Evaluation of Yield-Related Morphological, Physiological, Agronomic, and Nutrient Uptake Traits of Grain Sorghum Varieties in the Kerala Region (India)
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Attributes
2.2. Agronomic Traits and Yield
2.3. Physiological Measurements
2.4. Nutrient Content in Plants and Uptake from the Soil
2.5. Statistical Analysis
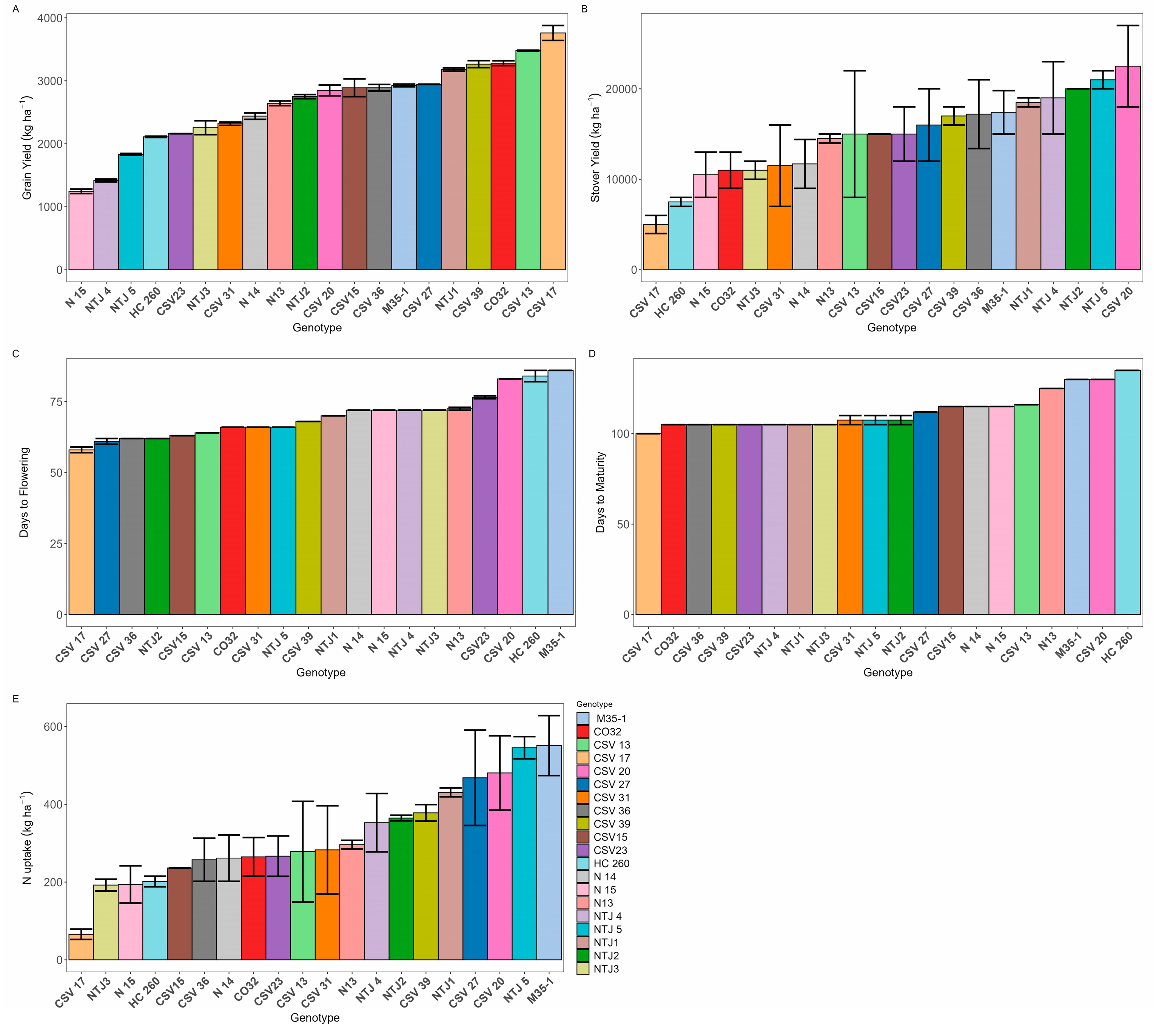
3. Results
3.1. Trait Variation
3.2. Influence of Leaf Morphology and Physiology on Grain and Stover Yield
3.3. Trait Correlation Analysis
3.4. Contribution of Component Traits
4. Discussion
4.1. Determinants of Increased Yield in Sorghum
4.2. Influence of Leaf Morphology and Physiology on Yield
4.3. Screening Varieties for Water Use Efficiency in Southern Indian Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAS | Days after sowing |
| DTF | Days to 50% flowering |
| DTM | Days to maturity |
| GWP | Grain weight per panicle |
| GY | Grain yield |
| HI | Harvest index |
| iWUE | Intrinsic water use efficiency |
| K | Potassium |
| LAD | Leaf area duration |
| LAI | Leaf area index |
| LN | Number of leaves |
| LW | Leaf width |
| N | Nitrogen |
| P | Phosphorous |
| PH | Plant height |
| PL | Panicle length |
| RC | Relative chlorophyll |
| SY | Stover yield |
| Tleaf | Leaf temperature |
| TW | Test weight |
| ΔT | Leaf temperature differential |
| ΦNPQ | Non-photochemical quenching |
| ΦPSII | Quantum yield of photosystem II |
References
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Gornall, J.; Betts, R.; Burke, E.; Clark, R.; Camp, J.; Willett, K.; Wiltshire, A. Implications of climate change for agricultural productivity in the early twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2973–2989. [Google Scholar] [CrossRef] [PubMed]
- Challinor, A.; Wheeler, T.; Garforth, C.; Craufurd, P.; Kassam, A. Assessing the vulnerability of food crop systems in Africa to climate change. Clim. Change 2007, 83, 381–399. [Google Scholar] [CrossRef]
- Hochman, Z.; Horan, H.; Reddy, D.R.; Sreenivas, G.; Tallapragada, C.; Adusumilli, R.; Gaydon, D.; Singh, K.K.; Roth, C.H. Smallholder farmers managing climate risk in India: 1. Adapting to a variable climate. Agric. Syst. 2017, 150, 54–66. [Google Scholar] [CrossRef]
- Gezie, M. Farmer’s response to climate change and variability in Ethiopia: A review. Cogent Food Agric. 2019, 5, 1613770. [Google Scholar] [CrossRef]
- Cohn, A.S.; Newton, P.; Gil, J.D.; Kuhl, L.; Samberg, L.; Ricciardi, V.; Manly, J.R.; Northrop, S. Smallholder agriculture and climate change. Annu. Rev. Environ. Resour. 2017, 42, 347–375. [Google Scholar] [CrossRef]
- Acevedo, M.; Pixley, K.; Zinyengere, N.; Meng, S.; Tufan, H.; Cichy, K.; Bizikova, L.; Isaacs, K.; Ghezzi-Kopel, K.; Porciello, J. A scoping review of adoption of climate-resilient crops by small-scale producers in low-and middle-income countries. Nat. Plants 2020, 6, 1231–1241. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Pret, V.; Falconnier, G.N.; Affholder, F.; Corbeels, M.; Chikowo, R.; Descheemaeker, K. Farm resilience to climatic risk. A review. Agron. Sustain. Dev. 2025, 45, 10. [Google Scholar] [CrossRef]
- Reddy, B.V.; Ramesh, S.; Reddy, P.S.; Kumar, A.A.; Tropics, S.-A. Genetic enhancement for drought tolerance in sorghum. Plant Breed. Rev. 2009, 31, 189–222. [Google Scholar]
- Watson-Lazowski, A.; Ghannoum, O. Chapter 9 The Outlook for C4 Crops in Future Climate Scenarios. In Photosynthesis, Respiration, and Climate Change; Springer: Berlin/Heidelberg, Germany, 2021; pp. 251–281. [Google Scholar]
- Hossain, M.S.; Islam, M.N.; Rahman, M.M.; Mostofa, M.G.; Khan, M.A.R. Sorghum: A prospective crop for climatic vulnerability, food and nutritional security. J. Agric. Food Res. 2022, 8, 100300. [Google Scholar] [CrossRef]
- Regassa, T.H.; Wortmann, C.S. Sweet sorghum as a bioenergy crop: Literature review. Biomass Bioenerg. 2014, 64, 348–355. [Google Scholar] [CrossRef]
- Joshi, A.; Gami, R.; Patel, R.; Arvinth, S. Elucidation of gene action and combining ability for grain and fodder yield and contributing traits in sorghum [Sorghum bicolor (L.) Moench]. Electron. J. Plant Breed. 2022, 13, 75–82. [Google Scholar] [CrossRef]
- Mohammed, R.; Are, A.K.; Bhavanasi, R.; Munghate, R.S.; Kavi Kishor, P.B.; Sharma, H.C. Quantitative genetic analysis of agronomic and morphological traits in sorghum, Sorghum bicolor. Front. Plant Sci. 2015, 6, 159278. [Google Scholar] [CrossRef]
- Barbosa, M.A.M.; Kuki, K.N.; Bengala, P.S.P.; Pereira, E.d.S.; de Barros, A.F.; Montoya, S.G.; Pimentel, L.D. Phenological and physiological evaluation of first and second cropping periods of sorghum and maize crops. J. Agron. Crop Sci. 2020, 206, 263–276. [Google Scholar] [CrossRef]
- Hariprasanna, K.; Rakshit, S. Economic importance of sorghum. In The Sorghum Genome; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–25. [Google Scholar]
- Liang, G.; Chu, C.; Reddi, N.; Lin, S.; Dayton, A. Leaf Blade Areas of Grain Sorghum Varieties and Hybrids 1. Agron. J. 1973, 65, 456–459. [Google Scholar] [CrossRef]
- Pan, L.; George-Jaeggli, B.; Borrell, A.; Jordan, D.; Koller, F.; Al-Salman, Y.; Ghannoum, O.; Cano, F.J. Coordination of stomata and vein patterns with leaf width underpins water-use efficiency in a C4 crop. Plant Cell Environ. 2022, 45, 1612–1630. [Google Scholar] [CrossRef]
- Al-Salman, Y.; Ghannoum, O.; Cano, F.J. Elevated [CO2] negatively impacts C4 photosynthesis under heat and water stress without penalizing biomass. J. Exp. Bot. 2023, 74, 2875–2890. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Ul-Allah, S.; Siddique, K.H. Physiological and agronomic approaches for improving water-use efficiency in crop plants. Agric. Water Manag. 2019, 219, 95–108. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef]
- Cai, F.; Zhang, Y.; Mi, N.; Ming, H.; Zhang, S.; Zhang, H.; Zhao, X. Maize (Zea mays L.) Physiological Responses to Drought and Rewatering, and the Associations with Water Stress Degree. Agric. Water Manag. 2020, 241, 106379. [Google Scholar] [CrossRef]
- Tardieu, F.; Parent, B.; Caldeira, C.F.; Welcker, C. Genetic and physiological controls of growth under water deficit. Plant Physiol. 2014, 164, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yu, G.; Fu, Y.; Sun, X.; Li, Y.; Shi, P.; Wang, Y.; Zheng, Z. Effects of vegetation control on ecosystem water use efficiency within and among four grassland ecosystems in China. Glob. Change Biol. 2008, 14, 1609–1619. [Google Scholar] [CrossRef]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Al-Salman, Y.; Cano, F.J.; Pan, L.; Koller, F.; Piñeiro, J.; Jordan, D.; Ghannoum, O. Anatomical drivers of stomatal conductance in sorghum lines with different leaf widths grown under different temperatures. Plant Cell Environ. 2023, 46, 2142–2158. [Google Scholar] [CrossRef]
- Al-Salman, Y.; Cano, F.J.; Mace, E.; Jordan, D.; Groszmann, M.; Ghannoum, O. High water use efficiency due to maintenance of photosynthetic capacity in sorghum under water stress. J. Exp. Bot. 2024, 75, 6778–6795. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Zhu, T.; Fonseca De Lima, C.F.; De Smet, I. The heat is on: How crop growth, development, and yield respond to high temperature. J. Exp. Bot. 2021, 72, 7359–7373. [Google Scholar] [CrossRef]
- Elangovan, M.; Prabhakar, P.; Chandra Sekara Reddy, D. Characterization and evaluation of Sorghum [Sorghum bicolor (L.) Moench] germplasm from Karnataka, India. Karnataka J. Agric. Sci. 2007, 20, 840–842. [Google Scholar]
- Boomiraj, K.; Wani, S.P.; Agrawal, P. Impact of climate change on dryland Sorghum in India. In Use of High Science Tools in Integrated Watershed Management, Proceedings of the National Symposium; International Crops Research Institute for the Semi-Arid Tropics (ICRI-SAT): Hyderabad, India, 2011. [Google Scholar] [CrossRef]
- Rakshit, S.; Gomashe, S.S.; Ganapathy, K.; Elangovan, M.; Ratnavathi, C.; Seetharama, N.; Patil, J. Morphological and molecular diversity reveal wide variability among sorghum Maldandi landraces from India. J. Plant Biochem. Biotech. 2012, 21, 145–156. [Google Scholar] [CrossRef]
- Kapanigowda, M.H.; Perumal, R.; Djanaguiraman, M.; Aiken, R.M.; Tesso, T.; Prasad, P.V.; Little, C.R. Genotypic variation in sorghum [Sorghum bicolor (L.) Moench] exotic germplasm collections for drought and disease tolerance. SpringerPlus 2013, 2, 650. [Google Scholar] [CrossRef] [PubMed]
- Maruthamuthu, E.; Venkatesh, K.; Bellundagi, A.; Pandey, S.; Pandey, C.D. Assessment of variability in sorghum [Sorghum bicolor (L.) Moench] germplasm for agro-morphological traits. Electron. J. Plant Breed. 2022, 13, 488–497. [Google Scholar] [CrossRef]
- Seetharam, K.; Ganesamurthy, K. Characterization of sorghum genotypes for yield and other agronomic traits through genetic variability and diversity analysis. Electron. J. Plant Breed. 2013, 4, 1073–1079. [Google Scholar]
- Elsahookie, M.; Cheyed, S. Estimating sorghum leaf area by measuring one leaf length. Iraq. J. Agric. Sci. 2014, 45, 1–5. [Google Scholar]
- Peltonen-Sainio, P.; Forsman, K.; Poutala, T. Crop Management Effects on Pre-and Post-Anthesis Changes in Leaf Area Index and Leaf Area Duration and their Contribution to Grain Yield and Yield Components in Spring Cereals. J. Agron. Crop Sci. 1997, 179, 47–61. [Google Scholar] [CrossRef]
- Summerfield, R.; Lawn, R.; Qi, A.e.a.; Ellis, R.; Roberts, E.; Chay, P.; Brouwer, J.; Rose, J.; Shanmugasundaram, S.; Yeates, S.J. Towards the reliable prediction of time to flowering in six annual crops. II. Soyabean (Glycine max). Exp. Agric. 1993, 29, 253–289. [Google Scholar] [CrossRef]
- Teixeira, T.P.M.; Pimentel, L.D.; dos Santos Dias, L.A.; da Costa Parrella, R.A.; da Paixão, M.Q.; Biesdorf, E.M. Redefinition of sweet sorghum harvest time: New approach for sampling and decision-making in field. Ind. Crops Prod. 2017, 109, 579–586. [Google Scholar] [CrossRef]
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P. MultispeQ Beta: A tool for large-scale plant phenotyping connected to the open PhotosynQ network. R. Soc. Open Sci. 2016, 3, 160592. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India. Pvt. Ltd.: New Delhi, India, 1973; 498p, ISBN 978-93-83692-35-4. [Google Scholar]
- Gopinath, P.P.; Parsad, R.; Joseph, B.; Adarsh, V.S. GRAPES: General Rshiny Based Analysis Platform Empowered by Statistics. 2020. Available online: https://www.kaugrapes.com/home (accessed on 10 March 2022).
- Hariprasanna, K.; Suresh, P.; Amasiddha, B.; Deepika, C.; Dalvi, U.; Chavan, U.; Jadhav, A.; Ratnavathi, C.; Venkateswarlu, R. Report of the AICRP on Sorghum Coordinating Team; All India Coordinated Research Project on Sorghum, Indian Institute of Millets Research (IIMR): Hyderabad, India, 2018; 78p, Available online: https://www.millets.res.in/aicsip17/reports/rb/AICRP_Sorghum_Coordinating_Team_report.pdf (accessed on 10 March 2022).
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef]
- Yin, X.; Schapendonk, A.H.; Struik, P.C. Exploring the optimum nitrogen partitioning to predict the acclimation of C3 leaf photosynthesis to varying growth conditions. J. Exp. Bot. 2019, 70, 2435–2447. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, E.; Ceccanti, C.; Guidi, L.; Landi, M. Role of beneficial elements in plants: Implications for the photosynthetic process. Photosynthetica 2021, 59, 349–360. [Google Scholar] [CrossRef]
- Choudhary, S.; Mutava, R.N.; Shekoofa, A.; Sinclair, T.R.; Prasad, P.V. Is the stay-green trait in sorghum a result of transpiration sensitivity to either soil drying or vapor pressure deficit? Crop Sci. 2013, 53, 2129–2134. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Lu, Y.; Liao, Y.; Nie, J.; Yuan, X.; Chen, F. Use of a leaf chlorophyll content index to improve the prediction of above-ground biomass and productivity. PeerJ 2019, 6, e6240. [Google Scholar] [CrossRef] [PubMed]
- Sales, C.R.; Wang, Y.; Evers, J.B.; Kromdijk, J. Improving C4 photosynthesis to increase productivity under optimal and suboptimal conditions. J. Exp. Bot. 2021, 72, 5942–5960. [Google Scholar] [CrossRef]
- Vadez, V.; Deshpande, S.P.; Kholova, J.; Hammer, G.L.; Borrell, A.K.; Talwar, H.S.; Hash, C.T. Stay-green quantitative trait loci’s effects on water extraction, transpiration efficiency and seed yield depend on recipient parent background. Functional Plant Biol. 2011, 38, 553–566. [Google Scholar] [CrossRef]
- Parker, G.G. Tamm review: Leaf Area Index (LAI) is both a determinant and a consequence of important processes in vegetation canopies. For. Ecol. Manag. 2020, 477, 118496. [Google Scholar] [CrossRef]
- Blancon, J.; Buet, C.; Dubreuil, P.; Tixier, M.-H.; Baret, F.; Praud, S. Maize green leaf area index dynamics: Genetic basis of a new secondary trait for grain yield in optimal and drought conditions. Theor. Appl. Genet. 2024, 137, 68. [Google Scholar] [CrossRef]
- Richards, R. Manipulation of leaf area and its effect on grain yield in droughted wheat. Aust. J. Agric. Res. 1983, 34, 23–31. [Google Scholar] [CrossRef]
- Kantolic, A.G.; Mercau, J.L.; Slafer, G.A.; Sadras, V.O. Simulated yield advantages of extending post-flowering development at the expense of a shorter pre-flowering development in soybean. Field Crops Res. 2007, 101, 321–330. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; De Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Worland, B.; Robinson, N.; Jordan, D.; Schmidt, S.; Godwin, I. Post-anthesis nitrate uptake is critical to yield and grain protein content in Sorghum bicolor. J. Plant Physiol. 2017, 216, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Jadav, N.J.; Kumar, D.; Raval, C.H.; Chaudhari, D.; Chaudhary, N. Effect of different nutrient management practices on growth, yield attributes and yield of transplanted pearl millet (Pennisetum glaucum L.). Int. J. Plant Soil Sci. 2021, 33, 260–266. [Google Scholar] [CrossRef]
- Meena, L.; Meena, S.L. Production potential, nutrient uptake, economics and soil properties as influenced by fodder sorghum (Sorghum bicolor) cultivars, nitrogen levels and FYM under semiarid condition of Rajasthan. Range Manag. Agrofor. 2012, 33, 171–176, ISSN: 2249-5231. [Google Scholar]
- Velikova, V.; Arena, C.; Izzo, L.G.; Tsonev, T.; Koleva, D.; Tattini, M.; Roeva, O.; De Maio, A.; Loreto, F. Functional and structural leaf plasticity determine photosynthetic performances during drought stress and recovery in two Platanus orientalis populations from contrasting habitats. Int. J. Mol. Sci. 2020, 21, 3912. [Google Scholar] [CrossRef]
- Al-Salman, Y.; Ghannoum, O.; Cano, F.J. Midday water use efficiency in sorghum is linked to faster stomatal closure rate, lower stomatal aperture and higher stomatal density. Plant J. 2023, 115, 1661–1676. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. Adv. Agron. 2019, 156, 109–157. [Google Scholar] [CrossRef]
- Kenney, A.M.; McKay, J.K.; Richards, J.H.; Juenger, T.E. Direct and indirect selection on flowering time, water-use efficiency (WUE, δ13C), and WUE plasticity to drought in Arabidopsis thaliana. Ecol. Evol. 2014, 4, 4505–4521. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.; Rebetzke, G.; Farquhar, G. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef]
- Cano, F.J.; Sharwood, R.E.; Cousins, A.B.; Ghannoum, O. The role of leaf width and conductances to CO2 in determining water use efficiency in C4 grasses. New Phytol. 2019, 223, 1280–1295. [Google Scholar] [CrossRef]
- Al-Salman, Y. Determinants of Leaf Water Use Efficiency in the C4 Crop Sorghum bicolor. Ph.D. Thesis, Western Sydney University, Sydney, Australia, 2021. [Google Scholar] [CrossRef]
- Zhi, X.; Hammer, G.; Borrell, A.; Tao, Y.; Wu, A.; Hunt, C.; van Oosterom, E.; Massey-Reed, S.R.; Cruickshank, A.; Potgieter, A.B. Genetic basis of sorghum leaf width and its potential as a surrogate for transpiration efficiency. Theor. Appl. Genet. 2022, 135, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, D. Agronomic and physiological responses of sorghum, maize and pearl millet to irrigation. Field Crops Res. 1995, 42, 57–67. [Google Scholar] [CrossRef]
- Zhi, X.; Tao, Y.; Jordan, D.; Borrell, A.; Hunt, C.; Cruickshank, A.; Potgieter, A.; Wu, A.; Hammer, G.; George-Jaeggli, B. Genetic control of leaf angle in sorghum and its effect on light interception. J. Exp. Bot. 2022, 73, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Effects of shading on morphology, physiology and grain yield of winter wheat. Eur. J. Agron. 2010, 33, 267–275. [Google Scholar] [CrossRef]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic efficiency in sun and shade plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef]
- Condon, A.G. Drying times: Plant traits to improve crop water use efficiency and yield. J. Exp. Bot. 2020, 71, 2239–2252. [Google Scholar] [CrossRef]





| Treatments/Varieties | Center of Collection | Pedigree/Parentage/Other Features |
|---|---|---|
| CSV 27 | ICAR-IIMR | GJ 35 × E 35-1 Dual-purpose kharif variety, resistant to grain moulds, non-lodging and non-shattering |
| CSV 31 | ICAR-IIMR | SPV 462 × SPV 1329 Kharif variety, resistant to grain mould, resistant to anthracnose and leaf blight |
| NTJ2 | RARS Nandyal, Andhra Pradesh | Nandyal Tella Jona–2, white sorghum variety |
| CO32 | TNAU | (APK 1 × M35-1) |
| CSV23 | ICAR-IIMR | SPV 861 × SU 248 |
| CSV15 | ICAR-IIMR | SPV 475 × SPV462 |
| CSV 20 | ICAR-IIMR | SPV 946 × Kh89-246 |
| CSV 36 | ICAR-IIMR | SPV1231 × NSV13, kharif variety |
| CSV 39 | ICAR-IIMR | SPV 772 × SPV 1754, kharif variety |
| NTJ 5 | RARS Nandyal | Nandyal Tella Jona–5, white sorghum variety |
| M35-1 | UAS Dharwad | Selection from maldandi landraces |
| CSV 17 | ICAR-IIMR | SPV 946 × SPV 772 |
| CSV 13 | ICAR-IIMR | (IS12622 × 555) × IS 3612 × E35-1-52 |
| N13 | RARS, Nandyal | Yellow sorghum variety |
| NTJ 4 | RARS, Nandyal | NTJ 1 × CMS3 |
| NTJ1 | RARS, Nandyal | Nandyal Tella Jona–1 |
| N 15 | RARS Nandyal | Rabi sorghum |
| N 14 | RARS Nandyal | Yellow sorghum variety |
| NTJ3 | RARS Nandyal | MJ 2092 × POD 24 |
| HC 260 | CCS HAU, Hisar | SPV 103 × PC 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anija Hari Kumar, S.; Chacko Thomas, U.; Al-Salman, Y.; Cano, F.J.; Stephen, R.; Pillai, P.S.; Ghannoum, O. Evaluation of Yield-Related Morphological, Physiological, Agronomic, and Nutrient Uptake Traits of Grain Sorghum Varieties in the Kerala Region (India). Agronomy 2025, 15, 2320. https://doi.org/10.3390/agronomy15102320
Anija Hari Kumar S, Chacko Thomas U, Al-Salman Y, Cano FJ, Stephen R, Pillai PS, Ghannoum O. Evaluation of Yield-Related Morphological, Physiological, Agronomic, and Nutrient Uptake Traits of Grain Sorghum Varieties in the Kerala Region (India). Agronomy. 2025; 15(10):2320. https://doi.org/10.3390/agronomy15102320
Chicago/Turabian StyleAnija Hari Kumar, Swathy, Usha Chacko Thomas, Yazen Al-Salman, Francisco Javier Cano, Roy Stephen, P. Shalini Pillai, and Oula Ghannoum. 2025. "Evaluation of Yield-Related Morphological, Physiological, Agronomic, and Nutrient Uptake Traits of Grain Sorghum Varieties in the Kerala Region (India)" Agronomy 15, no. 10: 2320. https://doi.org/10.3390/agronomy15102320
APA StyleAnija Hari Kumar, S., Chacko Thomas, U., Al-Salman, Y., Cano, F. J., Stephen, R., Pillai, P. S., & Ghannoum, O. (2025). Evaluation of Yield-Related Morphological, Physiological, Agronomic, and Nutrient Uptake Traits of Grain Sorghum Varieties in the Kerala Region (India). Agronomy, 15(10), 2320. https://doi.org/10.3390/agronomy15102320







