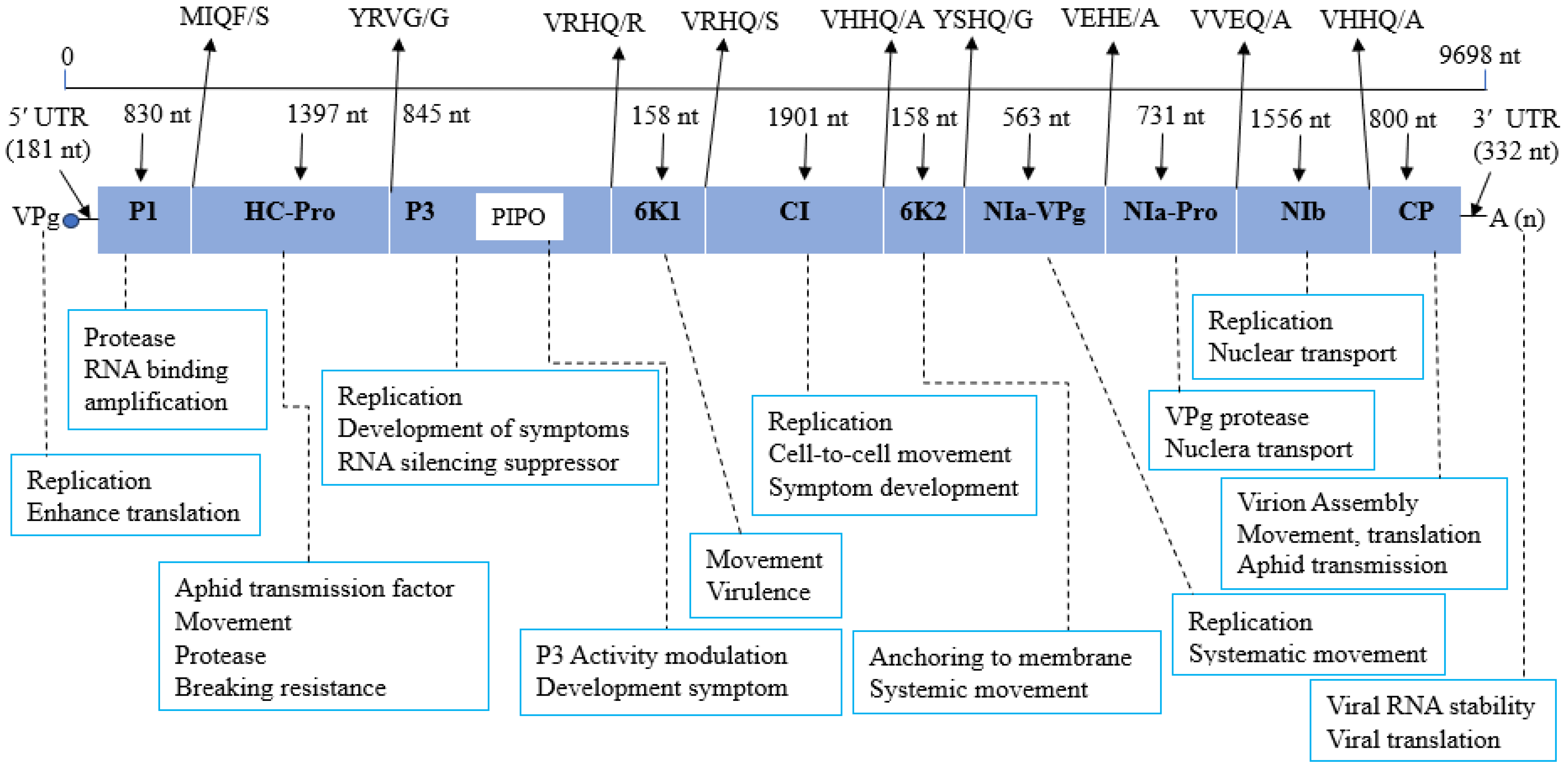
| PVY Strain | Genome Size (nt) | GC Content (%) | No. of Amino Acids | No. of aa Substitutions | Similarity % | GenBank Protein ID | Reference |
|---|---|---|---|---|---|---|---|
| NCBI RefSeq | 9704 | 42.15 | 3063 | - | - | NP_056759.1 | [58] |
| O | 9698 | 42.18 | 3081 | 123 | 97.0 | AAB50573.1 | [72] |
| 9649 | 41.97 | 3061 | 104 | 97.9 | AFS60379.1 | [73] | |
| NTN | 9700 | 41.46 | 3061 | 305 | 95.2 | AAN87843.1 | [74] |
| Z-NTN | 9646 | 41.84 | 3061 | 246 | 95.7 | AQK38488.1 | [75] |
| N-Wi | 9691 | 42.37 | 3061 | 172 | 96.7 | AQK38489.1 | |
| N | 9701 | 41.16 | 3061 | 302 | 95.2 | CAA66472.1 | [76] |
| C | 9699 | 41.88 | 3061 | 181 | 96.8 | ACD84569.1 | [77] |
| 9703 | 41.77 | 3062 | 204 | 96.3 | CAI65400.1 | [69] | |
| (N)W | 9698 | 42.04 | 3061 | 122 | 97.6 | ABQ53158.1 | [78] (unpublished) |
| Wilga | 9699 | 42.33 | 3061 | 968 | 97.1 | CAJ34850.1 | [69,79] |
| Wilga 156 | 9699 | 42.11 | 3061 | 208 | 96.5 | CAI64042.1 | [69] |
| nnp | 9699 | 41.73 | 3061 | 237 | 95.8 | AAO83661.2 | [80] |
| MV175 (isolate) | 9699 | 42.38 | 3061 | 239 | 94.6 | CCE46024.1 | [81] |
| MV99 (isolate) | 9699 | 42.41 | 3061 | 178 | 96.8 | CCE46023.1 |
| Genomic Location (nt) | Key Protein | Coordinates (aa) | Length (aa) |
|---|---|---|---|
| 182–1012 | P1 | 20–296 | 276 |
| 1010–2407 | HC–Pro | 296–761 | 465 |
| 2405–2659 | PIPO | 761–845 | 84 |
| 2657–3502 | P3 | 845–1126 | 281 |
| 3500–3658 | 6K1 | 1126–1178 | 52 |
| 3656–5557 | CI | 1178–1811 | 633 |
| 5558–5716 | 6K2 | 1812–1864 | 52 |
| 5714–6277 | NIa–VPg | 1864–2051 | 187 |
| 6278–7009 | Nia–Pro | 2052–2295 | 243 |
| 7010–8566 | Nib | 2296–2814 | 518 |
| 8567–9367 | CP | 2815–3081 | 266 |
| 1–181 | 5′ UTR | - | - |
| 9366–9698 | 3′ UTR | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebte, A.; Nagy, E.; Taller, J. Harnessing Genomics and Transcriptomics to Combat PVY Resistance in Potato: From Gene Discovery to Breeding Applications. Agronomy 2025, 15, 2611. https://doi.org/10.3390/agronomy15112611
Chebte A, Nagy E, Taller J. Harnessing Genomics and Transcriptomics to Combat PVY Resistance in Potato: From Gene Discovery to Breeding Applications. Agronomy. 2025; 15(11):2611. https://doi.org/10.3390/agronomy15112611
Chicago/Turabian StyleChebte, Abreham, Erzsébet Nagy, and János Taller. 2025. "Harnessing Genomics and Transcriptomics to Combat PVY Resistance in Potato: From Gene Discovery to Breeding Applications" Agronomy 15, no. 11: 2611. https://doi.org/10.3390/agronomy15112611
APA StyleChebte, A., Nagy, E., & Taller, J. (2025). Harnessing Genomics and Transcriptomics to Combat PVY Resistance in Potato: From Gene Discovery to Breeding Applications. Agronomy, 15(11), 2611. https://doi.org/10.3390/agronomy15112611





