Homogalacturonan Methylesterification and Cell Wall Regulation: Integrating Biochemistry, Mechanics, and Developmental Signaling for Crop Improvement
Abstract
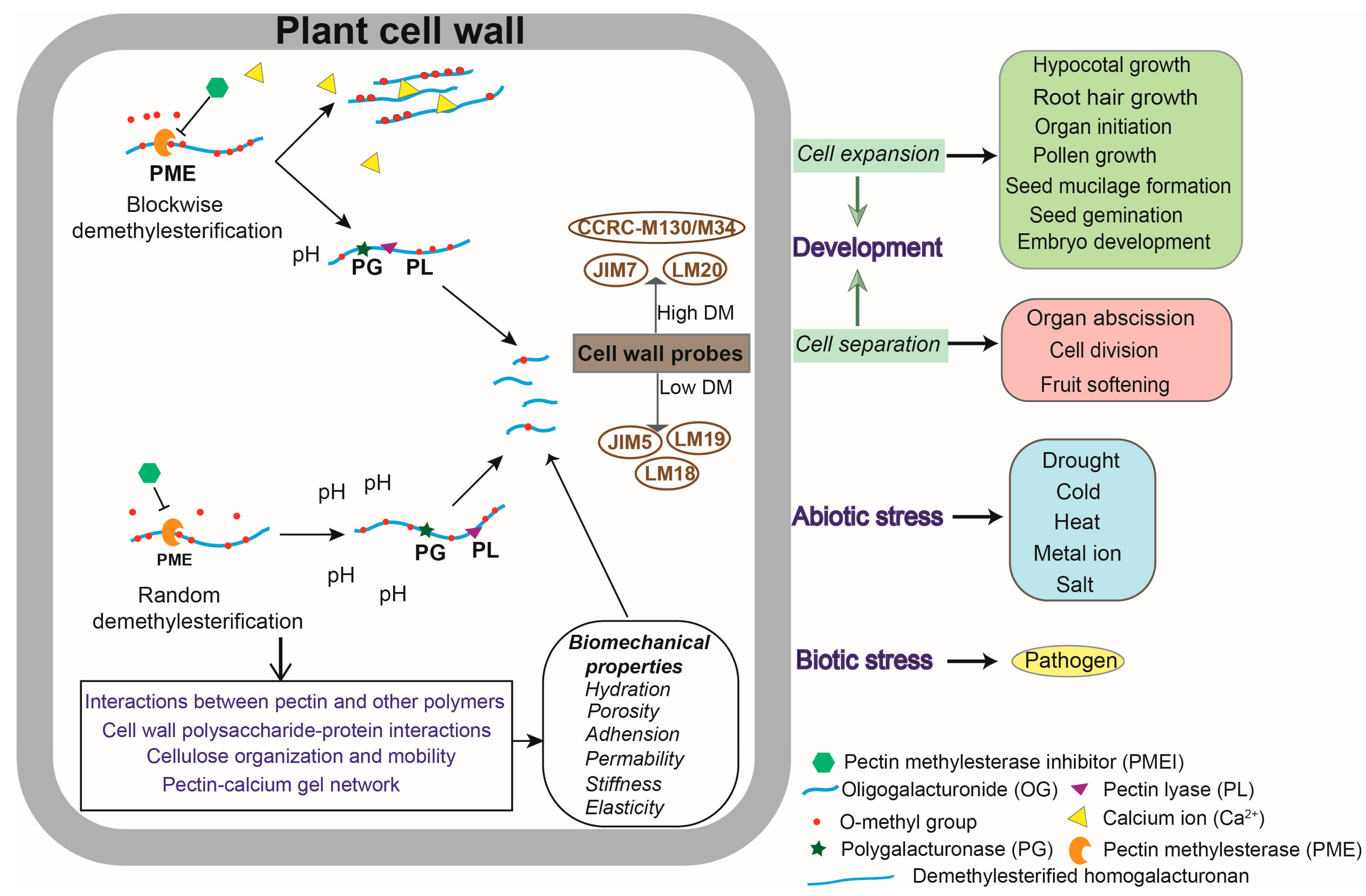
1. Introduction
2. Regulation of HG Methylesterification
2.1. PMEs and PMEIs
2.2. Transcriptional and Post-Transcriptional Control
| Regulator | Type | Target Gene(s)/Mechanism | Regulatory Effect on DM | Tissue/Process | Reference |
|---|---|---|---|---|---|
| STK (MADS-domain) | TF | PMEI6 | Activates PMEI6 expression, increasing DM | Seed coat mucilage | [17] |
| MYB52 | TF | PMEI6, PMEI14 | Activates PMEI expression, increasing DM | Seed coat mucilage | [18] |
| LUH | TF | PME | Activates PME expression, decreasing DM | Seed coat mucilage | [19] |
| ERF4 | TF | PMEI | Represses PMEI expression, decreasing DM | Seed coat mucilage; fruit | [21] |
| ETTIN | TF | PME/PMEI | Activates PME activity, decreasing DM | Gynoecium morphogenesis (Arabidopsis) | [22] |
| SlBES1; FvMYB79 | TF | PMEU1 | Represses PMEU1 expression, increasing DM | Fruit softening | [24,25] |
| FLYING SAUCER1 (FLY1) | Transmembrane RING E3 ubiquitin ligase | Regulates PME recycling within endomembrane system | Modulates DM | Arabidopsis seed coat mucilage | [26] |
| FLY1 homolog | Transmembrane RING E3 ubiquitin ligase | Redundant function in PME regulation | Modulates DM | Mucilage formation | [27] |
| Mucilage-Defect-1 (MUD1) | Nuclear-localized E3 ubiquitin ligase | Modulates stability of TFs (MYB52, LUH) and PME/PMEI regulators (SBT1.7, PMEI6, PMEI14) | Influences DM | Seed coat mucilage | [28] |
2.3. Hormone Regulation
| Hormone | Regulatory Effect | Target(s) | Physiological Context | Reference |
|---|---|---|---|---|
| Auxin | Promotes or inhibits demethylesterification | PME, PMEI | Phyllotactic patterning, root emergence, hypocotyl elongation | [34,35,36,37] |
| ABA | Induces PME expression | PME34, PME53 | Heat stress response | [29,30] |
| Ethylene | Represses or activates PMEI expression | PMEI genes | Fruit ripening | [31,32] |
| GA | Increases PME expression | PME genes | Hypocotyl elongation | [33] |
| BR | Represses PMEU1 expression | PME genes | Fruit softening | [25] |
2.4. Apoplastic pH
3. HG Methylesterification and CW Mechanics
3.1. Biomechanical Models and Pectin-Cellulose Interactions
3.2. PME/PMEI-Mediated Wall Regulation
3.3. Integration with Hormone Signaling and Feedback Loops
3.4. Integration with Other CW Components and Signaling
4. HG Methylesterification in Development and Environmental Interactions
4.1. Cell Growth
4.2. HG Methylesterification in Tip Growth and Morphogenesis
4.2.1. Localized PME/PMEI Activity in Tip-Growing Cells
4.2.2. Apical-Basal Gradients and Wall Mechanics
4.2.3. Integration with Plant Morphogenesis
4.3. Organ Initiation, and Seed Germination
4.4. HG in the Middle Lamella (ML) Influences Plant Development
4.5. Plant Resistance to Abiotic Stresses
4.6. Plant Resistance to Pathogens and Insects
| Biological Process/Context | Plant/Tissue | PME/PMEI/Enzyme Involved | HG Methylesterification Change | Outcome/Phenotype | Reference |
|---|---|---|---|---|---|
| Cell elongation/growth | Arabidopsishypocotyls | AtPMEI1, AtPMEI2, AtPME17 | ↑ DM | Promotes cell elongation | [33,74,75] |
| Shade avoidance | Arabidopsishypocotyls | - | ↑ DM | Required for shade-induced elongation | [77] |
| Organogenesis/leaf growth | Arabidopsis | CGR2 (HGMT) | ↑ DM | Expanded leaves, enhanced organ growth | [79] |
| Seed germination | Arabidopsis seeds | PMEI5 | ↑ DM | Accelerates germination | [95] |
| Seed coat mucilage release | Arabidopsis | PME isoform | ↓ DM | Enables mucilage release | [96] |
| Tip growth (pollen tube/root hairs) | Arabidopsis/tobacco | NtPPME1, PMEs/PMEIs | Localized DM gradients | Sustained pulsatile growth | [92,93] |
| Fruit softening | Strawberry/loquat | PMEs/PMEIs | ↓ DM | Softening of fruit/tissue | [103,104] |
| Cold stress tolerance | Arabidopsis/Chorispora bungeana | CbPMEI1, AtPMEI13 | ↑ DM—freezing resistance decreased | Reduced freezing tolerance | [108] |
| Heat stress tolerance | Arabidopsis | PME53/PME34 | Maintains Ca2+-HG crosslinks | Supports stomatal movement and thermotolerance | [29,30] |
| Drought tolerance | Arabidopsis/Populus tomentosa | PtoPME35 | ↓ DM | Reduced stomatal opening, enhanced drought tolerance | [109] |
| Heavy metal stress | Rice roots | PMEs | ↓ DM | Cd sequestration in cell walls | [110,111] |
| Salt stress | Arabidopsis | AtPMEI13/AtPMEI17 | ↑ or ↓ DM depending on context | Modulates salt tolerance | [108,112] |
| Pathogen resistance | Arabidopsis | AtPMEI13/AtPME17 | Localized DM changes | Modulates Botrytis susceptibility/resistance, aphid behavior | [115,121] |
| DAMP/OG signaling | Arabidopsis/strawberry | PMEs/FaPE1 | ↓ DM (OGs) | Activates defense signaling (MAPK, JA, SA pathways) | [118,124] |
| PRR activation | Arabidopsis | AtPME3 | Required for WAK activity | Pattern-triggered immunity activation | [126] |
5. HG Methylesterification as a Target for Crop Improvement and Breeding
6. Future Perspectives
6.1. Molecular Regulation, Enzymatic Mechanisms, and Structural Complexity
6.2. Crosstalk in CW Remodeling
6.3. Analytical Tools and Technological Advances
6.4. Comparative Insights Beyond Arabidopsis
| Species | No. of PME Genes | No. of PMEI Genes | Key References |
|---|---|---|---|
| Arabidopsis thaliana | 66 | 71 | [135] |
| Oryza sativa (rice) | 43 | 49 | [136,137] |
| Solanum lycopersicum (tomato) | 57 | 48 | [138,139] |
| Glycine max (soybean) | 127 | 170 | [140,141] |
| Zea mays (maize) | 43 | 49 | [142] |
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CW | Cell wall |
| HG | Homogalacturonan |
| RG-I | Rhamnogalacturonan I |
| RG-II | Rhamnogalacturonan II |
| PMEs | Pectin methylesterases |
| PMEIs | PME inhibitors |
| TFs | Transcription factors |
| DM | Degree of methylesterification |
| CWI | Cell wall integrity |
| GalA | Galacturonic acid residues |
| A-SCM | Arabidopsis seed coat mucilage |
| STK | SEEDSTICK |
| LUH | LEUNIG_HOMOLOG |
| BLH | BEL1-Like homeodomain |
| ERF | Ethylene response factor |
| BLR | BELLRINGER |
| FLY1 | FLYING SAUCER1 |
| MUD1 | Mucilage-Defect-1 |
| ABA | Abscisic acid |
| BRs | Brassinosteroids |
| GA | Gibberellic acid |
| BES1 | BRI1-EMS-SUPPRESSOR1 |
| OGs | Oligogalacturonides |
| ssNMR | Solid-state nuclear magnetic resonance (ssNMR) |
| PG | Polygalacturonase |
| APRX | Apoplastic isoperoxidase |
| PL | Pectate lyase |
| HGMT | Homogalacturonan methyltransferase |
| NPL | Nep1-like protein |
| AFM | Atomic force microscopy |
| NtLLG4 | LORELEI-like glycosylphosphatidylinositol-anchored protein 4 |
| ROS | Reactive oxygen species |
| HMS | HIGHLY METHYL ESTERIFIED SEEDS |
| TCJ | Tricellular junction |
| ML | Middle lamella |
| Cd | Cadmium |
| DAMPs | Damage-associated molecular patterns |
| MAPK | Mitogen-activated protein kinase |
| PAMPs | Pathogen-associated molecular patterns |
| PRRs | Pattern-recognition receptors |
| WAK2 | Wall associated kinase 2 |
| WAK1 | Wall associated kinase 1 |
| SA | Salicylic acid |
| FER | FERONIA |
| PTI | Pattern-triggered immunity |
| TBR | Trichome birefringence |
| MAS | Marker-assisted selection |
| GWAS | Genome-wide association study |
| UCI | Unilateral cross-incompatibility |
| RNAi | RNA interference |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| FTIR | Fourier transform infrared spectroscopy |
| ML | Machine learning |
| AI | Artificial intelligence |
| JA | Jasmonic acid |
References
- Anderson, C.T.; Kieber, J.J. Dynamic construction, perception, and remodeling of plant cell walls. Annu. Rev. Plant Biol. 2020, 71, 39–69. [Google Scholar] [CrossRef]
- Sterling, J.D.; Quigley, H.F.; Orellana, A.; Mohnen, D. The catalytic site of the pectin biosynthetic enzyme α-1,4-galacturonosyltransferase is located in the lumen of the Golgi. Plant Physiol. 2001, 127, 360–371. [Google Scholar] [CrossRef]
- Willats, W.G.; Orfila, C.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.J.; Voragen, A.G.; Marcus, S.E.; Christensen, T.M.; Mikkelsen, J.D.; Murray, B.S.; et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls: Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 2001, 276, 19404–19413. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Wang, W.; Zhang, Q.; Jia, W. Cell wall integrity signaling in fruit ripening. Int. J. Mol. Sci. 2023, 24, 4054. [Google Scholar] [CrossRef]
- Coculo, D.; Lionetti, V. The plant invertase/pectin methylesterase inhibitor superfamily. Front. Plant Sci. 2022, 13, 863892. [Google Scholar] [CrossRef]
- Jolie, R.P.; Duvetter, T.; Van Loey, A.M.; Hendrickx, M.E. Pectin methylesterase and its proteinaceous inhibitor: A review. Carbohydr. Res. 2010, 345, 2583–2595. [Google Scholar] [CrossRef]
- Micheli, F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Wormit, A.; Usadel, B. The multifaceted role of pectin methylesterase inhibitors (PMEIs). Int. J. Mol. Sci. 2018, 19, 2878. [Google Scholar] [CrossRef]
- Xu, F.; Gonneau, M.; Faucher, E.; Habrylo, O.; Lefebvre, V.; Domon, J.M.; Martin, M.; Sénéchal, F.; Peaucelle, A.; Pelloux, J.; et al. Biochemical characterization of pectin methylesterase inhibitor 3 from Arabidopsis thaliana. Cell Surf. 2022, 8, 100080. [Google Scholar] [CrossRef]
- Bonavita, A.; Carratore, V.; Ciardiello, M.A.; Giovane, A.; Servillo, L.; D’Avino, R. Influence of pH on the structure and function of kiwi pectin methylesterase inhibitor. J. Agric. Food Chem. 2016, 64, 5866–5876. [Google Scholar] [CrossRef] [PubMed]
- Kotnala, B.; N, S.M.; Vasu, P. Purification and characterization of a salt-dependent pectin methylesterase from Carica papaya fruit mesocarp–exocarp tissue. J. Food Sci. 2018, 83, 2062–2070. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Chen, T.; Xing, L.; Xu, K.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Regulatory mechanisms underlying the maintenance of homeostasis in Pyropia haitanensis under hypersaline stress conditions. Sci. Total Environ. 2019, 662, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, F.; L’Enfant, M.; Domon, J.-M.; Rosiau, E.; Crépeau, M.J.; Surcouf, O.; Esquivel-Rodriguez, J.; Marcelo, P.; Mareck, A.; Guérineau, F.; et al. Tuning of pectin methylesterification: PECTIN METHYLESTERASE INHIBITOR 7 modulates the processive activity of co-expressed pectin methylesterase 3 in a pH-dependent manner. J. Biol. Chem. 2015, 290, 23320–23335. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Huang, L.; Zhang, Z.; Shi, Q.; Hu, J.; He, G.; Guo, X.; Shi, H.; Liang, L. Uncovering the interactions between PME and PMEI at the gene and protein levels: Implications for the design of specific PMEI. J. Mol. Model. 2023, 29, 286. [Google Scholar] [CrossRef]
- Ezquer, I.; Mizzotti, C.; Nguema-Ona, E.; Gotté, M.; Beauzamy, L.; Viana, V.E.; Dubrulle, N.; Costa de Oliveira, A.; Caporali, E.; Koroney, A.S.; et al. The developmental regulator SEEDSTICK controls structural and mechanical properties of the Arabidopsis seed coat. Plant Cell 2016, 28, 2478–2492. [Google Scholar] [CrossRef]
- Shi, D.; Ren, A.; Tang, X.; Qi, G.; Xu, Z.; Chai, G.; Hu, R.; Zhou, G.; Kong, Y. MYB52 negatively regulates pectin demethylesterification in seed coat mucilage. Plant Physiol. 2018, 176, 2737–2749. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Tehseen, M.; Doblin, M.S.; Pettolino, F.A.; Wilson, S.M.; Bacic, A.; Golz, J.F. The transcriptional regulator LEUNIG_HOMOLOG regulates mucilage release from the Arabidopsis testa. Plant Physiol. 2011, 156, 46–60. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Wang, X.; Pei, S.; Kong, Y.; Hu, R.; Zhou, G. Transcription factors BLH2 and BLH4 regulate demethylesterification of homogalacturonan in seed mucilage. Plant Physiol. 2020, 183, 96–111. [Google Scholar] [CrossRef]
- Ding, A.; Tang, X.; Yang, D.; Wang, M.; Ren, A.; Xu, Z.; Hu, R.; Zhou, G.; O’Neill, M.; Kong, Y. ERF4 and MYB52 transcription factors play antagonistic roles in regulating homogalacturonan de-methylesterification in Arabidopsis seed coat mucilage. Plant Cell 2021, 33, 381–403. [Google Scholar] [CrossRef]
- Andres-Robin, A.; Reymond, M.C.; Dupire, A.; Battu, V.; Dubrulle, N.; Mouille, G.; Lefebvre, V.; Pelloux, J.; Boudaoud, A.; Traas, J.; et al. Evidence for the regulation of gynoecium morphogenesis by ETTIN via cell wall dynamics. Plant Physiol. 2018, 178, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Louvet, R.; Johansen, J.N.; Salsac, F.; Morin, H.; Fournet, F.; Belcram, K.; Gillet, F.; Höfte, H.; Laufs, P.; et al. The transcription factor BELLRINGER modulates phyllotaxis by regulating the expression of a pectin methylesterase in Arabidopsis. Development 2011, 138, 4733–4741. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Mo, X.; Wen, C.; Gao, Z.; Chen, X.; Xue, C. FvMYB79 positively regulates strawberry fruit softening via transcriptional activation of FvPME38. Int. J. Mol. Sci. 2021, 23, 101. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Liang, D.; Zhang, M.; Jia, C.; Qi, M.; Liu, Y.; Shao, Z.; Meng, F.; Hu, S.; et al. SlBES1 promotes tomato fruit softening through transcriptional inhibition of PMEU1. iScience 2021, 24, 102926. [Google Scholar] [CrossRef]
- Voiniciuc, C.; Dean, G.H.; Griffiths, J.S.; Kirchsteiger, K.; Hwang, Y.T.; Gillett, A.; Dow, G.; Western, T.L.; Estelle, M.; Haughn, G.W. Flying saucer1 is a transmembrane RING E3 ubiquitin ligase that regulates the degree of pectin methylesterification in Arabidopsis seed mucilage. Plant Cell 2013, 25, 944–959. [Google Scholar] [CrossRef]
- Kunieda, T.; Hara-Nishimura, I.; Demura, T.; Haughn, G.W. Arabidopsis FLYING SAUCER 2 functions redundantly with FLY1 to establish normal seed coat mucilage. Plant Cell Physiol. 2020, 61, 308–317. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, C.; Wang, M.; Ding, A.; Chai, G.; Sun, Y.; Zhou, G.; Yang, D.; Kong, Y. MUD1, a RING-v E3 ubiquitin ligase, has an important role in the regulation of pectin methylesterification in Arabidopsis seed coat mucilage. Plant Physiol. Biochem. 2021, 168, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Wu, H.C.; Wang, Y.D.; Liu, C.H.; Lin, C.C.; Luo, D.L.; Jinn, T.L. PECTIN METHYLESTERASE34 contributes to heat tolerance through its role in promoting stomatal movement. Plant Physiol. 2017, 174, 748–763. [Google Scholar] [CrossRef]
- Wu, H.C.; Yu, S.Y.; Wang, Y.D.; Jinn, T.L. Guard cell-specific pectin METHYLESTERASE53 is required for abscisic acid-mediated stomatal function and heat response in Arabidopsis. Front. Plant Sci. 2022, 13, 836151. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, W.; Geng, W.; Zhao, S.; Pan, Y.; Fan, G.; Zhang, S.; Wang, Y.; Liao, K. Transcriptome analysis insight into ethylene metabolism and pectinase activity of apricot (Prunus armeniaca L.) development and ripening. Sci. Rep. 2021, 11, 13569. [Google Scholar] [CrossRef]
- Srivastava, S.; Gupta, S.M.; Sane, A.P.; Nath, P. Isolation and characterization of ripening related pectin methylesterase inhibitor gene from banana fruit. Physiol. Mol. Biol. Plants 2012, 18, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, P.; McCann, M.C.; Roberts, K. Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol. 2007, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Peaucelle, A. Mechano-chemical aspects of organ formation in Arabidopsis thaliana: The relationship between auxin and pectin. PLoS ONE 2013, 8, e57813. [Google Scholar] [CrossRef]
- Jobert, F.; Soriano, A.; Brottier, L.; Casset, C.; Divol, F.; Safran, J.; Lefebvre, V.; Pelloux, J.; Robert, S.; Péret, B. Auxin triggers pectin modification during rootlet emergence in white lupin. Plant J. 2022, 112, 1127–1140. [Google Scholar] [CrossRef]
- Hocq, L.; Pelloux, J.; Lefebvre, V. Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci. 2017, 22, 20–29. [Google Scholar] [CrossRef]
- Schoenaers, S.; Balcerowicz, D.; Breen, G.; Hill, K.; Zdanio, M.; Mouille, G.; Holman, T.J.; Oh, J.; Wilson, M.H.; Nikonorova, N.; et al. The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Curr. Biol. 2018, 28, 722–732. [Google Scholar] [CrossRef]
- Jonsson, K.; Lathe, R.S.; Kierzkowski, D.; Routier-Kierzkowska, A.L.; Hamant, O.; Bhalerao, R.P. Mechanochemical feedback mediates tissue bending required for seedling emergence. Curr. Biol. 2021, 31, 1154–1164.e1153. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, B.; Du, F.; Guo, X.; Tian, C.; Hu, J.; Lü, S.; Long, M.; Zhang, L.; Wang, Y.; et al. A crosstalk between auxin and brassinosteroid regulates leaf shape by modulating growth anisotropy. Mol. Plant 2021, 14, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, F.; Habrylo, O.; Hocq, L.; Domon, J.M.; Marcelo, P.; Lefebvre, V.; Pelloux, J.; Mercadante, D. Structural and dynamical characterization of the pH-dependence of the pectin methylesterase-pectin methylesterase inhibitor complex. J. Biol. Chem. 2017, 292, 21538–21547. [Google Scholar] [CrossRef] [PubMed]
- Hocq, L.; Sénéchal, F.; Lefebvre, V.; Lehner, A.; Domon, J.M.; Mollet, J.C.; Dehors, J.; Pageau, K.; Marcelo, P.; Guérineau, F.; et al. Combined experimental and computational approaches reveal distinct pH dependence of pectin methylesterase inhibitors. Plant Physiol. 2017, 173, 1075–1093. [Google Scholar] [CrossRef]
- Barbez, E.; Dünser, K.; Gaidora, A.; Lendl, T.; Busch, W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, E4884–E4893. [Google Scholar] [CrossRef]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef]
- Ferrari, S.; Galletti, R.; Pontiggia, D.; Manfredini, C.; Lionetti, V.; Bellincampi, D.; Cervone, F.; De Lorenzo, G. Transgenic expression of a fungal endo-polygalacturonase increases plant resistance to pathogens and reduces auxin sensitivity. Plant Physiol. 2008, 146, 669–681. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol. 2012, 158, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Cosgrove, D.J. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol. 2012, 158, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Pérez García, M.; Zhang, Y.; Hayes, J.; Salazar, A.; Zabotina, O.A.; Hong, M. Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 2011, 50, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, S.E.; Schols, H.A. Characterisation of pectin-xylan complexes in tomato primary plant cell walls. Carbohydr. Polym. 2018, 197, 269–276. [Google Scholar] [CrossRef]
- Broxterman, S.E.; Schols, H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018, 192, 263–272. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, P.; Martinez-Sanz, M.; Bonilla, M.R.; Wang, D.; Gilbert, E.P.; Stokes, J.R.; Gidley, M.J. Cellulose-pectin composite hydrogels: Intermolecular interactions and material properties depend on order of assembly. Carbohydr. Polym. 2017, 162, 71–81. [Google Scholar] [CrossRef]
- Du, J.; Kirui, A.; Huang, S.; Wang, L.; Barnes, W.J.; Kiemle, S.N.; Zheng, Y.; Rui, Y.; Ruan, M.; Qi, S.; et al. Mutations in the pectin methyltransferase QUASIMODO2 influence cellulose biosynthesis and wall integrity in Arabidopsis. Plant Cell 2020, 32, 3576–3597. [Google Scholar] [CrossRef]
- Kirui, A.; Du, J.; Zhao, W.; Barnes, W.; Kang, X.; Anderson, C.T.; Xiao, C.; Wang, T. A pectin methyltransferase modulates polysaccharide dynamics and interactions in Arabidopsis primary cell walls: Evidence from solid-state NMR. Carbohydr. Polym. 2021, 270, 118370. [Google Scholar] [CrossRef]
- Phyo, P.; Wang, T.; Xiao, C.; Anderson, C.T.; Hong, M. Effects of pectin molecular weight changes on the structure, dynamics, and polysaccharide interactions of primary cell walls of Arabidopsis thaliana: Insights from solid-state NMR. Biomacromolecules 2017, 18, 2937–2950. [Google Scholar] [CrossRef]
- Francoz, E.; Ranocha, P.; Le Ru, A.; Martinez, Y.; Fourquaux, I.; Jauneau, A.; Dunand, C.; Burlat, V. Pectin demethylesterification generates platforms that anchor peroxidases to remodel plant cell wall domains. Dev. Cell 2019, 48, 261–276. [Google Scholar] [CrossRef]
- Carpin, S.; Crèvecoeur, M.; de Meyer, M.; Simon, P.; Greppin, H.; Penel, C. Identification of a Ca2+-pectate binding site on an apoplastic peroxidase. Plant Cell 2001, 13, 511–520. [Google Scholar] [CrossRef]
- Dauphin, B.G.; Ranocha, P.; Dunand, C.; Burlat, V. Cell-wall microdomain remodeling controls crucial developmental processes. Trends Plant Sci. 2022, 27, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Rydahl, M.G.; Hansen, A.R.; Kračun, S.K.; Mravec, J. Report on the current inventory of the toolbox for plant cell wall analysis: Proteinaceous and small molecular probes. Front. Plant Sci. 2018, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Vissenberg, K. Plant biology: Positive feedback between auxin and cell wall mechanics during apical hook formation. Cell Biol. 2021, 31, R306–R309. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, Y.; Wei, Z.; Liu, J.; Li, Y.; Ma, S.; Wang, Y.; Li, F.; Peng, J.; Wang, Z. Pectin methylesterase inhibitors GhPMEI53 and AtPMEI19 improve seed germination by modulating cell wall plasticity in cotton and Arabidopsis. J. Integr. Agric. 2024, 23, 3487–3505. [Google Scholar] [CrossRef]
- Leso, M.; Kokla, A.; Feng, M.; Melnyk, C.W. Pectin modifications promote haustoria development in the parasitic plant Phtheirospermum japonicum. Plant Physiol. 2023, 194, 229–242. [Google Scholar] [CrossRef]
- Wolf, S.; Mravec, J.; Greiner, S.; Mouille, G.; Höfte, H. Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr. Biol. 2012, 22, 1732–1737. [Google Scholar] [CrossRef]
- Wolf, S.; van der Does, D.; Ladwig, F.; Sticht, C.; Kolbeck, A.; Schürholz, A.K.; Augustin, S.; Keinath, N.; Rausch, T.; Greiner, S.; et al. A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 15261–15266. [Google Scholar] [CrossRef]
- Li, Z.; Sela, A.; Fridman, Y.; Garstka, L.; Höfte, H.; Savaldi-Goldstein, S.; Wolf, S. Optimal BR signalling is required for adequate cell wall orientation in the Arabidopsis root meristem. Development 2021, 148, dev199504. [Google Scholar] [CrossRef]
- Lorrai, R.; Erguvan, Ö.; Raggi, S.; Jonsson, K.; Široká, J.; Tarkowská, D.; Novák, O.; Griffiths, J.; Jones, A.M.; Verger, S.; et al. Cell wall integrity modulates HOOKLESS1 and PHYTOCHROME INTERACTING FACTOR4 expression controlling apical hook formation. Plant Physiol. 2024, 196, 1562–1578. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, G.; Xiao, J.; He, X.; Jiang, H.; Zhang, Z.; Zhang, Q.; Li, K.; Zhang, S.; Shi, X.; et al. AlphaFold-guided redesign of a plant pectin methylesterase inhibitor for broad-spectrum disease resistance. Mol. Plant 2024, 17, 1344–1368. [Google Scholar] [CrossRef]
- Bai, Y.; Ji, Y.; Jiang, Z.; Li, Z.; Liu, Z.; Zhang, Q.; Zhang, Y.; Jiang, C.; Yang, A.; Cheng, L.; et al. A pectin methylesterase inhibitor NtPMEI21 negatively regulates resistance to brown spot disease in Nicotiana tabacum L. Plant Sci. 2025, 359, 112635. [Google Scholar] [CrossRef]
- Huerta, A.I.; Sancho-Andrés, G.; Montesinos, J.C.; Silva-Navas, J.; Bassard, S.; Pau-Roblot, C.; Kesten, C.; Schlechter, R.; Dora, S.; Ayupov, T.; et al. The WAK-like protein RFO1 acts as a sensor of the pectin methylation status in Arabidopsis cell walls to modulate root growth and defense. Mol. Plant 2023, 16, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.; Li, J.; Wang, M.; Wang, X.; Zhang, H.; Chen, L.; Wu, T. Transcriptomic analysis of Camellia japonica to scale insects infestation and functional characterization of pectin methylesterase gene CjPME28 and polygalacturonase gene CjPG1. Plant Cell Rep. 2025, 44, 186. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, J.; Kemppainen, M.; Delhomme, N.; Shutava, I.; Zhou, J.; Takahashi, J.; Pardo, A.G.; Lundberg-Felten, J. Laccaria bicolor pectin methylesterases are involved in ectomycorrhiza development with Populus tremula × Populus tremuloides. New Phytologist. 2022, 236, 639–655. [Google Scholar] [CrossRef]
- Su, C.; Zhang, G.; Rodriguez-Franco, M.; Wietschorke, J.; Liang, P.; Yang, W.; Uhler, L.; Li, X.; Ott, T. Transcellular progression of infection threads in Medicago truncatula roots is controlled by locally confined cell wall modifications. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hocher, V.; Alloisio, N.; Auguy, F.; Fournier, P.; Doumas, P.; Pujic, P.; Gherbi, H.; Queiroux, C.; Da Silva, C.; Wincker, P.; et al. Transcriptomics of actinorhizal symbioses reveals homologs of the whole common symbiotic signaling cascade. Plant Physiol. 2011, 156, 700–711. [Google Scholar] [CrossRef]
- Lionetti, V.; Raiola, A.; Camardella, L.; Giovane, A.; Obel, N.; Pauly, M.; Favaron, F.; Cervone, F.; Bellincampi, D. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 2007, 143, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, F.; Graff, L.; Surcouf, O.; Marcelo, P.; Rayon, C.; Bouton, S.; Mareck, A.; Mouille, G.; Stintzi, A.; Höfte, H.; et al. Arabidopsis PECTIN METHYLESTERASE17 is co-expressed with and processed by SBT3.5, a subtilisin-like serine protease. Ann. Bot. 2014, 114, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.; Van Orden, J.; Wolf, S.; Vissenberg, K.; Delacourt, J.; Ndong, Y.A.; Pelloux, J.; Bischoff, V.; Urbain, A.; Mouille, G.; et al. A role for pectin demethylesterification in a developmentally regulated growth acceleration in dark-grown Arabidopsis hypocotyls. New Phytol. 2010, 188, 726–739. [Google Scholar] [CrossRef]
- Sénéchal, F.; Robinson, S.; Van Schaik, E.; Trévisan, M.; Saxena, P.; Reinhardt, D.; Fankhauser, C. Pectin methylesterification state and cell wall mechanical properties contribute to neighbor proximity-induced hypocotyl growth in Arabidopsis. Plant Direct 2024, 8, e584. [Google Scholar] [CrossRef]
- Reem, N.T.; Chambers, L.; Zhang, N.; Abdullah, S.F.; Chen, Y.; Feng, G.; Gao, S.; Soto-Burgos, J.; Pogorelko, G.; Bassham, D.C.; et al. Post-Synthetic Reduction of Pectin Methylesterification Causes Morphological Abnormalities and Alterations to Stress Response in Arabidopsis thaliana. Plants 2020, 9, 1558. [Google Scholar] [CrossRef]
- Weraduwage, S.; Kim, S.J.; Renna, L.; CAnozie, F.; DSharkey, T.; Brandizzi, F. Pectin Methylesterification Impacts the Relationship between Photosynthesis and Plant Growth. Plant Physiol. 2016, 171, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Lin, Y.J.; Tian, P.; Yu, X.Z. Exogenous proline regulates pectin demethylation by rescuing pectin methylesterase functioning of cell wall from Cr (VI) toxicity in rice plants. Chem. Biol. Technol. Agric. 2024, 11, 80. [Google Scholar] [CrossRef]
- Tian, G.W.; Chen, M.H.; Zaltsman, A.; Citovsky, V. Pollen-specific pectin methylesterase involved in pollen tube growth. Dev. Biol. 2006, 294, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Röckel, N.; Wolf, S.; Kost, B.; Rausch, T.; Greiner, S. Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J. 2008, 53, 133–143. [Google Scholar] [CrossRef]
- Sénéchal, F.; Mareck, A.; Marcelo, P.; Lerouge, P.; Pelloux, J. Arabidopsis PME17 Activity can be Controlled by Pectin Methylesterase Inhibitor4. Plant Signal Behav. 2015, 10, e983351. [Google Scholar] [CrossRef]
- Cascallares, M.; Setzes, N.; Marchetti, F.; López, G.A.; Distéfano, A.M.; Cainzos, M.; Zabaleta, E.; Pagnussat, G.C. A Complex Journey: Cell Wall Remodeling, Interactions, and Integrity During Pollen Tube Growth. Front. Plant Sci. 2020, 11, 599247. [Google Scholar] [CrossRef]
- Holdaway-Clarke, T.L.; Weddle, N.M.; Kim, S.; Robi, A.; Parris, C.; Kunkel, J.G.; Hepler, P.K. Effect of extracelular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. J. Exp. Bot. 2003, 54, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chebli, Y.; Geitmann, A. Cellular growth in plants requires regulation of cell wall biochemistry. Curr. Opin. Cell. Biol. 2017, 44, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Vavylonis, D.; Durachko, D.M.; Cosgrove, D.J. Nanoscale movements of cellulose microfibrils in primary cell walls. Nat. Plants 2017, 3, 17056. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Nanoscale structure, mechanics and growth of epidermal cell walls. Curr. Opin. Plant Biol. 2018, 46, 77–86. [Google Scholar] [CrossRef]
- Peaucelle, A.; Wightman, R.; Höfte, H. The control of growth symmetry breaking in the Arabidopsis hypocotyl. Curr. Biol. 2015, 25, 1746–1752. [Google Scholar] [CrossRef]
- Haas, K.T.; Wightman, R.; Meyerowitz, E.M.; Peaucelle, A. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 2020, 367, 1003–1007. [Google Scholar] [CrossRef]
- Somoza, S.C.; Boccardo, N.A.; Santin, F.; Sede, A.R.; Wengier, D.L.; Boisson-Dernier, A.; Muschietti, J.P. Arabidopsis RALF4 rapidly halts pollen tube growth by increasing ROS and decreasing calcium cytoplasmic tip levels. Biomolecules 2024, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Wang, H.; Jiang, Y.; Wang, Z.; Chen, Z.; Liu, C.; Yang, Z.; Gao, J.; Jiang, L.; Zhao, L.; et al. NtLLG4-mediated unconventional polar exocytosis of NtPPME1 coordinates cell wall rigidity and membrane dynamics to control pollen tube integrity. Sci. Adv. 2025, 11, eadw4550. [Google Scholar] [CrossRef] [PubMed]
- Hocq, L.; Guinand, S.; Habrylo, O.; Voxeur, A.; Tabi, W.; Safran, J.; Fournet, F.; Domon, J.M.; Mollet, J.C.; Pilard, S.; et al. The exogenous application of AtPGLR, an endo-polygalacturonase, triggers pollen tube burst and repair. Plant J. 2020, 103, 617–633. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.A.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Höfte, H. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef]
- Müller, K.; Levesque-Tremblay, G.; Bartels, S.; Weitbrecht, K.; Wormit, A.; Usadel, B.; Haughn, G.; Kermode, A.R. Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol. 2013, 161, 305–316. [Google Scholar] [CrossRef]
- Levesque-Tremblay, G.; Müller, K.; Mansfield, S.D.; Haughn, G.W. HIGHLY METHYL ESTERIFIED SEEDS is a pectin methyl esterase involved in embryo development. Plant Physiol. 2015, 167, 725–737. [Google Scholar] [CrossRef]
- Wachsman, G.; Zhang, J.; Moreno-Risueno, M.A.; Anderson, C.T.; Benfey, P.N. Cell wall remodeling and vesicle trafficking mediate the root clock in Arabidopsis. Science 2020, 370, 819–823. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, X.; Peng, G.; Liu, M.; Zhang, S.; Chen, M.; Liao, S.; Wei, X.; Xu, P.; Tan, X.; et al. Methylesterification of cell-wall pectin controls the diurnal flower-opening times in rice. Mol. Plant 2022, 15, 956–972. [Google Scholar] [CrossRef]
- Singh Yadav, A.; Roeder, A.H.K. An optimized live imaging and multiple cell layer growth analysis approach using Arabidopsis sepals. Front. Plant Sci. 2024, 15, 1449195. [Google Scholar] [CrossRef]
- Moreno, S.R.; Lenz, M.O.; Meyerowitz, E.M.; Locke, J.C.W.; Jönsson, H. Single-nucleus transcriptomics resolves differentiation dynamics between shoot stem cells and primary stem. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zamil, M.S.; Geitmann, A. The middle lamella—More than a glue. Phys. Biol. 2017, 14, 015004. [Google Scholar] [CrossRef]
- Lionetti, V.; Cervone, F.; De Lorenzo, G. A lower content of de-methylesterified homogalacturonan improves enzymatic cell separation and isolation of mesophyll protoplasts in Arabidopsis. Phytochemistry 2015, 112, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Guan, S.C.; Chen, J.Q.; Wen, C.J.; Cai, J.F.; Chen, X. Genome wide identification and functional characterization of strawberry pectin methylesterases related to fruit softening. BMC Plant Biol. 2020, 20, 13. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.; Yan, H.; Wang, H.; Wu, D.; Grierson, D.; Chen, K. The calcium-mediated homogalacturonan pectin complexation in cell walls contributes the firmness increase in loquat fruit during postharvest storage. J. Adv. Res. 2023, 49, 47–62. [Google Scholar] [CrossRef]
- Barnes, W.J.; Zelinsky, E.; Anderson, C.T. Polygalacturonase activity promotes aberrant cell separation in the quasimodo2 mutant of Arabidopsis thaliana. Cell Surf. 2022, 8, 100069. [Google Scholar] [CrossRef]
- Grandjean, C.; Voxeur, A.; Chabout, S.; Jobert, F.; Gutierrez, L.; Pelloux, J.; Mouille, G.; Bouton, S. Fine-tuning and remodeling of pectins play a key role in the maintenance of cell adhesion. Front. Plant Physiol. 2024, 2, 1441158. [Google Scholar] [CrossRef]
- Solecka, D.; Zebrowski, J.; Kacperska, A. Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann. Bot. 2008, 101, 521–530. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zhang, Q.; Zhang, Y.; Ou, X.; An, L.; Feng, H.; Zhao, Z. A cold-induced pectin methyl-esterase inhibitor gene contributes negatively to freezing tolerance but positively to salt tolerance in Arabidopsis. J. Plant Physiol. 2018, 222, 67–78. [Google Scholar] [CrossRef]
- Yang, W.; Ruan, M.; Xiang, M.; Deng, A.; Du, J.; Xiao, C. Overexpression of a pectin methylesterase gene PtoPME35 from Populus tomentosa influences stomatal function and drought tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2020, 523, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, Y.; Huang, H.; Zhan, J.; Wang, K.; Li, T. The predominant role of pectin in binding Cd in the root cell wall of a high Cd accumulating rice line (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2020, 206, 111210. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yu, H.; Wu, Y.; Huang, H.; Zhang, X.; Ye, D.; Wang, Y.; Zheng, Z.; Li, T. Nitric oxide amplifies cadmium binding in root cell wall of a high cadmium-accumulating rice (Oryza sativa L.) line by promoting hemicellulose synthesis and pectin demethylesterification. Ecotoxicol. Environ. Saf. 2022, 234, 113404. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.; Long, S.; Zhao, C. Maintenance of cell wall integrity under high salinity. Int. J. Mol. Sci. 2021, 22, 3260. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; He, H.; Fang, L.; Zhang, A. Pectin methylesterase31 positively regulates salt stress tolerance in Arabidopsis. Biochem. Biophys. Res. Commun. 2018, 496, 497–501. [Google Scholar] [CrossRef]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef]
- Del Corpo, D.; Fullone, M.R.; Miele, R.; Lafond, M.; Pontiggia, D.; Grisel, S.; Kieffer-Jaquinod, S.; Giardina, T.; Bellincampi, D.; Lionetti, V. AtPME17 is a functional Arabidopsis thaliana pectin methylesterase regulated by its PRO region that triggers PME activity in the resistance to Botrytis cinerea. Mol. Plant Pathol. 2020, 21, 1620–1633. [Google Scholar] [CrossRef]
- Raiola, A.; Lionetti, V.; Elmaghraby, I.; Immerzeel, P.; Mellerowicz, E.J.; Salvi, G.; Cervone, F.; Bellincampi, D. Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol. Plant Microbe Interact. 2011, 24, 432–440. [Google Scholar] [CrossRef]
- Lionetti, V.; Fabri, E.; De Caroli, M.; Hansen, A.R.; Willats, W.G.; Piro, G.; Bellincampi, D. Three pectin methylesterase inhibitors protect cell wall integrity for Arabidopsis immunity to Botrytis. Plant Physiol. 2017, 173, 1844–1863. [Google Scholar] [CrossRef]
- Osorio, S.; Castillejo, C.; Quesada, M.A.; Medina-Escobar, N.; Brownsey, G.J.; Suau, R.; Heredia, A.; Botella, M.A.; Valpuesta, V. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J. 2008, 54, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Osorio, S.; Bombarely, A.; Giavalisco, P.; Usadel, B.; Stephens, C.; Aragüez, I.; Medina-Escobar, N.; Botella, M.A.; Fernie, A.R.; Valpuesta, V. Demethylation of oligogalacturonides by FaPE1 in the fruits of the wild strawberry Fragaria vesca triggers metabolic and transcriptional changes associated with defence and development of the fruit. J. Exp. Bot. 2011, 62, 2855–2873. [Google Scholar] [CrossRef]
- Lionetti, V.; Giancaspro, A.; Fabri, E.; Giove, S.L.; Reem, N.; Zabotina, O.A.; Blanco, A.; Gadaleta, A.; Bellincampi, D. Cell wall traits as potential resources to improve resistance of durum wheat against Fusarium graminearum. BMC Plant Biol. 2015, 15, 6. [Google Scholar] [CrossRef]
- Silva-Sanzana, C.; Celiz-Balboa, J.; Garzo, E.; Marcus, S.E.; Parra-Rojas, J.P.; Rojas, B.; Olmedo, P.; Rubilar, M.A.; Rios, I.; Chorbadjian, R.A.; et al. Pectin methylesterases modulate plant homogalacturonan status in defenses against the aphid Myzus persicae. Plant Cell 2019, 31, 1913–1929. [Google Scholar] [CrossRef]
- Tran, D.; Dauphin, A.; Meimoun, P.; Kadono, T.; Nguyen, H.T.H.; Arbelet-Bonnin, D.; Zhao, T.; Errakhi, R.; Lehner, A.; Kawano, T.; et al. Methanol induces cytosolic calcium variations, membrane depolarization and ethylene production in Arabidopsis and tobacco. Ann. Bot. 2018, 122, 849–860. [Google Scholar] [CrossRef]
- Hann, C.T.; Bequette, C.J.; Dombrowski, J.E.; Stratmann, J.W. Methanol and ethanol modulate responses to danger- and microbe-associated molecular patterns. Front. Plant Sci. 2014, 5, 550. [Google Scholar] [CrossRef]
- Voxeur, A.; Habrylo, O.; Guénin, S.; Miart, F.; Soulié, M.C.; Rihouey, C.; Pau-Roblot, C.; Domon, J.M.; Gutierrez, L.; Pelloux, J.; et al. Oligogalacturonide production upon Arabidopsis thaliana-Botrytis cinerea interaction. Proc. Natl. Acad. Sci. USA 2019, 116, 19743–19752. [Google Scholar] [CrossRef]
- Degli Esposti, C.; Guerrisi, L.; Peruzzi, G.; Giulietti, S.; Pontiggia, D. Cell wall bricks of defence: The case study of oligogalacturonides. Front. Plant Sci. 2025, 16, 1552926. [Google Scholar] [CrossRef]
- Kohorn, B.D.; Kohorn, S.L.; Saba, N.J.; Martinez, V.M. Requirement for pectin methyl esterase and preference for fragmented over native pectins for wall-associated kinase-activated, EDS1/PAD4-dependent stress response in Arabidopsis. J. Biol. Chem. 2014, 289, 18978–18986. [Google Scholar] [CrossRef]
- Lin, W.; Tang, W.; Pan, X.; Huang, A.; Gao, X.; Anderson, C.T.; Yang, Z. Arabidopsis pavement cell morphogenesis requires FERONIA binding to pectin for activation of ROP GTPase signaling. Curr. Biol. 2022, 32, 497–507.e494. [Google Scholar] [CrossRef]
- Ranf, S. Sensing of molecular patterns through cell surface immune receptors. Curr. Opin. Plant Biol. 2017, 38, 68–77. [Google Scholar] [CrossRef]
- Li, P.; Lu, Y.J.; Chen, H.; Day, B. The lifecycle of the plant immune system. CRC Crit. Rev. Plant Sci. 2020, 39, 72–100. [Google Scholar] [CrossRef]
- Zhong, K.; Zhang, P.; Wei, X.; Platre, M.P.; He, W.; Zhang, L.; Małolepszy, A.; Cao, M.; Hu, S.; Tang, S.; et al. Natural variation of TBR confers plant zinc toxicity tolerance through root cell wall pectin methylesterification. Nat. Commun. 2024, 15, 5823. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; Chen, Z.; Zhang, D.; Zhang, H.; Wang, H.; Zhang, Y.; Cai, D.; Liu, J.; Xiao, S.; et al. A PECTIN METHYLESTERASE gene at the maize Ga1 locus confers male function in unilateral cross-incompatibility. Nat. Commun. 2018, 9, 3678. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, K.; Zhang, H.; Wang, Q.; Zhao, L.; Liu, J.; Chen, H. A single silk- and multiple pollen-expressed PMEs at the Ga1 locus modulate maize unilateral cross-incompatibility. J. Integr. Plant Biol. 2023, 65, 1344–1355. [Google Scholar] [CrossRef]
- Bapat, A.R.; Scott, M.P. Pectin methylesterase activities in reproductive tissues of maize plants with different haplotypes of the Ga1 and Ga2 cross incompatibility systems. Plant Reprod. 2024, 37, 479–488. [Google Scholar] [CrossRef]
- Cheng, M.; Meng, F.; Qi, H.; Mo, F.; Wang, P.; Chen, X.; Wang, A. Escaping drought: The pectin methylesterase inhibitor gene Slpmei27 can significantly change drought resistance in tomato. Plant Physiol. Biochem. 2022, 192, 207–217. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, D.; Gao, W.; Li, Y.; Tan, J.; Zhang, X. A comparative genome analysis of PME and PMEI families reveals the evolution of pectin metabolism in plant cell walls. PLoS ONE. 2013, 8, e72082. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Nguyen, H.P.; Lee, C. Genome-wide identification and expression analysis of rice pectin methylesterases: Implication of functional roles of pectin modification in rice physiology. J. Plant Physiol. 2015, 183, 23–29. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Jeong, H.Y.; Jeon, S.H.; Kim, D.; Lee, C. Rice pectin methylesterase inhibitor 28 (OsPMEI28) encodes a functional PMEI and its overexpression results in a dwarf phenotype through increased pectin methylesterification levels. J. Plant Physiol. 2017, 208, 17–25. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, F.; Wu, X.; Li, H. Characterization of the tomato (Solanum lycopersicum) pectin methylesterases: Evolution, activity of isoforms and expression during fruit ripening. Front. Plant Sci. 2020, 11, 238. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Nguyen, H.P.; Eom, S.H.; Lee, C. Integrative analysis of pectin methylesterase (PME) and PME inhibitors in tomato (Solanum lycopersicum): Identification, tissue-specific expression, and biochemical characterization. Plant Physiol. Biochem. 2018, 132, 557–565. [Google Scholar] [CrossRef]
- Wang, J.; Ling, L.; Cai, H.; Guo, C. Gene-wide identification and expression analysis of the PMEI family genes in soybean (Glycine max). 3 Biotech 2020, 10, 335. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Wang, S.; Zhang, Q.; Yang, S. Genome-wide identification of PME genes, evolution and expression analyses in soybean (Glycine max L.). BMC Plant Biol. 2021, 21, 578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, H.; Qin, X.; Chen, K.; Zhao, J.; Zhao, Y.; Yue, B. Genome-wide identification, phylogeny and expression analysis of the PME and PMEI gene families in maize. Sci. Rep. 2019, 9, 19918. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Ortega-Salazar, I.B.; Blanco-Ulate, B. Homogalacturonan Methylesterification and Cell Wall Regulation: Integrating Biochemistry, Mechanics, and Developmental Signaling for Crop Improvement. Agronomy 2025, 15, 2641. https://doi.org/10.3390/agronomy15112641
Wang D, Ortega-Salazar IB, Blanco-Ulate B. Homogalacturonan Methylesterification and Cell Wall Regulation: Integrating Biochemistry, Mechanics, and Developmental Signaling for Crop Improvement. Agronomy. 2025; 15(11):2641. https://doi.org/10.3390/agronomy15112641
Chicago/Turabian StyleWang, Duoduo, Isabel B. Ortega-Salazar, and Barbara Blanco-Ulate. 2025. "Homogalacturonan Methylesterification and Cell Wall Regulation: Integrating Biochemistry, Mechanics, and Developmental Signaling for Crop Improvement" Agronomy 15, no. 11: 2641. https://doi.org/10.3390/agronomy15112641
APA StyleWang, D., Ortega-Salazar, I. B., & Blanco-Ulate, B. (2025). Homogalacturonan Methylesterification and Cell Wall Regulation: Integrating Biochemistry, Mechanics, and Developmental Signaling for Crop Improvement. Agronomy, 15(11), 2641. https://doi.org/10.3390/agronomy15112641







