Inter-Day Instability in Plant Sap Composition Undermines Single-Day Diagnostics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Measurements
2.4. Statistical Analyses
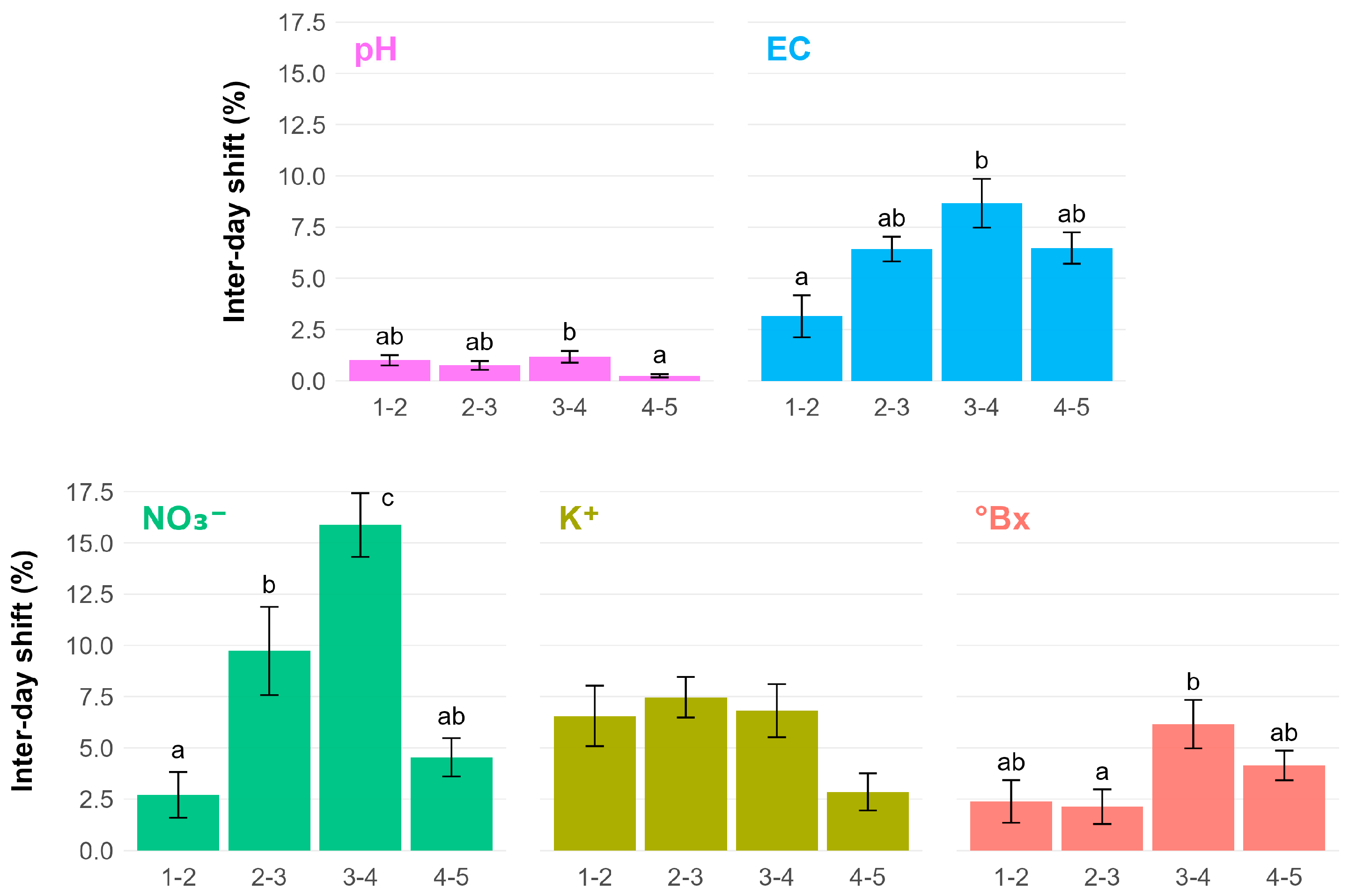
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| °Bx | Degree Brix (soluble solids) |
| BBCH | Phenological growth stage scale |
| EC | Electrical conductivity |
| ETc | Crop evapotranspiration |
| ISE | Ion-selective electrode |
| K+ | Potassium |
| LAI | Leaf area index |
| MRML | Most recently mature leaves |
| NO3− | Nitrate |
| VPD | Vapor pressure deficit |
References
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A Review of Methods for Sensing the Nitrogen Status in Plants: Advantages, Disadvantages and Recent Advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef]
- Tong, R.C.; Whitehead, C.S.; Fawole, O.A. Effects of Conventional and Bokashi Hydroponics on Vegetative Growth, Yield and Quality Attributes of Bell Peppers. Plants 2021, 10, 1281. [Google Scholar] [CrossRef]
- Rojas-Velázquez, Á.N.; Guillén-Castillo, O.I.; Alcalá-Jauregui, J.A.; Loredo-Osti, C.; Ramírez-Tobías, H.M.; Romero-Méndez, M.J.; Méndez-Cortés, H.; Hernández-Montoya, A. Effect of a Nitrogenous Nanocomposite on Leaching and N Content in Lettuce in Soil Columns. Discov. Nano 2023, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Mahal, N.K.; Miguez, F.E.; Sawyer, J.E.; Dong, L.; Schnable, P.S.; Castellano, M.J. Stalk Sap Nitrate Test as a Potential Tool for Nitrogen Fertilizer Recommendations for Maize. Field Crops Res. 2024, 310, 109330. [Google Scholar] [CrossRef]
- Kremper, R.; Juhász, E.K.; Novák, T.; Kincses, I.; Sándor, Z.; Tállai, M.; Béni, Á.; Szabó, A.; Szarvas, S.; Balla Kovács, A. Assessment of Spring Oat Nitrogen Supply Based on Plant Sap Nitrate Concentration and SPAD Values. Nitrogen 2025, 6, 19. [Google Scholar] [CrossRef]
- Olsen, J.K.; Lyons, D.J. Petiole Sap Nitrate Is Better than Total Nitrogen in Dried Leaf for Indicating Nitrogen Status and Yield Responsiveness of Capsicum in Subtropical Australia. Aust. J. Exp. Agric. 1994, 34, 835–843. [Google Scholar] [CrossRef]
- Pino, P.; Callejas, R.; Razeto, B.; Reginato, G. Petiole Extract Chemical Analysis to Evaluate Nutritional Status in Grapevine. Pesq. Agropec. Bras. 2012, 47, 111–117. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, X. Assessing Nitrogen Nutritional Status, Biomass and Yield of Cotton with NDVI, SPAD and Petiole Sap Nitrate Concentration. Exp. Agric. 2018, 54, 531–548. [Google Scholar] [CrossRef]
- Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B.; Farneselli, M.; Padilla, F.M. Assessing Crop N Status of Fertigated Vegetable Crops Using Plant and Soil Monitoring Techniques. Ann. Appl. Biol. 2015, 167, 387–405. [Google Scholar] [CrossRef]
- Roacho-Cortés, E.; Castellanos-Ramos, J.Z.; Etchevers, J.D. Field Diagnostic Techniques to Determine Nitrogen in Maize. Terra Latinoam. 2021, 39, e820. [Google Scholar] [CrossRef]
- Viana, M.C.M.; da Silva, I.P.; Freire, F.M.; Ferreira, M.M.; da Costa, É.L.; Mascarenhas, M.H.T.; Teixeira, M.F.F. Production and Nutrition of Irrigated Tanzania Guinea Grass in Response to Nitrogen Fertilization. Rev. Bras. Zootec. 2014, 43, 238–243. [Google Scholar] [CrossRef]
- Padilla, F.M.; Farneselli, M.; Gianquinto, G.; Tei, F.; Thompson, R.B. Monitoring Nitrogen Status of Vegetable Crops and Soils for Optimal Nitrogen Management. Agric. Water Manag. 2020, 241, 106356. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Y.; Li, M.; Ci, B.; Lu, X.; Feng, X.; Wen, S.; Ma, F. Effect of Different NPK Fertilization Timing Sequences Management on Soil-Petiole System Nutrient Uptake and Fertilizer Utilization Efficiency of Drip Irrigation Cotton. Sci. Rep. 2023, 13, 14287. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, K.H. Electrochemical Sensors for Sustainable Precision Agriculture—A Review. Front. Chem. 2022, 10, 848320. [Google Scholar] [CrossRef]
- da Silva, A.O.; Silva, D.J.; Bassoi, L.H.; de Melo Chaves, A.R. NO3−, K+, and Chlorophyll Index in Fertigated Grapevines in the Semi-Arid Region of Brazil. Sci. Agric. 2023, 80, e20210122. [Google Scholar] [CrossRef]
- Gallardo, M.; Peña-Fleitas, M.T.; Padilla, F.M.; Cedeño, J.; Thompson, R.B. Prescriptive-Corrective Irrigation and Macronutrient Management in Greenhouse Soil-Grown Tomato Using the VegSyst-DSS v2 Decision Support Tool. Horticulturae 2023, 9, 1128. [Google Scholar] [CrossRef]
- Parks, S.E.; Irving, D.E.; Milham, P.J. A Critical Evaluation of On-Farm Rapid Tests for Measuring Nitrate in Leafy Vegetables. Sci. Hortic. 2012, 134, 1–6. [Google Scholar] [CrossRef]
- Incrocci, L.; Massa, D.; Pardossi, A. New Trends in the Fertigation Management of Irrigated Vegetable Crops. Horticulturae 2017, 3, 37. [Google Scholar] [CrossRef]
- Riechelman, B.; Postma, R.; Specken, J.; de Haan, J. Critical Nutrient Concentrations of Arable Crops. In Literature Study on the Usability of Critical Concentrations to Diagnose Nutrient Deficiency and/or Steer Fertiliser Application (Rapport 1792.N.20); Nutriënten Management Instituut BV: Wageningen, The Netherlands, 2021; p. 42. [Google Scholar]
- Esteves, E.; Locatelli, G.; Bou, N.A.; Ferrarezi, R.S. Sap Analysis: A Powerful Tool for Monitoring Plant Nutrition. Horticulturae 2021, 7, 426. [Google Scholar] [CrossRef]
- Silva-Pumarada, G.; Di Gioia, F. Assessing the Reliability of Portable Ion-Selective Electrodes Proposed for the On-Farm Management of Nutrient Solutions and Fertigation of Horticultural Crops. Acta Hortic. 2023, 1377, 801–807. [Google Scholar] [CrossRef]
- Sonneveld, C.; De Bes, S.S. Relationship between Analytical Data of Plant Sap and Dried Material of Glasshouse Crops. Commun. Soil Sci. Plant Anal. 1983, 14, 75–87. [Google Scholar] [CrossRef]
- Burns, I.G.; Hutsby, W. Choice of Leaf for Estimation of K Status by Analysis of Petiole Sap. J. Sci. Food Agric. 1986, 37, 115–128. [Google Scholar] [CrossRef]
- Alt, D.; Füll, A.M. Control of the Nitrogen Status of Lettuce by Nitrate Analysis of Plant Sap. Acta Hortic. 1988, 222, 23–27. [Google Scholar] [CrossRef]
- Wira, A.B.; Jamil, A.Z.; Armizatul, S.A.H. Effect of Varying Nitrogen Levels on Plant Sap Characteristics and Growth Performance of Tomato (Lycopercisum esculentum var. baccarat). J. Trop. Agric. Food Sci. 2013, 41, 183–191. [Google Scholar]
- Yosoff, S.F.; Mohamed, M.T.M.; Parvez, A.; Ahmad, S.H.; Ghazali, F.M.; Hassan, H. Production System and Harvesting Stage Influence on Nitrate Content and Quality of Butterhead Lettuce. Bragantia 2015, 74, 322–330. [Google Scholar] [CrossRef]
- Cadahía López, C. El Análisis de Savia Como Índice de Fertilización Para las Plantas de Tomate. Ph.D. Thesis, Universidad Central, Madrid, Spain, 1964; p. 228. [Google Scholar]
- Azuara, P.; García López de Sa, M.E.; Hernando, V. The Effect of Light Intensity on the Concentration of Bioelements in the Sap of the Tomato Plant (Lycopersicon esculentum L.). J. Plant Nutr. 1982, 5, 111–121. [Google Scholar] [CrossRef]
- Palenski, F.J.; Kemp, P.D. Validity of the Nitrate Test Strip Technique for Use on Cereals. Agric. Soc. Proc. N. Z. 1989, 19, 35–42. [Google Scholar]
- Vitosh, M.L.; Silva, G.H. Factors Affecting Potato Petiole Sap Nitrate Tests. Commun. Soil Sci. Plant Anal. 1996, 27, 1137–1152. [Google Scholar] [CrossRef]
- He, Y.; Terabayashi, S.; Namiki, T. The Effects of Leaf Position and Time of Sampling on Nutrient Concentration in the Petiole Sap from Tomato Plants Cultured Hydroponically. J. Jpn. Soc. Hortic. Sci. 1998, 67, 331–336. [Google Scholar] [CrossRef]
- Raynal, C.; Cousin, I. Petiolar Sap Nitrate as a Guide in the Fertilization of Strawberry. Acta Hortic. 1997, 439, 753–762. [Google Scholar] [CrossRef]
- Nagarajah, S. A Petiole Sap Test for Nitrate and Potassium in Sultana Grapevines. Aust. J. Grape Wine Res. 1999, 5, 56–60. [Google Scholar] [CrossRef]
- Studstill, D.W.; Simonne, E.H.; Hutchinson, C.M.; Hochmuth, R.C.; Dukes, M.D.; Davis, W.E. Petiole Sap Testing Sampling Procedures for Monitoring Pumpkin Nutritional Status. Commun. Soil Sci. Plant Anal. 2003, 34, 2355–2362. [Google Scholar] [CrossRef]
- Llanderal, A.; García-Caparrós, P.; Segura, M.L.; Contreras, J.I.; Lao, M.T. Nutritional Changes in Petiole Sap over Space and Time in a Tomato Crop Greenhouse. J. Plant Nutr. 2019, 42, 1205–1217. [Google Scholar] [CrossRef]
- Scaife, A.; Stevens, K.L. Monitoring Sap Nitrate in Vegetable Crops: Comparison of Test Strips with Electrode Methods, and Effects of Time of Day and Leaf Position. Commun. Soil Sci. Plant Anal. 1983, 14, 761–771. [Google Scholar] [CrossRef]
- Rosen, C.J.; Errebhi, M.; Wang, W. Testing Petiole Sap for Nitrate and Potassium: A Comparison of Several Analytical Procedures. HortScience 1996, 31, 1173–1176. [Google Scholar] [CrossRef]
- Arunachalam, V.; Fernandes, C.M.; Salgaonkar, D.C. Quick Method to Quantify the Potassium and Sodium Content Variation in Leaves of Banana Varieties. Anal. Sci. 2020, 36, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Martins, M.J.; Sprey, L.; Maurício, A.; Rosa, C.; Faria, J.; Martins, M.B.; de Sousa, M.L.; Santos, R.; de Sousa, R.M.; et al. Analysis of Petiole Sap Nutrients Using Rapid and Standard Methods and Its Relation to Leaf Analysis of Fertilized Malus domestica cv. Gala. Horticulturae 2024, 10, 36. [Google Scholar] [CrossRef]
- Peña-Fleitas, M.T.; Gallardo, M.; Padilla, F.M.; Rodríguez, A.; Thompson, R.B. Use of a Portable Rapid Analysis System to Measure Nitrate Concentration of Nutrient and Soil Solution, and Plant Sap in Greenhouse Vegetable Production. Agronomy 2021, 11, 819. [Google Scholar] [CrossRef]
- Ulissi, V.; Antonucci, F.; Benincasa, P.; Farneselli, M.; Tosti, G.; Guiducci, M.; Tei, F.; Costa, C.; Pallottino, F.; Pari, L.; et al. Nitrogen Concentration Estimation in Tomato Leaves by VIS-NIR Non-Destructive Spectroscopy. Sensors 2011, 11, 6411–6424. [Google Scholar] [CrossRef]
- Simonne, E.; Hochmuth, R. An Overview of Fertilization and Irrigation Management in the Conventional and Certified Organic Production of Vegetable Crops in Florida. Horticulturae 2016, 2, 7. [Google Scholar] [CrossRef]
- Silva Filho, J.B.; Fontes, P.C.R.; Ferreira, J.F.S.; Cecon, P.R.; Crutchfield, E. Optimal Nutrient Solution and Dose for the Yield of Nuclear Seed Potatoes under Aeroponics. Agronomy 2022, 12, 2820. [Google Scholar] [CrossRef]
- Hochmuth, G.; Hochmuth, R. Plant Petiole Sap-Testing for Vegetable Crops (CIR1144); UF/IFAS Extension: Gainesville, FL, USA, 2022; p. 6. [Google Scholar]
- García Monroy, A.; Barrios Díaz, J.M.; Barrios Díaz, B.; Medina, E.J.; Vázquez Cruz, F. Monitoreo del Estado Nutrimental del Cultivo de Jitomate en Invernadero (COMEII-23036). In Proceedings of the VIII Congreso Nacional y I Congreso Internacional de Riego, Drenaje y Biosistemas, Coahuila, Mexico, 4–6 October 2023; p. 9. [Google Scholar]
- Vialet-Chabrand, S.; Matthews, J.S.A.; Simkin, A.J.; Raines, C.A.; Lawson, T. Importance of Fluctuations in Light on Plant Photosynthetic Acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant Responses to Rising Vapor Pressure Deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Ruan, H. Response of Plants to Water Stress: A Meta-Analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef]
- Jiao, X.; Yu, X.; Ding, J.; Du, Q.; Zhang, J.; Song, X.; Bai, P.; Li, J. Effects of Rising VPD on the Nutrient Uptake, Water Status and Photosynthetic System of Tomato Plants at Different Nitrogen Applications under Low Temperature. Sci. Hortic. 2022, 304, 111335. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zeng, Z.L.; Shi, Z.M.; Wang, J.H.; Huang, W. Variation in Photosynthetic Efficiency under Fluctuating Light between Rose Cultivars and Its Potential for Improving Dynamic Photosynthesis. Plants 2023, 12, 1186. [Google Scholar] [CrossRef]
- Pantoja-Benavides, A.D.; Garces-Varon, G.; Restrepo-Díaz, H. Foliar Cytokinins or Brassinosteroids Applications Influence the Rice Plant Acclimatization to Combined Heat Stress. Front. Plant Sci. 2022, 13, 983276. [Google Scholar] [CrossRef]
- Ugarte Barco, F.A.; Zhiñin-Huachun, I.A.; Hernández Pérez, R. Biostimulant Influence on Morphological and Agrochemical Characters in Banana (Musa AAA cv. Williams). Terra Latinoam. 2022, 40, e1456. [Google Scholar] [CrossRef]
- Díaz-Vázquez, F.A.; Sandoval-Rangel, A. Influence of Mulching and Poultry Manure on Wild Tomato (Solanum lycopersicum var. cerasiforme (Dunal)) Production. Ecosist. Recur. Agropecu. 2023, 10, e3605. [Google Scholar] [CrossRef]
- Casado Alcalá, M. Estudio de la Fertilización y Nutrición del Fresón Mediante el Análisis de Savia. Ph.D. Thesis, Universidad Complutense, Madrid, Spain, 1974; p. 218. [Google Scholar]
- Bonoan, R.R. An Assessment of the Nitrogen Fertilizer Requirements of Winter Cabbages (Brassica oleracea var. capitata L.). Ph.D. Thesis, Massey University, Auckland, New Zealand, 1990; p. 257. [Google Scholar]
- Abarza, E.; Raynal, C. Tests et Analyses de Laboratoire: Le Nutrichek® à l’étude. Infos-Ctifl 2003, 196, 38–43. [Google Scholar]
- Delgado, J.A.; Follett, R.F. Sap Test to Determine Nitrate-Nitrogen Concentrations in Aboveground Biomass of Winter Cover Crops. Commun. Soil Sci. Plant Anal. 1998, 29, 545–559. [Google Scholar] [CrossRef]
- Lee, M.J.; Rhee, H.C.; Choi, G.L.; Oh, S.S.; Lee, J.T.; Lee, J.G. Rapid Analysis of Nitrate Concentration in Different Growth Stages and Plant Parts of Paprika Leaf Using On-Site Rapid Detection Kit. Prot. Hortic. Plant Fact. 2017, 26, 333–339. [Google Scholar] [CrossRef]
- Prasad, M.; Spiers, T.M. A Rapid Nitrate Sap Test for Outdoor Tomatoes. Sci. Hortic. 1985, 25, 211–215. [Google Scholar] [CrossRef]
- Coltman, R.R. Yields of Greenhouse Tomatoes to Maintain Specific Petiole Sap Nitrate Levels. HortScience 1988, 23, 148–151. [Google Scholar] [CrossRef]
- Hochmuth, G.J.; Hochmuth, R.C.; Donley, M.E.; Hanlon, E.A. Eggplant Yield in Response to Potassium Fertilization on Sandy Soil. HortScience 1993, 28, 1002–1005. [Google Scholar] [CrossRef]
- White, J.M.; Tyson, R.V.; Hanlon, E.A.; Hochmuth, G.J.; Neal, C.A. Plant Petiole Sap Testing for Nitrogen and Potassium in Sweet Corn Grown on Mineral Soil. Proc. Fla. State Hortic. Soc. 1996, 109, 149–151. [Google Scholar]
- Rodríguez, A.; Peña-Fleitas, M.T.; Padilla, F.M.; Gallardo, M.; Thompson, R.B. Petiole Sap Nitrate Concentration to Assess Crop Nitrogen Status of Greenhouse Sweet Pepper. Sci. Hortic. 2021, 285, 110157. [Google Scholar] [CrossRef]
- Matthäus, D.; Gysi, C. Plant-Sap Analysis in Vegetables—A Tool to Decide on Nitrogen Top Dressing. Acta Hortic. 2001, 563, 93–102. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. In BBCH Monograph; Julius Kühn-Institut: Quedlinburg, Germany, 2018; p. 204. [Google Scholar]
- da Silva, J.R.; de Alvarenga, F.V.; Boaretto, R.M.; Lopes, J.R.S.; Quaggio, J.A.; Coletta Filho, H.D.; Mattos, D. Following the Effects of Micronutrient Supply in HLB-Infected Trees: Plant Responses and ‘Candidatus Liberibacter asiaticus’ Acquisition by the Asian Citrus Psyllid. Trop. Plant Pathol. 2020, 45, 597–610. [Google Scholar] [CrossRef]
- Meesters, C.; Cialdella, L.; Ingels, R.; Jacquemyn, H.; Lievens, B. Cultivar-Dependent Effects of Plant-Beneficial Fungi on Plant Nutrient Composition and Feeding Damage by Nesidiocoris tenuis. Plant Soil 2023, 492, 177–190. [Google Scholar] [CrossRef]
- Hochmuth, G.J. Efficiency Ranges for Nitrate-Nitrogen and Potassium for Vegetable Petiole Sap Quick Tests. HortTechnology 1994, 4, 218–222. [Google Scholar] [CrossRef]
- Rosen, C.J.; Eliason, R. Nutrient Management for Commercial Fruit & Vegetable Crops in Minnesota; University of Minnesota Extension Service: St. Paul, MN, USA, 2005; p. 38. [Google Scholar]
- Schulbach, K.; Smith, R.; Hartz, T.; Jackson, L. Guide to Nitrogen Quick-Test for Vegetables with the “Cardy” Nitrate Meter; California Department of Food and Agriculture: Sacramento, CA, USA, 2007; p. 11. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers. An Introduction to Nutrient Management, 8th ed.; Pearson India: Tamil Nadu, India, 2017; p. 520. [Google Scholar]
- Santa Cruz, J.; Calbucheo, D.; Valdebenito, S.; Cáceres, C.; Castillo, P.; Aguilar, M.; Hernández, I.; Allendes, H.; Vidal, K.; Peñaloza, P. Crushed, Squeezed, or Pressed? How Extraction Methods Influence Sap Analysis. Agronomy, 2025; submitted. [Google Scholar] [CrossRef]
- Hartz, T.K.; Smith, R.F.; Schulbach, K.F.; LeStrange, M. On-Farm Nitrogen Tests Improve Fertilizer Efficiency, Protect Groundwater. Calif. Agric. 1994, 48, 29–32. [Google Scholar] [CrossRef]
- Panique, E.; Kelling, K.A.; Schulte, E.E. Establishment of Potassium Tissue and Sap Critical Values; University of Wisconsin-Madison: Madison, WI, USA, 1996; p. 16. [Google Scholar]
- Altland, J.E.; Gilliam, C.H.; Keever, G.J.; Edwards, J.H.; Sibley, J.L.; Fare, D.C. Rapid Determination of Nitrogen Status in Pansy. HortScience 2003, 38, 537–541. [Google Scholar] [CrossRef]
- Selk, G.E.; LeValley, R.C.; Highfill, G.A.; Buchanan, D.S. Comparing the Accuracy of the Cardy Portable Nitrate Meter with Laboratory Analysis of Nitrate Concentrations in Summer Annual Forages; Oklahoma Agricultural Experiment Station: Stillwater, OK, USA, 2004; p. 4. [Google Scholar]
- Giannothanasis, E.; Cedeño, J.; Ntatsi, G.; Thompson, R.B.; Savvas, D. Developing and Validating a Modelling Approach Linked with Ion Selective Electrodes to Control Pollution of Water Resources from Greenhouse Crops. J. Environ. Manag. 2025, 387, 125792. [Google Scholar] [CrossRef]
- Thompson, R.B.; Fernández Fernández, M.M.; Cánovas Fernández, G.; Gallardo, M. Aplicaciones Prácticas de Sistemas de Análisis Rápido de Nutrientes Para Mejorar el Manejo del Nitrógeno en Cultivos de Invernadero. In Mejora en la Eficiencia del Uso de Agua y Fertilizantes en Agricultura; Gázquez, J.C., Ed.; Cajamar Caja Rural: Almería, Spain, 2018; pp. 181–202. [Google Scholar]
- Blidi, S.; Granholm, K.; Sokalski, T.; Mousavi, Z.; Lewenstam, A.; Leito, I.; Bobacka, J. Long-Time Evaluation of Solid-State Composite Reference Electrodes. Membranes 2022, 12, 569. [Google Scholar] [CrossRef]
- Peña-Fleitas, M.T.; Grasso, R.; Gallardo, M.; Padilla, F.M.; de Souza, R.; Rodríguez, A.; Thompson, R.B. Sample Temperature Affects Measurement of Nitrate with a Rapid Analysis Ion Selective Electrode System Used for N Management of Vegetable Crops. Agronomy 2022, 12, 3031. [Google Scholar] [CrossRef]
- Kurtz, C.; Pauletti, V.; Gervini de Menezes Júnior, F.O.; Mora, C. Nitrogen for Diagnosis by Chlorophyll Index and Nitrate in Sap for Onion in Direct Seeding System. Rev. Thema 2022, 21, 92–114. [Google Scholar] [CrossRef]
- Li, Q.; Denison, J.; Gluck, M.; Liu, G. Comparison of SPAD-Based Leaf Greenness and Paralleled Petiole Sap Nitrate Concentrations for Monitoring Potato Vine Nitrogen Status. Veg. Res. 2023, 3, 30. [Google Scholar] [CrossRef]
- Maltais-Landry, G.; Buchanan, C.; Longanecker, J. Using Processed Fertilizers or Composted Poultry Manure Results in Similar Yields but Contrasting Nutrient Budgets in Organic Cabbage Production. J. Plant Nutr. 2023, 46, 2462–2472. [Google Scholar] [CrossRef]
- Nascente, A.S.; Cobucci, T.; Brasil, V.; dos A. Reis, R. Cotton, Bean, and Soybean Yield and Nutrient Redistribution in Leaf Sap in Response to Organic Molecules Complexed Fertilizers. Aust. J. Crop Sci. 2023, 17, 74–82. [Google Scholar] [CrossRef]
- Loehwing, W.F. Effects of Insolation and Soil Characteristics on Tissue Fluid Reaction in Wheat. Plant Physiol. 1930, 5, 293–305. [Google Scholar] [CrossRef]
- Ingalls, R.A.; Shive, J.W. Relation of H-Ion Concentration of Tissue Fluids to the Distribution of Iron in Plants. Plant Physiol. 1931, 6, 103–125. [Google Scholar] [CrossRef]
- Justes, E.; Meynard, J.M.; Mary, B.; Plénet, D. Diagnosis Using Stem Base Extract: JUBIL Method. In Diagnosis of the Nitrogen Status in Crops; Lemaire, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 163–187. [Google Scholar]
- Papastylianou, I. Diurnal Variation of Nitrate Concentration in Cereals Grown under Rainfed Mediterranean Conditions. Commun. Soil Sci. Plant Anal. 1995, 26, 1121–1131. [Google Scholar] [CrossRef]
- Capstaff, N.M.; Domoney, C.; Miller, A.J. Real-Time Monitoring of Rhizosphere Nitrate Fluctuations under Crops Following Defoliation. Plant Methods 2021, 17, 11. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Ali, M.A.; Dong, L.; Wang, X.; Archontoulis, S.V.; Schnable, J.C.; Castellano, M.J. Continuous in Situ Soil Nitrate Sensors: The Importance of High-Resolution Measurements across Time and a Comparison with Salt Extraction-Based Methods. Soil Sci. Soc. Am. J. 2021, 85, 677–690. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Broyer, T.C. General Nature of the Process of Salt Accumulation by Roots with Description of Experimental Methods. Plant Physiol. 1936, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Smeal, D.; Arnold, R.N.; Gregory, E.J. Potato Nitrogen Management by Monitoring Petiole Nitrate Level. J. Plant Nutr. 1996, 19, 1405–1412. [Google Scholar] [CrossRef]
- Coltman, R.R. Sampling Considerations for Nitrate Quick Tests of Greenhouse-Grown Tomatoes. J. Amer. Soc. Hortic. Sci. 1987, 112, 922–927. [Google Scholar] [CrossRef]
- Gustafson, F.G. Diurnal Changes in the Acidity of Bryophyllum calycinum. J. Gen. Physiol. 1925, 7, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L. Plant Sap Analysis as a Monitoring Technique for Tomatoes in Rockwool. Acta Hortic. 1988, 221, 403–411. [Google Scholar] [CrossRef]
- MacKerron, D.K.L.; Young, M.W.; Davies, H.V. A Critical Assessment of the Value of Petiole Sap Analysis in Optimizing the Nitrogen Nutrition of the Potato Crop. Plant Soil 1995, 172, 247–260. [Google Scholar] [CrossRef]
- Janeiro Cid, R. Análisis Químico del Extracto Celular de Peciolo en Fresa Mediante Laboratorios Portátiles. Master’s Thesis, Colegio de Postgraduados, Texcoco, Mexico, 2014; p. 50. [Google Scholar]
- Llanderal, A.; García-Caparrós, P.; Pérez-Alonso, J.; Contreras, J.I.; Segura, M.L.; Reca, J.; Lao, M.T. Approach to Petiole Sap Nutritional Diagnosis Method by Empirical Model Based on Climatic and Growth Parameters. Agronomy 2020, 10, 188. [Google Scholar] [CrossRef]
- Santa Cruz, J.; Calbucheo, D.; Valdebenito, S.; Vásquez, V.; Aguilar, M.; Peñaloza, P.; Allendes, H.; Vidal, K. Forgotten Science: How 1900s Studies Challenge the Current Research on Sap Analysis. Int. J. Agric. Biol. Eng. 2025, 18, 317–318. [Google Scholar] [CrossRef]
- López Núñez, R.; García, I.; Cabrera, F.; Murillo, M.; Roca, M.; Martin, F. Nitrato en Pecíolo de Fresa. Horticultura 1999, 136, 17–22. [Google Scholar]
- Brust, G.J. Using Nitrate-N Petiole Sap-Testing for Better Nitrogen Management in Vegetable Crops; University of Maryland Extension: College Park, MD, USA, 2008; p. 9. [Google Scholar]
- Hochmuth, G.J.; Maynard, D.; Vavrina, C.; Hanlon, E.; Simonne, E. Plant Tissue Analysis and Interpretation for Vegetable Crops in Florida (HS964); UF/IFAS Extension: Gainesville, FL, USA, 2018; p. 48. [Google Scholar]
| Parameter | Extraction Method | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|---|
| pH | Citrus squeezer | 6.33 ± 0.02 | 6.27 ± 0.03 | 6.35 ± 0.03 | 6.28 ± 0.04 | 6.26 ± 0.03 |
| Potato press | 6.31 ± 0.03 | 6.26 ± 0.03 | 6.28 ± 0.02 | 6.20 ± 0.02 | 6.19 ± 0.02 | |
| Garlic press | 6.25 ± 0.01 | 6.22 ± 0.03 | 6.19 ± 0.02 | 6.21 ± 0.03 | 6.24 ± 0.03 | |
| Juicer | 6.49 ± 0.01 | 6.45 ± 0.02 | 6.47 ± 0.03 | 6.34 ± 0.02 | 6.35 ± 0.01 | |
| Garlic grinder | 6.31 ± 0.01 | 6.18 ± 0.02 | 6.26 ± 0.02 | 6.19 ± 0.03 | 6.20 ± 0.02 | |
| EC (dS/m) | Citrus squeezer | 9.20 ± 0.10 | 9.07 ± 0.20 | 9.47 ± 0.13 | 10.29 ± 0.16 | 9.67 ± 0.12 |
| Potato press | 9.48 ± 0.13 | 8.95 ± 0.18 | 9.53 ± 0.13 | 10.70 ± 0.18 | 9.72 ± 0.10 | |
| Garlic press | 8.96 ± 0.14 | 8.97 ± 0.09 | 9.71 ± 0.14 | 10.19 ± 0.18 | 9.63 ± 0.11 | |
| Juicer | 9.69 ± 0.17 | 9.22 ± 0.17 | 9.79 ± 0.17 | 10.57 ± 0.24 | 10.08 ± 0.15 | |
| Garlic grinder | 9.52 ± 0.19 | 9.16 ± 0.09 | 9.79 ± 0.13 | 10.72 ± 0.12 | 9.96 ± 0.11 | |
| NO3− (mmol/L) | Citrus squeezer | 52.07 ± 1.71 | 50.23 ± 4.35 | 52.30 ± 1.96 | 61.29 ± 5.29 | 58.53 ± 4.16 |
| Potato press | 56.91 ± 2.19 | 56.91 ± 5.63 | 51.61 ± 1.99 | 61.98 ± 4.22 | 57.37 ± 2.75 | |
| Garlic press | 56.68 ± 3.56 | 60.37 ± 5.66 | 55.07 ± 3.77 | 61.52 ± 4.75 | 64.98 ± 2.63 | |
| Juicer | 65.44 ± 2.79 | 66.82 ± 6.54 | 60.83 ± 3.06 | 68.66 ± 5.98 | 67.05 ± 5.76 | |
| Garlic grinder | 67.74 ± 3.30 | 68.66 ± 5.56 | 56.68 ± 3.18 | 66.59 ± 7.36 | 68.43 ± 2.08 | |
| K+ (mmol/L) | Citrus squeezer | 63.21 ± 2.28 | 68.69 ± 1.97 | 62.11 ± 1.74 | 64.30 ± 1.03 | 68.32 ± 2.07 |
| Potato press | 65.40 ± 2.29 | 67.96 ± 3.10 | 62.48 ± 2.00 | 69.05 ± 1.12 | 68.32 ± 2.07 | |
| Garlic press | 61.38 ± 1.37 | 68.32 ± 4.01 | 62.48 ± 1.10 | 67.96 ± 1.46 | 66.13 ± 2.33 | |
| Juicer | 66.13 ± 2.26 | 69.42 ± 2.92 | 64.30 ± 2.26 | 67.23 ± 1.65 | 68.32 ± 2.72 | |
| Garlic grinder | 65.40 ± 1.35 | 67.96 ± 2.89 | 65.40 ± 1.66 | 69.78 ± 1.55 | 67.96 ± 2.00 | |
| Bx (°) | Citrus squeezer | 4.67 ± 0.04 | 4.79 ± 0.06 | 4.84 ± 0.13 | 5.09 ± 0.13 | 4.99 ± 0.10 |
| Potato press | 4.81 ± 0.04 | 4.89 ± 0.09 | 5.07 ± 0.20 | 5.21 ± 0.07 | 5.04 ± 0.06 | |
| Garlic press | 4.73 ± 0.09 | 5.03 ± 0.09 | 5.01 ± 0.09 | 5.29 ± 0.09 | 4.97 ± 0.11 | |
| Juicer | 5.04 ± 0.08 | 5.10 ± 0.16 | 4.87 ± 0.14 | 5.26 ± 0.07 | 5.04 ± 0.06 | |
| Garlic grinder | 5.13 ± 0.09 | 5.10 ± 0.06 | 5.06 ± 0.10 | 5.54 ± 0.21 | 5.24 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santa Cruz, J.; Calbucheo, D.; Valdebenito, S.; Cortés, J.; Gautier, C.; Amín, A.; Hernández, I.; Allendes, H.; Peñaloza, P. Inter-Day Instability in Plant Sap Composition Undermines Single-Day Diagnostics. Agronomy 2025, 15, 2509. https://doi.org/10.3390/agronomy15112509
Santa Cruz J, Calbucheo D, Valdebenito S, Cortés J, Gautier C, Amín A, Hernández I, Allendes H, Peñaloza P. Inter-Day Instability in Plant Sap Composition Undermines Single-Day Diagnostics. Agronomy. 2025; 15(11):2509. https://doi.org/10.3390/agronomy15112509
Chicago/Turabian StyleSanta Cruz, Javier, Diego Calbucheo, Samuel Valdebenito, Javiera Cortés, Constanza Gautier, Aanisa Amín, Ignacia Hernández, Hernán Allendes, and Patricia Peñaloza. 2025. "Inter-Day Instability in Plant Sap Composition Undermines Single-Day Diagnostics" Agronomy 15, no. 11: 2509. https://doi.org/10.3390/agronomy15112509
APA StyleSanta Cruz, J., Calbucheo, D., Valdebenito, S., Cortés, J., Gautier, C., Amín, A., Hernández, I., Allendes, H., & Peñaloza, P. (2025). Inter-Day Instability in Plant Sap Composition Undermines Single-Day Diagnostics. Agronomy, 15(11), 2509. https://doi.org/10.3390/agronomy15112509






