Effect of Sowing Date and Low-Temperature Seed Germination on Rapeseed Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
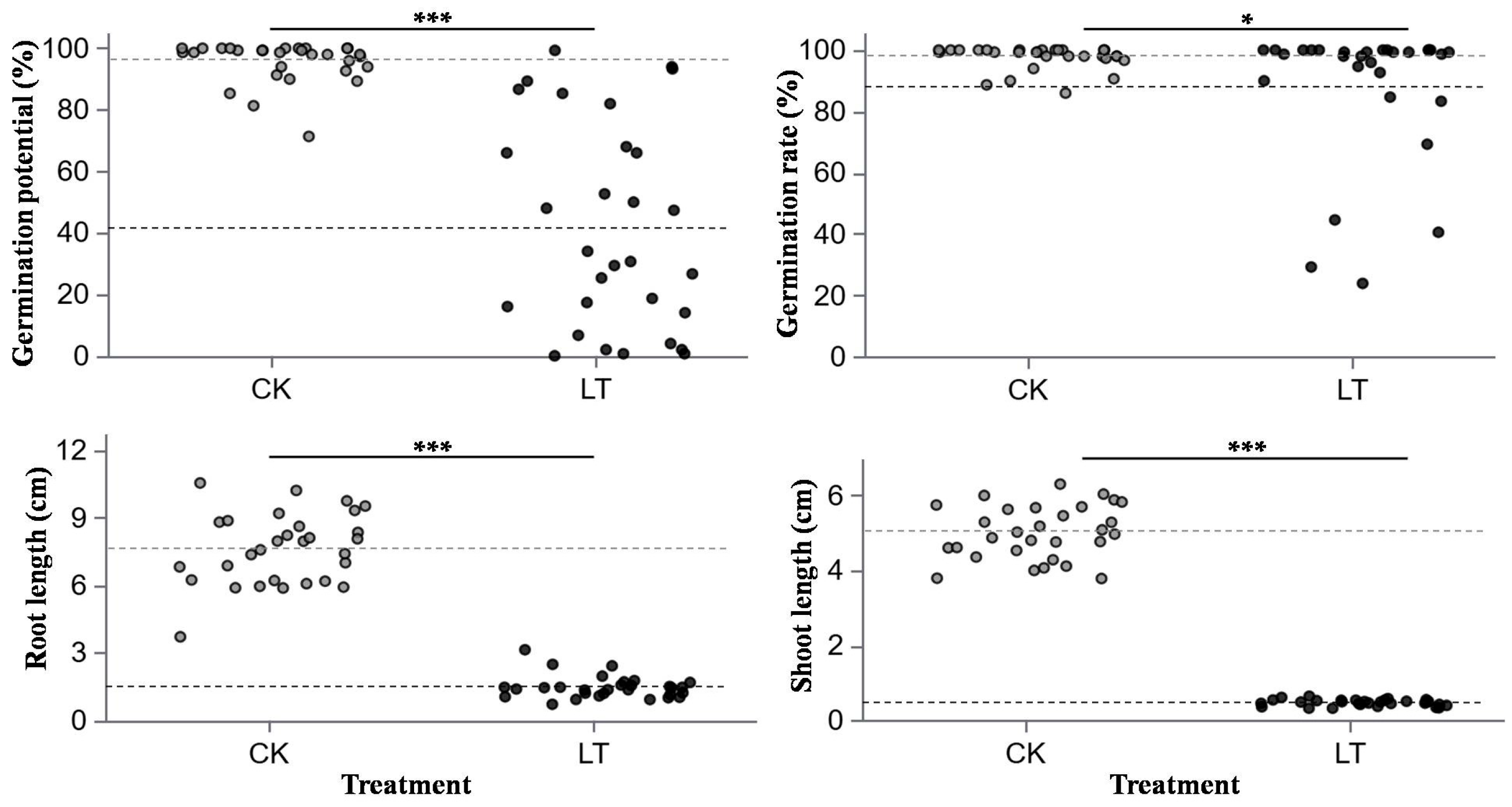
2.2. Seed Germination
- Normal temperature (CK): 20 °C/14 °C (11 h/13 h dark/dark).
- Low temperature (LT): 12 °C/6 °C (11 h/13 h dark/dark).
- Germination potential (GP, %) was calculated as the ratio of the number of germinated seeds on the third day after imbibition to the total number of seeds.
- Germination rate (GR, %) was calculated as the ratio of the number of germinated seeds on the tenth day after imbibition to the total number of seeds.
- The root length (RL) and shoot length (SL) were measured as the mean of 10 randomly selected seedlings per Petri dish after 10 days of incubation.
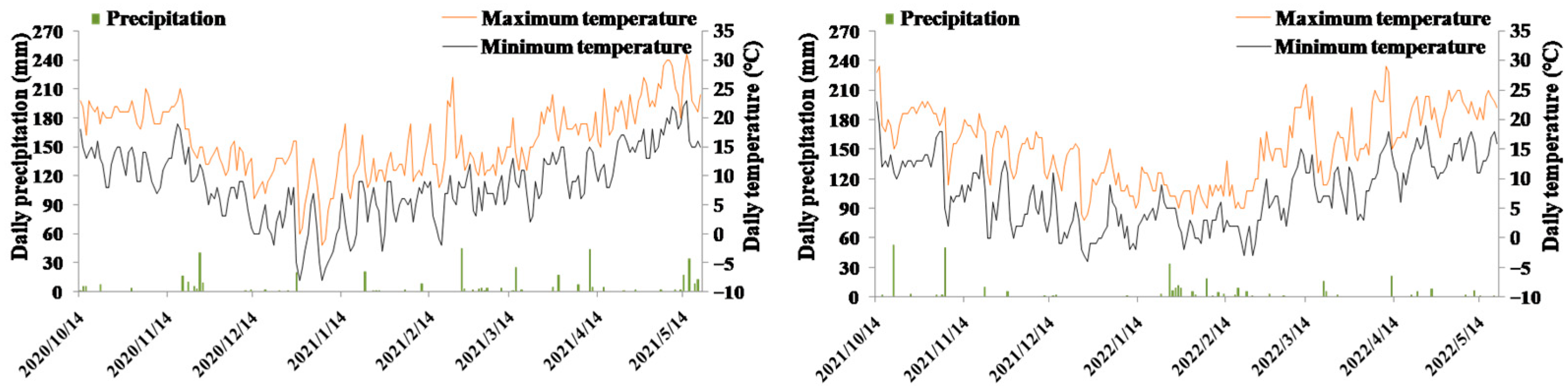
2.3. Field Experimental Design and Management
2.4. Statistical Analysis
3. Results
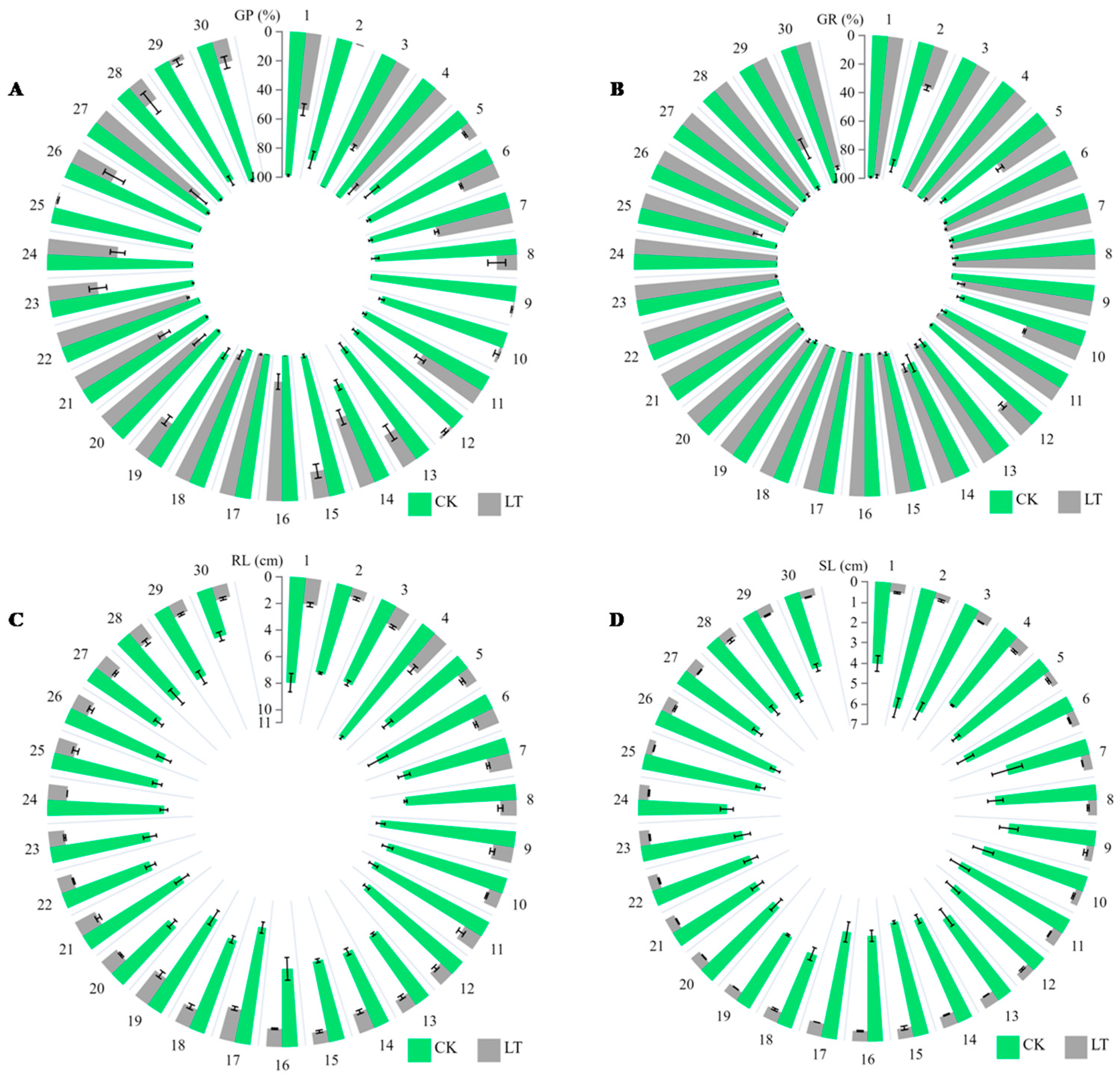
3.1. Effects of Temperature Treatments on Rapeseed Germination
3.2. Overwintering Survival Rates of Rapeseed in Response to Sowing Dates
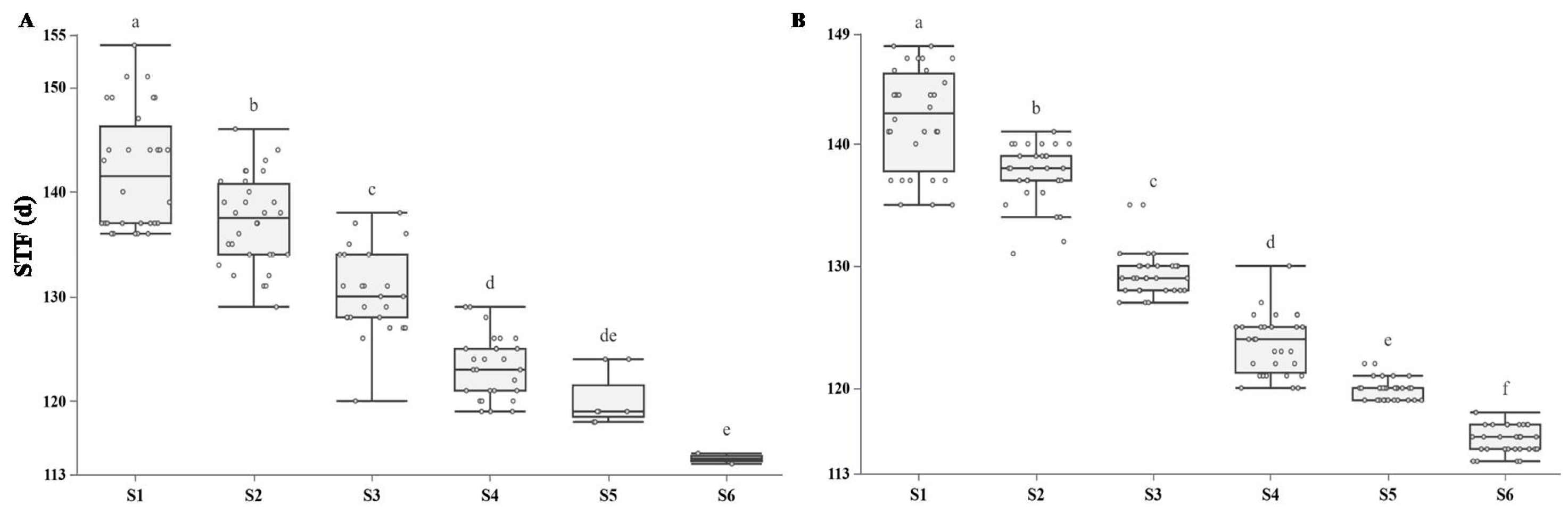
3.3. The Responses of Rapeseed Flowering Stage to Sowing Dates
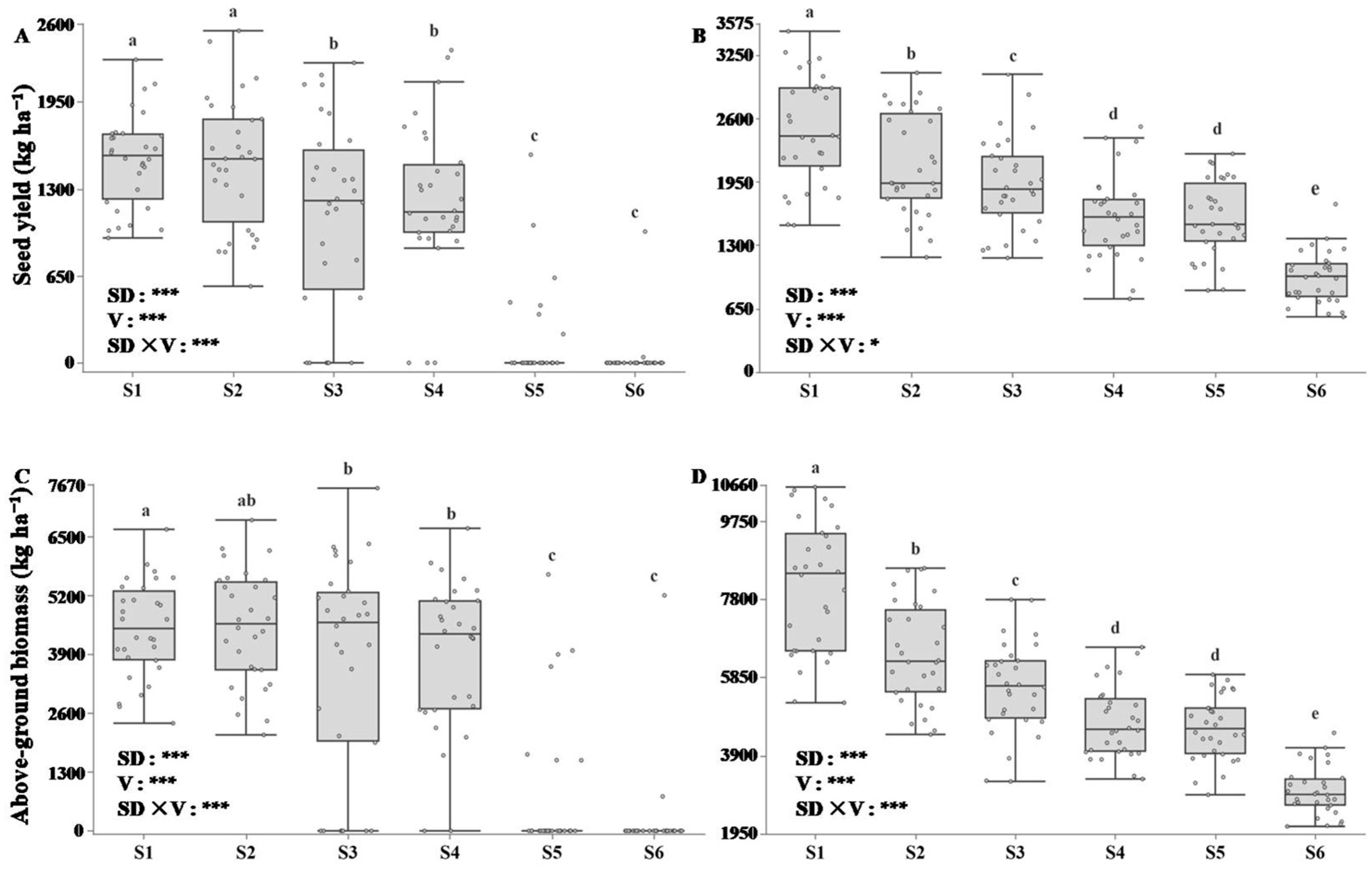
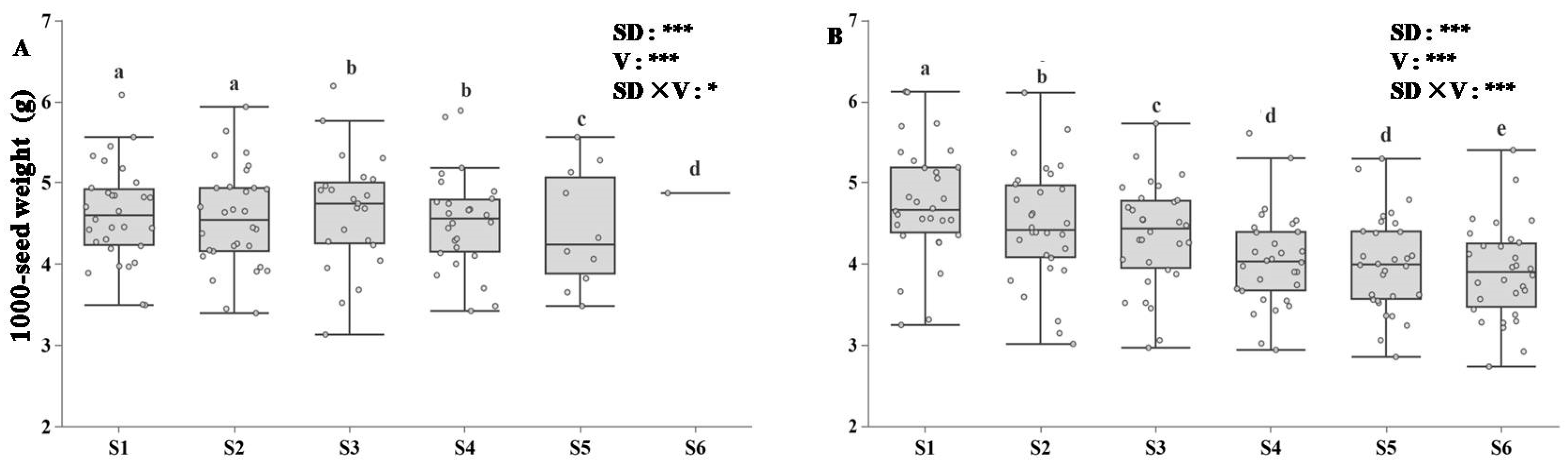
3.4. Yield per Plant in Response to Sowing Dates
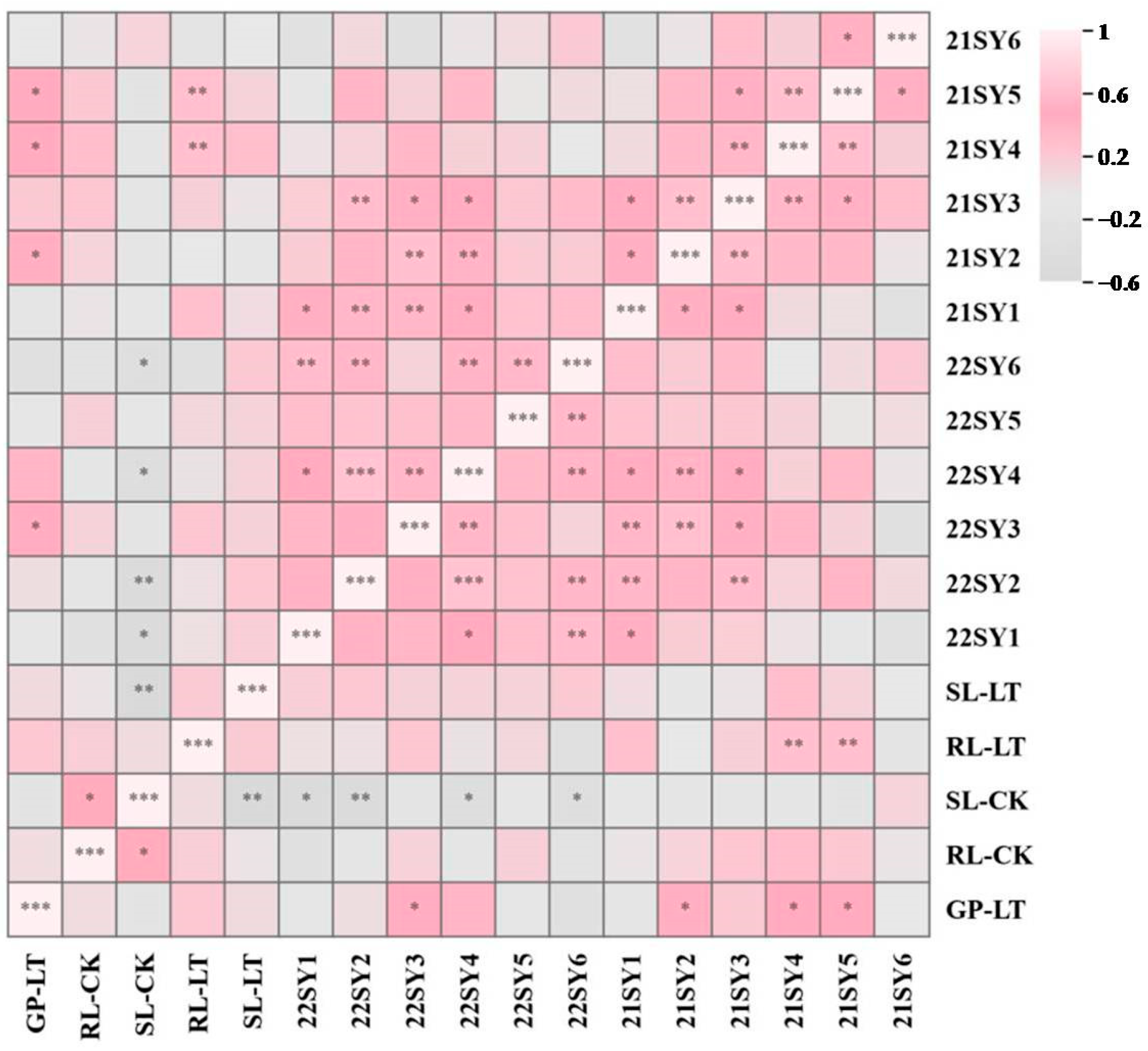
3.5. Correlation Analysis of Rapeseed Yield and Seed Germination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Q.; Hua, W.; Yin, Y.; Zhang, X.; Liu, L.; Shi, J.; Zhao, Y.; Qin, L.; Chen, C.; Wang, H. Rapeseed research and production in China. Crop. J. 2017, 5, 127–135. [Google Scholar] [CrossRef]
- Tian, Z.; Ji, Y.; Xu, H.; Qiu, H.; Sun, L.; Zhong, H.; Liu, J. The potential contribution of growing rapeseed in winter fallow fields across Yangtze River Basin to energy and food security in China. Resour. Conserv. Recycl. 2021, 164, 105159. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, W.; Jiang, M.; Yang, L.; Zhou, X. QTL mapping for low temperature germination in rapeseed. Sci. Rep. 2021, 11, 23382. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cheng, T.; Hu, L. Effect of wide–narrow row arrangement and plant density on yield and radiation use efficiency of mechanized direct-seeded canola in Central China. Field Crops Res. 2015, 172, 42–52. [Google Scholar] [CrossRef]
- Li, F.; Xiong, C.; Gu, J.J.; Cao, X.; Wang, S.S.; Hu, W.; Zhou, Z.G.; Chen, B.L. Effects of late sowing on yield, quality, photo-synthetic source succession and load ability characteristics of rape. Sci. Agric. Sin. 2024, 57, 4673–4685. [Google Scholar]
- Cao, X.; Huang, J.; Zhou, G.; Deng, N. A review of rice–rapeseed cropping system in China: Towards sustainable development. Crop. Environ. 2025, 4, 192–202. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, L.; Redden, B.; Yan, G. Identification of fast and slow germination accessions of Brassica napus L. for genetic studies and breeding for early vigour. Crop Pasture Sci. 2015, 66, 481–491. [Google Scholar] [CrossRef]
- Zhu, J.F.; Zhang, J.Y.; Yang, L.Y.; Jiang, M.Y.; Jiang, J.X.; Li, Y.L.; Wang, W.R.; Zhou, X.R. Identification of rape (Brassica napus L.) germplasm resources with low temperature germination tolerance. Acta Agric. Shanghai 2019, 35, 22–27. [Google Scholar]
- Qin, Z.; Lyu, J.; Teng, Z.; Meng, S.; Peng, Y.; Yuan, D.; Duan, M.; Zhang, J.; Ye, N. ABA biosynthesis rather than ABA catabolism is induced by low temperature and inhibits seed germination by activating OsTPP3. Crop J. 2025, 13, 752–763. [Google Scholar] [CrossRef]
- Xian, M.; Luo, T.; Khan, M.N.; Hu, L.; Xu, Z. Identifying differentially expressed genes associated with tolerance against low temperature stress in Brassica napus through transcriptome analysis. Int. J. Agric. Biol. 2017, 19, 273–281. [Google Scholar] [CrossRef]
- Luo, T.; Sheng, Z.; Zhang, C.; Li, Q.; Liu, X.; Qu, Z.; Xu, Z. Seed characteristics affect low-temperature stress tolerance performance of rapeseed (Brassica napus L.) during seed germination and seedling emergence stages. Agronomy 2022, 12, 1969. [Google Scholar] [CrossRef]
- Hang, M.; Dai, R.; Zhang, S.; Jiang, H. Alleviation effects of seed soaking with H2O2 on seed germination in rape under low temperature stress. Nanjing Nongye Daxue Xuebao 2017, 40, 963–970. [Google Scholar]
- Li, M.Q.; Zhou, Y.; Luo, D.; Wang, L.H.; Cheng, Y.; Ding, X.Y.; Zeng, L.; Gao, C.L.; Lan, X.J.; Zou, X.L.; et al. Identification and selection of late sowing tolerant rapeseed (Brassica napus L.) accessions. Chin. J. Oil Crop Sci. 2023, 45, 1238–1246. [Google Scholar]
- Sghaier, A.H.; Tarnawa, Á.; Khaeim, H.; Kovács, G.P.; Gyuricza, C.; Kende, Z. The Effects of temperature and water on the seed germination and seedling development of rapeseed (Brassica napus L.). Plants 2022, 11, 2819. [Google Scholar] [CrossRef]
- Xie, Z.; Kong, J.; Tang, M.; Luo, Z.; Li, D.; Liu, R.; Feng, S.; Zhang, C. Modelling winter rapeseed (Brassica napus L.) growth and yield under different sowing dates and densities using AquaCrop model. Agronomy 2023, 13, 367. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, T.; Liu, J.; Xian, M.; Yuan, J.; Hu, L.; Xu, Z. Evaluation of the low-temperature tolerance of rapeseed genotypes at the germination and seedling emergence stages. Crop. Sci. 2019, 59, 1709–1717. [Google Scholar] [CrossRef]
- Nykiforuk, C.L.; Johnson-Flanagan, A.M. Storage reserve mobilization during low temperature germination and early seedling growth in Brassica napus. Plant Physiol. Biochem. 1999, 37, 939–947. [Google Scholar] [CrossRef]
- Boter, M.; Calleja-Cabrera, J.; Carrera-Castaño, G.; Wagner, G.; Hatzig, S.V.; Snowdon, R.J.; Legoahec, L.; Bianchetti, G.; Bouchereau, A.; Nesi, N.; et al. An integrative approach to analyze seed germination in Brassica napus. Front. Plant Sci. 2019, 10, 1342. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Yang, P.; Hu, L.; Xu, Z. Comprehensive evaluation of low temperature tolerance in rapeseed during germination and emergence periods. Crops 2015, 5, 116–122. [Google Scholar]
- Rezaei, E.E.; Siebert, S.; Ewert, F. Climate and management interaction cause diverse crop phenology trends. Agric. For. Meteorol. 2017, 233, 55–70. [Google Scholar] [CrossRef]
- Rapacz, M. Physiological effects of winter rape (Brassica napus var. oleifera) prehardening to frost. II. growth, energy partitioning and water status during cold acclimation. J. Agron. Crop. Sci. 1998, 181, 81–87. Available online: https://en.ifr-pan.edu.pl/public/files/publication/rapaczjanowiak_1998_2.pdf (accessed on 20 October 2025). [CrossRef]
- Ozer, H. Sowing date and nitrogen rate effects on growth, yield and yield components of two summer rapeseed cultivars. Eur. J. Agron. 2003, 19, 453–463. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Hao, M.; Wang, N.; Wen, Q.-H.; Mei, F.-J.; Khan, A.; Wang, B.-Z.; Zhang, X.-L.; Wang, W.; et al. Climate suitability determines optimal yielding of dryland maize: A validation on timely sowing as an ancient wisdom. Field Crop. Res. 2025, 328, 109920. [Google Scholar] [CrossRef]
- Wu, F.; Guo, S.; Huang, W.; Han, Y.; Wang, Z.; Feng, L.; Wang, G.; Li, X.; Lei, Y.; Yang, B.; et al. Adaptation of cotton production to climate change by sowing date optimization and precision resource management. Ind. Crops Prod. 2023, 203, 117167. [Google Scholar] [CrossRef]
- Arduini, I.; Ercoli, L.; Mariotti, M.; Masoni, A. Sowing date affect spikelet number and grain yield of durum wheat. Cereal Res. Commun. 2009, 37, 469–478. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Cheng, S.; Fan, P.; Zhou, N.-B.; Xing, Z.-P.; Hu, Y.-J.; Xu, F.-F.; Guo, B.-W.; Wei, H.-Y.; Zhang, H.-C. Effects of sowing date and ecological points on yield and the temperature and radiation resources of semi-winter wheat. J. Integr. Agric. 2023, 22, 1366–1380. [Google Scholar] [CrossRef]
- Goffman, F.D.; Alonso, A.P.; Schwender, J.; Shachar-Hill, Y.; Ohlrogge, J.B. Light enables a very high efficiency of carbon storage in developing embryos of rapeseed. Plant Physiol. 2005, 138, 2269–2279. [Google Scholar] [CrossRef] [PubMed]

| No. | Name | Origin | No. | Name | Origin | No. | Name | Origin |
|---|---|---|---|---|---|---|---|---|
| 1 | Huyou21 | Shanghai | 11 | Fengyou9 | Henan | 21 | Fengyou10 | Henan |
| 2 | ZS11 | Hubei | 12 | D4818 | Guizhou | 22 | Zheyou50 | Zhejiang |
| 3 | 3406 | Shanghai | 13 | 98033 | Jiangshu | 23 | Qingza4 | Qinghai |
| 4 | 3409 | Shanghai | 14 | Zhong11-P073 | Hubei | 24 | 3453 | Shanghai |
| 5 | Qinyou7 | Shanxi | 15 | 3439 | Shanghai | 25 | HF08 | Shanghai |
| 6 | 3417 | Shanghai | 16 | M417 | Zhejiang | 26 | 3456 | Shanghai |
| 7 | 3426 | Shanghai | 17 | Zheza0903 | Zhejiang | 27 | 3462 | Shanghai |
| 8 | YH800 | Jiangshu | 18 | 86155 | Hubei | 28 | 3465 | Shanghai |
| 9 | P7089 | Hubei | 19 | Huyou039 | Shanghai | 29 | 3471 | Shanghai |
| 10 | Qinyou99 | Shanxi | 20 | 3445 | Shanghai | 30 | ShuYJ-3 | Jiangshu |
| Season (Year) | Total Rainfall (mm) | Mean Air Temp (°C) | Min Air Temp (°C) | Max Air Temp (°C) | Mean Soil Temp at 5 cm (°C) * |
|---|---|---|---|---|---|
| 2020–2021 | 394.2 | 12.4 | –8 | 31 | 15.4 (October → 7.8 December) |
| 2021–2022 | 350.1 | 13.1 | –4 | 29 | 15.9 (October → 8.1 December) |
| Genotype (No.) | GP (%) CK | GP (%) LT | RL (cm) CK | RL (cm) LT | SL (cm) CK | SL (cm) LT | GP Reduction (%) |
|---|---|---|---|---|---|---|---|
| 3409 (4) | 96 ± 2 | 85 ± 3 | 8.1 ± 0.4 | 2.5 ± 0.3 | 5.0 ± 0.2 | 0.6 ± 0.1 | 11.5 |
| M417 (16) | 97 ± 1 | 82 ± 4 | 7.4 ± 0.5 | 2.1 ± 0.2 | 4.8 ± 0.3 | 0.5 ± 0.1 | 15.5 |
| Zheza0903 (17) | 98 ± 1 | 88 ± 2 | 7.9 ± 0.3 | 1.9 ± 0.2 | 5.5 ± 0.2 | 0.8 ± 0.1 | 10.2 |
| 86155 (18) | 95 ± 2 | 84 ± 3 | 7.1 ± 0.3 | 1.6 ± 0.2 | 5.0 ± 0.3 | 0.6 ± 0.1 | 11.6 |
| 3445 (20) | 97 ± 1 | 80 ± 3 | 7.0 ± 0.4 | 1.7 ± 0.2 | 4.9 ± 0.3 | 0.5 ± 0.1 | 17.5 |
| Zheyou50 (22) | 98 ± 2 | 83 ± 3 | 8.0 ± 0.4 | 1.8 ± 0.2 | 5.2 ± 0.2 | 0.6 ± 0.1 | 15.3 |
| 3462 (27) | 96 ± 1 | 81 ± 4 | 7.6 ± 0.5 | 1.5 ± 0.2 | 5.1 ± 0.3 | 0.5 ± 0.1 | 15.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Lei, L.; Meng, X.; Li, H.; Wang, W. Effect of Sowing Date and Low-Temperature Seed Germination on Rapeseed Yield. Agronomy 2025, 15, 2545. https://doi.org/10.3390/agronomy15112545
Zhu J, Lei L, Meng X, Li H, Wang W. Effect of Sowing Date and Low-Temperature Seed Germination on Rapeseed Yield. Agronomy. 2025; 15(11):2545. https://doi.org/10.3390/agronomy15112545
Chicago/Turabian StyleZhu, Jifeng, Lei Lei, Xianmin Meng, Hongwei Li, and Weirong Wang. 2025. "Effect of Sowing Date and Low-Temperature Seed Germination on Rapeseed Yield" Agronomy 15, no. 11: 2545. https://doi.org/10.3390/agronomy15112545
APA StyleZhu, J., Lei, L., Meng, X., Li, H., & Wang, W. (2025). Effect of Sowing Date and Low-Temperature Seed Germination on Rapeseed Yield. Agronomy, 15(11), 2545. https://doi.org/10.3390/agronomy15112545





