Comparative Drought Response of Solanum melongena, S. macrocarpon, S. dasyphyllum, and S. melongena × S. dasyphyllum Interspecific Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
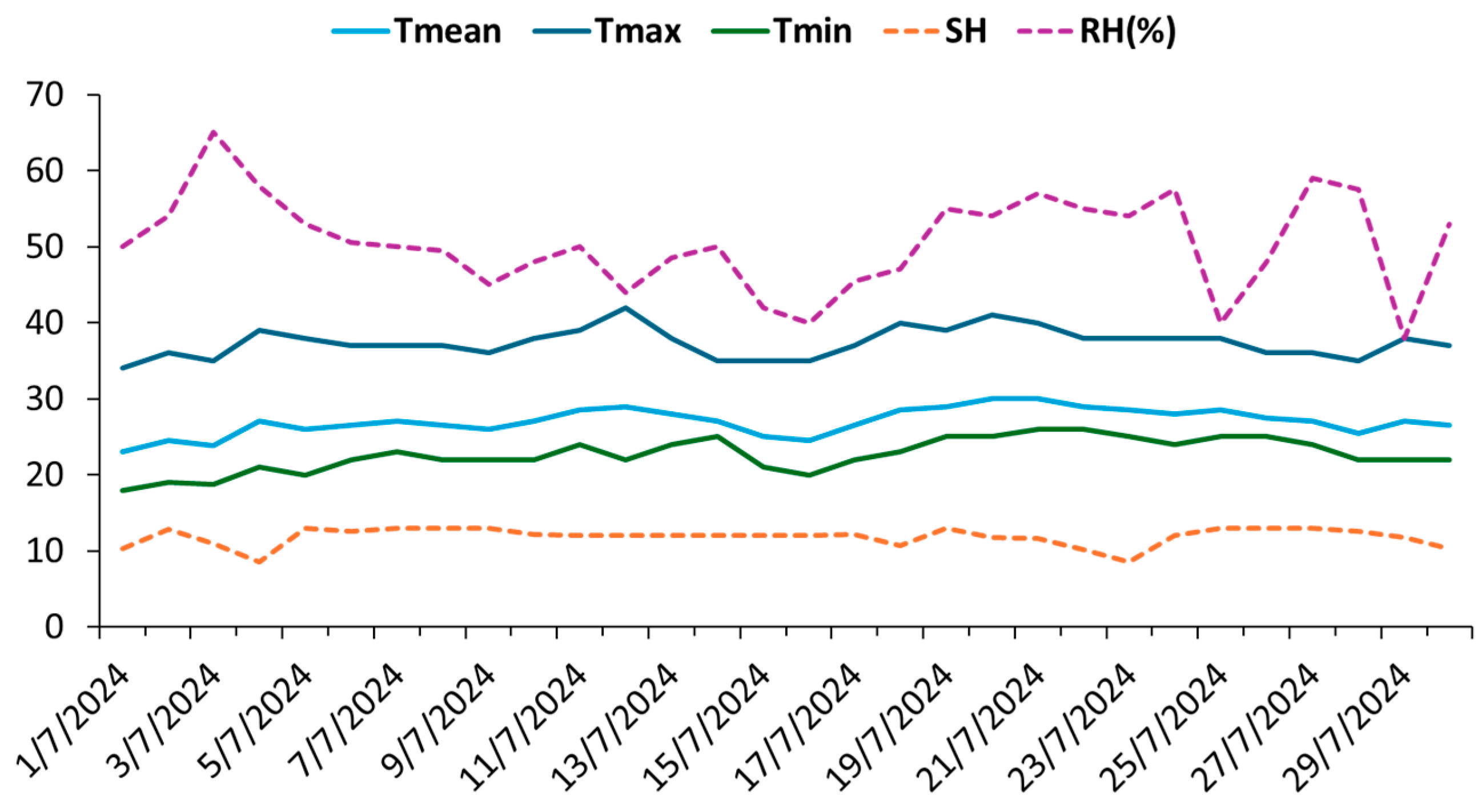
2.2. Experimental Site and Conditions
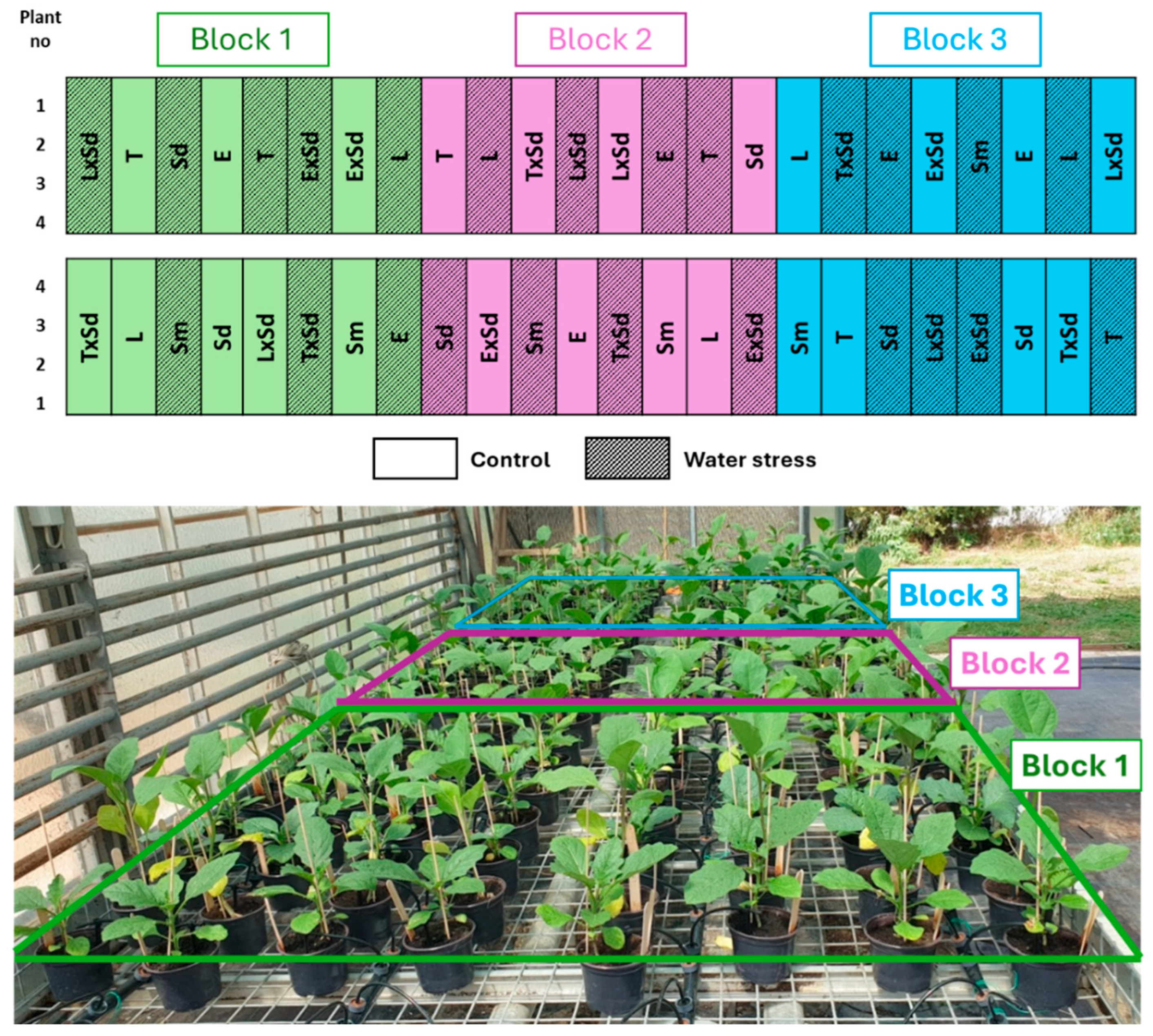
2.3. Experimental Design and Irrigation Treatments
2.4. Measurements of Agronomic Traits
2.5. Measurements of Physiological Traits
2.6. Mid-Parent and Better-Parent Heterosis
2.7. Statistical Analysis
3. Results
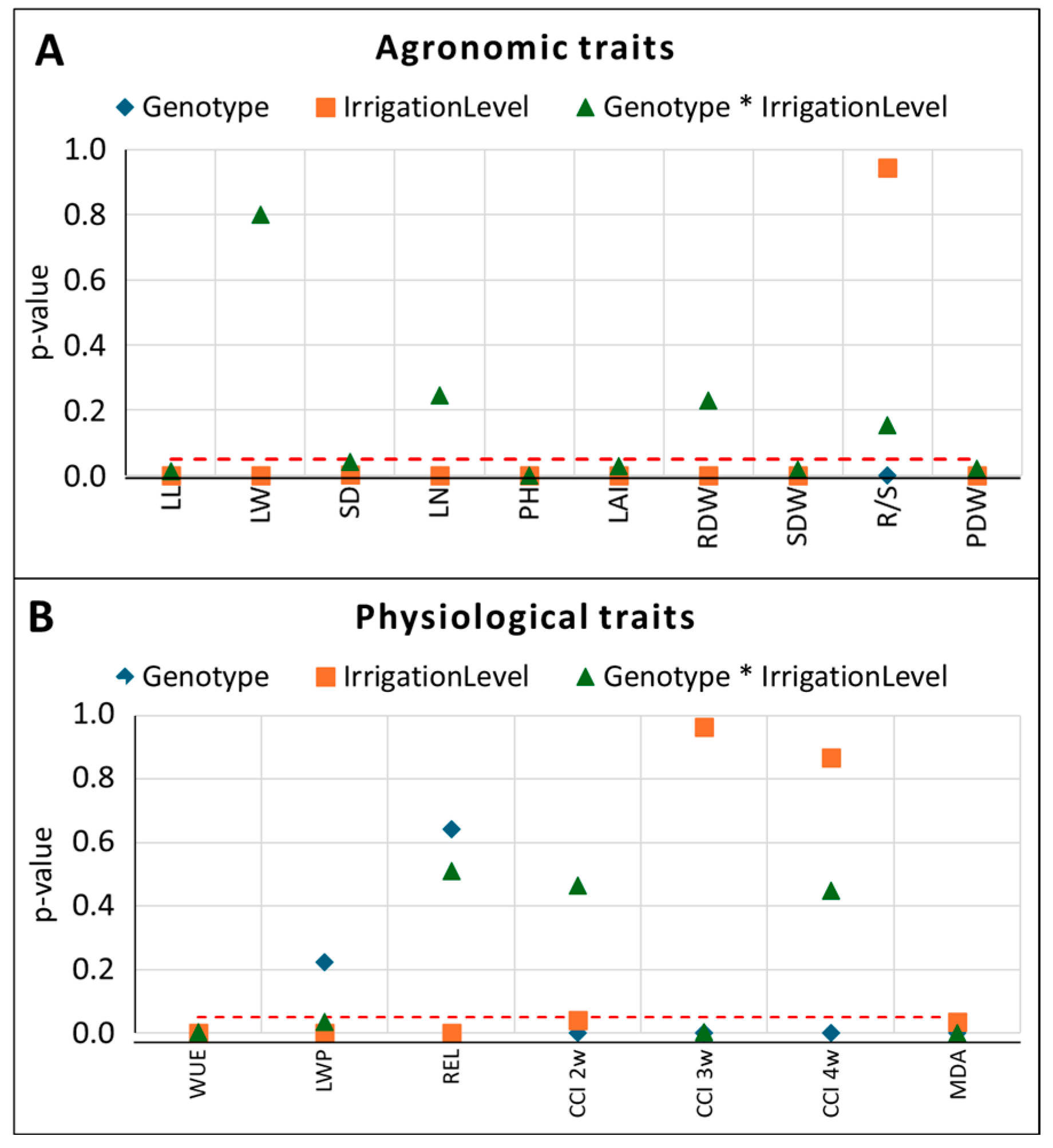
3.1. Main Effects and Interactions
3.1.1. Effects on Agronomic Traits
3.1.2. Effects on Physiological Traits
3.2. Agronomic Traits
3.2.1. Leaf Dimensions
3.2.2. Stem Diameter
3.2.3. Leaf Number
3.2.4. Plant Height
3.2.5. Leaf Area Index (LAI)
3.2.6. Root Dry Weight (RDW)
3.2.7. Shoot Dry Weight (SDW)
3.2.8. Plant Dry Weight (PDW)
3.2.9. Root-to-Shoot Ratio
3.3. Physiological Traits
3.3.1. Water Use Efficiency (WUE)
3.3.2. Leaf Water Potential (LWP)
3.3.3. Relative Electrolyte Leakage (REL)
3.3.4. Chlorophyll Content Index (CCI)
3.3.5. Malondialdehyde Content (MDA)
3.4. Heterosis of the Interspecific Hybrids Under Control and Water Stress Conditions
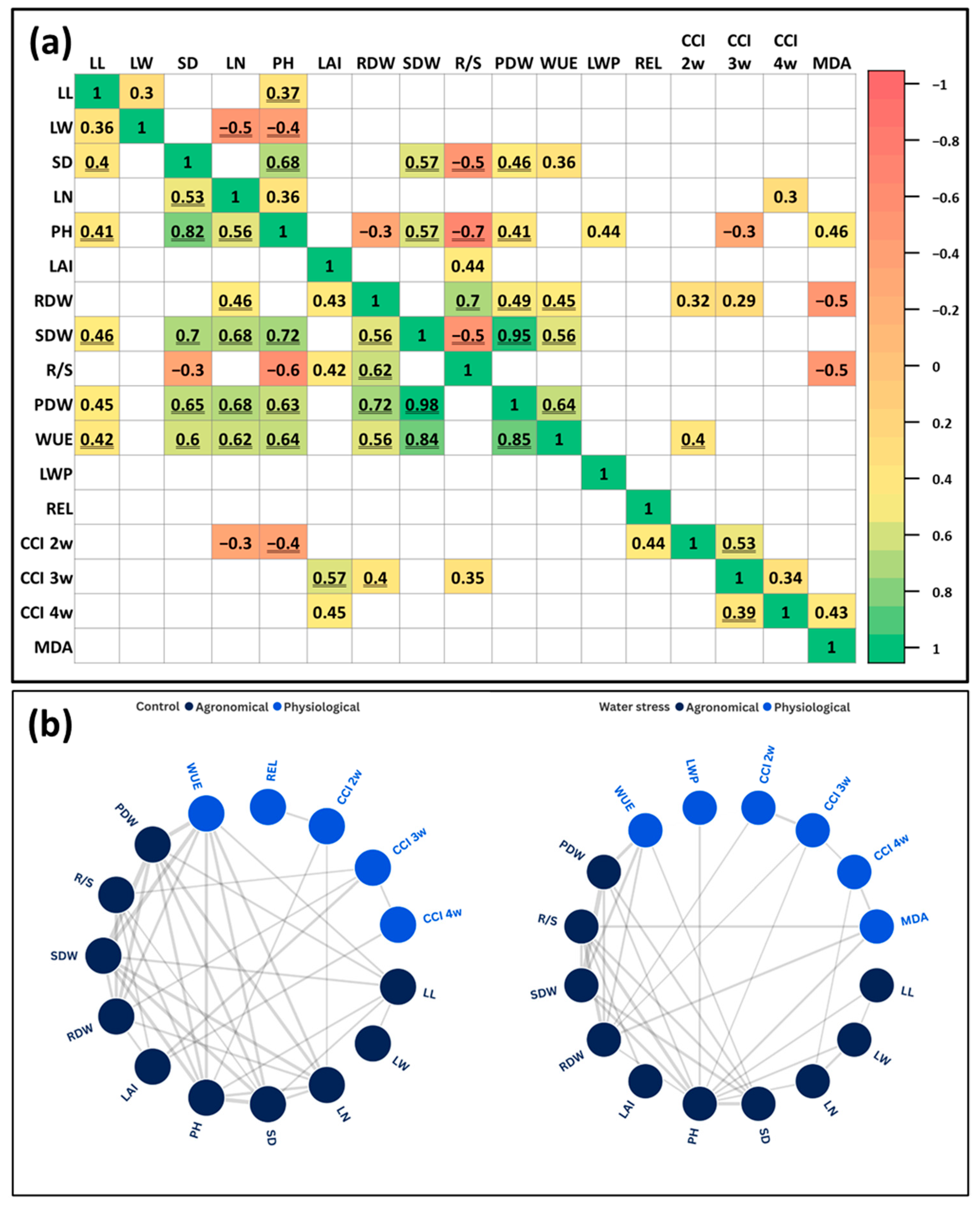
3.5. Correlation Analysis
3.5.1. Correlations Under Full Irrigation Conditions
3.5.2. Correlations Under Deficit Irrigation Conditions
3.6. Principal Component Analysis (PCA)
4. Discussion
4.1. Genotypic Responses to Water Stress
4.2. S. macrocarpon and S. dasyphyllum Have Different Drought Avoidance Adaptations
4.3. Heterosis Was Retained Under Different Irrigation Conditions
4.4. Water Stress Shifted Correlation Networks
4.5. Implications for Eggplant Breeding
4.6. Policy Implications and Future Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Trait | Statistic | Source of Variation | |||||
|---|---|---|---|---|---|---|---|
| Genotype | Irrigation Level | Genotype × Irrigation Level | Block | Error | Total | ||
| LL 1 | Sum of Squares | 105.367 | 96.436 | 25.512 | 5.497 | 171.760 | 404.572 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 15.052 | 96.436 | 3.645 | 2.749 | 1.363 | ||
| F | 11.042 | 70.744 | 2.674 | 2.016 | |||
| Sig. | 0.000 | 0.000 | 0.013 | 0.137 | |||
| LW | Sum of Squares | 66.248 | 54.025 | 7.282 | 6.102 | 241.900 | 375.557 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 9.464 | 54.025 | 1.040 | 3.051 | 1.920 | ||
| F | 4.930 | 28.140 | 0.542 | 1.589 | |||
| Sig. | 0.000 | 0.000 | 0.801 | 0.208 | |||
| SD | Sum of Squares | 38.811 | 4.161 | 5.773 | 1.050 | 48.180 | 97.975 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 5.544 | 4.161 | 0.825 | 0.525 | 0.382 | ||
| F | 14.500 | 10.882 | 2.157 | 1.373 | |||
| Sig. | 0.000 | 0.001 | 0.042 | 0.257 | |||
| LN | Sum of Squares | 81.624 | 34.132 | 4.790 | 1.417 | 65.210 | 187.173 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 11.661 | 34.132 | 0.684 | 0.709 | 0.518 | ||
| F | 22.531 | 65.950 | 1.322 | 1.369 | |||
| Sig. | 0.000 | 0.000 | 0.245 | 0.258 | |||
| PH | Sum of Squares | 2072.389 | 359.764 | 129.609 | 3.228 | 590.700 | 3155.690 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 296.056 | 359.764 | 18.516 | 1.614 | 4.688 | ||
| F | 63.151 | 76.740 | 3.949 | 0.344 | |||
| Sig. | 0.000 | 0.000 | 0.001 | 0.709 | |||
| LAI | Sum of Squares | 7.568 | 11.031 | 1.153 | 0.009 | 1.840 | 21.602 |
| Df | 7 | 1 | 7 | 2 | 30 | 47 | |
| Mean Square | 1.081 | 11.031 | 0.165 | 0.005 | 0.061 | ||
| F | 17.628 | 179.856 | 2.687 | 0.075 | |||
| Sig. | 0.000 | 0.000 | 0.028 | 0.928 | |||
| RDW | Sum of Squares | 2.974 | 3.271 | 0.752 | 0.416 | 9.980 | 17.393 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 0.425 | 3.271 | 0.107 | 0.208 | 0.079 | ||
| F | 5.363 | 41.298 | 1.357 | 2.624 | |||
| Sig. | 0.000 | 0.000 | 0.229 | 0.077 | |||
| SDW | Sum of Squares | 37.793 | 49.591 | 10.903 | 1.660 | 77.369 | 177.317 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 5.399 | 49.591 | 1.558 | 0.830 | 0.614 | ||
| F | 8.793 | 80.762 | 2.537 | 1.351 | |||
| Sig. | 0.000 | 0.000 | 0.018 | 0.263 | |||
| R/S | Sum of Squares | 0.150 | 0.000 | 0.025 | 0.008 | 0.000 | 0.182 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 0.021 | 0.000 | 0.004 | 0.004 | 0.002 | ||
| F | 9.326 | 0.005 | 1.557 | 1.672 | |||
| Sig. | 0.000 | 0.944 | 0.154 | 0.192 | |||
| PDW | Sum of Squares | 47.915 | 77.029 | 16.024 | 3.218 | 116.848 | 261.034 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 6.845 | 77.029 | 2.289 | 1.609 | 0.927 | ||
| F | 7.381 | 83.063 | 2.468 | 1.735 | |||
| Sig. | 0.000 | 0.000 | 0.021 | 0.181 | |||
| WUE | Sum of Squares | 8.556 | 4.279 | 2.483 | 0.702 | 13.576 | 29.596 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 1.222 | 4.279 | 0.355 | 0.351 | 0.108 | ||
| F | 11.344 | 39.715 | 3.292 | 3.257 | |||
| Sig. | 0.000 | 0.000 | 0.003 | 0.042 | |||
| LWP | Sum of Squares | 63.692 | 254.206 | 102.379 | 16.977 | 321.839 | 759.094 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 9.099 | 254.206 | 14.626 | 8.489 | 2.554 | ||
| F | 3.562 | 99.522 | 5.726 | 3.323 | |||
| Sig. | 0.002 | 0.000 | 0.000 | 0.039 | |||
| REL | Sum of Squares | 998.511 | 3129.379 | 1666.648 | 287.118 | 12,904.992 | 18,986.648 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 142.644 | 3129.379 | 238.093 | 143.559 | 102.421 | ||
| F | 1.393 | 30.554 | 2.325 | 1.402 | |||
| Sig. | 0.214 | 0.000 | 0.029 | 0.250 | |||
| CCI 2 w | Sum of Squares | 1975.019 | 190.004 | 278.484 | 508.714 | 4781.287 | 7733.508 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 282.146 | 190.004 | 39.783 | 254.357 | 37.947 | ||
| F | 7.435 | 5.007 | 1.048 | 6.703 | |||
| Sig. | 0.000 | 0.027 | 0.401 | 0.002 | |||
| CCI 3 w | Sum of Squares | 9265.481 | 0.230 | 1336.596 | 238.336 | 7293.472 | 18,134.115 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 1323.640 | 0.230 | 190.942 | 119.168 | 57.885 | ||
| F | 22.867 | 0.004 | 3.299 | 2.059 | |||
| Sig. | 0.000 | 0.950 | 0.003 | 0.132 | |||
| CCI 4 w | Sum of Squares | 4615.424 | 1.876 | 618.025 | 273.606 | 10,940.877 | 16,449.808 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 659.346 | 1.876 | 88.289 | 136.803 | 86.832 | ||
| F | 7.593 | 0.022 | 1.017 | 1.575 | |||
| Sig. | 0.000 | 0.883 | 0.423 | 0.211 | |||
| MDA | Sum of Squares | 5.945 | 0.360 | 6.592 | 0.012 | 10.787 | 23.696 |
| Df | 7 | 1 | 7 | 2 | 126 | 143 | |
| Mean Square | 0.849 | 0.360 | 0.942 | 0.006 | 0.086 | ||
| F | 9.921 | 4.205 | 10.999 | 0.072 | |||
| Sig. | 0.000 | 0.042 | 0.000 | 0.931 | |||
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 October 2025).
- Chapman, M.A. Eggplant Breeding and Improvement for Future Climates. In Genomic Designing of Climate-Smart Vegetable Crops; Springer International Publishing: Cham, Switzerland, 2020; pp. 257–276. [Google Scholar] [CrossRef]
- Daunay, M.-C. Eggplant. In Vegetables II Fabaceae, Liliaceae, Solanaceae, and Umbelliferae; Prohens-Tomás, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 163–220. ISBN 9780521872218. [Google Scholar]
- Gebrechorkos, S.H.; Sheffield, J.; Vicente-Serrano, S.M.; Funk, C.; Miralles, D.G.; Peng, J.; Dyer, E.; Talib, J.; Beck, H.E.; Singer, M.B.; et al. Warming Accelerates Global Drought Severity. Nature 2025, 642, 628–635. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Flores-Saavedra, M.; Gramazio, P.; Vilanova, S.; Mircea, D.M.; Ruiz-González, M.X.; Vicente, Ó.; Prohens, J.; Plazas, M. Introgressed Eggplant Lines with the Wild Solanum incanum Evaluated under Drought Stress Conditions. J. Integr. Agric. 2025, 24, 2203–2216. [Google Scholar] [CrossRef]
- Sarker, B.C.; Hara, M.; Uemura, M. Proline Synthesis, Physiological Responses and Biomass Yield of Eggplants during and after Repetitive Soil Moisture Stress. Sci. Hortic. 2005, 103, 387–402. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; Eaton, T.E. Eggplant (Solanum melongena L.) Plant Growth and Fruit Yield as Affected by Drip Irrigation Rate. HortScience 2015, 50, 1709–1714. [Google Scholar] [CrossRef]
- Hannachi, S.; Signore, A.; Adnan, M.; Mechi, L. Single and Associated Effects of Drought and Heat Stresses on Physiological, Biochemical and Antioxidant Machinery of Four Eggplant Cultivars. Plants 2022, 11, 2404. [Google Scholar] [CrossRef]
- Kadoglidou, K.I.; Anthimidou, E.; Krommydas, K.; Papa, E.; Karapatzak, E.; Tsivelika, N.; Irakli, M.; Mellidou, I.; Xanthopoulou, A.; Kalivas, A. Effect of Biostimulants on Drought Tolerance of Greenhouse-Grown Tomato. Horticulturae 2025, 11, 601. [Google Scholar] [CrossRef]
- Argento, S.; Treccarichi, S.; Arena, D.; Rizzo, G.F.; Branca, F. Exploitation of a Grafting Technique for Improving the Water Use Efficiency of Eggplant (Solanum melongena L.) Grown in a Cold Greenhouse in Mediterranean Climatic Conditions. Agronomy 2023, 13, 2705. [Google Scholar] [CrossRef]
- Consentino, B.B.; Sabatino, L.; Vultaggio, L.; Rotino, G.L.; La Placa, G.G.; D’Anna, F.; Leto, C.; Iacuzzi, N.; De Pasquale, C. Grafting Eggplant Onto Underutilized Solanum Species and Biostimulatory Action of Azospirillum Brasilense Modulate Growth, Yield, NUE and Nutritional and Functional Traits. Horticulturae 2022, 8, 722. [Google Scholar] [CrossRef]
- Ji, T.; Guo, X.; Wu, F.; Wei, M.; Li, J.; Ji, P.; Wang, N.; Yang, F. Proper Irrigation Amount for Eggplant Cultivation in a Solar Greenhouse Improved Plant Growth, Fruit Quality and Yield by Influencing the Soil Microbial Community and Rhizosphere Environment. Front. Microbiol. 2022, 13, 981288. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Wakchaure, G.C.; Kumar, D.; Kumar, S.; Gawhale, B.J.; Meena, K.K.; Sawant, C.P.; Singh, D.K.; Paramasivam, S.K.; Minhas, P.S. Grafting Wild Rootstocks as a Climate-Resilient Strategy to Enhance Productivity, Quality and Tolerance in Eggplant under Variable Water Stress Induced by Deficit Irrigation. Agric. Water Manag. 2025, 314, 109492. [Google Scholar] [CrossRef]
- Rotino, G.L.; Perri, E.; Acciarri, N.; Sunseri, F.; Arpaia, S. Development of Eggplant Varietal Resistance to Insects and Diseases via Plant Breeding. Adv. Hortic. Sci. 1997, 11, 193–201. [Google Scholar]
- Prohens, J.; Plazas, M.; Raigón, M.D.; Seguí-Simarro, J.M.; Stommel, J.R.; Vilanova, S. Characterization of Interspecific Hybrids and First Backcross Generations from Crosses between Two Cultivated Eggplants (Solanum melongena and S. aethiopicum Kumba Group) and Implications for Eggplant Breeding. Euphytica 2012, 186, 517–538. [Google Scholar] [CrossRef]
- Rizza, F.; Mennella, G.; Collonnier, C.; Sihachakr, D.; Kashyap, V.; Rajam, M.V.; Presterà, M.; Rotino, G.L. Androgenic Dihaploids from Somatic Hybrids between Solanum melongena and S. aethiopicum Group Gilo as a Source of Resistance to Fusarium Oxysporum f. Sp. Melongenae. Plant Cell Rep. 2002, 20, 1022–1032. [Google Scholar] [CrossRef]
- Prabhu, M.; Natarajan, S.; Veeraragavathatham, D.; Pugalendhi, L. The Biochemical Basis of Shoot and Fruit Borer Resistance in Interspecific Progenies of Brinjal (Solanum melongena). EurAsian J. Biosci. 2009, 57, 50–57. [Google Scholar] [CrossRef]
- Mennella, G.; Rotino, G.L.; Fibiani, M.; D’Alessandro, A.; Francese, G.; Toppino, L.; Cavallanti, F.; Acciarri, N.; Lo Scalzo, R. Characterization of Health-Related Compounds in Eggplant (Solanum melongena L.) Lines Derived from Introgression of Allied Species. J. Agric. Food Chem. 2010, 58, 7597–7603. [Google Scholar] [CrossRef]
- Pang, X.; Chen, J.; Li, L.; Huang, W.; Liu, J. Deciphering Drought Resilience in Solanaceae Crops: Unraveling Molecular and Genetic Mechanisms. Biology 2024, 13, 1076. [Google Scholar] [CrossRef]
- Prohens, J.; Gramazio, P.; Plazas, M.; Dempewolf, H.; Kilian, B.; Díez, M.J.; Fita, A.; Herraiz, F.J.; Rodríguez-Burruezo, A.; Soler, S.; et al. Introgressiomics: A New Approach for Using Crop Wild Relatives in Breeding for Adaptation to Climate Change. Euphytica 2017, 213, 158. [Google Scholar] [CrossRef]
- Cebeci, E. Comparative Evaluation of Eggplant Genotypes with Their Wild Relatives under Gradually Increased Drought Stress. Bragantia 2024, 83, e20230246. [Google Scholar] [CrossRef]
- Fita, A.; Fioruci, F.; Plazas, M.; Rodriguez-Burruezo, A.; Prohens, J. Drought Tolerance among Accessions of Eggplant and Related Species. Bull. UASVM Hortic. 2015, 72, 461–462. [Google Scholar] [CrossRef]
- Khapte, P.S.; Changan, S.S.; Kumar, P.; Singh, T.H.; Singh, A.K.; Rane, J.; Reddy, K.S. Deciphering Desiccation Tolerance in Wild Eggplant Species: Insights from Chlorophyll Fluorescence Dynamics. BMC Plant Biol. 2024, 24, 702. [Google Scholar] [CrossRef]
- Opoku, V.A.; Adu, M.O.; Asare, P.A.; Asante, J.; Hygienus, G.; Andersen, M.N. Rapid and Low-Cost Screening for Single and Combined Effects of Drought and Heat Stress on the Morpho-Physiological Traits of African Eggplant (Solanum aethiopicum) Germplasm. PLoS ONE 2024, 19, e0295512. [Google Scholar] [CrossRef]
- Plazas, M.; González-Orenga, S.; Nguyen, H.T.; Morar, I.M.; Fita, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Growth and Antioxidant Responses Triggered by Water Stress in Wild Relatives of Eggplant. Sci. Hortic. 2022, 293, 110685. [Google Scholar] [CrossRef]
- Villanueva, G.; Plazas, M.; Gramazio, P.; Moya, R.D.; Prohens, J.; Vilanova, S. Evaluation of Three Sets of Advanced Backcrosses of Eggplant with Wild Relatives from Different Gene Pools under Low N Fertilization Conditions. Hortic. Res. 2023, 10, uhad141. [Google Scholar] [CrossRef]
- Bletsos, F.; Roupakias, D.; Tsaktsira, M.; Scaltsoyjannes, A. Production and Characterization of Interspecific Hybrids between Three Eggplant (Solanum melongena L.) Cultivars and Solanum macrocarpon L. Sci. Hortic. 2004, 101, 11–21. [Google Scholar] [CrossRef]
- García-Fortea, E.; Gramazio, P.; Vilanova, S.; Fita, A.; Mangino, G.; Villanueva, G.; Arrones, A.; Knapp, S.; Prohens, J.; Plazas, M. First Successful Backcrossing towards Eggplant (Solanum melongena) of a New World Species, the Silverleaf Nightshade (S. elaeagnifolium), and Characterization of Interspecific Hybrids and Backcrosses. Sci. Hortic. 2019, 246, 563–573. [Google Scholar] [CrossRef]
- Gramazio, P.; Prohens, J.; Plazas, M.; Mangino, G.; Herraiz, F.J.; Vilanova, S. Development and Genetic Characterization of Advanced Backcross Materials and An Introgression Line Population of Solanum incanum in a S. melongena Background. Front. Plant Sci. 2017, 8, 1477. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, B.; Prohens, J.; Gramazio, P.; Kouassi, A.B.; Vilanova, S.; Galán-Ávila, A.; Herraiz, F.J.; Kouassi, A.; Seguí-Simarro, J.M.; Plazas, M. Development of Backcross Generations and New Interspecific Hybrid Combinations for Introgression Breeding in Eggplant (Solanum melongena). Sci. Hortic. 2016, 213, 199–207. [Google Scholar] [CrossRef]
- Plazas, M.; Vilanova, S.; Gramazio, P.; Rodriguez-Burruezo, A.; Fita, A.; Herraiz, F.J.; Ranil, R.; Fonseka, R.; Niran, L.; Fonseka, H.; et al. Interspecific Hybridization between Eggplant and Wild Relatives from Different Genepools. J. Am. Soc. Hortic. Sci. 2016, 141, 34–44. [Google Scholar] [CrossRef]
- Toppino, L.; Ribolzi, S.; Shaaf, S.; Rossini, L.; Boyaci, H.; Çalışkan, S.; Unlu, A.; Bassolino, L.; Irdani, T.; Fadda, S.; et al. Introgression Breeding in Eggplant from S. tomentosum and Genetic Mapping of Novel Key Agronomical Traits. In Proceedings of the 17th EUCARPIA Meeting on Genetics and Breeding of Capsicum and Eggplant; Véronique Lefebvre and Marie-Christine Daunay, Avignon, France, 11–13 September 2019; Daunay, M.-C., Ed.; Institut National de la Recherche Agronomique (INRA): Avignon, France, 2019; p. 232. [Google Scholar]
- Cebeci, E.; Boyaci, H.; Kıran, S.; Ellialtioglu, S. Assessment Results of Salinity Stressed F2 Population Originated from Interspecific Hybridization of Eggplant with Wild Relative S. incanum L. Hortic. Stud. 2024, 41, 50–59. [Google Scholar] [CrossRef]
- Cebeci, E.; Boyacı, H.; Doğan, Y.; Toppino, L.; Rotino, G. Determination of Heat and Drought Tolerant Lines in Segregating Populations Produced by Interspecific Crosses in Eggplant. Ekin J. Crop Breed. Genet. 2023, 9, 81–90. [Google Scholar]
- Mangino, G.; Plazas, M.; Vilanova, S.; Prohens, J.; Gramazio, P. Performance of a Set of Eggplant (Solanum melongena) Lines with Introgressions from Its Wild Relative S. incanum under Open Field and Screenhouse Conditions and Detection of QTLs. Agronomy 2020, 10, 467. [Google Scholar] [CrossRef]
- Villanueva, G.; Rosa-Martínez, E.; Şahin, A.; García-Fortea, E.; Plazas, M.; Prohens, J.; Vilanova, S. Evaluation of Advanced Backcrosses of Eggplant with Solanum elaeagnifolium Introgressions under Low N Conditions. Agronomy 2021, 11, 1770. [Google Scholar] [CrossRef]
- Bletsos, F.A.; Olympios, C.M. Rootstocks and Grafting of Tomatoes, Peppers and Eggplants for Soil-Borne Disease Resistance, Improved Yield and Quality. Eur. J. Plant Sci. Biotechnol. 2008, 2, 62–73. [Google Scholar]
- Gisbert, C.; Prohens, J.; Raigón, M.D.; Stommel, J.R.; Nuez, F. Eggplant Relatives as Sources of Variation for Developing New Rootstocks: Effects of Grafting on Eggplant Yield and Fruit Apparent Quality and Composition. Sci. Hortic. 2011, 128, 14–22. [Google Scholar] [CrossRef]
- Gisbert, C.; Prohens, J.; Nuez, F. Performance of Eggplant Grafted onto Cultivated, Wild and Hybrid Materials of Eggplant and Tomato. Int. J. Plant Prod 2011, 5, 367–380. [Google Scholar]
- Krommydas, K.; Mavromatis, A.; Bletsos, F.; Roupakias, D. Suitability of CMS-Based Interspecific Eggplant (Solanum melongena L.) Hybrids as Rootstocks for Eggplant Grafting. J. Agric. Ecol. Res. Int. 2018, 15, 1–15. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; D’Anna, F.; Palazzolo, E.; Mennella, G.; Rotino, G.L. Hybrids and Allied Species as Potential Rootstocks for Eggplant: Effect of Grafting on Vigour, Yield and Overall Fruit Quality Traits. Sci. Hortic. 2018, 228, 81–90. [Google Scholar] [CrossRef]
- González-Orenga, S.; Plazas, M.; Ribera, E.; Pallotti, C.; Boscaiu, M.; Prohens, J.; Vicente, O.; Fita, A. Transgressive Biochemical Response to Water Stress in Interspecific Eggplant Hybrids. Plants 2023, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, A.B.; Kouassi, K.B.A.; Sylla, Z.; Plazas, M.; Fonseka, R.M.; Kouassi, A.; Fonseka, H.; N’guetta, A.S.P.; Prohens, J. Genetic Parameters of Drought Tolerance for Agromorphological Traits in Eggplant, Wild Relatives, and Interspecific Hybrids. Crop Sci. 2021, 61, 55–68. [Google Scholar] [CrossRef]
- Flores-Saavedra, M.; Plazas, M.; Vilanova, S.; Prohens, J.; Gramazio, P. Induction of Water Stress in Major Solanum Crops: A Review on Methodologies and Their Application for Identifying Drought Tolerant Materials. Sci. Hortic. 2023, 318, 112105. [Google Scholar] [CrossRef]
- Daunay, M.-C.; Salinier, J.; Aubriot, X. Crossability and Diversity of Eggplants and Their Wild Relatives. In The Eggplant Genome; Springer International Publishing: Cham, Switzerland, 2019; ISBN 9783319992082. [Google Scholar]
- Kadoglidou, K.; Malkoyannidis, C.; Radoglou, K.; Eleftherohorinos, I.; Constantinidou, H.-I.A. Pronamide Effects on Physiology and Yield of Sugar Beet. Weed Sci. 2008, 56, 457–463. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Xanthopoulou, A.; Kalyvas, A.; Mellidou, I. Utilization of Tomato Landraces to Improve Seedling Performance under Salt Stress. Stresses 2021, 1, 238–252. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap Pressure in Vascular Plants: Negative Hydrostatic Pressure Can Be Measured in Plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Matsi, T.; Kamou, N.; Avdouli, D.; Mellidou, I.; Karamanoli, K. Decoding the Potential of a New Pseudomonas Putida Strain for Inducing Drought Tolerance of Tomato (Solanum lycopersicum) Plants through Seed Biopriming. J. Plant Physiol. 2022, 271, 153658. [Google Scholar] [CrossRef] [PubMed]
- Flourish. Available online: https://flourish.studio/ (accessed on 1 August 2025).
- Tani, E.; Kizis, D.; Markellou, E.; Papadakis, I.; Tsamadia, D.; Leventis, G.; Makrogianni, D.; Karapanos, I. Cultivar-Dependent Responses of Eggplant (Solanum melongena L.) to Simultaneous Verticillium Dahliae Infection and Drought. Front. Plant Sci. 2018, 9, 1181. [Google Scholar] [CrossRef]
- Figàs, M.; Prohens, J.; Raigón, M.; Fernández-de-Córdova, P.; Fita, A.; Soler, S. Characterization of a Collection of Local Varieties of Tomato (Solanum lycopersicum L.) Using Conventional Descriptors and the High-Throughput Phenomics Tool Tomato Analyzer. Genet. Resour. Crop Evol. 2015, 62, 189–204. [Google Scholar] [CrossRef]
- Abbas, K.; Li, J.; Gong, B.; Lu, Y.; Wu, X.; Lü, G.; Gao, H. Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones. Int. J. Mol. Sci. 2023, 24, 13876. [Google Scholar] [CrossRef]
- Delfin, E.F.; Drobnitch, S.T.; Comas, L.H. Plant Strategies for Maximizing Growth during Water Stress and Subsequent Recovery in Solanum melongena L. (Eggplant). PLoS ONE 2021, 16, e0256342. [Google Scholar] [CrossRef]
- Kiran, S.; Baysal Furtana, G. Responses of Eggplant Seedlings to Combined Effects of Drought and Salinity Stress: Effects on Photosynthetic Pigments and Enzymatic and Non-Enzymatic Antioxidants. Gesunde Pflanz. 2023, 75, 2579–2590. [Google Scholar] [CrossRef]
- Plazas, M.; Nguyen, H.T.; González-Orenga, S.; Fita, A.; Vicente, O.; Prohens, J.; Boscaiu, M. Comparative Analysis of the Responses to Water Stress in Eggplant (Solanum melongena) Cultivars. Plant Physiol. Biochem. 2019, 143, 72–82. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root Traits Contributing to Plant Productivity under Drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of Root System Architecture by DEEPER ROOTING 1 Increases Rice Yield under Drought Conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Flores-Saavedra, M.; Plazas, M.; Gramazio, P.; Vicente, O.; Vilanova, S.; Prohens, J. Growth and Antioxidant Responses to Water Stress in Eggplant MAGIC Population Parents, F1 Hybrids and a Subset of Recombinant Inbred Lines. BMC Plant Biol. 2024, 24, 560. [Google Scholar] [CrossRef]
- Parveen, A.; Rai, G.K.; Bagati, S.; Rai, P.K.; Singh, P. Morphological, Physiological, Biochemical and Molecular Responses of Plants to Drought Stress. In Abiotic Stress Tolerance Mechanisms in Plants; CRC Press: Boca Raton, FL, USA, 2021; pp. 321–339. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium Application in Two Methods Promotes Drought Tolerance in Solanum lycopersicum Plant by Inducing the Antioxidant Defense System. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- David-Rogeat, N.; Broadley, M.R.; Stavridou, E. Heat and Salinity Stress on the African Eggplant F1 Djamba, a Kumba Cultivar. Front. Plant Sci. 2024, 15, 1323665. [Google Scholar] [CrossRef]
- Watts, S.; Kariyat, R. Morphological Characterization of Trichomes Shows Enormous Variation in Shape, Density and Dimensions across the Leaves of 14 Solanum Species. AoB Plants 2021, 13, plab071. [Google Scholar] [CrossRef] [PubMed]
- Haliński, Ł.P.; Paszkiewicz, M.; Gołebiowski, M.; Stepnowski, P. The Chemical Composition of Cuticular Waxes from Leaves of the Gboma Eggplant (Solanum macrocarpon L.). J. Food Compos. Anal. 2012, 25, 74–78. [Google Scholar] [CrossRef]
- Galdon-Armero, J.; Fullana-Pericas, M.; Mulet, P.A.; Conesa, M.A.; Martin, C.; Galmes, J. The Ratio of Trichomes to Stomata Is Associated with Water Use Efficiency in Solanum lycopersicum (Tomato). Plant J. 2018, 96, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, S.H.; do Amaral Júnior, A.T.; Vergara-Diaz, O.; Gracia-Romero, A.; Fernandez-Gallego, J.A.; Chang-Espino, M.C.; Buchaillot, M.L.; Rezzouk, F.Z.; de Lima, V.J.; Serret, M.D.; et al. Heterosis and Reciprocal Effects for Physiological and Morphological Traits of Popcorn Plants under Different Water Conditions. Agric. Water Manag. 2022, 261, 107371. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, Z.; Wang, S.; Yang, J.; Wang, L.; Jia, T.; Li, J.; Wang, H.; Song, S.; Lu, Y.; et al. Molecular Mechanisms of Heterosis under Drought Stress in Maize Hybrids Zhengdan7137 and Zhengdan7153. Front. Plant Sci. 2024, 15, 1487639. [Google Scholar] [CrossRef] [PubMed]
- Mejaya, M.J.; Suhartina, S.; Purwantoro, P.; Soehendi, R.; Trustinah, T.; Indriani, F.C.; Susanto, G.W.A.; Baliadi, Y.; Sulistyo, A.; Sholihin, S.; et al. Drought Tolerant Index and Heterosis Level of Soybean {Glycine max (L.) Merrill} Genotypes. Scientifica 2025, 2025, 1213004. [Google Scholar] [CrossRef]
- Abdelmula, A.A.; Link, W.; Von Kittlitz, E.; Stelling, D. Heterosis and Inheritance of Drought Tolerance in Faba Bean, Vicia faba L. Plant Breed. 1999, 118, 485–490. [Google Scholar] [CrossRef]
- Razi, K.; Suresh, P.; Mahapatra, P.P.; Al Murad, M.; Venkat, A.; Notaguchi, M.; Bae, D.W.; Prakash, M.A.S.; Muneer, S. Exploring the Role of Grafting in Abiotic Stress Management: Contemporary Insights and Automation Trends. Plant Direct 2024, 8, e70021. [Google Scholar] [CrossRef]
- Mulyana, A.; Purwoko, B.S.; Dewi, I.S.; Maharijaya, A. Comparison of Six Anther Culture Methods for the Production of Doubled Haploids in Eggplant (Solanum melongena L.). Euphytica 2023, 219, 44. [Google Scholar] [CrossRef]
- Krommydas, K.S.; Tzikalios, Z.; Madesis, P.; Bletsos, F.A.; Mavromatis, A.; Roupakias, D. Development and Fertility Restoration of CMS Eggplant Lines Carrying the Cytoplasm of Solanum violaceum. J. Agric. Sci. 2016, 8, 10–26. [Google Scholar] [CrossRef]



| Trait | Abbreviation | Unit |
|---|---|---|
| Agronomic | ||
| Leaf length | LL | cm |
| Leaf width | LW | cm |
| Stem diameter | SD | mm |
| Leaf number | LN | |
| Plant height | PH | cm |
| Leaf area index | LAI | |
| Root dry weight | RDW | g |
| Shoot dry weight | SDW | g |
| Root-to-shoot ratio | R/S | |
| Plant dry weight | PDW | g |
| Physiological | ||
| Water use efficiency | WUE | g L−1 |
| Leaf water potential | LWP | bar |
| Relative electrolyte leakage | REL | |
| Chlorophyll content index | CCI | |
| Malondialdehyde | MDA | μmol g−1 |
| Genotype | Treatment | LL 1 | LW | SD | LN | PH | LAI | RDW | SDW | R/S | PDW | WUE | LWP | REL | CCI 2 w | CCI 3 w | CCI 4 w | MDA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | Control | 19.08 b,c | 9.75 a–d | 9.04 a,b | 11.67 b | 29.25 b,c | 1.31 b,c | 1.13 d,e | 5.59 c,d | 0.20 c,d | 6.72 c,d | 2.45 d | −9.17 e–g | 44.91 ns | 29.45 d | 35.52 f–h | 56.85 a–d | 4.08 c–e |
| WS | 16.75 d,e | 8.33 c,d | 8.10 b–e | 10.17 c,d | 24.75 d | 0.65 f,g | 0.57 f | 4.04 e,f | 0.15 d | 4.61 e | 2.44 d | −14.67 b–d | 76.90 ns | 32.10 d,e | 33.50 g,h | 55.47 a–e | 7.75 a | |
| Change (%) | −12.21 * | −14.56 * | −10.4 ns | −12.85 * | −15.38 * | −50.38 * | −49.56 * | −27.73 * | −25.0 ns | −31.4 * | −0.41 ns | −59.98 * | 71.23 * | 9.00 ns | −5.69 ns | −2.43 ns | 89.95 * | |
| ExSd | Control | 18.83 b–d | 10.58 a | 8.71 b,c | 13.50 a | 28.42 b,c | 1.98 a | 1.75 a | 7.07 a,b | 0.25 a–c | 8.81 a,b | 2.91 a–d | −12.17 b–f | 65.40 ns | 35.92 c–e | 53.10 d | 64.78 a | 3.72 c–f |
| WS | 15.92 e,f | 8.40 b–d | 8.22 b–e | 11.33 b,c | 20.83 e,f | 1.37 b,c | 1.05 d,e | 4.64 d,e | 0.22 c,d | 5.68 d,e | 3.33 a | −13.83 b–d | 68.78 ns | 36.78 b–e | 46.27 d–f | 56.67 a–d | 3.75 c–f | |
| Change (%) | −15.45 * | −20.6 * | −5.63 ns | −16.07 * | −26.71 * | −30.81 * | −40.0 * | −34.28 * | −12.0 ns | −35.53 * | 14.43 ns | −13.64 ns | 5.17 ns | 2.39 ns | −12.86 * | −12.52 ns | 0.81 ns | |
| L | Control | 19.50 b | 10.58 a | 9.84 a | 11.83 b | 36.83 a | 1.21 b–d | 1.06 d,e | 6.92 a,b | 0.15 d | 7.98 a–c | 2.64 b–d | −10.25 d–g | 56.57 ns | 29.33 e | 48.67 d,e | 47.85 d–g | 4.17 c–e |
| WS | 18.58 b–d | 8.08 d,e | 8.38 b–d | 9.67 d | 27.58 c | 0.54 g | 0.72 e,f | 4.60 d,e | 0.16 d | 5.32 d,e | 3.12 a,b | −19.17 a | 68.52 ns | 36.10 b–e | 44.12 d–h | 49.30 b–f | 4.09 c–e | |
| Change (%) | −4.72 ns | −23.63 * | −14.84 * | −18.26 * | −25.12 * | −55.37 * | −32.08 * | −33.53 * | 6.67 | −33.33 * | 18.18 ns | −87.02 * | 21.12 ns | 23.08 ns | −9.35 ns | 3.03 ns | −1.92 ns | |
| LxSd | Control | 18.33 b–d | 9.92 a–d | 7.96 c–e | 12.50 a,b | 22.08 d–f | 1.84 a | 1.74 a | 6.58 a–c | 0.27 a–c | 8.32 a,b | 2.99 a–c | −11.67 c–g | 56.51 ns | 30.43 d,e | 67.80 b | 63.98 a,b | 5.01 b |
| WS | 15.75 e,f | 8.08 d,e | 7.66 d–g | 11.33 b,c | 19.25 fg | 0.93 d–f | 1.15 c,d | 4.73 d,e | 0.24 b,c | 5.89 d,e | 3.10 a,b | −14.50 b–d | 88.40 ns | 32.07 d,e | 54.82 c,d | 63.17 a,b,c | 3.42 e,f | |
| Change (%) | −14.08 * | −18.55 ns | −3.77 ns | −9.36 * | −12.82 * | −49.46 * | −33.91 * | −28.12 * | −11.11 ns | −29.21 * | 3.68 ns | −24.25 * | 56.43 * | 5.39 ns | −19.14 * | −1.27 ns | −31.74 * | |
| Sd | Control | 16.75 d,e | 11.32 a | 6.77 g,h | 10.17 c,d | 11.92 h | 1.13 c–e | 1.18 c,d | 4.31 e,f | 0.27 a–c | 5.48 d,e | 1.61 e | −12.75 b–f | 60.08 ns | 36.87 b–e | 32.73 h | 48.13 c–g | 4.14 c–e |
| WS | 15.57 e,f | 10.29 a–c | 6.20 h | 9.00 d,e | 9.79 h | 0.86 e,f | 1.07 d,e | 3.29 f | 0.33 a | 4.36 e | 2.55 c,d | −15.00 b,c | 73.00 ns | 34.10 d,e | 34.40 g,h | 41.29 e–g | 2.45 g | |
| Change (%) | −7.04 ns | −9.1 ns | −8.42 ns | −11.5 * | −17.87 * | −23.89 * | −9.32 ns | −23.49 ns | 22.22 ns | −20.44 ns | 58.39 * | −17.65 * | 21.50 ns | −7.51 ns | 5.1 ns | −14.21 ns | −40.82 * | |
| Sm | Control | 19.75 b | 10.75 a | 6.92 f–h | 9.00 d,e | 12.83 h | 1.91 a | 1.27 b–d | 4.36 e,f | 0.28 a–c | 5.63 d,e | 1.86 e | −8.83 fg | 63.47 ns | 46.28 a,b | 64.27 b,c | 52.28 a–e | 4.40 b,c |
| WS | 16.17 e,f | 10.08 a–c | 7.64 d–g | 8.50 e | 12.08 h | 0.86 e,f | 1.10 d,e | 4.05 e,f | 0.27 a–c | 5.15 e | 3.02 a–c | −13.67 b–e | 66.08 ns | 51.33 a | 82.58 a | 56.75 a–d | 4.29 b–d | |
| Change (%) | −18.13 * | −6.23 ns | 10.4 ns | −5.56 ns | −5.85 ns | −54.97 * | −14.17 ns | −7.11 ns | −3.57 ns | −8.53 ns | 62.37 * | −52.78 * | 4.11 ns | 10.91 ns | 28.49 * | 8.55 ns | −2.5 ns | |
| T | Control | 22.08 a | 10.33 a,b | 8.69 b,c | 11.67 b | 31.33 b | 1.51 b | 1.63 a,b | 7.52 a | 0.22 c,d | 9.15 a | 3.03 a–c | −7.33 g | 52.97 ns | 40.23 b–d | 36.70 f–h | 33.95 g | 3.99 c–e |
| WS | 17.08 c–e | 8.00 d,e | 8.35 b–d | 9.83 d | 24.50 d | 0.79 fg | 1.00 d,e | 4.64 d,e | 0.21 c,d | 5.64 d,e | 3.30 a | −16.50 a,b | 59.21 ns | 40.88 b–d | 37.57 e–h | 36.88 fg | 3.15 f | |
| Change (%) | −22.64 * | −22.56 * | −3.8 ns | −15.77 * | −21.80 * | −47.68 * | −38.65 * | −38.3 * | −4.55 ns | −38.36 * | 8.91 ns | −125.1 * | 11.78 ns | 1.62 ns | 2.37 ns | 8.63 ns | −21.05 * | |
| TxSd | Control | 17.25 c–e | 8.42 b–d | 7.82 c–f | 13.17 a | 22.33 d,e | 1.88 a | 1.55 a–c | 6.13 b,c | 0.25 a–c | 7.68 b,c | 2.54 c,d | −11.50 c–g | 59.70 ns | 34.25 d,e | 44.78 d–g | 46.45 d–g | 5.01 b |
| WS | 14.25 f | 6.42 e | 7.32 e–g | 11.75 b | 17.83 g | 1.34 b,c | 1.08 d,e | 3.67 e,f | 0.32 a,b | 4.75 e | 3.20 a | −13.67 b–e | 83.60 ns | 45.62 a–c | 51.00 d | 57.77 a–d | 3.52 d–f | |
| Change (%) | −17.39 * | −23.75 * | −6.39 ns | −10.78 * | −20.15 * | −28.72 * | −30.32 * | −40.13 * | 28.0 ns | −38.15 * | 25.98 * | −18.87 * | 40.03 * | 33.2 * | 13.89 ns | 24.37 * | −29.74 * |
| MPH | BPH | ||||||
|---|---|---|---|---|---|---|---|
| Trait | Treatment | ExSd | LxSd | TxSd | ExSd | LxSd | TxSd |
| LL 1 | Control | 5.11 | 1.13 | −11.15 * | −1.31 | −6.00 | −21.88 * |
| WS | −1.49 | −7.76 * | −12.71 * | −4.96 * | −15.23 * | −16.57 * | |
| LW | Control | 0.43 | −9.41 | −22.22 * | −6.54 | −12.37 | −25.62 * |
| WS | −9.77 * | −12.03 | −29.8 * | −18.37 * | −21.48 * | −37.61 * | |
| SD | Control | 10.18 * | −4.15 | 1.23 | −3.65 | −19.11 * | −9.91 * |
| WS | 14.97 * | 5.08 | 0.62 | 1.48 | −8.59 * | −12.34 * | |
| LN | Control | 23.63 * | 13.64 * | 20.6 * | 15.68 * | 5.66 | 12.85 * |
| WS | 18.21 * | 21.37 * | 24.8 * | 11.41 | 17.17 * | 19.53 * | |
| PH | Control | 38.06 * | −9.42 | 3.26 | −2.84 | −40.05 * | −28.73 * |
| WS | 20.61 * | 3.02 | 4.0 | −15.84 * | −30.20 * | −27.22 * | |
| LAI | Control | 62.3 * | 57.26 * | 42.42 * | 51.15 * | 52.07 * | 24.50 * |
| WS | 81.46 * | 32.86 * | 62.42 * | 59.30 * | 8.14 * | 55.81 * | |
| RDW | Control | 51.52 * | 55.36 * | 10.32 | 48.31 * | 47.46 * | −4.91 |
| WS | 28.05 * | 28.49 | 4.35 | −1.87 | 7.48 | 0.93 | |
| SDW | Control | 42.77 * | 17.29 * | 3.72 | 26.30 * | −4.91 | −18.48 * |
| WS | 26.6 * | 19.9 | −7.44 | 14.85 | 2.83 | −20.91 | |
| R/S | Control | 6.38 | 28.57 * | 2.04 | −7.41 | 0.00 | −7.41 |
| WS | −8.33 | −2.04 | 18.52 * | −33.33 * | −27.27 * | −3.03 | |
| PDW | Control | 44.43 * | 23.63 * | 4.99 | 31.10 * | 4.26 | −16.07 * |
| WS | 26.64 * | 21.69 | −5.0 | 23.21 * | 10.71 | −15.78 | |
| WUE | Control | 43.35 * | 40.71 * | 9.48 | 18.78 * | 13.26 * | −16.17 * |
| WS | 33.47 * | 9.35 | 9.4 | 30.59 * | −0.64 | −3.03 | |
| LWP | Control | 11.04 | 1.48 | 14.54 | −4.55 | −8.47 * | −9.80 |
| WS | −6.77 | −15.13 * | −13.21 | −7.80 | −24.36 * | −17.15 * | |
| REL (%) | Control | 24.58 * | −3.11 | 5.62 | 8.85 | −5.94 | −0.63 |
| WS | −8.23 | 24.93 * | 26.47 * | −10.56 | 21.10 | 14.52 | |
| CCI 2 w | Control | 8.32 | −8.07 | −11.15 | −2.58 | −17.47 | −14.86 |
| WS | 11.12 | −8.63 | 21.69 * | 7.86 | −11.16 | 11.59 | |
| CCI 3w | Control | 55.6 * | 66.58 * | 28.99 * | 49.49 * | 39.31 * | 22.02 * |
| WS | 36.29 * | 39.63 * | 41.73 * | 34.51 * | 24.25 * | 35.75 * | |
| CCI 4w | Control | 23.41 * | 33.32 * | 13.18 | 13.95 | 32.93 * | −3.49 |
| WS | 17.14 | 39.46 | 47.81 * | 2.16 | 28.13 * | 39.91 * | |
| MDA | Control | −9.49 | 20.58 * | 23.25 * | −10.14 | 20.14 * | 21.01 * |
| WS | −26.47 * | 4.59 * | 25.71 * | −51.61 * | −16.38 * | 11.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krommydas, K.; Papa, E.; Gaitani, P.; Papadopoulou, A.; Mellidou, I.; Bouloumpasi, E.; Kadoglidou, K.I. Comparative Drought Response of Solanum melongena, S. macrocarpon, S. dasyphyllum, and S. melongena × S. dasyphyllum Interspecific Hybrids. Agronomy 2025, 15, 2516. https://doi.org/10.3390/agronomy15112516
Krommydas K, Papa E, Gaitani P, Papadopoulou A, Mellidou I, Bouloumpasi E, Kadoglidou KI. Comparative Drought Response of Solanum melongena, S. macrocarpon, S. dasyphyllum, and S. melongena × S. dasyphyllum Interspecific Hybrids. Agronomy. 2025; 15(11):2516. https://doi.org/10.3390/agronomy15112516
Chicago/Turabian StyleKrommydas, Konstantinos, Eleni Papa, Panagiota Gaitani, Anastasia Papadopoulou, Ifigeneia Mellidou, Elisavet Bouloumpasi, and Kalliopi I. Kadoglidou. 2025. "Comparative Drought Response of Solanum melongena, S. macrocarpon, S. dasyphyllum, and S. melongena × S. dasyphyllum Interspecific Hybrids" Agronomy 15, no. 11: 2516. https://doi.org/10.3390/agronomy15112516
APA StyleKrommydas, K., Papa, E., Gaitani, P., Papadopoulou, A., Mellidou, I., Bouloumpasi, E., & Kadoglidou, K. I. (2025). Comparative Drought Response of Solanum melongena, S. macrocarpon, S. dasyphyllum, and S. melongena × S. dasyphyllum Interspecific Hybrids. Agronomy, 15(11), 2516. https://doi.org/10.3390/agronomy15112516






