Yield Potential of Silage Sorghum: Cultivar Differences in Biomass Production, Plant Height, and Tillering Under Contrasting Soil Conditions in Central Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
- Obora (49.0255714° N, 16.6188814° E): Gleyic Fluvisol Clayic; heavy-textured, medium organic carbon content, neutral pH.
- Písky (49.0177722° N, 16.5917347° E): Arenic Chernozem; sandy-gravel substrate, lower water retention, weakly acidic, medium organic carbon content.
2.2. Cultural Practices, Treatments, and Varieties
2.3. Data Collection
2.3.1. Meteorological and Soil Conditions
2.3.2. Soil Chemical Parameters
2.3.3. Plant Trait Measurements
Plant Height and Number of Tillers
Dry Matter Content and Dry Aboveground Biomass Yield
2.4. Statistical Analysis
3. Results
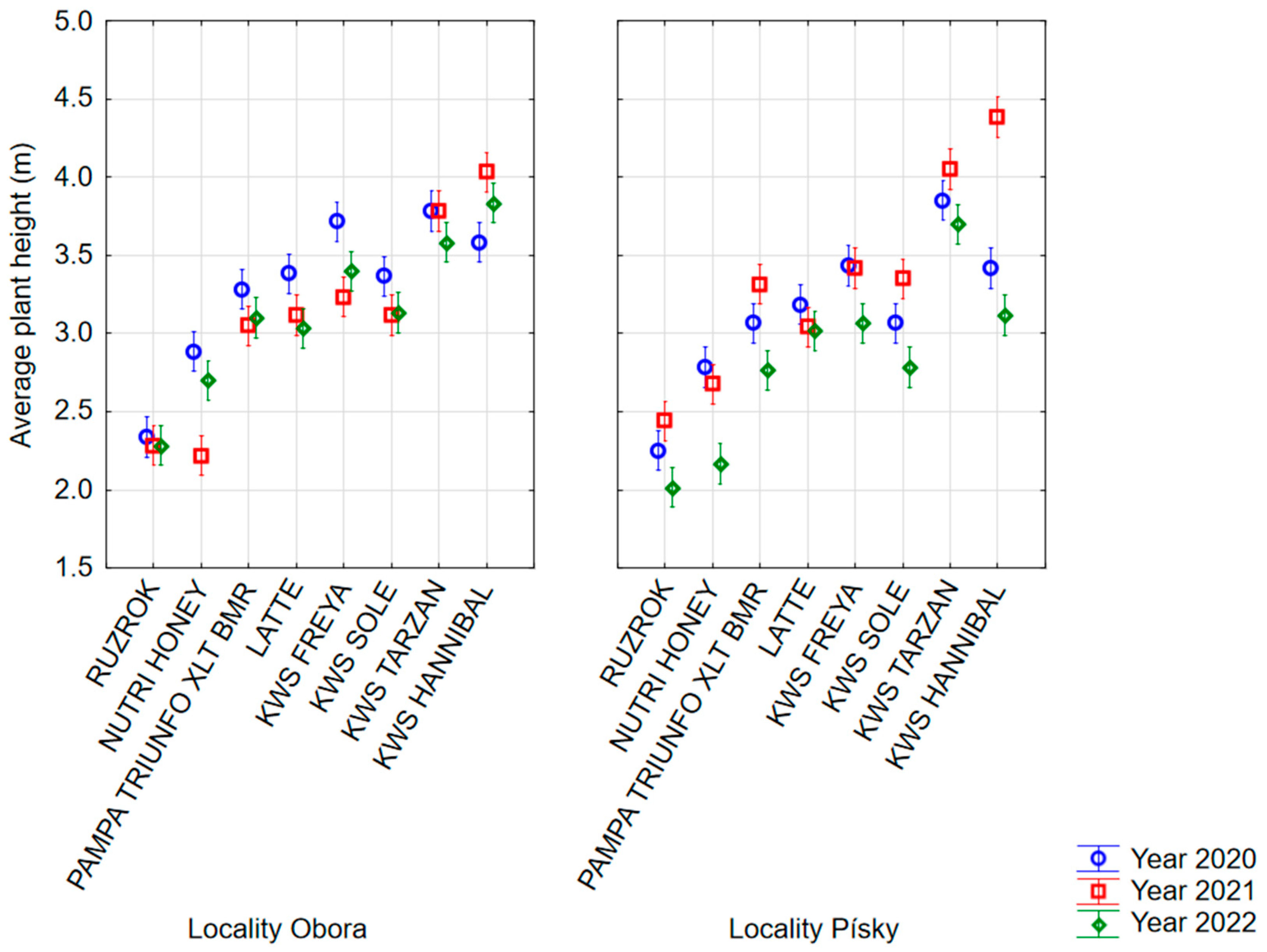
3.1. Plant Height
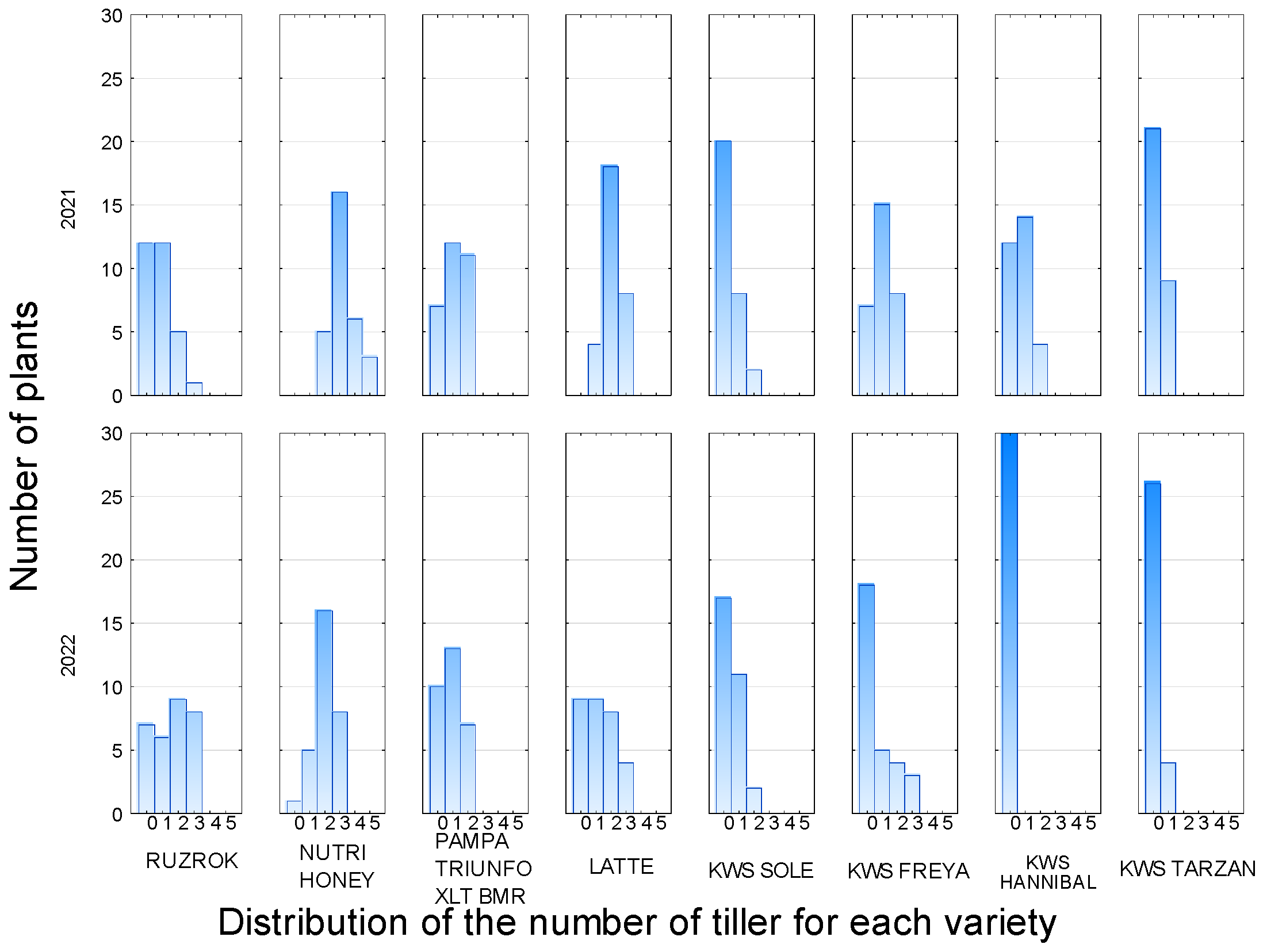
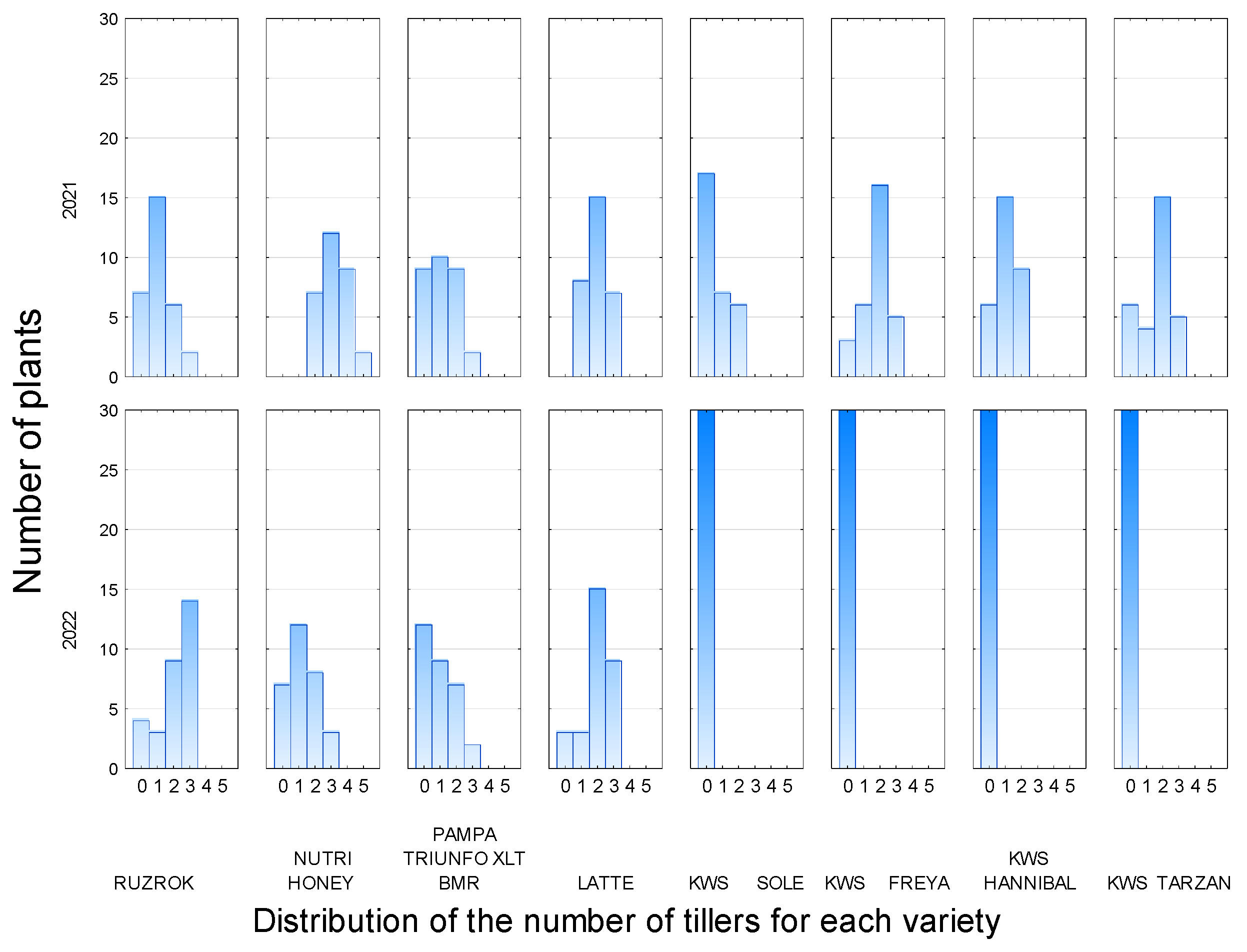
3.2. Number and Distribution of Tillers
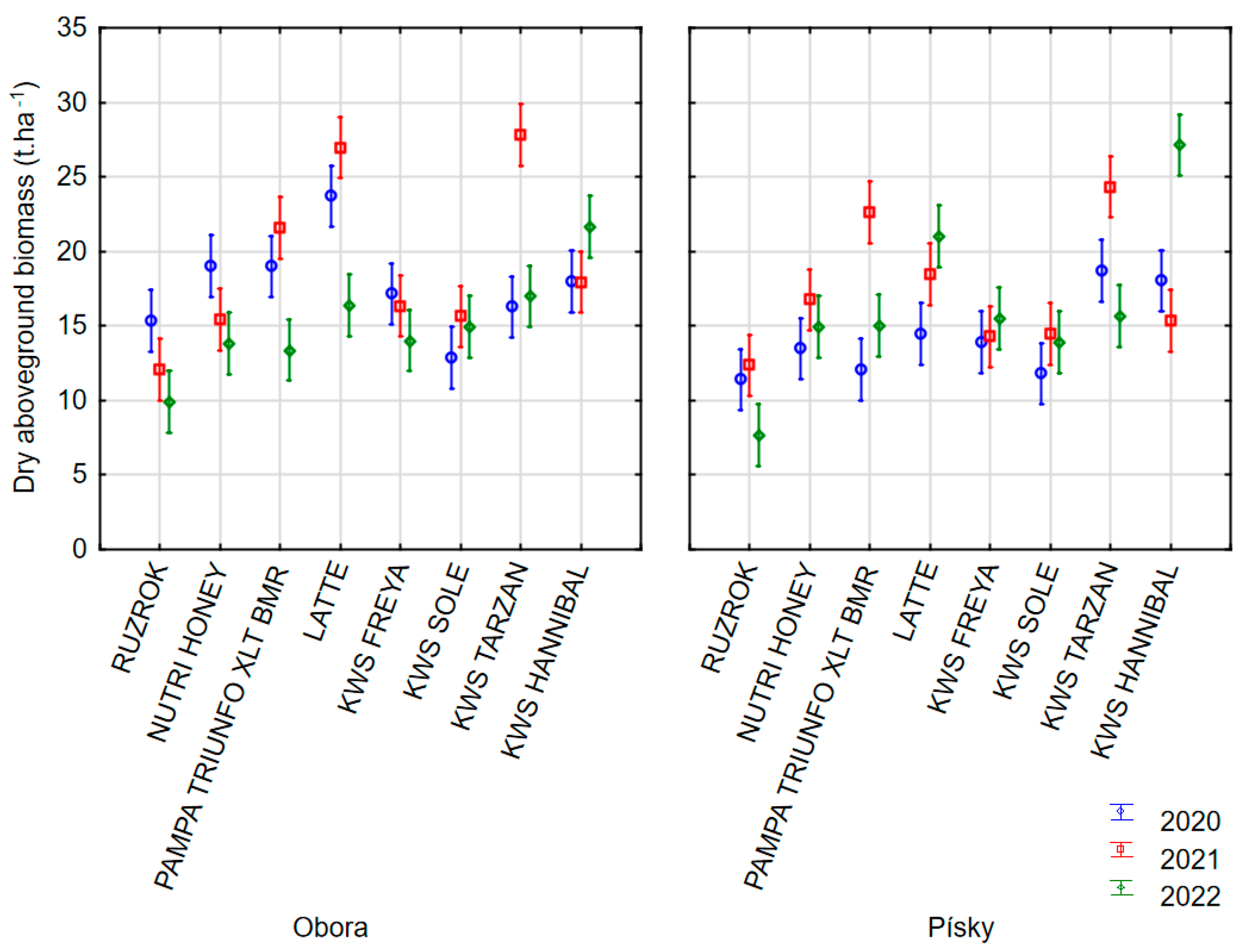
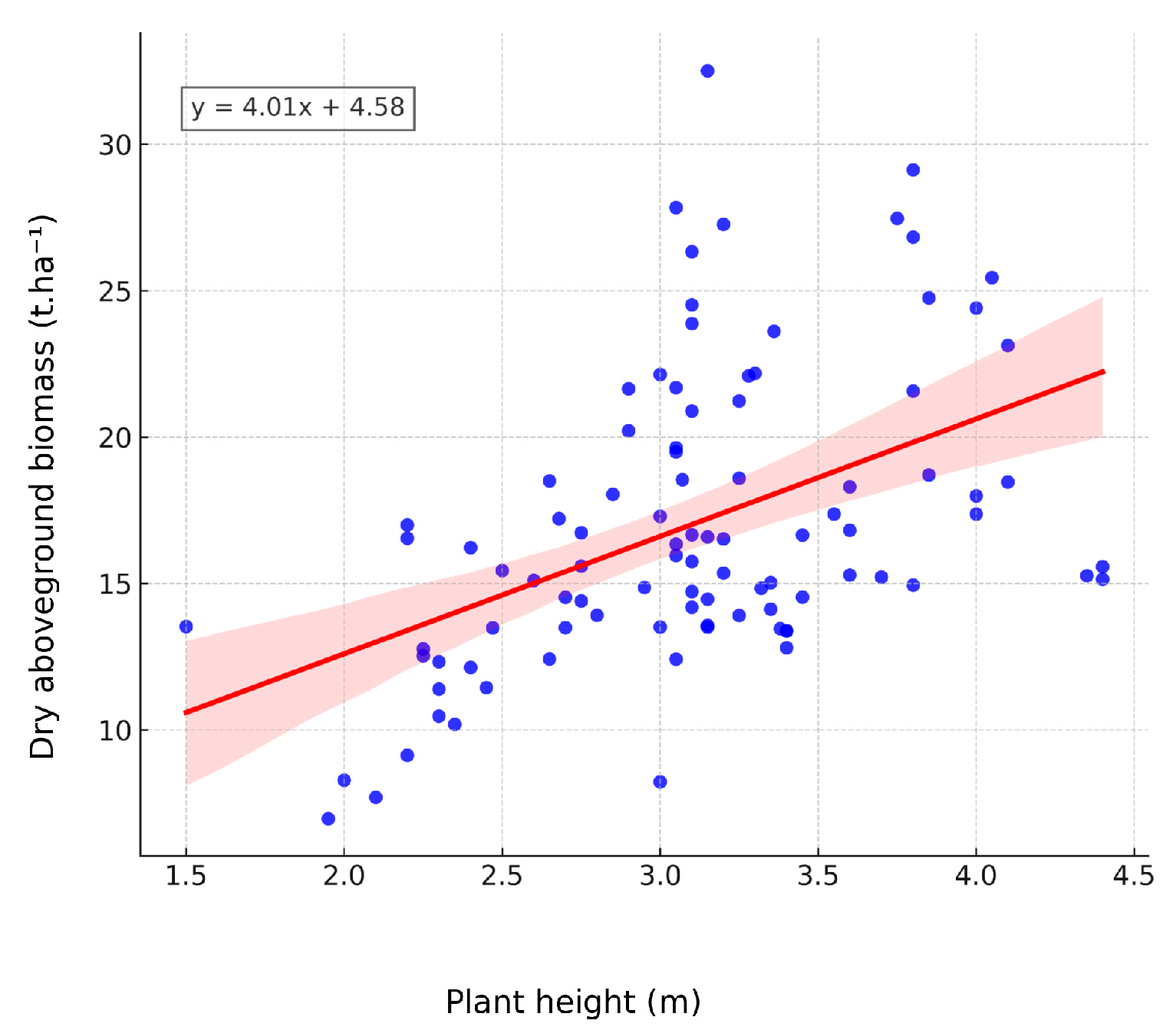
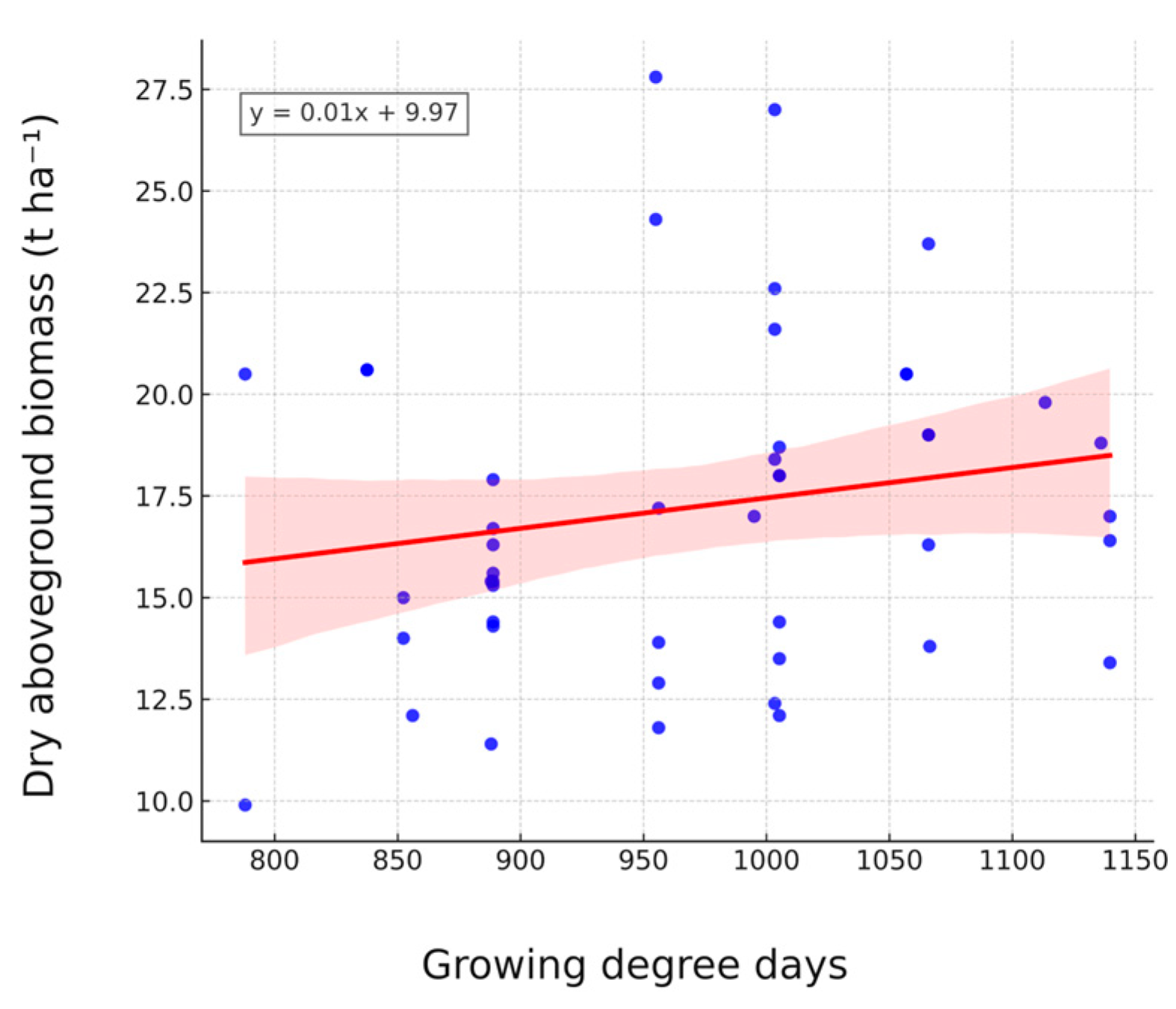
3.3. Dry Aboveground Biomass Yield
4. Discussion
4.1. Plant Height
4.2. Number of Tillers
4.3. Dry Matter Content and Dry Aboveground Biomass Yield
4.4. Climatic Drivers and Genotype × Environment Interactions
4.5. Agronomic Traits: No Single Driver of Yield Instability
4.6. Practical Implications for Cultivar Selection
4.7. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV | ultraviolet |
| C4 | plants with high biomass production that use a special photosynthetic process |
| P | phosphorus |
| K | potassium |
| Mg | magnesium |
| Ca | calcium |
| pH | hydrogen exponent |
| Cox | oxidizable carbon |
| DM | dry matter |
| DABY | dry aboveground biomass yield |
| HSD | honestly significant difference |
| CI | confidence interval |
| SS | sum of squares |
| DF | degrees of freedom |
| MS | mean square |
| GDD | growing degree day |
References
- Taylor, J.R.N. Sorghum and millets: Taxonomy, history, distribution, and production. In Sorghum and Millets: Chemistry, Technology, and Nutritional Attributes, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; Woodhead Publishing and AACC International Press: Cambridge, UK, 2018; pp. 1–19. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 6 August 2025).
- Trnka, M.; Balek, J.; Brázdil, R.; Dubrovský, M.; Eitzinger, J.; Hlavinka, P.; Chuchma, F.; Možný, M.; Prášil, I.T.; Růžek, P.; et al. Posun agroklimatických podmínek Česka v posledních 60 letech. Zpr. Agrobase 2021, 33, 28–30. [Google Scholar]
- Orság, M.; Fischer, M.; Trnka, M.; Brotan, J.; Pozníková, G.; Žalud, Z. Trends in Air Temperature and Precipitation in South-eastern Czech Republic, 1961–2020. Acta Univ. Agric. Silvic. Mendel. Brun. 2022, 70, 283–294. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Singh, J.; Rai, S.; Baig, M.J.; Biradar, N.; Kumar, A.; Verma, O.P.S. Water requirement estimates of feed and fodder production for Indian livestock vis a vis livestock water productivity. Indian J. Anim. Sci. 2014, 84, 1090–1094. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.E.; Ullah, F.; Ben-Hur, A.; Reddy, A.S.N. Transcriptome Analysis of Drought-Resistant and Drought-Sensitive Sorghum (Sorghum bicolor) Genotypes in Response to PEG-Induced Drought Stress. Int. J. Mol. Sci. 2020, 21, 772. [Google Scholar] [CrossRef]
- Busta, L.; Schmitz, E.; Kosma, D.K.; Schnable, J.C.; Cahoon, E.B. A co-opted steroid synthesis gene, maintained in sorghum but not maize, is associated with a divergence in leaf wax chemistry. Proc. Natl. Acad. Sci. USA 2021, 118, e2022982118. [Google Scholar] [CrossRef]
- Wani, S.P.; Albrizio, R.; Rao, V.N. Sorghum. In Crop Yield Response to Water, 1st ed.; Steduto, P., Hsiao, T.C., Fereres, E., Raes, D., Eds.; FAO: Rome, Italy, 2012; pp. 144–151. [Google Scholar]
- Emendack, Y.Y.; Burke, J.J.; Sanchez, J.; Echevarria, H.L.; Hayes, C.M. Agro-morphological characterization of diverse sorghum lines for pre- and post-flowering drought tolerance. Aust. J. Crop Sci. 2018, 12, 135–150. [Google Scholar] [CrossRef]
- Thomas, H.; Ougham, H. The stay-green trait. J. Exp. Bot. 2014, 65, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Habyarimana, E.; Bonardi, P.; Laureti, D.; di Bari, V.; Cosentino, S.; Lorenzoni, C. Multilocational evaluation of biomass sorghum hybrids under two stand densities and variable water supply in Italy. Ind. Crops Prod. 2004, 20, 3–9. [Google Scholar] [CrossRef]
- Kožnarová, V.; Klabzuba, J. Recommendation of World Meteorological Organization to describing meteorological or climatological conditions. Plant Soil Environ. 2002, 4, 190–192. [Google Scholar] [CrossRef]
- StatSoft Inc STATISTICA (Data Analysis Software System), Version, 1.4. StatSoft Inc: Tulsa, OK, USA. Available online: http://www.statsoft.com (accessed on 6 August 2025).
- Hermuth, J.; Kosová, K. Characterization of the first Czech sorghum variety Ruzrok tested in Czech Republic. Czech J. Genet. Plant Breed. 2017, 53, 37–44. [Google Scholar] [CrossRef]
- Kanbar, A.; Flubacher, N.; Hermuth, J.; Kosová, K.; Horn, T.; Nick, P. Mining Sorghum Biodiversity—Potential of Dual-Purpose Hybrids for Bio-Economy. Diversity 2021, 13, 192. [Google Scholar] [CrossRef]
- Sowiński, J.; Szydełko, E. Growth rate and yields of a sorghum-sudangrass hybrid variety grown on a light and a medium-heavy soil as affected by cutting management and seeding rate. Pol. J. Agron. 2011, 4, 23–28. [Google Scholar] [CrossRef]
- van Oosterom, E.J.; Hammer, G.; Kim, H.K.; McLean, G.; Deifel, K. Plant design features that improve grain yield of cereals under drought. In Proceedings of the 14th Australian Society of Agronomy Conference, Gosford, Australia, 21–25 September 2008; Unkovich, M., Ed.; The Regional Institute: Gosford, Australia, 2008. [Google Scholar]
- Kamau, R.M.; Cheruiyot, E.K.; Karori, S.M. Phenotypic expressions of diverse Sorghum bicolor [L.] Moench genotypes exposed to cold and warm temperatures. Afr. J. Agric. Res. 2022, 18, 1121–1139. [Google Scholar] [CrossRef]
- Maulana, F.; Tesso, T.T. Cold temperature episode at seedling and flowering stages reduces growth and yield components in sorghum. Crop Sci. 2013, 53, 564–574. [Google Scholar] [CrossRef]
- Keskin, B.; Akdeniz, H.; Temel, S.; Eren, B. Determination of Agricultural Characteristics of Some Silage Sorghum and Sudan Grass Varieties Grown as Second Product. YYU J. Agric. Sci. 2018, 28, 412–418. [Google Scholar] [CrossRef]
- Hermuth, J.; Janovská, D.; Strašil, Z.; Usťak, S.; Hýsek, J. Čirok obecný—Sorghum bicolor (L.) Moench: Možnosti využití v podmínkách České republiky, 1st ed.; Výzkumný ústav rostlinné výroby: Praha-Ruzyně, Česká Republika, 2012; pp. 26–31. [Google Scholar]
- Štěpánek, P. Nabídka Čiroku Pro Rok 2018. Available online: https://www.agromanual.cz/cz/clanky/ochrana-rostlin-a-pestovani/osivo-a-sadba-1/nabidka-ciroku-pro-rok-2018 (accessed on 6 August 2025).
- Wang, X.; Hunt, C.; Cruickshank, A.; Mace, E.; Hammer, G.; Jordan, D. The impacts of flowering time and tillering on grain yield of sorghum hybrids across diverse environments. Agronomy 2020, 10, 135. [Google Scholar] [CrossRef]
- Chen, K.; Feng, L.; Shoujun, S. Research status and prospect of QTL mapping for tillers and other traits in forage sorghum. Mol. Plant Breed. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Liu, R.; Finlayson, S.A. Sorghum tiller bud growth is repressed by contact with the overlying leaf. Plant Cell Environ. 2019, 42, 2120–2132. [Google Scholar] [CrossRef]
- Ikanović, J.; Janković, S.; Popović, V.; Rakić, S.; Dražić, G.; Živanović, L.J.; Kolarić, L.J. The effect of nitrogen fertilizer rates on green biomass and dry matter yield of sorghum sp. at different growth stages. Biotechnol. Anim. Husb. 2014, 30, 743–749. [Google Scholar] [CrossRef]
- McLean, G.; Kholová, J.; van Oosterom, E. Modelling the dynamics and phenotypic consequences of tiller outgrowth and cessation in sorghum. Silico Plants 2023, 5, diad019. [Google Scholar] [CrossRef]
- Kim, H.K.; Luquet, D.; van Oosterom, E.; Dingkuhn, M.; Hammer, G. Regulation of tillering in sorghum: Genotypic effects. Ann. Bot. 2010, 106, 69–78. [Google Scholar] [CrossRef]
- Alam, M.M.; Hammer, G.L.; van Oosterom, E.J.; Cruickshank, A.; Hunt, C.; Jordan, D.R. A physiological framework to explain genetic and environmental regulation of tillering in sorghum. New Phytol. 2014, 203, 155–167. [Google Scholar] [CrossRef]
- Barbier, F.F.; Dun, E.A.; Kerr, S.C.; Chabikwa, T.G.; Beveridge, C.A. An update on the signals controlling shoot branching. Trends Plant Sci. 2019, 24, 220–236. [Google Scholar] [CrossRef]
- Lafarge, T.A.; Hammer, G.L. Predicting plant leaf area production: Shoot assimilate accumulation and partitioning, and leaf area ratio, are stable for a wide range of sorghum population densities. Field Crops Res. 2002, 77, 137–151. [Google Scholar] [CrossRef]
- Lafarge, T.A.; Hammer, G.L. Tillering in grain sorghum over a wide range of population densities: Modelling dynamics of tiller fertility. Ann. Bot. 2002, 90, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ertan, A.; Hazim, S.T. Hydrocyanic acid content, forage yield and some quality features of two Sorghum-sudan grass hybrid cultivars under different nitrogen doses in Thrace, Turkey. Curr. Trends Nat. Sci. 2019, 8, 55–62. [Google Scholar]
- Miron, J.; Solomon, R.; Adin, G.; Nir, U.; Nikbachat, M.; Yosef, E.; Carmi, A.; Weinberg, Z.G.; Kipnis, T.; Zuckerman, E.; et al. Effects of harvest stage and re-growth on yield, composition, ensilage and in vitro digestibility of new forage sorghum varieties. J. Sci. Food Agric. 2006, 86, 140–147. [Google Scholar] [CrossRef]
- Hermuth, J.; Janovská, D.; Hlásná Čepková, P.; Usťak, S.; Strašil, Z.; Dvořáková, Z. Sorghum and Foxtail Millet—Promising Crops for the Changing Climate in Central Europe. In Alternative Crops and Cropping Systems, 1st ed.; Konvalina, P., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Moura, M.M.A.; Pires, D.A.A.; Costa, R.F.; Tolentino, D.C.; Rigueira, J.P.S.; Junqueira, E.C.S. Nutritional value of sorghum silages. Acta Sci. Anim. Sci. 2017, 39, 137–142. [Google Scholar] [CrossRef]
- Kubeš, P. Vliv Různé Šířky Řádků na Výnos Biomasy a Obsah Sušiny při Pěstování Čiroku. Master’s Thesis, Jihočeská Univerzita v Českých Budějovicích, České Budějovice, Czech Republic, 2016. [Google Scholar]
- Povolný, M.; Hampl, B. Výsledky Zkoušek Užitné Hodnoty ze Sklizně 2014. Čirok. ÚKZÚZ, Národní Odrůdový Úřad: Brno, Czech Republic, 2015. Available online: http://eagri.cz/public/web/file/399243/ZUH_Cirok_2014.pdf (accessed on 13 June 2024).
- Ngidi, A.; Shimelis, H.; Abady, S.; Chaplot, V.; Figlan, S. Genetic variation and association of yield, yield components, and carbon storage in sorghum (Sorghum bicolor [L.] Moench) genotypes. BMC Genom Data 2024, 74, 12. [Google Scholar] [CrossRef]
- Islam, T.M.T.; Sedgley, R.H. Evidence of a ‘uniculm effect’ in spring wheat (Triticum aestivum L.) in a Mediterranean environment. Euphytica 1981, 30, 277–282. [Google Scholar] [CrossRef]
- Hammer, G.; Cooper, M.; Tardieu, F.; Welch, S.; Walsh, B.; van Eeuwijk, F.; Chapman, S.; Podlich, D. Models for navigating biological complexity in breeding improved crop plants. Trends Plant Sci. 2006, 11, 587–593. [Google Scholar] [CrossRef]
- Guerra, J.V.S.; Parella, R.A.C.; Cavallin, I.C.; de Carvalho, A.J.; Portugal, A.F.; Anjos, J.R.N.; Guedes, F.L.; Godinho, V.P.C. Agronomic performance, adaptability and stability of biomass sorghum genotypes in different regions of Brazil, using the Annicchiarico method. Cienc. Rural 2025, 55, e20230681. [Google Scholar] [CrossRef]
- Atim, J.; Kaweesi, T.; Hutmacher, R.B.; Putnam, D.H.; Pedraza, J.; de Ben, C.M.; Schramm, T.; Angeles, J.; Clark, N.E.; Dahlberg, J.A. Optimizing Sorghum for California: A Multi-Location Evaluation of Biomass Yield, Feed Quality, and Biofuel Feedstock Potential. Agronomy 2024, 14, 2866. [Google Scholar] [CrossRef]
- Dolapčev Rakić, A.; Prodanović, S.; Sikora, V.; Vasiljević, S.; Župunski, V.; Jevtić, R.; Uhlarik, A. Potential for Enhancing Forage Sorghum Yield and Yield Components in a Changing Pannonian Climate. Agriculture 2025, 15, 1439. [Google Scholar] [CrossRef]
- Rosa, M.A.B.; Tardin, F.D.; Souza, J.M.D.S.; Dos Santos, J.A.P.; Macedo, T.D.F.; Santos, J.; de Freitas, M.H.; Todescatto, F.; Da Costa, R.A.P.; Figueiredo, J.E.F.; et al. Characterization of forage, sweet and biomass sorghum for agronomic performance and ensilability. Rev. Bras. Milho Sorgo 2022, 21, e1239. [Google Scholar] [CrossRef]
- Markos, S.; Simion, T.; Samuel, T.; Yildiz, F. Evaluation of improved lowland sorghum (Sorghum bicolor (L.) Moench) varieties in Southern Ethiopia. Cogent Food Agric. 2020, 6, 1727624. [Google Scholar] [CrossRef]
- Frantová, N.; Rábek, M.; Porčová, L.; Jovanović, I.; Širůček, P.; Lukas, V.; Hájek, J.; Elzner, P.; Holková, L.; Smutná, P.; et al. Monitoring Drought Tolerance Mechanisms of Sorghum and Maize Under Unevenly Distributed Precipitation. Int. J. Plant Prod. 18. [CrossRef]
- Clarke, S.J.; McLean, J.; George-Jaeggli, B.; McLean, G.; De Voil, P.; Eyre, J.X.; Rodriguez, D. Understanding the diversity in yield potential and stability among commercial sorghum hybrids can inform crop designs. Field Crops Res. 2019, 230, 84–97. [Google Scholar] [CrossRef]
- Birhanu, C.; Mekbib, F.; Lule, D.; Bekeko, Z.; Girma, G.; Tirfessa, A.; Ayana, G.; Nida, H.; Mengiste, T. Genotype by environment interactions and stability for grain yield and other agronomic traits in selected sorghum genotypes in Ethiopia. Agrosyst. Geosci. Environ. 2024, 7, e20544. [Google Scholar] [CrossRef]
- Ouédraogo, N.; Sawadogo, A.P.W.; Koala, M.; Kouraogo, I.; Nébié, B.; Gracen, V. Genotype x Environment Interaction and Stability of Heading Time and Grain Yield of 12 Open Pollinated Sorghum Varieties across Different Agro-ecological Zones in Burkina Faso. J. Exp. Agric. Int. 2024, 46, 310–322. [Google Scholar] [CrossRef]
- Mwamahonje, A.; Mdindikasi, Z.; Mchau, D.; Mwenda, E.; Sanga, D.; Garcia-Oliveira, A.L.; Ojiewo, C.O. Advances in Sorghum Improvement for Climate Resilience in the Global Arid and Semi-Arid Tropics: A Review. Agronomy 2024, 14, 3025. [Google Scholar] [CrossRef]
- Behera, P.P.; Singode, A.; Bhat, B.V.; Borah, N.; Verma, H.; Supriya, P.; Sarma, R.N. Identifying genetic determinants of forage sorghum [Sorghum bicolor (Moench)] adaptation through GWAS. BMC Plant Biol. 2024, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Riaz, A.; Jiang, J.; Li, X.; Uzair, M.; Mishra, P.; Zeb, A.; Zhang, J.; Singh, R.P.; Luo, L.; et al. Advancing Climate-Resilient Sorghum: The Synergistic Role of Plant Biotechnology and Microbial Interactions. Rice 2025, 18, 41. [Google Scholar] [CrossRef] [PubMed]


| Month | Year 2020 | Year 2021 | Year 2022 | Period 1961–1990 | Period 1991–2020 |
|---|---|---|---|---|---|
| IV. | 10.6 | 7.6 | 8.4 | 9.6 | 11.0 |
| V. | 13.0 | 12.7 | 16.1 | 14.6 | 15.6 |
| VI. | 18.3 | 20.9 | 20.3 | 17.7 | 19.2 |
| VII. | 19.9 | 21.2 | 20.7 | 19.3 | 20.9 |
| VIII. | 21.3 | 18.0 | 21.4 | 18.6 | 20.6 |
| IX. | 16.0 | 15.6 | 14.0 | 14.7 | 15.4 |
| average | 16.2 | 16.2 | 16.8 | 15.8 | 17.1 |
| Month | Year 2020 | Year 2021 | Year 2022 | Period 1961–1990 | Period 1991–2020 |
|---|---|---|---|---|---|
| IV. | 10.8 | 13.0 | 15.2 | 32.2 | 27.8 |
| V. | 81.8 | 84.8 | 43.0 | 62.8 | 52.2 |
| VI. | 128.6 | 59.8 | 54.6 | 68.6 | 61.7 |
| VII. | 44.0 | 45.6 | 98.8 | 57.1 | 68.9 |
| VIII. | 97.6 | 175.8 | 86.0 | 54.3 | 61.1 |
| IX. | 87.2 | 22.0 | 33.6 | 35.5 | 53.9 |
| sum | 444.6 | 401.0 | 331.2 | 311.5 | 325.7 |
| Locality | Nutrient Content (mg kg−1) | pH | Cox | |||
|---|---|---|---|---|---|---|
| P | K | Mg | Ca | |||
| Obora | 88.2 | 154.0 | 316.0 | 4358 | 7.06 | 1.33 |
| Písky | 114.9 | 166.0 | 189.0 | 1967 | 6.47 | 1.42 |
| Source | SS | DF | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Year | 1.754 | 2 | 0.877 | 71.6 | 0.000 |
| Locality | 0.222 | 1 | 0.222 | 18.1 | 0.000 |
| Variety | 34.418 | 7 | 4.917 | 401.6 | 0.000 |
| Year*Locality | 1.831 | 2 | 0.916 | 74.8 | 0.000 |
| Year*Variety | 2.442 | 14 | 0.174 | 14.2 | 0.000 |
| Locality*Variety | 0.321 | 7 | 0.046 | 3.7 | 0.001 |
| Year*Locality*Variety | 0.818 | 14 | 0.058 | 4.8 | 0.000 |
| Location | Obora | Písky | ||
|---|---|---|---|---|
| Year | 2021 | 2022 | 2021 | 2022 |
| Variety | Distribution of Tillers Occurrence (%) | |||
| RUZROK | 77 | 87 | 60 | 77 |
| NUTRI HONEY | 100 | 77 | 100 | 100 |
| PAMPA TRIUNFO XLT BMR | 70 | 60 | 77 | 67 |
| LATTE | 100 | 90 | 100 | 70 |
| KWS SOLE | 43 | 0 | 33 | 43 |
| KWS FREYA | 90 | 0 | 77 | 40 |
| KWS HANNIBAL | 80 | 0 | 60 | 0 |
| KWS TARZAN | 80 | 0 | 30 | 13 |
| Source | SS | DF | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Year | 188.12 | 2 | 94.06 | 29.09 | 0.000 |
| Locality | 68.23 | 1 | 68.23 | 21.10 | 0.000 |
| Variety | 1266.83 | 7 | 180.98 | 55.96 | 0.000 |
| Variety*Locality | 69.31 | 7 | 9.90 | 3.06 | 0.006 |
| Variety*Year | 737.68 | 14 | 52.69 | 16.29 | 0.000 |
| Locality*Year | 135.23 | 2 | 67.2 | 20.91 | 0.000 |
| Variety*Locality*Year | 272.79 | 14 | 19.48 | 6.03 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porčová, L.; Frantová, N.; Rábek, M.; Jovanović, I.; Smutný, V.; Řiháček, M.; Mrkvicová, E. Yield Potential of Silage Sorghum: Cultivar Differences in Biomass Production, Plant Height, and Tillering Under Contrasting Soil Conditions in Central Europe. Agronomy 2025, 15, 2352. https://doi.org/10.3390/agronomy15102352
Porčová L, Frantová N, Rábek M, Jovanović I, Smutný V, Řiháček M, Mrkvicová E. Yield Potential of Silage Sorghum: Cultivar Differences in Biomass Production, Plant Height, and Tillering Under Contrasting Soil Conditions in Central Europe. Agronomy. 2025; 15(10):2352. https://doi.org/10.3390/agronomy15102352
Chicago/Turabian StylePorčová, Lenka, Nicole Frantová, Michal Rábek, Ivana Jovanović, Vladimír Smutný, Michal Řiháček, and Eva Mrkvicová. 2025. "Yield Potential of Silage Sorghum: Cultivar Differences in Biomass Production, Plant Height, and Tillering Under Contrasting Soil Conditions in Central Europe" Agronomy 15, no. 10: 2352. https://doi.org/10.3390/agronomy15102352
APA StylePorčová, L., Frantová, N., Rábek, M., Jovanović, I., Smutný, V., Řiháček, M., & Mrkvicová, E. (2025). Yield Potential of Silage Sorghum: Cultivar Differences in Biomass Production, Plant Height, and Tillering Under Contrasting Soil Conditions in Central Europe. Agronomy, 15(10), 2352. https://doi.org/10.3390/agronomy15102352











