Abstract
Pearl millet is primarily grown under rainfed conditions in Sub-Saharan Africa. Early droughts are prevalent in the Sahel region, where pearl millet is widely cultivated, and they severely impact pearl millet growth and productivity by affecting plant stand and reducing plant density in the field. Consequently, genetic improvement for early drought tolerance is a promising strategy to enhance productivity in these regions. This study aims to identify pearl millet lines that are tolerant to water stress at the seedling stage by assessing various water-stress-tolerance traits. Two hundred pearl millet inbred lines were screened for drought tolerance by inducing water stress with polyethylene glycol 6000 (PEG 6000) in the laboratory. The experiment was repeated in the greenhouse using pot screening. The experimental design was an alpha lattice with 10 entries × 20 blocks in two replications. Four treatments (0 g/L, 115 g/L, 235 g/L, 289 g/L) were applied in the laboratory: one control and three concentrations of PEG 6000. Control and stress were applied in the greenhouse. Data were collected on germination rate and growth parameters, including root and seedling length, leaf length and width, and chlorophyll content. Results revealed significant differences among the pearl millet inbred lines under both drought and well-watered conditions. The inbred lines IP-16403 and IP-18062 were the most tolerant in both the greenhouse and laboratory. Water stress significantly reduced plant growth, although an increase in root length was observed in some lines. The number of days to 50% emergence was positively and strongly correlated with survival time (+0.45), while leaf width was negatively correlated with survival time (−0.29) and water stress tolerance (−0.37). The drought-tolerant and drought-susceptible pearl millet inbred lines identified in this study provide valuable genetic resources for enhancing pearl millet productivity in arid and semi-arid environments, especially in the face of unpredictable climate variability.
1. Introduction
Pearl millet (Pennisetum glaucum [L.] R., Br.) is a crucial staple for humans and livestock in the semi-arid and arid regions of Sub-Saharan West Africa (WA) and South Asia. It is an exceptionally heat- and drought-tolerant cereal crop, playing a vital role in food security in the Sahel region of West Africa [1,2].
Despite its importance, pearl millet productivity in West Africa is low (651 kg/ha) compared to the global average yield (1008 kg/ha) and the yield in India (1239 kg/ha) [3,4]. There is a significant yield gap between the realized yield and the biological potential of pearl millet, as evidenced by the yields of 4 to 5 tons per hectare achieved in northwestern India during the summer season [3,4]. This yield gap can be attributed to biotic and abiotic constraints, as well as socio-economic challenges. The main biotic constraints to pearl millet production include Striga hermonthica, birds, downy mildew, head miner, and the use of low-yielding cultivars [5,6]. Water stress and low soil fertility are the primary abiotic constraints affecting millet production [5,6].
In Sub-Saharan Africa, pearl millet is predominantly grown in regions characterized by low and erratic rainfall and in sandy soils with low organic matter and poor water retention capacity. In these areas, farmers typically sow pearl millet before or just after the first rain. Due to the scarcity of rain, pearl millet can experience early drought stress if the season’s first rains are far apart [7]. Over the last two decades, early drought has been more frequent (24%) compared to late drought stress (19%) [8]. Early drought stress can cause yield losses of 43% compared to the 25% yield loss of late drought stress [8]. This highlights that early drought is a critical constraint in West African Sahelian farming systems. The adverse effects of early drought stress have been reported on the germination, growth parameters, and panicle initiation of pearl millet [9,10]. Current climate models predict more variable rainfall patterns from year to year in Sub-Saharan Africa, leading to more extreme weather events [7]. The occurrence of drought, especially at the beginning of the rainy season, is likely to become more frequent. Overall, climate change is expected to reduce pearl millet yields in the region due to increased drought stress [7].
Water stress reduces plant growth by negatively affecting various physiological and biochemical processes, such as photosynthesis, respiration, translocation, ion uptake, carbohydrate synthesis, nutrient metabolism, and growth factor production [11,12]. Therefore, it is essential to identify the most effective adaptation traits to enhance drought resilience through breeding approaches. While significant research has been conducted on terminal drought in pearl millet [13,14,15,16,17], there is limited work on early drought stress. Root architecture and seedling vigour may contribute to early drought tolerance in pearl millet. This study aimed to identify early-drought-tolerant pearl millet germplasm by screening a large number of inbred lines from West African origins.
2. Materials and Methods
2.1. Genetic Materials
This study was conducted in 2022 at the Institute of Environment and Agricultural Research (INERA, Ouagadougou, Burkina Faso) at the research centre of Kamboinse (CREAF/K) in Burkina Faso, which is at 12°28′ latitude N and 1°32′ longitude W and an altitude of 296 m, and was repeated in 2023 between July and August. The Kamboinse research station is in the Sudanian zone of Burkina Faso.
The plant materials used were 200 pearl millet inbred lines, including 143 Pearl Millet Germplasm Association Panel (PMiGAP) introduced from the international crop research institute for semi-arid tropics (ICRISAT, Tillaberi, Niger) and 57 from INERA (Ouagadougou, Burkina Faso).
2.2. Experimental Design
For the laboratory screening, the experimental design is an alpha lattice of 10 entries × 20 blocks in two replicates with four treatments, including three polyethylene glycol 6000 (PEG 6000 MW) concentrations, namely 115 g/L (treatment 1), 235 g/L (treatment 2), 289 g/L (treatment 3), and the control (0 g/L). These different PEG 6000 concentrations correspond to osmotic pressures of 0, −3, −7.5, and −10 bars, respectively [18]. The 0 bars is the control (T0), −3 bars is treatment 1 (T1), −7.5 bars is treatment 2 (T2), and −10 bars is treatment 3 (T3). Each treatment consists of two replicates. Each of the PEG 6000 concentrations was prepared by mixing the corresponding amount of PEG 6000 with 1 L of sterile distilled water. For two hours, this mixture was placed on magnetic stirrers for PEG 6000 homogenization in water. PEG 6000 indicates osmotic water stress, as it reduces water uptake without physical damage to plants [19]. Ten seeds of each pearl millet line were placed on Whatman paper, and appropriate moisture was provided to allow seed germination. Double-layer Whatman paper was placed in 7 cm diameter Petri dishes and moistened with either 7 mL of distilled water for the control or 7 mL of different PEG 6000 concentrations corresponding to each treatment. To reduce evaporation and contamination, the Petri dishes were sealed with sterilized parafilm. Before sowing, the seeds’ surfaces were sterilized using 70% alcohol for 30 s, and they were then placed in 5% calcium hypochlorite for 30 min. After sterilization, the seeds were rinsed several times with pure sterile water before being placed in the Petri dishes. After sowing, the Petri dishes were placed in a growing chamber for four days at 25 °C temperature with a relative air humidity of 70%.
For the greenhouse screening, an alpha lattice of 10 entries × 20 blocks with three replications was used as well both for the control and water-stress-imposed treatment. Two treatments were used for this screening. Indeed, pots with a volume of 750 mL were used in two treatments (T0 and T1). In the control (T0), the pots were watered to field capacity every 48 h until the end of the experiment. For the treatment T1, pots were watered at field capacity the day before sowing, and water stress was imposed from the first day of sowing to determine the capacity of germination and the survival time of the lines. Each pot was filled with 700 g of soil with a maximum water retention capacity of 39.4 mL per 100 g of soil. The soil’s water retention capacity was determined by the centrifugation method [20]. The experiment lasted one month (July to August).
2.3. Data Collection
For the laboratory screening, data were collected at the leaf initiation stage (10 days after treatment) on the germination percentage (PGE) [21]; root length (LCR), as the distance between the base and the root apex; seedling length (LOP), measured from the base to the apex; root/seedling ratio (RRP); and plant vigour index (IVP). Parameters were measured on all seedlings present in each Petri dish 10 days after treatment. A seed was considered germinated when the radicle reached 2 mm [21]. The seedling vigour index was calculated according to the formula proposed by [22].
IVP = [(Seedling length + Root length) × Germination percentage]/100
During greenhouse screening, several variables were measured, such as the number of days to 50% emergence (NJE), which represents the number of days from the sowing date to the emergence of 50% of the seeds per pot, the number of leaves (NOF) six days after sowing (DAS), the first leaf length (LoPF) measured from the sheath to its apex at the tenth DAS, the first leaf width (LaPF) measured at the widest point of the leaf at the eighth DAS, the seedling chlorophyll content (TChlo) measured using the SPAD chlorophyll metre (on a scale from 0.0 to 99.9) by averaging the values taken from three (towards the base, in the middle, and towards the end of the leaf) different locations on the same leaf on the same seedling, the emergence vigour score (SVL), the drought sensitivity score (SSS), and the survival time of each line (DUS). The vigour score was taken by a visual observation of all the pots. It was then converted into a quantitative variable by assigning scores 1, 2, and 3 according to whether the seedlings were vigorous or not. Score 1 refers to vigorous seedlings (lines), score 2 to moderately vigorous seedlings, and score 3 to non-vigorous seedlings. This variable was evaluated at the fourth DAS [23].
The drought sensitivity score (SSS) was also taken by visual observation of all the pots [23]. It was then converted into a quantitative variable by assigning scores 1, 2, and 3 according to the degree of sensitivity of the seedlings. Score 1 was given to drought-tolerant inbred lines (seedlings whose leaves were still green with no sign of stress), score 2 was moderately tolerant (seedlings whose leaves had started to bend but were still standing upright), and score 3 for drought-sensitive seedlings (seedlings that had practically lost their water and had wilted leaves). This variable was measured on the fourth DAS.
2.4. Data Analysis
A single analysis of variance (p < 0.01 and p < 0.05) was performed first to determine the significant differences between the lines within and between treatments for the laboratory and the greenhouse screening. Then, a combined analysis of variance was performed using Breeding View software to determine the significance of the reaction of lines to the different levels of water stress imposed in the laboratory using the rank order method [24]. In the greenhouse, tolerant lines were identified based on their survival time. This method was based on the set of variables measured: the mean rank, the standard deviation of the rank, the sum of the ranks, and the final rank. A path coefficient analysis and correlations were carried out to determine the variables’ contribution to the inbred lines’ survival time. The principal component analysis was carried out to determine the variable association.
3. Results
3.1. Analysis of Variance for Traits Measured During Laboratory and Greenhouse Screenings
The analysis of variance shows significant differences between the pearl millet inbred lines for all traits measured in the laboratory except RRP in T2 and LOP and RRP in T3 (Table 1). In the greenhouse, the results revealed significant differences among the inbred lines for all traits except leaf number (Table 2).

Table 1.
Mean square of the lines screened in the laboratory for water stress under four treatments.

Table 2.
Mean square of the lines under greenhouse screening.
The AMMI analysis showed a highly significant difference between the line × treatment (L × T) interaction for the percentage of germination (PEG), the seedling length (LOP), the root length (LCR), the ratio between root and seedling length (RRP), and the seedling vigour index (IVP) (Table 3).

Table 3.
Results of analysis of variance for laboratory screening using the AMMI model.
3.2. Effect of PEG 6000 on Pearl Millet Germination
In this study, the different concentrations of PEG 6000 have affected the germination of pearl millet inbred lines seed (Table 3). Germination capacity varied significantly between treatments (33.33% at −10 bar to 100% at 0 bar). The average germination percentage was 84% under normal conditions, with extremes of 0 and 100 (Figure 1A). Under stress conditions, the average value was 83%, with minimum and maximum values of 5 and 100, respectively (Figure 1B).
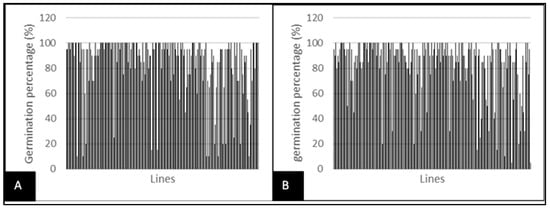
Figure 1.
Variability of lines for germination percentage depending on water stress. (A) germination percentage for the control treatment; (B) germination percentage under water stress.
3.3. Effect of Imposed Water Stress on Seedling Growth
Root and seedling length were all affected by drought, but the effects were most marked on the shoots and above-ground parts of the plant (Figure 2B or Figure 3B). Shoot parameters will therefore also help the breeder to select water-stress-tolerant genotypes. In the present investigation, the root length significantly declined with increased external water potential (Table 3, Figure 2). Consequently, all treatments caused a decrease in root elongation in all genotypes compared to their controls. Similarly, shoot length decreased significantly with increasing external water stress (Table 3, Figure 3). Nevertheless, primary root elongation was observed in about ten lines under stressed conditions, compared to controls. Pearl millet inbred lines IP-13363 and IP-18062 recorded 60 cm and 138 cm root lengths under water stress, compared to 56 cm and 136 cm under normal conditions.
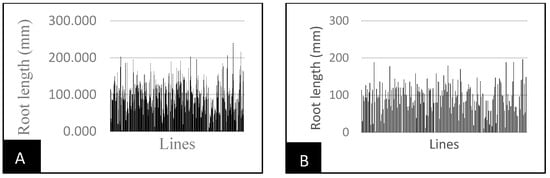
Figure 2.
Line variability in root length depending on water stress. (A) Root length of control treatment; (B) root length under water stress.
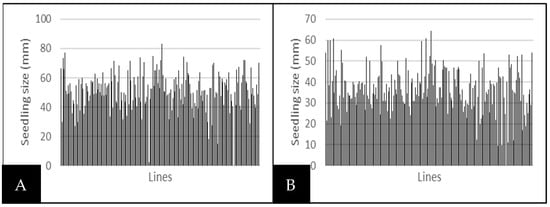
Figure 3.
Line variability in seedling height depending on water stress. (A) Seedling height in control treatment; (B) seedling height under water stress.
Root length ranged from 0 to 240 mm, with an average of 91 mm under normal conditions (Figure 2A), and from 0 to 289 mm, with an average of 93 mm under stress conditions (Figure 2B). Seedling height ranged from 0 to 100 mm, with an average of 51 mm under normal conditions (Figure 3A), while under stress conditions, it varied from 0 to 64 mm, with an average of 36 mm (Figure 3B).
3.4. Water Stress Pot Screening in Greenhouse for Effects on Seedlings (Leaf Length and Width, Shoot Length, and Number of Leaves)
In the greenhouse, survival time varied from 16 to 27 days with an average of 21 days (Table 2). Seedling height ranged from 36 to 159 mm, with an average of 90 mm, and the width of the first leaf varied from 2 to 5.5 mm, with an average of 3.7 mm (Table 2). The average number of leaves was 2, with a minimum and maximum of 1 and 3, respectively (Table 2). Figure 4 shows the condition of the lines on the 14th DAS in the greenhouse. Some have practically wilted, while others show no signs of wilting due to water stress.
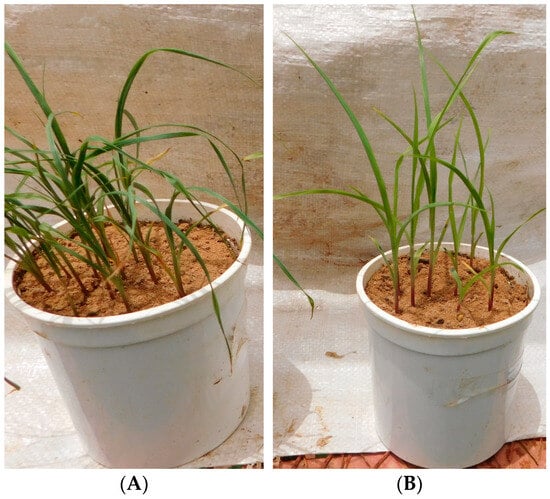
Figure 4.
Variability of inbred lines with respect to deficient water stress in the greenhouse. (A) Sensitive inbred line; (B) tolerant inbred line.
3.5. Relationship Between Plant Survival and Water Stress Tolerance Parameters
The results of the correlations and the path coefficient analysis using survival time as the dependent variable for indirect selection are presented in Figure 5 and Table 4. The path coefficient analysis shows that variables such as number of days to 50% seedling emergence (r = 0.624), leaf chlorophyll content (r = 0.104), and leaf length (r = 0.010) have a direct positive effect on pearl millet lines’ survival time. Other variables such as the number of leaves (r = −0.506), vigour at emergence (r = −0.607), seedling height (r = −0.036), leaf width (r = −0.764), root length (r = −0.000), and root–plant ratio (r = −0.246) had a direct negative effect on the survival time of pearl millet lines (Figure 5, Table 4). The number of days to 50% emergence recorded the highest positive path coefficient result (b = 0.624, SE = 0.170, p = 0.000), so an increase in the 50% emergence date resulted in an increase of one day in survival time. In contrast, leaf width recorded the highest negative path coefficient (b = −0.764, SE= 0.298, p = 0.010), so an increase of one unit area in leaf width resulted in a decrease of one day in survival time.
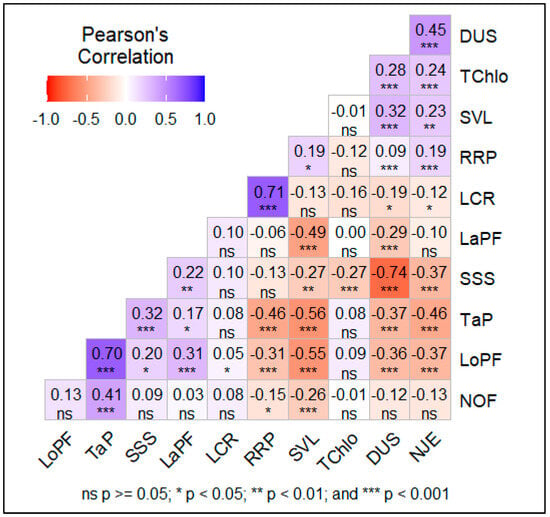
Figure 5.
Phenotypic correlations among measured traits. DUS = line survival time, LCR = root length, LaPF = width of first leaf, LoPF = length of first leaf, NJE = number of days to 50% emergence, NOF = number of leaves, SSS = drought sensitivity score, SVL = vigour at emergence score, TChlo = chlorophyll content, TaP = seedling size, RRP = root/seedling ratio.

Table 4.
Sequential regression analysis of juvenile water-stress-tolerance traits on survival time in pearl millet.
3.6. Variable Association
The first two principal components (PCAs) explain 56.06% and 62.99% of the total variability in the greenhouse and laboratory, respectively (Figure 6a,b). PCA 1, explaining 37.07% of the variation in the greenhouse, is positively associated with seedling length (r = 17.95), leaf length (r = 12.60), leaf width (r = 6.134), susceptibility to drought (r = 11.93), and the seedling survival time (r = 15.27). On the other hand, PCA 2, explaining 20.90% of total variation, is negatively correlated with seedling vigour (r = 18.066), number of days to the emergence of 50% of seedlings (r = 5.04), leaf chlorophyll content (r = 18.25) and seedling–root ratio (r = 2.79).
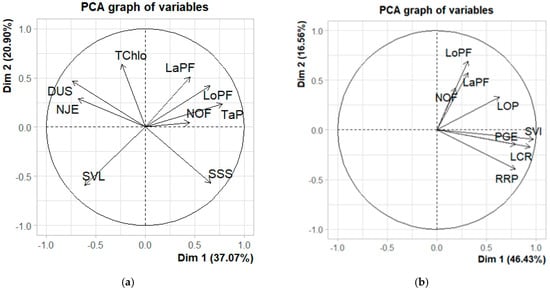
Figure 6.
Discriminant traits in the greenhouse (a) and in the laboratory (b).
In the laboratory experiment, the root length (r = 22.78), seedling vigour index (r = 24.57), and germination percentage (16.36) contribute most to PCA 1, which explains 46.43% of total variation. PCA2, which explains 16.56% of total variability, is more associated with the leaf width (r = 20.54), leaf length (r = 31.34), and total number of leaves (r = 13.89).
3.7. Pearl Millet Seedlings’ Responses to Post-Emergence Water Stress
In this study, lines IP-11763 and IP-19334 were the most tolerant in the pot screening in the greenhouse, with a survival time of 27 days each, while the IKMLS1 20582 and IKMLS1 18012 lines were the most susceptible, with a survival time of 16 days (Table 5). The tolerant lines generally had a higher chlorophyll content than the susceptible lines, with 50% more days of survival than the susceptible lines. Lines that developed more leaves were consistently more susceptible than the tolerant inbred lines.

Table 5.
Rank (R), mean rank (), the standard deviation of ranks (SDR), and the sum of ranks (RS) of the indicators of tolerance to post-emergence water stress for the ten most tolerant and ten most sensitive lines.
In the lab screening using PEG 6000, IKMLS1 18 047 (RS = 44.00), IP-16403 (RS = 47.51), IP-8172 (RS = 50.95), IKMLS1 18 039 (RS = 53.31), and IP-18062 (RS = 55.43) were the most tolerant lines, while IP-3757 (RS = 210.91), IKMLS1 20 134 (RS = 212.72), IKMS1 20 882 (RS = 214.54), IKMLS1 18 001 (RS = 215.15), and IKMLS1 18 046 (RS = 221.42) were the most susceptible (Table 5). The tolerant lines were distinguished from the susceptible ones by their more developed root lengths and a higher vigour index (Table 5).
4. Discussion
The analysis of variance showed the presence of large variability within the pearl millet inbred lines in their response to water stress in both the greenhouse and the laboratory screenings. Water stress significantly reduced the expression of all the traits in the laboratory. In sorghum, most morphological and physiological traits at the seedling stage are affected by water stress [25,26,27].
4.1. Effect of PEG 6000 on Pearl Millet Germination
The germination percentage decreased as the concentration of PEG 6000 increased. These results are in line with those of [28,29], who demonstrated in their work that water potential had a negative impact on germination percentage in wheat and rice, respectively. This could be explained by the contrasting concentration between the interior of the grain and the middle due to the osmotic phenomenon. This inhibition of germination results from tissue hydration issues, which have repercussions on the radicle elongation process [30] and water molecule penetration into the grains, hindering osmotic adjustment [31]. Such a result has been reported by numerous authors and on various crops such as sunflower [32] and pearl millet [33].
The tolerance to water stress during the first growing stage is a key adaptation trait [34]. The seedling stage is important in the crop life cycle, and drought tolerance during this stage makes a crop stable [35]. Furthermore, seed germination is a selection criterion considered for water stress tolerance [36,37]. Water stress affected shoot growth more than root growth.
4.2. Effect of PEG 6000 on Pearl Millet Seedling Development
In about ten lines, the roots increased in length under stress compared to the control. A similar finding was reported in several studies [38,39,40,41]. This was confirmed by the root–shoot ratio, which increased under water stress. According to [42], water stress induces an increase in the primary roots and the root–shoot ratio of plants, unlike shoots. The elongation of the primary root is an adaptive response to water stress. Pearl millet lines that survived water stress longer had longer primary roots than pearl millet lines that survived water stress for a shorter time, suggesting that the tolerant lines respond to water stress by developing their root system faster. According to [8], there is a positive correlation between primary root growth and early drought tolerance in pearl millet. In fact, roots are likely to detect the water deficiency in the soil and respond to it because it is at this level that the water–plant contact is established [43,44]. Root length at the seedling stage provides a fair estimate of root growth in the field [26,45]. According to [46], the long-root trait is associated with maintaining plant productivity under drought conditions. The decrease in shoot growth under lab screening with PEG 6000 may be due to there being less water absorption and decreased osmotic potential created by the PEG 6000 [47]. According to [48], the decrease in shoot growth is due to a restricted cell division and enlargement, as water stress directly reduces growth by decreasing cell division and elongation. According to [49], water deficit causes a delay in plant growth. This is reflected in a reduction in stem height and width development, and a reduction in the number of leaves and the leaf area. References [50,51] reported that water stress reduces shoot growth. This could be an adaptative mechanism of pearl millet to reduce water loss through transpiration. According to [52], the reduction in above-ground growth is an adaptive capacity necessary for the survival of plants exposed to abiotic stress. Leaf area is an important determinant of transpiration. One of the first reactions of plants to water deficit is to reduce their leaf area. This reduction is one of the plant’s responses to dehydration. It helps conserve water resources. This helps the plant survive [53]. Leaf area gradually determines the quantities of water used by the plant in the form of transpiration and the quantities of carbon fixed photosynthetically. It also determines the plant’s resistance to drought, as it has been shown that the greater the leaf area, the more water will be lost compared to a smaller leaf area [54]. Survival time and chlorophyll content are positively correlated. This would mean that lines that are able to photosynthesize under water stress are the most tolerant.
The seedling vigour index (IVP) measures crop stress tolerance at the seedling stage by considering germination percentage and shoot and root lengths together. In general, IVP was significantly reduced by PEG-induced drought stress, but this variable increased for some lines under water-deficit stress compared to the control. This would mean that these lines performed better under PEG 6000-induced drought stress and would be considered the most tolerant. A similar observation was reported by [55] on lettuce, ref. [56] on pearl millet, ref. [57] on wheat, and [22] on barley. Furthermore, the results of the analysis show that tolerant lines develop fewer leaves, but these leaves are also less broad and less lengthy. These characteristics would enable tolerant lines to limit their water loss through transpiration and have a longer survival time.
5. Conclusions
Germination and seedling growth are the most important and water-stress-sensitive stages in a plant’s life cycle. This study provided information on the response of 200 pearl millet lines to early drought tolerance. We found that there is a large variability in pearl millet seedlings’ drought tolerance. Findings from this study proved that water stress at the seedling stage slowed down growth in pearl millet. Rapid root development was found to be the main adaptative trait in early drought, and genotypes that developed faster the root system withstood water stress for longer. Two lines, namely IP-16403 and IP-18062, were highly tolerant to water stress both in pot screening in the greenhouse and in laboratory screening using PEG 6000. These lines are sources of donors of seedling drought tolerance that can be used in pre-breeding and trait market discovery as well. On the other hand, two pearl millet inbred lines, namely IKMLS1 18018 and IP-8426, proved to be very sensitive in the lab and in the greenhouse. These lines are a source of drought tolerance donors for seedlings that can be used for pre-selection and trait market discovery. This study also showed that the number of days to 50% sowing and the root length had a direct positive effect on the survival time of the lines, while leaf width had direct adverse effects on survival time. The selected tolerant parents will be used as donors to improve elite varieties of pearl millet. In any case, this study must be continued in the field for confirmation of the status of the identified tolerant/susceptible lines.
Author Contributions
Conceptualization, L.V.B., A.R. and I.D.; methodology, L.V.B., K.S., P.B. and I.D.; formal analysis, L.V.B. and A.R.; writing—original draft preparation, L.V.B.; writing—review and editing, L.V.B., A.R. and I.D.; supervision, K.S. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Strengthening Networks and Institutional Capacities in Plant Breeding for the Development of Resilient Crops Meeting the Needs of West African Farm-ers—ABEE project, which was funded by the European Union through the West and Central African Council for Agricultural Research and Development.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank WECARD for the research grant and INERA for making available the lab and the greenhouse for this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Debieu, M.; Sine, B.; Passot, S.; Grondin, A.; Akata, E.; Gangashetty, P.; Vadez, V.; Gantet, P.; Foncéka, D.; Cournac, L.; et al. Response to early drought stress and identification of QTLs controlling biomass production under drought in pearl millet. PLoS ONE 2018, 13, e0201635. [Google Scholar] [CrossRef]
- Varshney, R.K.; Shi, C.; Thudi, M.; Mariac, C.; Wallace, J.; Qi, P.; Zhang, H.; Zhao, Y.; Wang, X.; Rathore, A.; et al. Pearl millet genome sequence provides a resource to improve agronomic traits in arid environments. Nat. Biotechnol. 2017, 35, 969–976. [Google Scholar] [CrossRef]
- FAOSTAT. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 August 2024).
- Yadav, O.P.; Gupta, S.K.; Govindaraj, M.; Sharma, R.; Varshney, R.K.; Srivastava, R.K.; Rathore, A.; Mahala, R.S. Genetic gains in pearl millet in India: Insights into historic breeding strategies and future perspective. Front. Plant Sci. 2021, 12, 645038. [Google Scholar] [CrossRef]
- Rouamba, A.; Shimelis, H.; Drabo, I.; Laing, M.; Gangashetty, P.; Mathew, I.; Mrema, E.; Shayanowako, A.I.T. Constraints to Pearl Millet (Pennisetum glaucum) Production and Farmers’ Approaches to Striga hermonthica Management in Burkina Faso. Sustainability 2021, 13, 8460. [Google Scholar] [CrossRef]
- Drabo, I.; Zangre, R.G.; Danquah, E.Y.; Ofori, K.; Witcombe, J.R.; Hash, C.T. Identifying farmers’ preferences and constraints to pearl millet production in the Sahel and North-Sudan zones of Burkina Faso. Ex. Agric. 2019, 55, 765–775. [Google Scholar] [CrossRef]
- Ma, R.; Sun, L.; Chen, X.; Mei, B.; Chang, G.; Wang, M.; Zhao, D. Proteomic Analyses Provide Novel Insights into Plant Growth and Ginsenoside Biosynthesis in Forest Cultivated Panax ginseng (F. ginseng). Front Plant Sci. 2016, 7, 1. [Google Scholar] [CrossRef]
- De la Fuente, C.; Grondin, A.; Sine, B.; Debieu, M.; Belin, C.; Hajjarpoor, A.; Atkinson, J.; Passot, S.; Salson, M.; Orjuela, J.; et al. Glutaredoxin regulation of primary root growth is associated with early drought stress tolerance in pearl millet. Elife 2024, 12, RP86169. Available online: https://elifesciences.org/reviewed-preprints/86169v2 (accessed on 16 May 2024). [CrossRef]
- Khayatnezhad, M.; Zaeifizadeh, M.; Gholamin, R. Investigation and selection index for drought stress. Aust. J. Basic Appl. Sci. 2010, 4, 4815–4822. [Google Scholar]
- Rauf, S. Breeding sunflower (Helianthus annuus L.) for drought tolerance. Commun. Biometry Crop Sci. 2008, 3, 29–44. [Google Scholar]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Panneerselvam, R. Soil salinity alters the morphology in Catharanthus roseus and its effects on endogenous mineral constituents. Eurasian J. Biosci. 2008, 2, 18–25. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Bidinger, F.R.; Mahalakshmi, V.; Rao, G.D.P. Assessment of drought resistance in pearl millet (Pennisetum americanum (L.) Leeke). II. Estim. Genotype Response stress. Aust. J. Agric. Res. 1987, 38, 49–59. [Google Scholar] [CrossRef]
- Hash, C.T.; Bramel-Cox, P.J. 113 Marker Applications in Pearl Millet. Available online: https://core.ac.uk/download/pdf/211014241.pdf (accessed on 7 August 2024).
- Howarth, C.J.; Yadav, R.S. Successful marker assisted selection for drought tolerance and disease resistance in pearl millet. Iger Innov. 2002, 6, 18–21. [Google Scholar]
- Serraj, R.; Hash, C.T.; Rizvi, S.M.H.; Sharma, A.; Yadav, R.S.; Bidinger, F.R. Recent advances in marker-assisted selection for drought tolerance in pearl millet. Plant Prod. Sci. 2005, 8, 334–337. [Google Scholar] [CrossRef]
- Serba, D.D.; Yadav, R.S. Genomic tools in pearl millet breeding for drought tolerance: Status and prospects. Front. Plant Sci. 2016, 7, 1724. [Google Scholar] [CrossRef] [PubMed]
- Hadas, A. Water Uptake and Germination of Leguniinous Seeds Under Changing External Water Potential in Osmotic Solutions. J. Exp. Bot. 1976, 27, 480–489. [Google Scholar] [CrossRef]
- Romo, S.; Labrador, E.; Dopico, B. Water stress-regulated gene expression in Cicer arietinum seedlings and plants. Plant Physiol. Biochem. 2001, 39, 1017–1026. [Google Scholar] [CrossRef]
- Revaillot, S.; Pouget, C.; Alvarez, G.; Fontaine, S. Mesurer la capacité de rétention en eau d’un sol par centrifugation: Une méthode fiable, facile et rapide à mettre en œuvre dans un laboratoire. NOV’AE-Ingénierie Savoir-Faire Innovants 2021, 107, 17–44. [Google Scholar]
- Sayar, R.; Bchini, H.; Mosbahi, M.; Khemira, H. Response of durum wheat (Triticum durum Desf.) growth to salt and drought stresses. Czech J. Genet. Plant Breed. 2010, 46, 54–63. [Google Scholar] [CrossRef]
- Hellal, F.A.; El-Shabrawi, H.M.; Abd El-Hady, M.; Khatab, I.A.; El-Sayed, S.A.A.; Abdelly, C. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J. Genet. Eng. Biotechnol. 2018, 16, 203–212. [Google Scholar] [CrossRef]
- Bazie, V.L. Evaluation of Pearl Millet (Pennisetum glaucum (L.) R. Br.) S3 Lines for Post-Emergence Water Stress Tolerance. Master’s Thesis, Joseph KI-ZERBO University, Ouagadougou, Burkina Faso, 2019. [Google Scholar]
- Farshadfar, E.; Rafiee, F.; Hasheminasab, H. Evaluation of genetic parameters of agronomic and morpho-physiological indicators of drought tolerance in bread wheat (Triticum aestivum L.) using diallel mating design. Aust. J. Crop Sci. 2013, 7, 268–275. [Google Scholar]
- Ali, M.A.; Abbas, A.; Awan, S.I.; Jabran, K.; Gardezi, S.D.A. Correlated response of various morpho-physiological characters with grain yield in sorghum landraces at different growth phases. J. Anim. Plant Sci. 2011, 21, 671–679. [Google Scholar]
- Ali, M.A.; Jabran, K.; Awan, S.I.; Abbas, A.; Zulkiffal, M.; Acet, T.; Farooq, J.; Rehman, A. Morpho-physiological diversity and its implications for improving drought tolerance in grain sorghum at different growth stages. Aust. J. Crop Sci. 2011, 5, 311–320. [Google Scholar]
- Bibi, A.; Sadaqat, H.; Tahir, M.; Akram, H.M. Screening of sorghum (Sorghum bicolor var Moench) for drought tolerance at seedling stage in polyethylene glycol. J. Anim. Plant Sci. 2012, 22, 671–678. [Google Scholar]
- Abido, W.A.E.; Zsombik, L. Effect of water stress on germination of some Hungarian wheat landraces varieties. Acta Ecol. Sin. 2018, 38, 422–428. [Google Scholar] [CrossRef]
- Shereen, A.; Khanzada, M.A.; Wahid Baloch, M.A.; Asma, A.; Shirazi, M.U.; Khan, M.A.; Arif, M. Effects of PEG induced water stress on growth and physiological responses of rice genotypes at seedling stage. Pak. J. Bot. 2019, 51, 2013–2021. Available online: http://pakbs.org/pjbot/paper_details.php?id=8146 (accessed on 10 May 2024). [CrossRef] [PubMed]
- Hegarty, T.W.; Ross, H.A. Differential Sensitivity to Moisture Stress of Seed Germination and Seedling Radicle Growth in Calabrese (Brassica oleracea var. italica) and Cress (Lepidium sativum). Ann. Bot. 1978, 42, 1003–1005. [Google Scholar] [CrossRef]
- Manohar, M.S. Effect of “Osmotic” Systems on Germination of Peas (Pisum sativum, L.). Planta 1966, 71, 81–86. [Google Scholar] [CrossRef]
- Ashraf, M.; Kausar, A.; Ashraf, M.Y. Alleviation of salt stress in pearl millet (Pennisetum glaucum (L.) R. Br.) through seed treatments. Agronomie 2003, 23, 227–234. [Google Scholar] [CrossRef]
- Chojnowski, M.; Corbineau, F.; Côme, D. Physiological and biochemical changes induced in sunflower seeds by osmopriming and subsequent drying, storage and aging. Seed Sci. Res. 1997, 7, 323–332. [Google Scholar] [CrossRef]
- Grouzis, M. Structure, Productivité et Dynamique des Systèmes Ecologiques Sahéliens (Mare d’Oursi, Burkina Faso). Ph.D. Thesis, Université de Paris-Sud, Orsay, France, 1987. Available online: https://www.theses.fr/1987PA112339 (accessed on 10 May 2024).
- Shitole, S.M.; Dhumal, K.N. Effect of water stress by polyethylene glycol 6000 and sodium chloride on seed germination and seedling growth of Cassia angustifolia. Int. J. Pharm. Sci. Res. 2012, 3, 528–531. [Google Scholar]
- Sy, A.; Grouzis, M.; Danthu, P. Seed germination of seven Sahelian legume species. J. Arid. Environ. 2001, 49, 875–882. [Google Scholar] [CrossRef]
- Lamia, H.; Naoufel, S.; Larbi, K.M.; Néjib, R.M. Effect of osmotic stress on Myrtus communis germination. Biologia 2012, 67, 132–136. [Google Scholar] [CrossRef]
- Bibi, A.; Sadaqat, H.A.; Akram, H.M.; Mohammed, M.I. Physiological Markers for Screening Sorghum (Sorghum bicolor) Germplasm under Water Stress Condition. Int. J. Agric. Biol. 2010, 12, 451–455. [Google Scholar]
- Okçu, G.; Kaya, M.; Atak, M. Effects of Salt and Drought Stresses on Germination and Seedling Growth of Pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar]
- Salih, A.A.; Ali, I.A.; Lux, A.; Luxová, M.; Cohen, Y.; Sugimoto, Y.; Inanaga, S. Rooting, Water Uptake, and Xylem Structure Adaptation to Drought of Two Sorghum Cultivars. Crop Sci. 1999, 39, 168–173. [Google Scholar] [CrossRef]
- Younis, M.E.; El-Shahaby, O.A.; Abo-Hamed, S.A.; Ibrahim, A.H. Effects of Water Stress on Growth, Pigments and 14CO2 Assimilation in Three Sorghum Cultivars. J. Agron. Crop Sci. 2000, 185, 73–82. [Google Scholar] [CrossRef]
- Mohi-Ud-Din, M.; Hossain, M.A.; Rohman, M.M.; Uddin, M.N.; Haque, M.S.; Ahmed, J.U.; Hossain, A.; Hassan, M.M.; Mostofa, M.G. Multivariate Analysis of Morpho-Physiological Traits Reveals Differential Drought Tolerance Potential of Bread Wheat Genotypes at the Seedling Stage. Plants 2021, 10, 879. [Google Scholar] [CrossRef]
- Khodarahmpour, Z. Effect of drought stress induced by polyethylene glycol (PEG) on germination indices in corn (Zea mays L.) hybrids. Afr. J. Biotechnol. 2011, 10, 18222–18227. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, R.G.; Mao, G.; Koczan, J.M. Identification of Drought Tolerance Determinants by Genetic Analysis of Root Response to Drought Stress and Abscisic Acid. Plant Physiol. 2006, 142, 1065–1074. [Google Scholar] [CrossRef]
- Rajendran, R.A.; Muthiah, A.R.; Manickam, A.; Shanmugasundaram, P.; Joel, A.J. Indices of drought tolerance in sorghum (Sorghum bicolor L. Moench) genotypes at early stages of plant growth. Res. J. Agric. Biol. Sci. 2011, 7, 42–46. [Google Scholar]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Kaydan, D.; Yagmur, M. Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCl. Afr. J. Biotechnol. 2008, 7, 2862. [Google Scholar]
- Kramer, P.J. Problems in Water Relations of Plants and Cells. In International Review of Cytol.; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 1983; Volume 85, pp. 253–286. Available online: https://www.sciencedirect.com/science/article/pii/S007476960862375X (accessed on 10 May 2024).
- Thakur, P.S.; Rai, V.K. Dynamics of amino acid accumulation of two differentially drought resistant Zea mays cultivars in response to osmotic stress. Environ. Exp. Bot. 1982, 22, 221–226. [Google Scholar] [CrossRef]
- Benmahioul, B.; Daguin, F.; Kaid-Harche, M. Effet du stress salin sur la germination et la croissance in vitro du pistachier (Pistacia vera L.). Comptes Rendus Biol. 2009, 332, 752–758. [Google Scholar] [CrossRef]
- Kuiper, D.; Schuit, J.; Kuiper, P.J.C. Actual cytokinin concentrations in plant tissue as an indicator for salt resistance in cereals. Plant Soil. 1990, 123, 243–250. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Lebon, E.; Pellegrino, A.; Tardieu, F.; Lecoeur, J. Shoot development in grapevine (Vitis vinifera) is affected by the modular branching pattern of the stem and intra-and inter-shoot trophic competition. Ann. Bot. 2004, 93, 263–274. [Google Scholar] [CrossRef]
- Belkharchouche, H.; Fellah, S.; Bouzerzour, H.; Benmahammed, A.; Chellal, N. Vigueur de Croissance, Translocation et Rendement en Grains du blé dur (Triticum durum desf) Sous Conditions Semi Arides. Available online: http://archives.univ-biskra.dz/handle/123456789/589 (accessed on 9 May 2025).
- Duman, I. Effects of Seed Priming with PEG or K3PO4 on Germination and Seedling Growth in Lettuce. 2006. Available online: https://scialert.net/abstract/?doi=pjbs.2006.923.928 (accessed on 8 May 2024).
- Radhouane, L. Response of Tunisian autochthonous pearl millet (Pennisetum glaucum (L.) R. Br.) to drought stress induced by polyethylene glycol (PEG) 6000. Afr. J. Biotechnol. 2007, 6, 1684–5315. Available online: https://www.ajol.info/index.php/ajb/article/view/57121 (accessed on 8 May 2024).
- Saha, R.R.; Hannan, A.; Nessa, A.; Malek, M.A.; Islam, M.R. Selection of drought tolerant wheat genotypes by osmotic stress imposed at germination and early seedling stage. SAARC J. Agric. 2017, 15, 177–192. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).