LSES1, Encoding a Member of the Casein Kinase 1 Family, Is Involved in the Regulation of Leaf Senescence in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Cytological Observation
2.3. Genetic Analysis
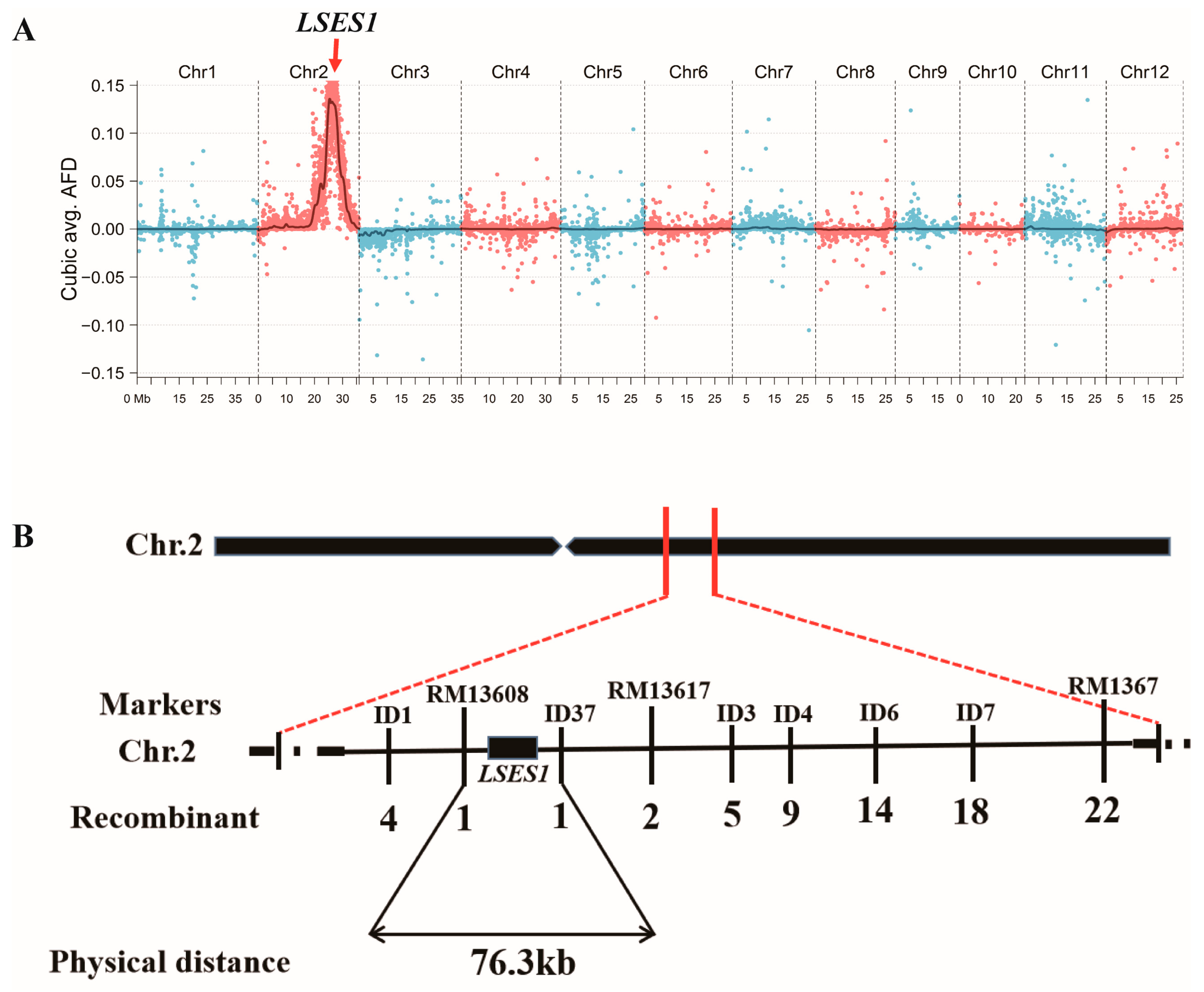
2.4. Preliminary Gene Mapping Based on BSA-Seq
2.5. Map-Based Cloning
2.6. Sequencing Verification of Candidate Gene Mutation Sites
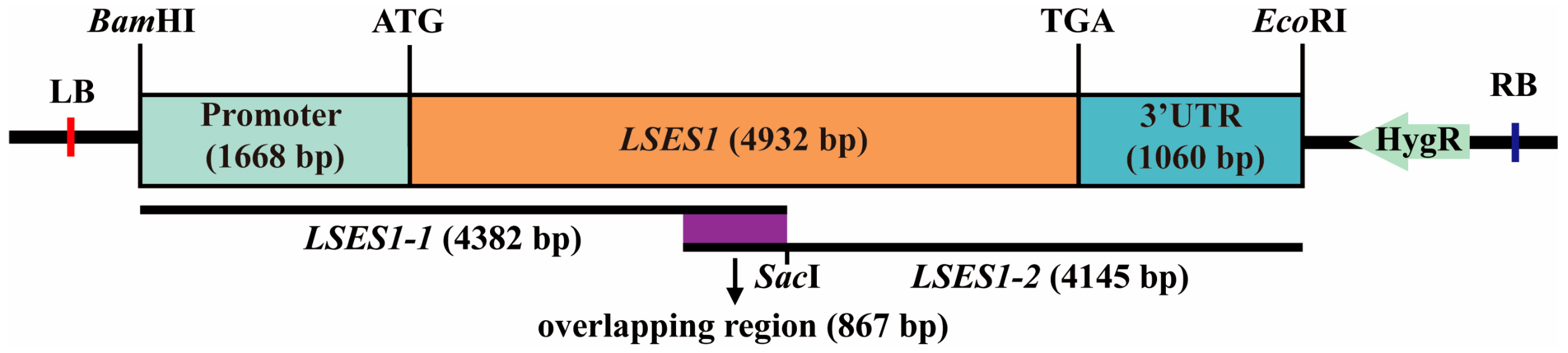
2.7. Vector Construction and Genetic Transformation
2.8. Phenotypic Identification of Transgenic Lines
2.9. Subcellular Localization
2.10. RT-qPCR Analysis
2.11. Statistical Analysis
3. Results
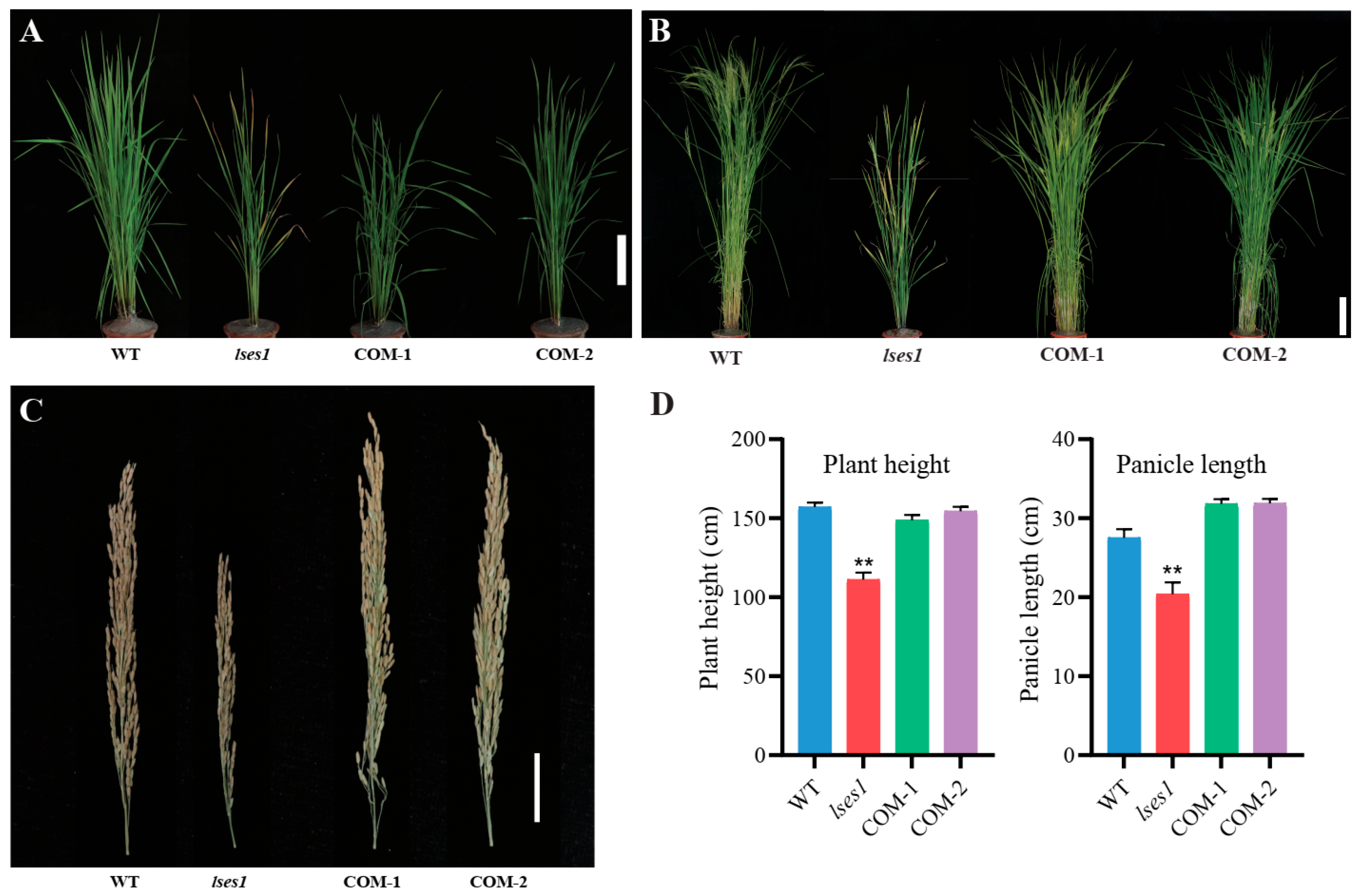
3.1. Phenotypic Characteristics of the lses1 Mutant
3.2. Ultrastructure of Mesophyll Cells
3.3. Genetic Analysis of the Leaf Phenotype in the lses1 Mutant
3.4. Preliminary Gene Mapping of LSES1
3.5. Map-Based Cloning of LSES1
3.6. Genetic Complementation Verification of LSES1
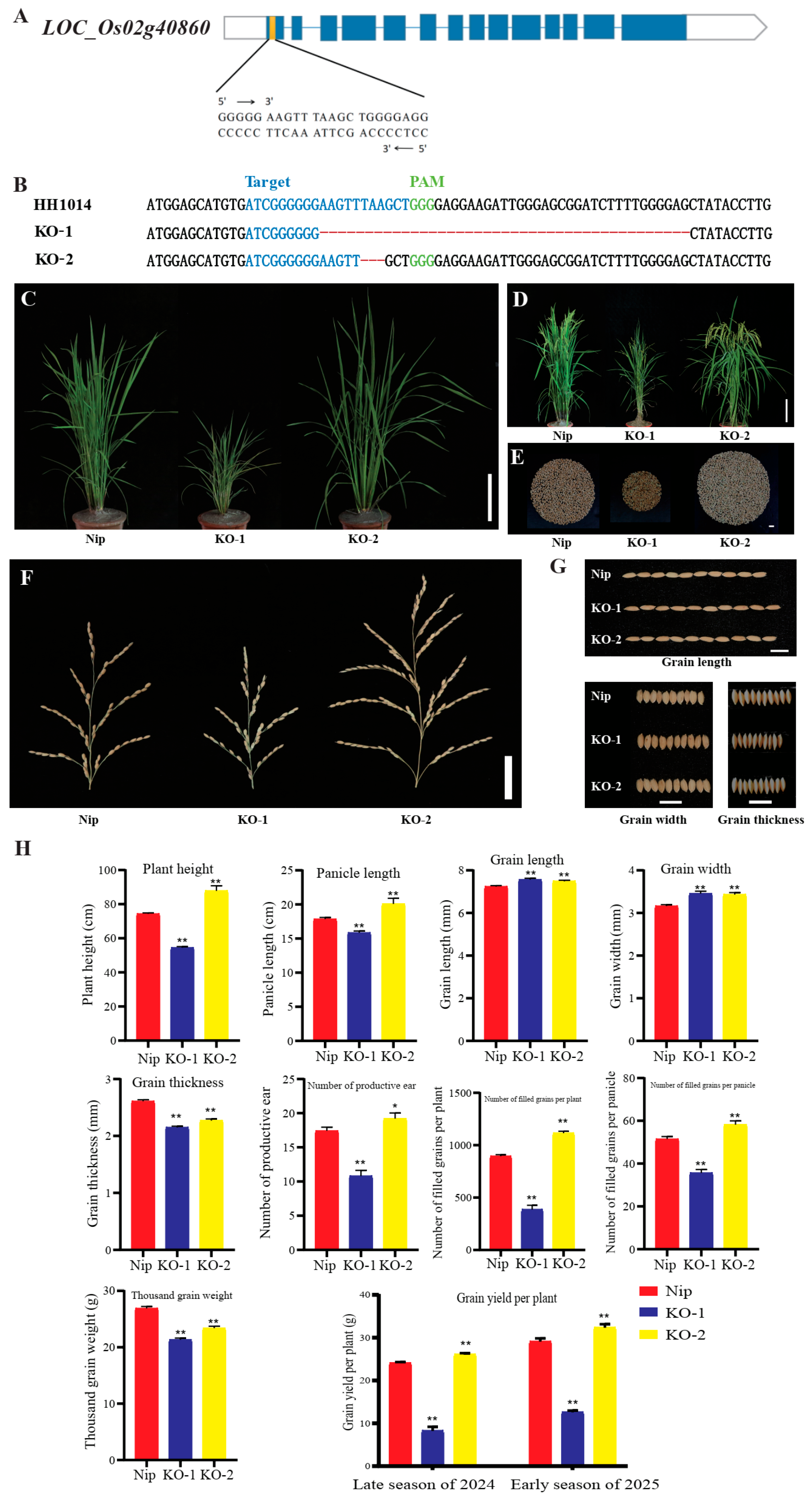
3.7. Validation of LSES1 Knockout and Acquisition of Improved Lines
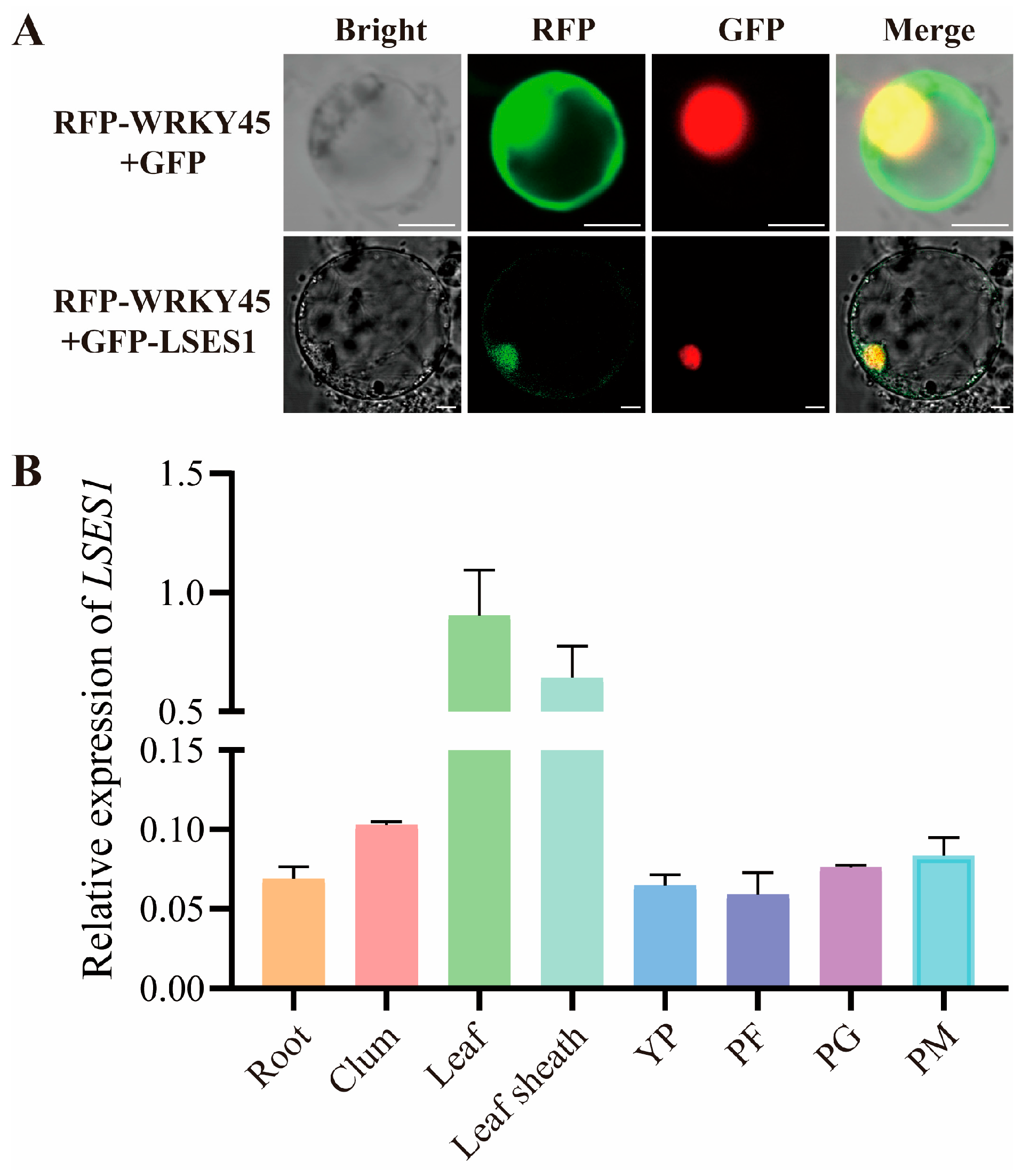
3.8. Subcellular Localization and Tissue-Specific Expression
4. Discussion
4.1. The lses1 Mutant Exhibits Obvious Physiological Characteristics of Premature Leaf Senescence and Excessive Starch Accumulation
4.2. LSES1 Is Involved in the Regulating of Leaf Senescence in Rice
4.3. LSES1 Likely Possesses Diverse Biological Regulatory Functions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEL | Arabidopsis EL1-like |
| LSES1 | Leaf Starch Excess and Senescence 1 |
| CK | Casein kinase |
| CRY2 | Cryptochrome 2 |
| ACS5 | ACC Synthase 5 |
| ETO1 | Ethylene Overproduction 1 |
| ACS7 | ACC Synthase 7 |
| SMXL | The suppressor of MAX2 1-like |
| SL | Strigolactone |
| MAX2 | More Axillary Growth 2 |
| CKL6 | Casein kinase-like 6 |
| PCD | Programmed cell death |
| O2 | Opaque2 |
| PPDK | Pyruvate phosphate dikinase |
| hbd2 | hybrid breakdown 2 |
| LTRPK1 | Low-temperature Response Protein Kinase 1 |
| LTG1 | Low-Temperature Growth 1 |
| DTG1 | Defective Tiller Growth 1 |
| ROS | Reactive oxygen species |
| MDA | Malondialdehyde |
| ABA | Abscisic acid |
| BSA-seq | Bulked segregant analysis sequencing |
| SSRs | Simple sequence repeats |
| InDel | Insertion–deletion |
| PCR | Polymerase chain reaction |
| COM | Complementary transformant |
| KO | Knockout |
| GFP | Green fluorescent protein |
| RFP | Red fluorescent protein |
| YP | Young panicles |
| PF | Panicles in the flowering stage |
| PG | Panicles in the grain-filling stage |
| PM | Panicles in the mature stage |
| CRISPR/Cas9 | Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 |
| RT-qPCR | Reverse-transcription quantitative PCR |
| TEM | Transmission electron microscopy |
| Chr | Chromosome |
| SNP | Single-nucleotide polymorphism |
| BiFC | Bimolecular fluorescence complementation |
| LCI | luciferase complementation imaging assay |
| GW2 | Grain Width 2 |
References
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf senescence: Progression, regulation, and application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Kossmann, J. Transitory and storage starch metabolism: Two sides of the same coin? Curr. Opin. Biotech. 2015, 32, 143–148. [Google Scholar] [CrossRef]
- Hirose, T.; Aoki, N.; Harada, Y.; Masaki, O.; Yoichi, H.; Ryu, O.; Miyao, A.; Hirohiko, H.; Tomio, T. Disruption of a rice gene for α-glucan water dikinase, OsGWD1, leads to hyperaccumulation of starch in leaves but exhibits limited effects on growth. Front. Plant Sci. 2013, 4, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.H.; Chen, X.L.; Du, D.; Xing, Y.D.; Zhang, T.Q.; Zhu, M.D.; Liu, M.M.; Zhu, X.Y.; Sang, X.C.; He, G.H. Identification and gene mapping of starch accumulation and early senescence leaf mutant esl9 in rice. Acta Agron. Sin. 2017, 43, 473–482. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.T.; Wang, X.W.; Xie, J.; Yin, W.Z.; Zhang, T.; Zhu, X.Y.; Yu, P.; Huang, J.Y.; Yang, Z.L.; He, G.H.; et al. Identification and gene mapping of an early senescent leaf mutant esl11 of rice. Crop Sci. 2018, 58, 1932–1941. [Google Scholar] [CrossRef]
- Lin, T.; Sun, L.; Gong, H.; Liu, L.; Zhao, Z.; Dong, H.; Wang, Y.; Jiang, L.; Wan, J. Identification and gene mapping of a premature leaf senescence 5 mutant with starch accumulation in rice leaves. J. Nanjing Agric. Univ. 2020, 43, 414–422. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, M.D.; Chen, X.L.; Zhu, X.Y.; Xing, Y.D.; Du, D.; Zhang, Y.Y.; Liu, M.M.; Zhang, Q.L.; Lu, X.; Peng, S.S.; et al. Identification and gene mapping of the starch accumulation and premature leaf senescence mutant ossac4 in rice. J. Integr. Agr. 2020, 19, 2150–2164. [Google Scholar] [CrossRef]
- Chu, X.; Lu, T.; Ye, H.; Wang, S.; Lin, H.; Wu, X.; He, R.; Yan, G.; Wang, Y.; Li, S.; et al. Cloning and functional analysis of leaf senescence gene LPS1 in Oryza sativa. Chin. J. Rice Sci. 2021, 35, 427–438. (In Chinese) [Google Scholar]
- Xie, Z.; Zhao, B.; Zhang, M.; Sang, X.; Zhao, F.; Feng, P.; He, G.; Zhu, X. Mutation of OsSAC3, encoding the xanthine dehydrogenase, caused early senescence in rice. Int. J. Mol. Sci. 2022, 23, 11053. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Qu, L.; Xue, H.W. Casein kinase 1 AELs promote senescence by enhancing ethylene biosynthesis through phosphorylating WRKY22 transcription factor. New Phytol. 2024, 244, 116–130. [Google Scholar] [CrossRef]
- Pinna, L.A.; Meggio, F. Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog. Cell Cycle Res. 1997, 3, 77–97. [Google Scholar]
- Chen, H.H.; Xue, H.W. Casein kinase 1 in plants. Chin. Bull. Life Sci. 2018, 30, 1184–1192. (In Chinese) [Google Scholar]
- Robinson, L.C.; Menold, M.M.; Garrett, S.; Culbertson, M.R. Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol. Cell Biol. 1993, 13, 2870–2881. [Google Scholar] [PubMed]
- Robinson, L.C.; Bradley, C.; Bryan, J.D.; Jerome, A.; Kweon, Y.; Panek, H.R. The Yck2 yeast casein kinase 1 isoform shows cell cycle-specific localization to sites of polarized growth and is required for proper septin organization. Mol. Biol. Cell 1999, 10, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The origins of protein phosphorylation. Nat. Cell Biol. 2002, 4, E127–E130. [Google Scholar] [CrossRef]
- Tan, S.T.; Dai, C.; Liu, H.T.; Xue, H.W. Arabidopsis casein kinase1 proteins CK1.3 and CK1.4 phosphorylate cryptochrome2 to regulate blue light signaling. Plant Cell 2013, 25, 2618–2632. [Google Scholar] [CrossRef]
- Tan, S.T.; Xue, H.W. Casein kinase 1 regulates ethylene synthesis by phosphorylating and promoting the turnover of ACS5. Cell Rep. 2014, 9, 1692–1702. [Google Scholar] [CrossRef]
- Su, X.; Xue, H.W. Plant-specific casein kinases phosphorylate and stabilize SMXL6/7/8 to suppress strigolactone signaling and promote shoot branching. Mol. Plant 2025, 18, 1458–1471. [Google Scholar] [CrossRef]
- Ben-Nissan, G.; Cui, W.; Kim, D.J.; Yang, Y.; Yoo, B.C.; Lee, J.Y. Arabidopsis casein kinase 1-like 6 contains a microtubule-binding domain and affects the organization of cortical microtubules. Plant Physiol. 2008, 148, 1897–1907. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Li, Y.; Zhang, R.; Yang, J.; Ma, H.; Min, L.; Zhang, X. Reversing anther thermotolerance by manipulating the cis-elements in the promoter of a high-temperature upregulated gene Casein Kinase I in upland cotton. Sci. China Life Sci. 2025, 68, 1558–1569. [Google Scholar] [CrossRef]
- Liao, L.; Huang, Y.; Wang, S.; Zhang, H.; Pan, J.; Long, Z.; Huang, Y.; Li, X.; Chen, D.; Yang, T. The CK1-Opaque2 module orchestrates endosperm filling and nutrient storage in maize seeds. Crop J. 2025, 13, 192–203. [Google Scholar] [CrossRef]
- Yamamoto, E.; Takashi, T.; Morinaka, Y.; Lin, S.Y.; Wu, J.Z.; Matsumoto, T.; Kitano, H.; Matsuoka, M.; Ashikari, M. Gain of deleterious function causes an autoimmune response and Bateson-Dobzhansky-Muller incompatibility in rice. Mol. Genet. Genom. 2010, 283, 305–315. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Z.H.; Luo, D.; Xue, H.W. Roles of OsCKI1, a rice casein kinase I, in root development and plant hormone sensitivity. Plant J. 2003, 36, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ji, S.X.; Fang, X.L.; Wang, Q.G.; Li, Z.; Yao, F.Y.; Hou, L.; Dai, S.J. Protein kinase LTRPK1 influences cold adaptation and microtubule stability in rice. J. Plant Growth Regul. 2013, 32, 483–490. [Google Scholar] [CrossRef]
- Lu, G.W.; Wu, F.Q.; Wu, W.X.; Wang, H.J.; Zheng, X.M.; Zhang, Y.H.; Chen, X.L.; Zhou, K.N.; Jin, M.N.; Cheng, Z.J.; et al. Rice LTG1 is involved in adaptive growth and fitness under low ambient temperature. Plant J. 2014, 78, 468–480. [Google Scholar] [CrossRef]
- Li, J.; Zhou, D.; Li, D.; Wang, G.; Qin, R.; Gong, C.; Chen, K.; Tong, Y.; Li, L.; Liu, K.; et al. The dual role of casein kinase 1, DTG1, in regulating tillering and grain size in rice. Crop J. 2024, 12, 1569–1582. [Google Scholar] [CrossRef]
- Gothandam, K.M.; Kim, E.S.; Cho, H.J.; Chung, Y.Y. OsPPR1, a pentatricopeptide repeat protein of rice is essential for the chloroplast biogenesis. Plant Mol. Biol. 2005, 58, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Tamiru, M.; Abe, A.; Yoshida, K.; Uemura, A.; Yaegashi, H.; Obara, T.; Oikawa, K.; Utsushi, H.; Kanzaki, E.; et al. MutMap accelerates breeding of a salt-tolerant rice cultivar. Nat. Biotechnol. 2015, 33, 445–449. [Google Scholar] [CrossRef]
- Oryza sativa Japonica Group IRGSP-1.0. Available online: http://rice.plantbiology.msu.edu/ (accessed on 14 July 2021).
- Edwards, K.; Johnstone, C.; Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991, 19, 1349. [Google Scholar] [CrossRef]
- Panaud, O.; Chen, X.; McCouch, S.R. Development of microsatellite markers and characterization of simple sequence length poly-morphism (SSLP) in rice (Oryza sativa L.). Mol. Gen. Genet. 1996, 252, 597–607. [Google Scholar]
- CRISPRdirect. Available online: http://crispr.dbcls.jp/ (accessed on 2 November 2022).
- Chen, Z.; Wang, Y.; Huang, R.; Zhang, Z.; Huang, J.; Yu, F.; Lin, Y.; Guo, Y.; Liang, K.; Zhou, Y.; et al. Integration of transcriptomic and proteomic analyses reveals several levels of metabolic regulation in the excess starch and early senescent leaf mutant lses1 in rice. BMC Plant Biol. 2022, 22, 137. [Google Scholar] [CrossRef] [PubMed]


| Hybrid Combination | Normal Phenotype Strain | Mutant Phenotype Strain | Total Number of Plants | χ2C |
|---|---|---|---|---|
| (3:1) | ||||
| lses1/Nip | 289 | 93 | 382 | 0.09 |
| Nip/lses1 | 206 | 66 | 272 | 0.08 |
| lses1/02428 | 162 | 51 | 213 | 0.13 |
| lses1/HZ | 214 | 75 | 289 | 0.12 |
| lses1/ZS97B | 229 | 73 | 302 | 0.11 |
| lses1/MH2155 | 194 | 67 | 261 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Zhang, Q.; Wei, X.; Chen, Z.; Xu, M.; Zhuang, M.; Huang, T.; Huang, R.; Guo, Y.; Liang, K.; et al. LSES1, Encoding a Member of the Casein Kinase 1 Family, Is Involved in the Regulation of Leaf Senescence in Rice. Agronomy 2025, 15, 2601. https://doi.org/10.3390/agronomy15112601
Chen F, Zhang Q, Wei X, Chen Z, Xu M, Zhuang M, Huang T, Huang R, Guo Y, Liang K, et al. LSES1, Encoding a Member of the Casein Kinase 1 Family, Is Involved in the Regulation of Leaf Senescence in Rice. Agronomy. 2025; 15(11):2601. https://doi.org/10.3390/agronomy15112601
Chicago/Turabian StyleChen, Fangyu, Qishen Zhang, Xinyu Wei, Zhiming Chen, Ming Xu, Mancheng Zhuang, Tinggu Huang, Rongyu Huang, Yuchun Guo, Kangjing Liang, and et al. 2025. "LSES1, Encoding a Member of the Casein Kinase 1 Family, Is Involved in the Regulation of Leaf Senescence in Rice" Agronomy 15, no. 11: 2601. https://doi.org/10.3390/agronomy15112601
APA StyleChen, F., Zhang, Q., Wei, X., Chen, Z., Xu, M., Zhuang, M., Huang, T., Huang, R., Guo, Y., Liang, K., & Jia, Q. (2025). LSES1, Encoding a Member of the Casein Kinase 1 Family, Is Involved in the Regulation of Leaf Senescence in Rice. Agronomy, 15(11), 2601. https://doi.org/10.3390/agronomy15112601






