Heat Stress Tolerance and Photosynthetic Responses to Transient Light Intensities of Greek Grapevine Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Photosynthetic Light Response Curves (LRCs)
2.3. Temperature Dependence Model
2.4. Chlorophyll Content Measurements
2.5. Chlorophyll Fluorescence Quenching Analysis
2.6. Leaf Transpirational Cooling and Thermoregulation
2.7. Leaf Morphology
2.8. Statistical Data Analysis
3. Results
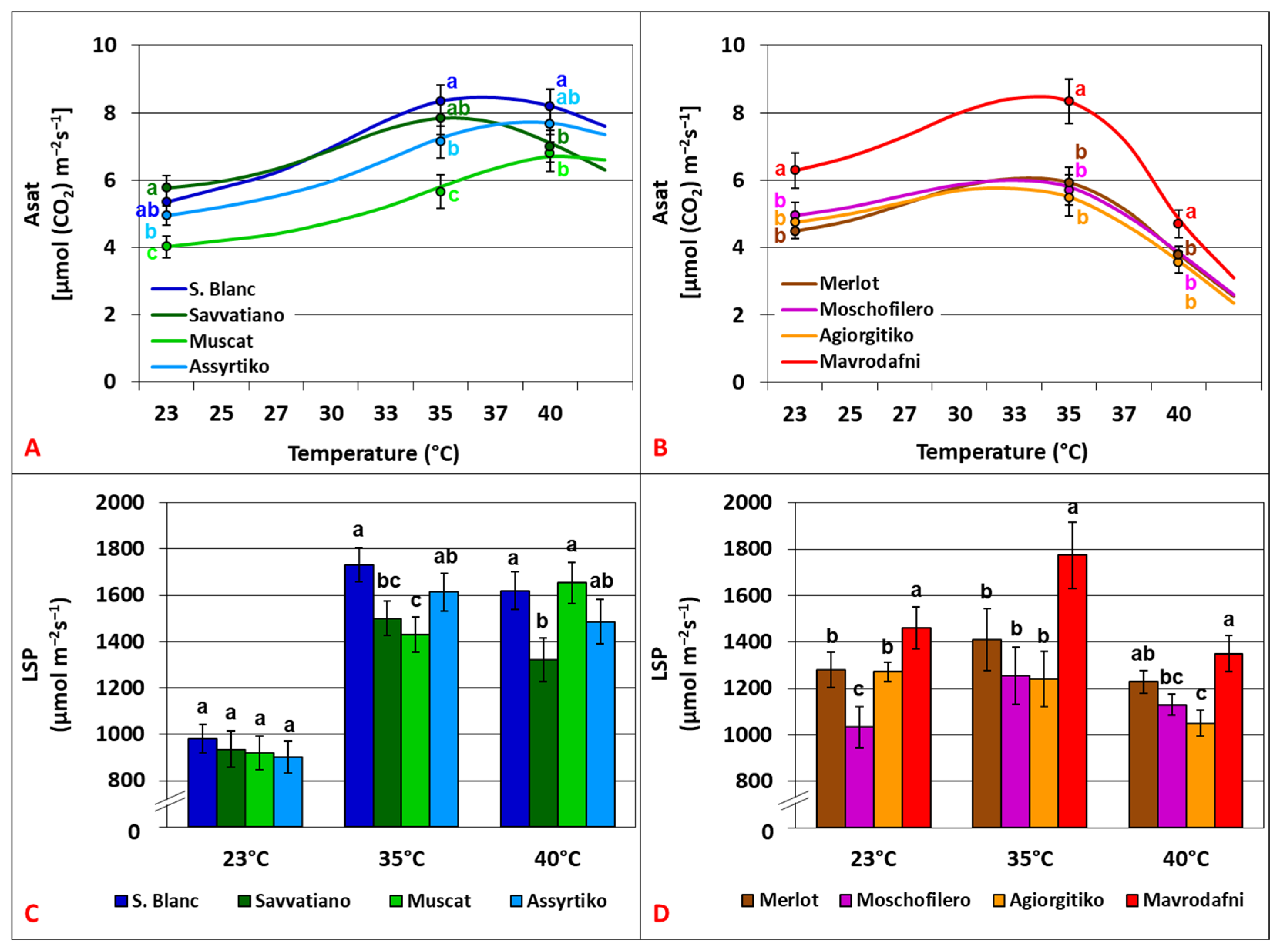
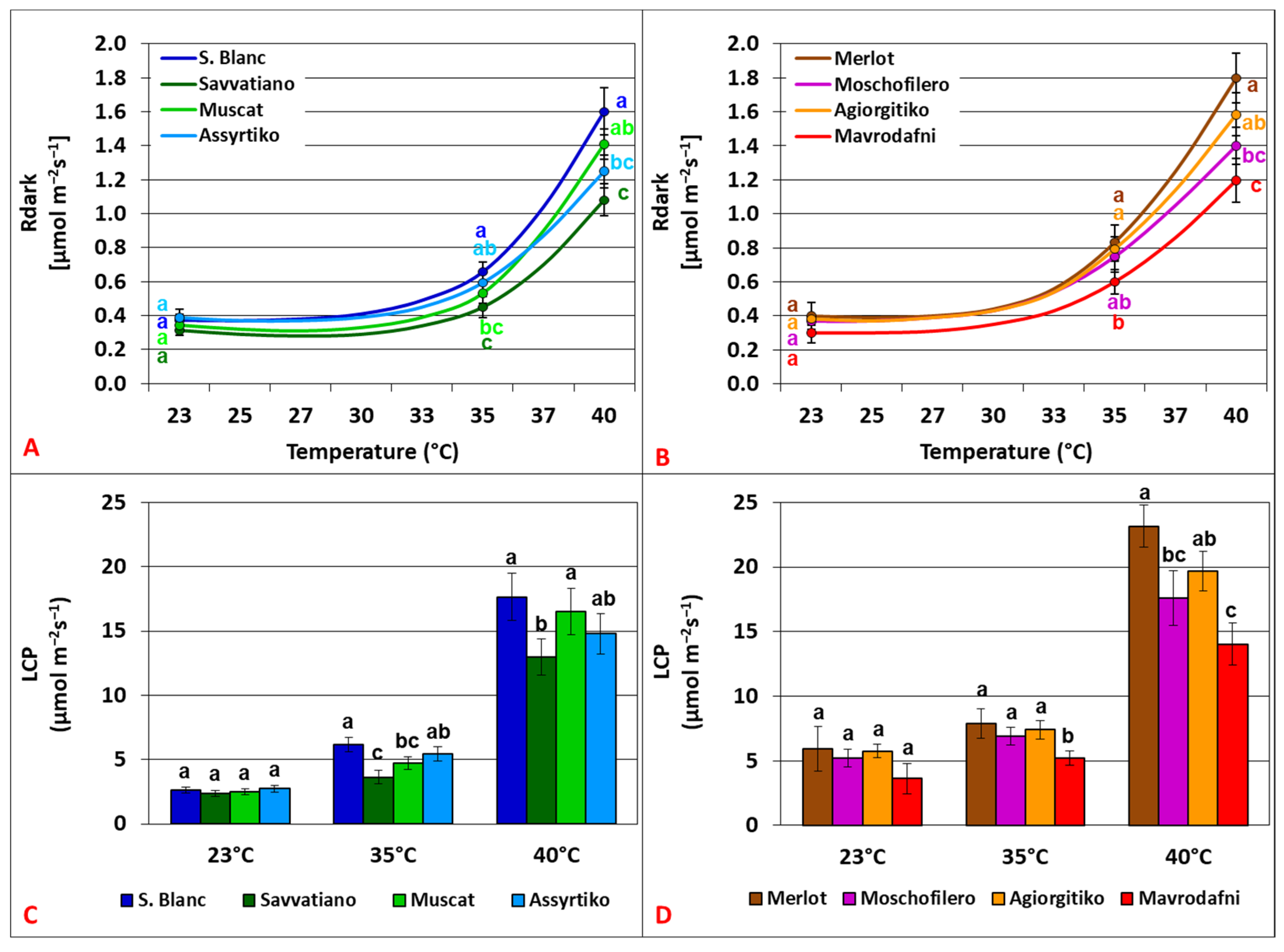
3.1. Temperature Dependence of Light Response Curves (LRCs) Parameters
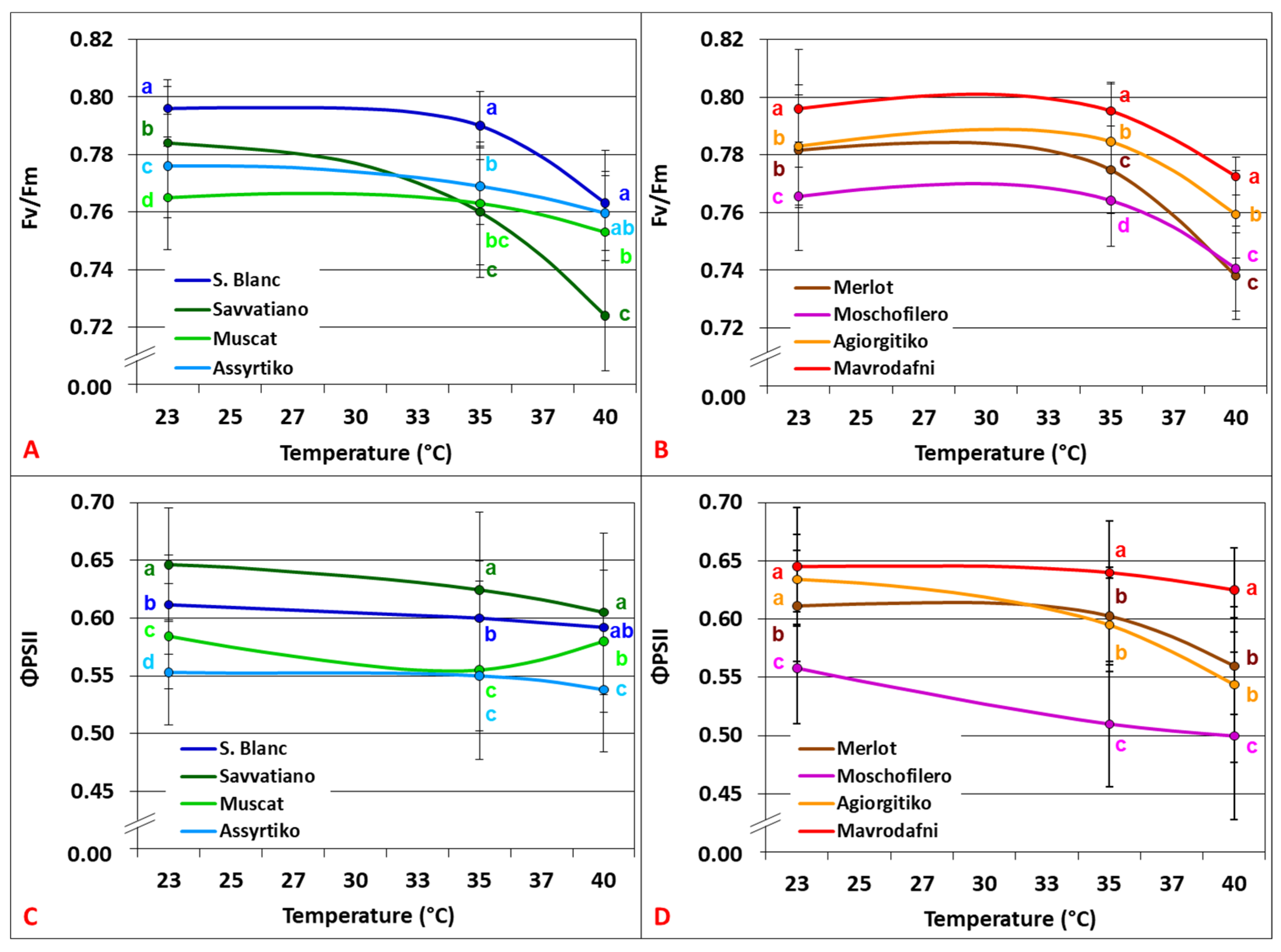
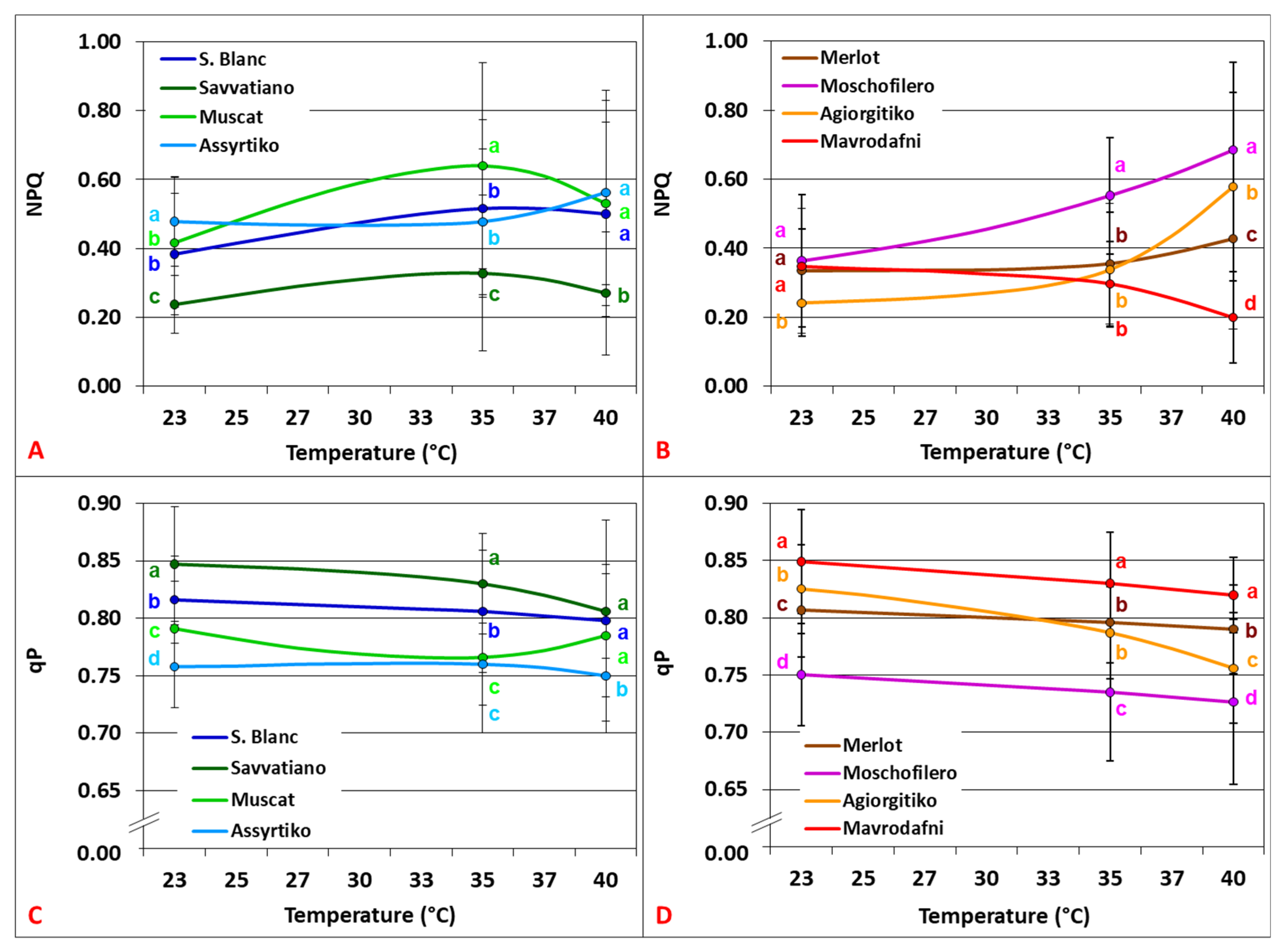
3.2. Temperature Dependence of Chlorophyll Fluorescence Parameters
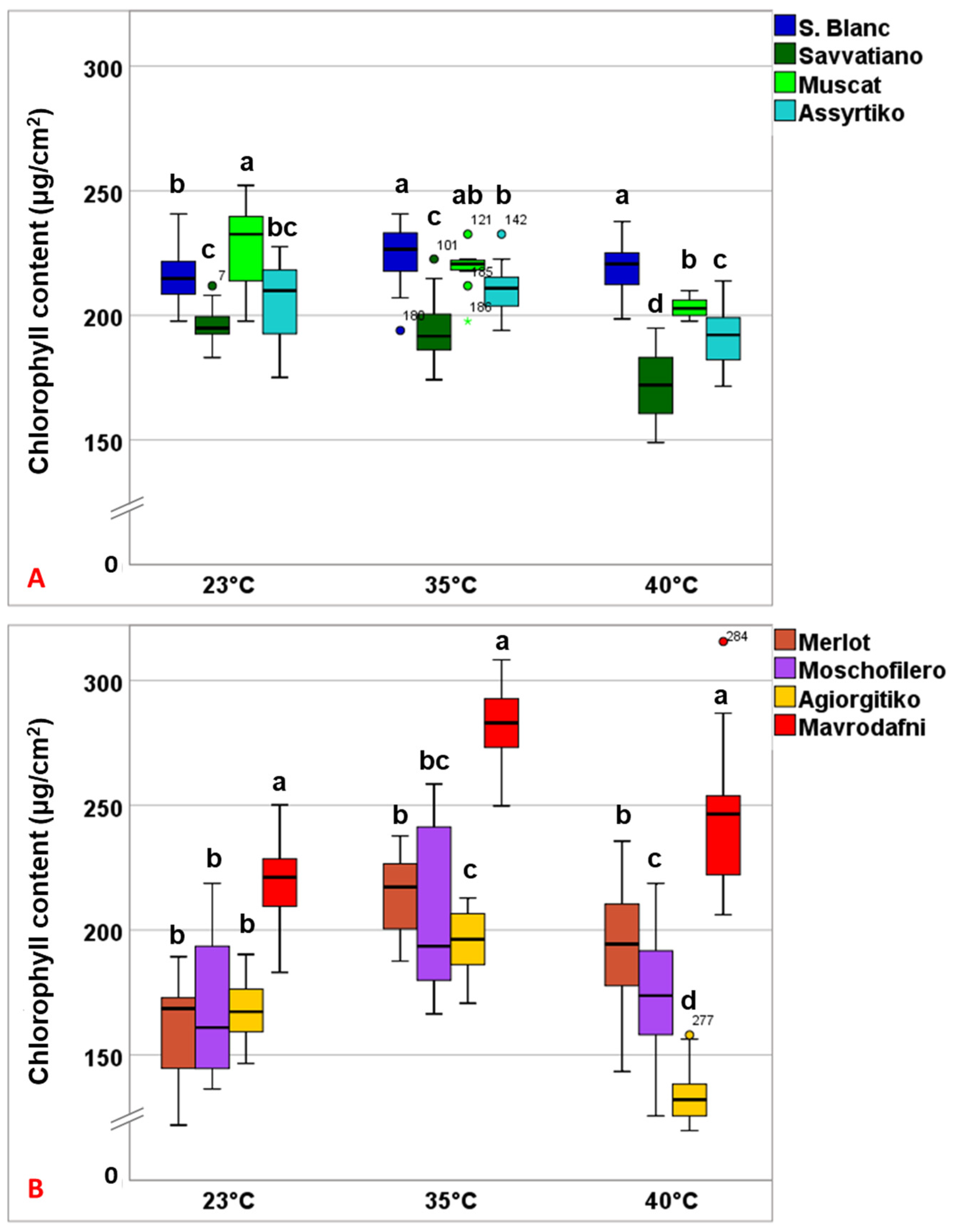
3.3. Temperature-Induced Changes in Chlorophyll Content
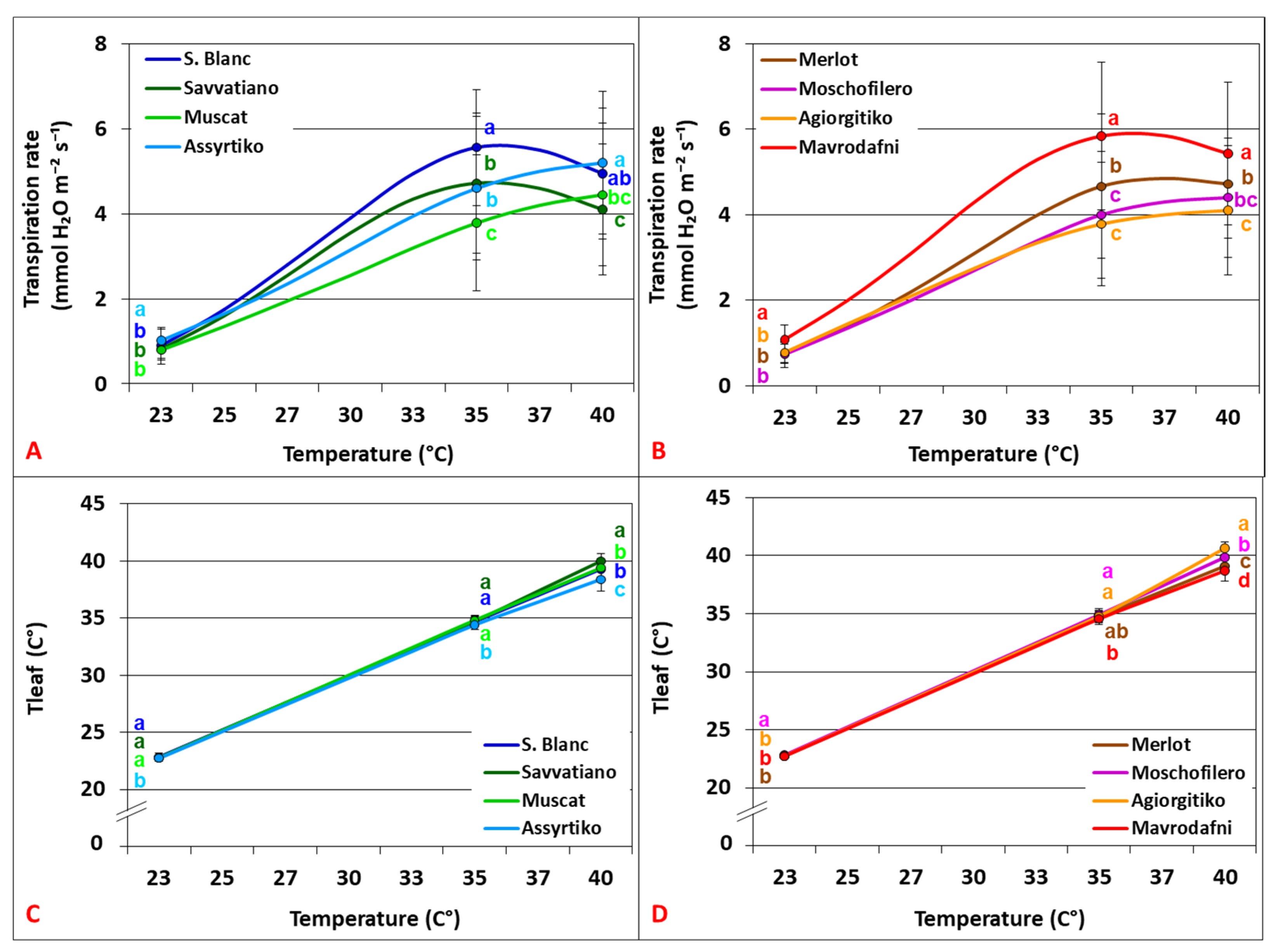
3.4. Leaf Transpirational Cooling and Thermoregulation
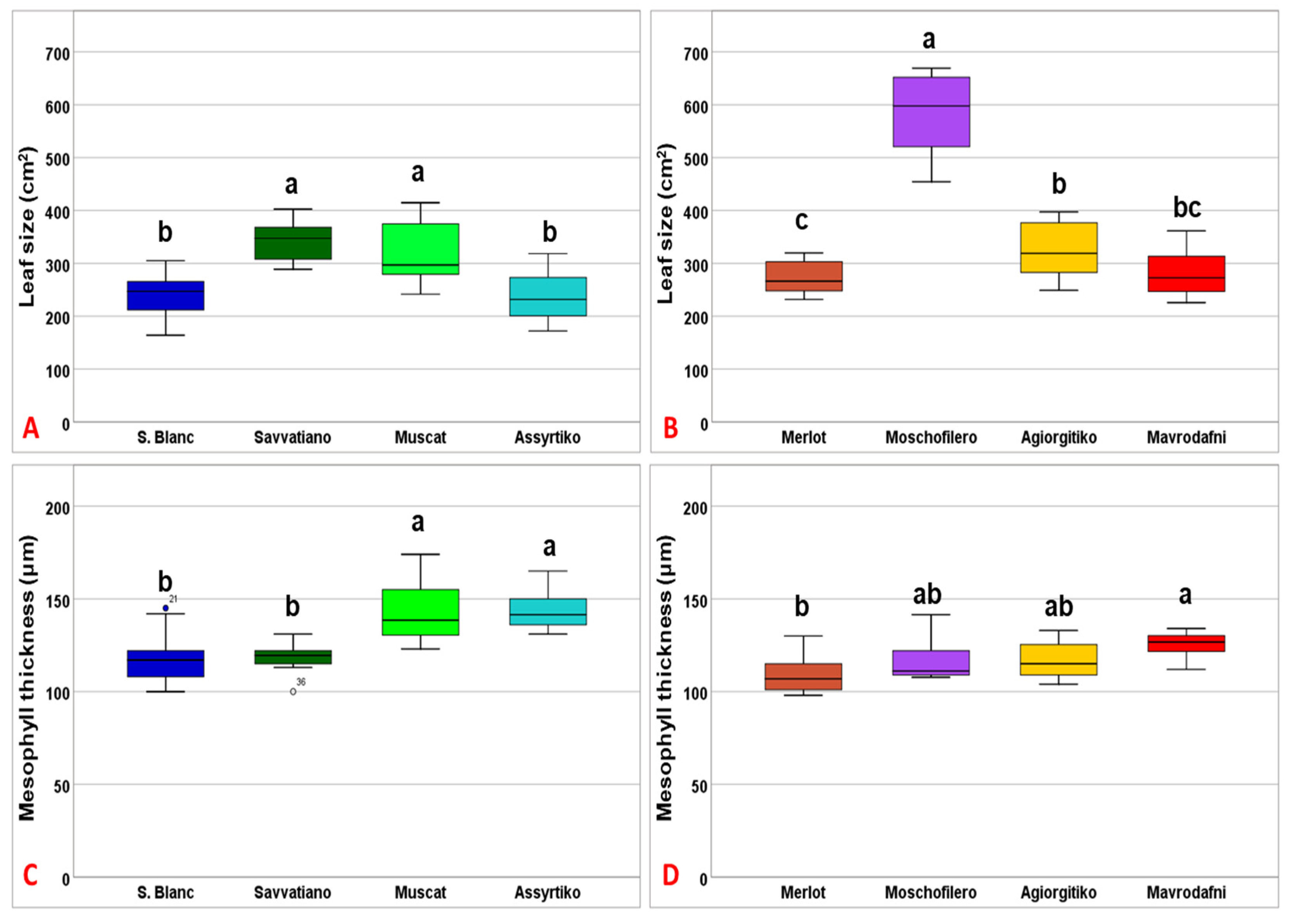
3.5. Leaf Traits Contributing to Thermotolerance
4. Discussion
4.1. Photosynthetic Dynamics Under Light-Saturated Conditions and Heat Stress
4.2. PSII Functionality and Photoprotective Responses Under Heat Stress
4.3. Chlorophyll Reduction as an Adaptive Response to Light and Heat Stress
4.4. The Thermodynamic Role of Leaf Structure and Transpirational Cooling to Light and Heat Stress Management
4.5. The Cultivar-Specific Performances Under Limited Light Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PSII | Photosystem II |
| Gs | Stomatal conductance |
| VPD | Vapour pressure deficit |
| LHCII | Light-harvesting complex |
| PSII-RCs | PSII reaction centers |
| LRCs | Light response curves |
| Asat | Light-saturated photosynthesis |
| Rdark | Dark respiration rate |
| LSP | Light saturation point |
| LCP | Light compensation point |
| An | Net photosynthetic rate |
| ɸ (I0) | Quantum yield at I = 0 [μmol(CO2) μmol (photon)–1] |
| I | Photosynthetic photon flux density [μmol (photon) m–2 s–1] |
| Agmax | Estimate of the maximum gross photosynthetic rate |
| Topt | Optimal temperature for photosynthesis |
| LCC | Leaf chlorophyll content |
| SV | Spad value |
| Fs | Steady-state fluorescence |
| Fm′ | Fluorescence in the light-adapted state |
| ΦPSII | Actual quantum efficiency of PSII |
| Fm | Maximum fluorescence |
| Fo | Minimum fluorescence |
| Fv/Fm | Maximum quantum efficiency of PSII |
| NPQ | Non-photochemical quenching |
| qP | Photochemical quenching |
| Fo’ | Minimum chlorophyll fluorescence in light-adapted leaf |
| Tleaf | Leaf temperature |
| E | Apparent transpiration |
| ROS | Reactive oxygen species. |
References
- Todaro, V.; D’Oria, M.; Secci, D.; Zanini, A.; Tanda, M.G. Climate change over the Mediterranean region: Local temperature and precipitation variations at five pilot sites. Water 2022, 14, 2499. [Google Scholar] [CrossRef]
- Nastos, P.; Saaroni, H. Living in Mediterranean cities in the context of climate change: A review. Int. J. Climatol. 2024, 44, 3169–3190. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine responses to heat stress and global warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef] [PubMed]
- Dinis, L.T.; Bernardo, S.; Yang, C.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Mediterranean viticulture in the context of climate change. Ciênc. Téc. Vitiviníc. 2022, 37, 139–158. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Mitigating heat wave and exposure damage to “Cabernet Sauvignon” wine grape with partial shading under two irrigation amounts. Front. Plant Sci. 2020, 11, 579192. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; Martínez de Toda, F. Strategies in vineyard establishment to face global warming in viticulture: A mini review. J. Sci. Food Agric. 2021, 101, 1261–1269. [Google Scholar] [CrossRef]
- Karim, M.A.; Fracheboud, Y.; Stamp, P. Effect of high temperature on seedling growth and photosynthesis of tropical maize genotypes. J. Agron. Crop Sci. 2000, 184, 217–223. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Havaux, M. Stress tolerance of photosystem II in vivo: Antagonistic effects of water, heat, and photoinhibition stresses. Plant Physiol. 1992, 100, 424–432. [Google Scholar] [CrossRef]
- Ghouil, H.; Montpied, P.; Epron, D.; Ksontini, M.; Hanchi, B.; Dreyer, E. Thermal optima of photosynthetic functions and thermostability of photochemistry in cork oak seedlings. Tree Physiol. 2003, 23, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, Q.; Ci, D.; Shao, X.; Zhang, D. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Kadir, S. Thermostability of photosynthesis of Vitis aestivalis and V. vinifera. J. Am. Soc. Hortic. Sci. 2006, 131, 476–483. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Kim, K.; Portis, A.R., Jr. Temperature dependence of photosynthesis in Arabidopsis plants with modifications in Rubisco activase and membrane fluidity. Plant Cell Physiol. 2005, 46, 522–530. [Google Scholar] [CrossRef]
- García-Cerdán, J.G.; Sveshnikov, D.; Dewez, D.; Jansson, S.; Funk, C.; Schröder, W.P. Antisense inhibition of the PsbX protein affects PSII integrity in the higher plant Arabidopsis thaliana. Plant Cell Physiol. 2009, 50, 191–202. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Matus, J.T.; Gomès, E.; Pascual, I. Toward understanding grapevine responses to climate change: A multi-stress and holistic approach. J. Exp. Bot. 2024, 76, 2949–2969. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef]
- Konrad, W.; Katul, G.; Roth-Nebelsick, A. Leaf temperature and its dependence on atmospheric CO2 and leaf size. Geol. J. 2021, 56, 866–885. [Google Scholar] [CrossRef]
- Galat Giorgi, E.; Sadras, V.O.; Keller, M.; Perez Peña, J. Interactive effects of high temperature and water deficit on Malbec grapevines. Aust. J. Grape Wine Res. 2019, 25, 345–356. [Google Scholar] [CrossRef]
- Soar, C.J.; Collins, M.J.; Sadras, V.O. Irrigated Shiraz vines (Vitis vinifera) upregulate gas exchange and maintain berry growth in response to short spells of high maximum temperature in the field. Funct. Plant Biol. 2009, 36, 801–814. [Google Scholar] [CrossRef]
- Gerganova, M.; Popova, A.V.; Stanoeva, D.; Velitchkova, M. Tomato plants acclimate better to elevated temperature and high light than to treatment with each factor separately. Plant Physiol. Biochem. 2016, 104, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yu, X.; Li, X.; Dos Santos, T.M.; Rosenqvist, E.; Ottosen, C.O. Combined high light and heat stress induced complex response in tomato with better leaf cooling after heat priming. Plant Physiol. Biochem. 2020, 151, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fraga, H.; García de Cortázar Atauri, I.; Malheiro, A.C.; Santos, J.A. Modelling climate change impacts on viticultural yield, phenology and stress conditions in Europe. Glob. Change Biol. 2016, 22, 3774–3788. [Google Scholar] [CrossRef]
- Koufos, G.C.; Mavromatis, T.; Koundouras, S.; Jones, G.V. Adaptive capacity of winegrape varieties cultivated in Greece to climate change: Current trends and future projections. OENO One 2020, 54, 1201–1219. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Abernethy, K.E.; Smart, R.E.; Wilson, R.J.; Maclean, I.M. Climate change impacts and adaptive strategies: Lessons from the grapevine. Glob. Change Biol. 2016, 22, 3814–3828. [Google Scholar] [CrossRef]
- Orduna, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Jones, G.V.; Alves, F. Impact of climate change on wine production: A global overview and regional assessment in the Douro Valley of Portugal. Int. J. Glob. Warm. 2012, 4, 383–406. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Ibañez, V.N.; Berli, F.J.; Masuelli, R.W.; Bottini, R.A.; Marfil, C.F. Influence of altitude and enhanced ultraviolet-B radiation on tuber production, seed viability, leaf pigments and morphology in the wild potato species Solanum kurtzianum Bitter & Wittm collected from an elevational gradient. Plant Sci. 2017, 261, 60–68. [Google Scholar] [CrossRef]
- Arias, L.A.; Berli, F.; Fontana, A.; Bottini, R.; Piccoli, P. Climate change effects on grapevine physiology and biochemistry: Benefits and challenges of high altitude as an adaptation strategy. Front. Plant Sci. 2022, 13, 835425. [Google Scholar] [CrossRef]
- Campbell, J.W.; Aarup, T. Photosynthetically available radiation at high latitudes. Limnol. Oceanogr. 1989, 34, 1490–1499. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Lobo, F.D.A.; De Barros, M.P.; Dalmagro, H.J.; Dalmolin, Â.C.; Pereira, W.E.; De Souza, E.C.; Vourlitis, G.L.; Rodríguez Ortíz, C.E. Fitting net photosynthetic light-response curves with Microsoft Excel—A critical look at the models. Photosynthetica 2013, 51, 445–456. [Google Scholar] [CrossRef]
- June, T.; Evans, J.R.; Farquhar, G.D. A simple new equation for the reversible temperature dependence of photosynthetic electron transport: A study on soybean leaf. Funct. Plant Biol. 2004, 31, 275–283. [Google Scholar] [CrossRef]
- Monje, O.A.; Bugbee, B. Inherent limitations of nondestructive chlorophyll meters: A comparison of two types of meters. HortScience 1992, 27, 69–71. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components–calculation of qP and Fv-/Fm-; without measuring Fo. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Martin, T.N.; Fipke, G.M.; Minussi Winck, J.E.; Márchese, J.A. ImageJ software as an alternative method for estimating leaf area in oats. Acta Agron. 2020, 69, 162–169. [Google Scholar] [CrossRef]
- Luo, D.; Huang, G.; Zhang, Q.; Zhou, G.; Peng, S.; Li, Y. Plasticity of mesophyll cell density and cell wall thickness and composition play a pivotal role in regulating plant growth and photosynthesis under shading in rapeseed. Ann. Bot. 2023, 132, 963–978. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Matthews, J.S.; Simkin, A.J.; Raines, C.A.; Lawson, T. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhang, Q.H.; Shuang, S.P.; Cun, Z.; Wu, H.M.; Chen, J.W. The responses of light reaction of photosynthesis to dynamic sunflecks in a typically shade-tolerant species Panax notoginseng. Front. Plant Sci. 2021, 12, 718981. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: Heidelberg, Germany, 2008; p. 27. [Google Scholar]
- Wang, X.Q.; Zeng, Z.L.; Li, Y.Y.; Huang, W. Photoinhibition and photosynthetic regulation in fluctuating light under compound stresses of drought and heat. Physiol. Plant. 2024, 176, 14406. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Zhang, L.; Liu, J. Heat stress, especially when coupled with high light, accelerates the decline of tropical seagrass (Enhalus acoroides) meadows. Mar. Pollut. Bull. 2023, 192, 115043. [Google Scholar] [CrossRef]
- Saewong, C.; Ow, Y.X.; Nualla-Ong, A.; Buapet, P. Comparative effects of heat stress on photosynthesis and oxidative stress in Halophila ovalis and Thalassia hemprichii under different light conditions. Mar. Environ. Res. 2024, 199, 106589. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Wang, H.; Yang, X.; Zhai, H.; Du, Y. Stimulation of cyclic electron flow around PSI as a response to the combined stress of high light and high temperature in grape leaves. Funct. Plant Biol. 2018, 45, 1038–1045. [Google Scholar] [CrossRef]
- Sun, Y.; Hao, G.; Gao, Y.; Du, Y.; Yang, X.; Zhai, H. Increasing cyclic electron flow mediated by NDH is related to heat tolerance under low light in grape leaves. PeerJ 2017, 5, 3147v1. [Google Scholar]
- Sun, Y.; Wang, Q.; Xiao, H.; Cheng, J. Low light facilitates cyclic electron flows around PSI to assist PSII against high temperature stress. Plants 2022, 11, 3537. [Google Scholar] [CrossRef]
- He, Y.; Yadav, V.; Bai, S.; Wu, J.; Zhou, X.; Zhang, W.; Han, S.; Wang, M.; Zeng, B.; Wu, X.; et al. Performance evaluation of new table grape varieties under high light intensity conditions based on the photosynthetic and chlorophyll fluorescence characteristics. Horticulturae 2023, 9, 1035. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Phenotyping of wheat cultivars for heat tolerance using chlorophyll a fluorescence. Funct. Plant Biol. 2012, 39, 936–947. [Google Scholar] [CrossRef]
- Van der Westhuizen, M.M.; Oosterhuis, D.M.; Berner, J.M.; Boogaers, N. Chlorophyll a fluorescence as an indicator of heat stress in cotton (Gossypium hirsutum L.). S. Afr. J. Plant Soil 2020, 37, 116–119. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Xie, M.; Zhao, Z.; Yang, L.; Liu, J.; Hou, D. Estimation of Fv/Fm in spring wheat using UAV-Based multispectral and RGB imagery with multiple machine learning methods. Agronomy 2023, 13, 1003. [Google Scholar] [CrossRef]
- Beris, E.; Venios, X.; Papachristos, D.; Ponchon, M.; Kontodimas, D.; Korkas, E.; Banilas, G.; Reineke, A. Grapevine Responses to the Entomopathogenic Fungi Beauveria bassiana and Isaria fumosorosea and the Effects of Salicylic Acid on Their Virulence Against the European Grapevine Moth, Lobesia botrana. Microorganisms 2025, 13, 1630. [Google Scholar] [CrossRef] [PubMed]
- Percival, G.C.; Percival, C.D. Evaluation of Heat Tolerance in Foliar Tissue of Acer Genotypes. Arboric. Urban For. 2024, 50, 157–168. [Google Scholar]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef]
- Nicol, L.; Nawrocki, W.J.; Croce, R. Disentangling the sites of non-photochemical quenching in vascular plants. Nat. Plants 2019, 5, 1177–1183. [Google Scholar] [CrossRef]
- Qiu, Q.; Sun, Y.; Guo, D.; Wang, L.; Pagay, V.; Wang, S. Heat Stress Downregulates Photosystem I Redox State on Leaf Photosynthesis in Grapevine. Agronomy 2025, 15, 948. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Chen, L.S.; Cheng, L. Both xanthophyll cycle-dependent thermal dissipation and the antioxidant system are up-regulated in grape (Vitis labrusca L. cv. Concord) leaves in response to N limitation. J. Exp. Bot. 2003, 54, 2165–2175. [Google Scholar] [CrossRef]
- Aazami, M.A.; Asghari-Aruq, M.; Hassanpouraghdam, M.B.; Ercisli, S.; Baron, M.; Sochor, J. Low temperature stress mediates the antioxidants pool and chlorophyll fluorescence in Vitis vinifera L. cultivars. Plants 2021, 10, 1877. [Google Scholar] [CrossRef]
- Chen, J.W.; Kuang, S.B.; Long, G.Q.; Yang, S.C.; Meng, Z.G.; Li, L.G.; Chen, Z.J.; Zhang, G.H. Photosynthesis, light energy partitioning, and photoprotection in the shade-demanding species Panax notoginseng under high and low level of growth irradiance. Funct. Plant Biol. 2016, 43, 479–491. [Google Scholar] [CrossRef]
- Singsaas, E.L.; Ort, D.R.; Delucia, E.H. Elevated CO2 effects on mesophyll conductance and its consequences for interpreting photosynthetic physiology. Plant Cell Environ. 2004, 27, 41–50. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Liu, M.; Fang, Y.L. Effects of heat stress on physiological indexes and ultrastructure of grapevines. Sci. Agric. Sin. 2020, 53, 1444–1458. [Google Scholar]
- Kolyva, F.; Rhizopoulou, S.; Meletiou-Christou, M.S.; Stratakis, E. Physiological characteristics of expanding and expanded leaves of Vitis vinifera L. cv. Assyrtiko in climate change conditions. Biol. Life Sci. Forum 2020, 4, 55. [Google Scholar] [CrossRef]
- Ferrini, F.; Mattii, G.B.; Nicese, F.P. Effect of temperature on key physiological responses of grapevine leaf. Am. J. Enol. Vitic. 1995, 46, 375–379. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, Z.Q.; Lee, K.W. Photosynthetic and physiological responses to high temperature in grapevine (Vitis vinifera L.) leaves during the seedling stage. J. Hortic. Sci. Biotechnol. 2017, 92, 2–10. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, Y.; Liu, S.; Wang, J.; Zhu, S.; Chen, G.; Hou, J.; Wang, C. Influence of high temperature on photosynthesis, antioxidative capacity of chloroplast, and carbon assimilation among heat-tolerant and heat-susceptible genotypes of nonheading Chinese cabbage. HortScience 2017, 52, 1464–1470. [Google Scholar] [CrossRef]
- Gonçalves, J.F.D.C.; Barreto, D.C.D.S.; Santos Junior, U.M.D.; Fernandes, A.V.; Sampaio, P.D.T.B.; Buckeridge, M.S. Growth, photosynthesis and stress indicators in young rosewood plants (Aniba rosaeodora Ducke) under different light intensities. Braz. J. Plant Physiol. 2005, 17, 325–334. [Google Scholar] [CrossRef]
- Ma, Z.; Li, S.; Zhang, M.; Jiang, S.; Xiao, Y. Light intensity affects growth, photosynthetic capability, and total flavonoid accumulation of Anoectochilus plants. HortScience 2010, 45, 863–867. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shen, J.; Takahashi, Y. Unraveling the molecular dynamics of thylakoids under light stress. Plant Cell Physiol. 2014, 55, 1203–1205. [Google Scholar] [CrossRef]
- Kirchhoff, H. Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos. Trans. R. Soc. B 2014, 369, 20130225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Tardy, F. Loss of chlorophyll with limited reduction of photosynthesis as an adaptive response of Syrian barley landraces to high-light and heat stress. Funct. Plant Biol. 1999, 26, 569–578. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Huang, X.; Xing, J.; Yao, J.; Yin, T.; Jiang, J.; Wang, P.; Xu, B. STAYGREEN-mediated chlorophyll a catabolism is critical for photosystem stability during heat-induced leaf senescence in perennial ryegrass. Plant Cell Environ. 2022, 45, 1412–1427. [Google Scholar] [CrossRef]
- MacMillan, P.; Teixeira, G.; Lopes, C.M.; Monteiro, A. The role of grapevine leaf morphoanatomical traits in determining capacity for coping with abiotic stresses: A review. Ciênc. Téc. Vitiviníc. 2021, 36, 75–88. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, L.; Zhu, S.; Hou, J.; Chen, G.; Liu, F.; Liu, S.; Wang, C. Influence of heat stress on leaf morphology and nitrogen–carbohydrate metabolisms in two wucai (Brassica campestris L.) genotypes. Acta Soc. Bot. Pol. 2017, 86, 3554. [Google Scholar] [CrossRef]
- Tserej, O.; Feeley, K.J. Variation in leaf temperatures of tropical and subtropical trees are related to leaf thermoregulatory traits and not geographic distributions. Biotropica 2021, 53, 868–878. [Google Scholar] [CrossRef]
- Slot, M.; Cala, D.; Aranda, J.; Virgo, A.; Michaletz, S.T.; Winter, K. Leaf heat tolerance of 147 tropical forest species varies with elevation and leaf functional traits, but not with phylogeny. Plant Cell Environ. 2021, 44, 2414–2427. [Google Scholar] [CrossRef]
- Tang, S.; Lin, X.; Li, W.; Guo, C.; Han, J.; Yu, L. Nutrient resorption responses of female and male Populus cathayana to drought and shade stress. Physiol. Plant. 2023, 175, 13980. [Google Scholar] [CrossRef] [PubMed]
- Salem-Fnayou, A.B.; Bouamama, B.; Ghorbel, A.; Mliki, A. Investigations on the leaf anatomy and ultrastructure of grapevine (Vitis vinifera) under heat stress. Microsc. Res. Tech. 2011, 74, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, J.; Zhao, T.H.; Cao, Y.; Wang, G.; Sun, B.; Yan, X.; Guo, W.; Li, M.H. The smaller the leaf is, the faster the leaf water loses in a temperate forest. Front. Plant Sci. 2019, 10, 58. [Google Scholar] [CrossRef]
- Leigh, A.; Sevanto, S.; Close, J.D.; Nicotra, A.B. The influence of leaf size and shape on leaf thermal dynamics: Does theory hold up under natural conditions? Plant Cell Environ. 2017, 40, 237–248. [Google Scholar] [CrossRef]
- Lusk, C.H.; Grierson, E.R.; Laughlin, D.C. Large leaves in warm, moist environments confer an advantage in seedling light interception efficiency. New Phytol. 2019, 223, 1319–1327. [Google Scholar] [CrossRef]
- Gago, P.; Conejero, G.; Martínez, M.C.; This, P.; Verdeil, J.L. Comparative anatomy and morphology of the leaves of grenache noir and syrah grapevine cultivars. S. Afr. J. Enol. Vitic. 2019, 40, 132–140. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Leaf size and leaf area index in Fagus sylvatica forests: Competing effects of precipitation, temperature, and nitrogen availability. Ecosystems 2008, 11, 655–669. [Google Scholar] [CrossRef]
- Tozer, W.C.; Rice, B.; Westoby, M. Evolutionary divergence of leaf width and its correlates. Am. J. Bot. 2015, 102, 367–378. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Kang, H.; Tang, Y. Reduction in leaf size at higher altitudes across 39 broad-leaved herbaceous species on the northeastern Qinghai-Tibetan Plateau. J. Plant Ecol. 2022, 15, 1227–1240. [Google Scholar] [CrossRef]
- Deva, C.R.; Urban, M.O.; Challinor, A.J.; Falloon, P.; Svitákova, L. Enhanced leaf cooling is a pathway to heat tolerance in common bean. Front. Plant Sci. 2020, 11, 19. [Google Scholar] [CrossRef]
- Guo, J.J.; Gong, X.W.; Hao, G.Y. Leaf Transpirational Cooling and Thermal Tolerance Vary Along the Spectrum of Iso-Anisohydric Stomatal Regulation in Sand-Fixing Shrubs. Plant Cell Environ. 2025, 48, 2053–2066. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Weiser, M.D.; McDowell, N.G.; Zhou, J.; Kaspari, M.; Helliker, B.R.; Enquist, B.J. The energetic and carbon economic origins of leaf thermoregulation. Nat. Plants 2016, 2, 16129. [Google Scholar] [CrossRef]
- Monteiro, M.V.; Blanuša, T.; Verhoef, A.; Hadley, P.; Cameron, R.W. Relative importance of transpiration rate and leaf morphological traits for the regulation of leaf temperature. Aust. J. Bot. 2016, 64, 32–44. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Zhang, H.; Fu, P.; Fan, Z. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 2017, 31, 2202–2211. [Google Scholar] [CrossRef]
- Millan, M.; Simonneau, T.; Coupe-Ledru, A.; Boulord, R.; Christophe, A.; Pallas, B. Relationships between leaf temperature, stomatal conductance and architecture: Potential impact on leaf burning among a range of genotypes in grapevine. OENO One 2023, 57, 345–359. [Google Scholar] [CrossRef]
- Porch, T.G.; Hall, A.E. Heat tolerance. In Genomics and Breeding for Climate-Resilient Crops; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 2, pp. 167–202. [Google Scholar]
- Kibler, C.L.; Trugman, A.T.; Roberts, D.A.; Still, C.J.; Scott, R.L.; Caylor, K.K.; Stella, J.C.; Singer, M.B. Evapotranspiration regulates leaf temperature and respiration in dryland vegetation. Agric. For. Meteorol. 2023, 339, 109560. [Google Scholar] [CrossRef]
- Venios, X.; Banilas, G.; Beris, E.; Biniari, K.; Korkas, E. Physiological Efficiency and Adaptability of Greek Indigenous Grapevine Cultivars Under Heat Stress and Elevated CO2: Insights into Photosynthetic Dynamics. Plants 2025, 14, 2518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, P.; Wang, J. Effects of light intensity and temperature on the photosynthesis characteristics and yield of lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, J.; Dayananda, B.; Li, J. Effect of light intensities on the photosynthesis, growth and physiological performances of two maple species. Front. Plant Sci. 2022, 13, 999026. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Reich, P.B. Leaf-level light compensation points in shade-tolerant woody seedlings. New Phytol. 2005, 166, 710–713. [Google Scholar] [CrossRef]
- Miccichè, D.; De Rosas, M.I.; Ferro, M.V.; Di Lorenzo, R.; Puccio, S.; Pisciotta, A. Effects of artificial canopy shading on vegetative growth and ripening processes of cv. Nero d’Avola (Vitis vinifera L.). Front. Plant Sci. 2023, 14, 1210574. [Google Scholar] [CrossRef]
- Louarn, G.; Dauzat, J.; Lecoeur, J.; Lebon, E. Influence of trellis system and shoot positioning on light interception and distribution in two grapevine cultivars with different architectures: An original approach based on 3D canopy modelling. Aust. J. Grape Wine Res. 2008, 14, 143–152. [Google Scholar] [CrossRef]
- Faralli, M.; Zanzotti, R.; Bertamini, M. Maintaining canopy density under summer stress conditions retains PSII efficiency and modulates must quality in Cabernet franc. Horticulturae 2022, 8, 679. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Villalobos-Soublett, E.; Garrido-Salinas, M.; Verdugo-Vásquez, N. Monofilament shading nets improved water use efficiency on high-temperature days in grapevines subjected to hyperarid conditions. Horticulturae 2024, 10, 176. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Lider, L.A. Influence of cluster exposure to the sun on the composition of Thompson Seedless fruit. Am. J. Enol. Vitic. 1968, 19, 175–184. [Google Scholar] [CrossRef]
- Cartechini, A.; Palliotti, A. Effect of shading on vine morphology and productivity and leaf gas exchange characteristics in grapevines in the field. Am. J. Enol. Vitic. 1995, 46, 227–234. [Google Scholar] [CrossRef]
- Chorti, E.; Guidoni, S.; Ferrandino, A.; Novello, V. Effect of different cluster sunlight exposure levels on ripening and anthocyanin accumulation in Nebbiolo grapes. Am. J. Enol. Vitic. 2010, 61, 23–30. [Google Scholar] [CrossRef]
- Caravia, L.; Collins, C.; Petrie, P.R.; Tyerman, S.D. Application of shade treatments during Shiraz berry ripening to reduce the impact of high temperature. Aust. J. Grape Wine Res. 2016, 22, 422–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venios, X.; Banilas, G.; Beris, E.; Biniari, K.; Korkas, E. Heat Stress Tolerance and Photosynthetic Responses to Transient Light Intensities of Greek Grapevine Cultivars. Agronomy 2025, 15, 2344. https://doi.org/10.3390/agronomy15102344
Venios X, Banilas G, Beris E, Biniari K, Korkas E. Heat Stress Tolerance and Photosynthetic Responses to Transient Light Intensities of Greek Grapevine Cultivars. Agronomy. 2025; 15(10):2344. https://doi.org/10.3390/agronomy15102344
Chicago/Turabian StyleVenios, Xenophon, Georgios Banilas, Evangelos Beris, Katerina Biniari, and Elias Korkas. 2025. "Heat Stress Tolerance and Photosynthetic Responses to Transient Light Intensities of Greek Grapevine Cultivars" Agronomy 15, no. 10: 2344. https://doi.org/10.3390/agronomy15102344
APA StyleVenios, X., Banilas, G., Beris, E., Biniari, K., & Korkas, E. (2025). Heat Stress Tolerance and Photosynthetic Responses to Transient Light Intensities of Greek Grapevine Cultivars. Agronomy, 15(10), 2344. https://doi.org/10.3390/agronomy15102344





