Purpureocillium takamizusanense: A New Entomopathogenic Fungus in the Americas and Its Pathogenicity Against the Cacao Black Bug, Antiteuchus tripterus (Hemiptera: Pentatomidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Fungi Isolation Process
2.3. Morphological and Molecular Characterization
2.4. Radial Growth and Sporulation
2.5. In Vitro Pathogenicity Tests
2.5.1. Antiteuchus tripterus Collection
2.5.2. Concentration–Mortality Bioassays
2.5.3. Time–Mortality Bioassay
2.6. Field Conditions Pathogenicity Tests
2.7. Insect Infection Confirmation
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.9. Statistical Analyses
3. Results
3.1. Molecular Characterization
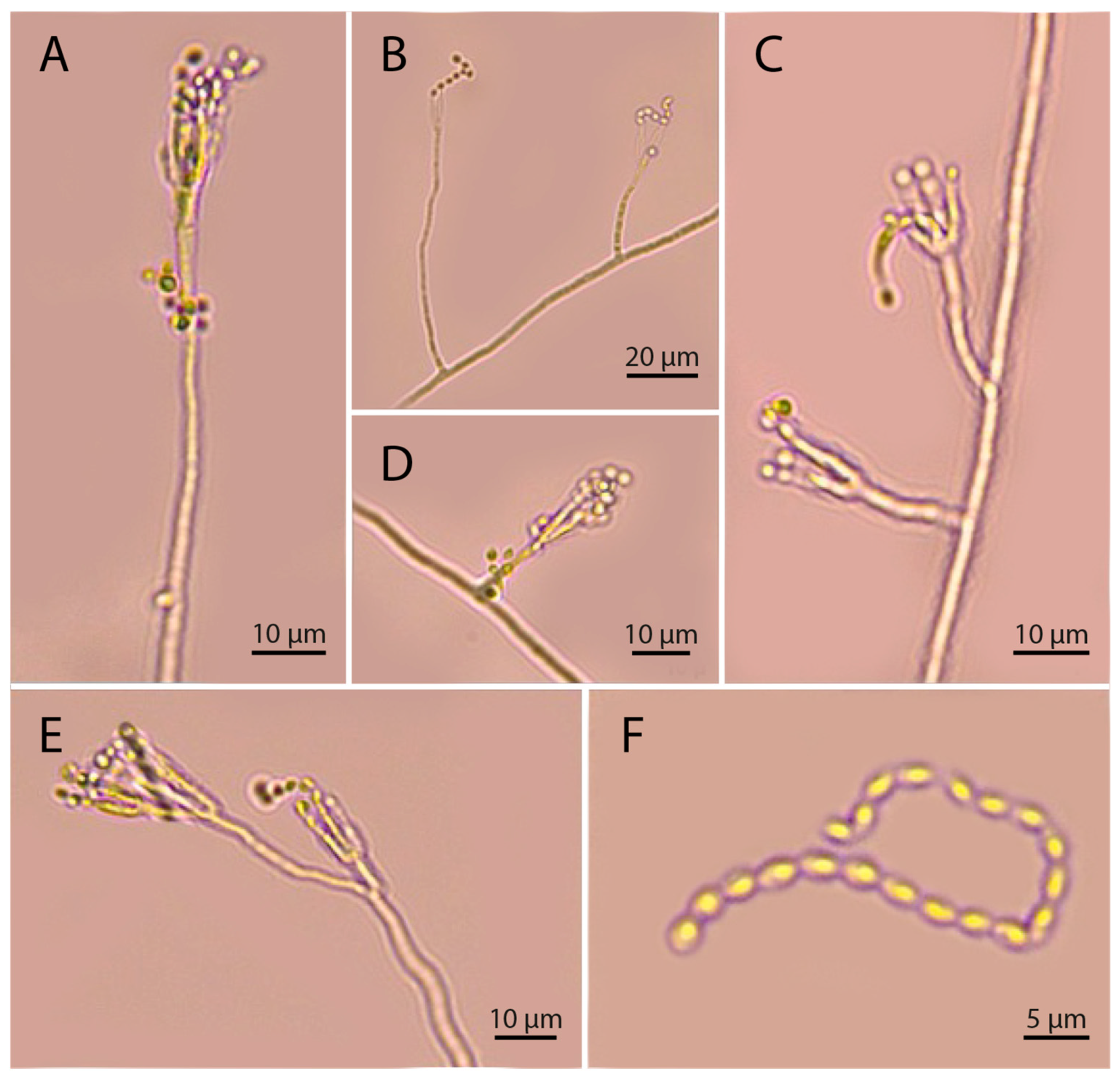
3.2. Morphological Characterization
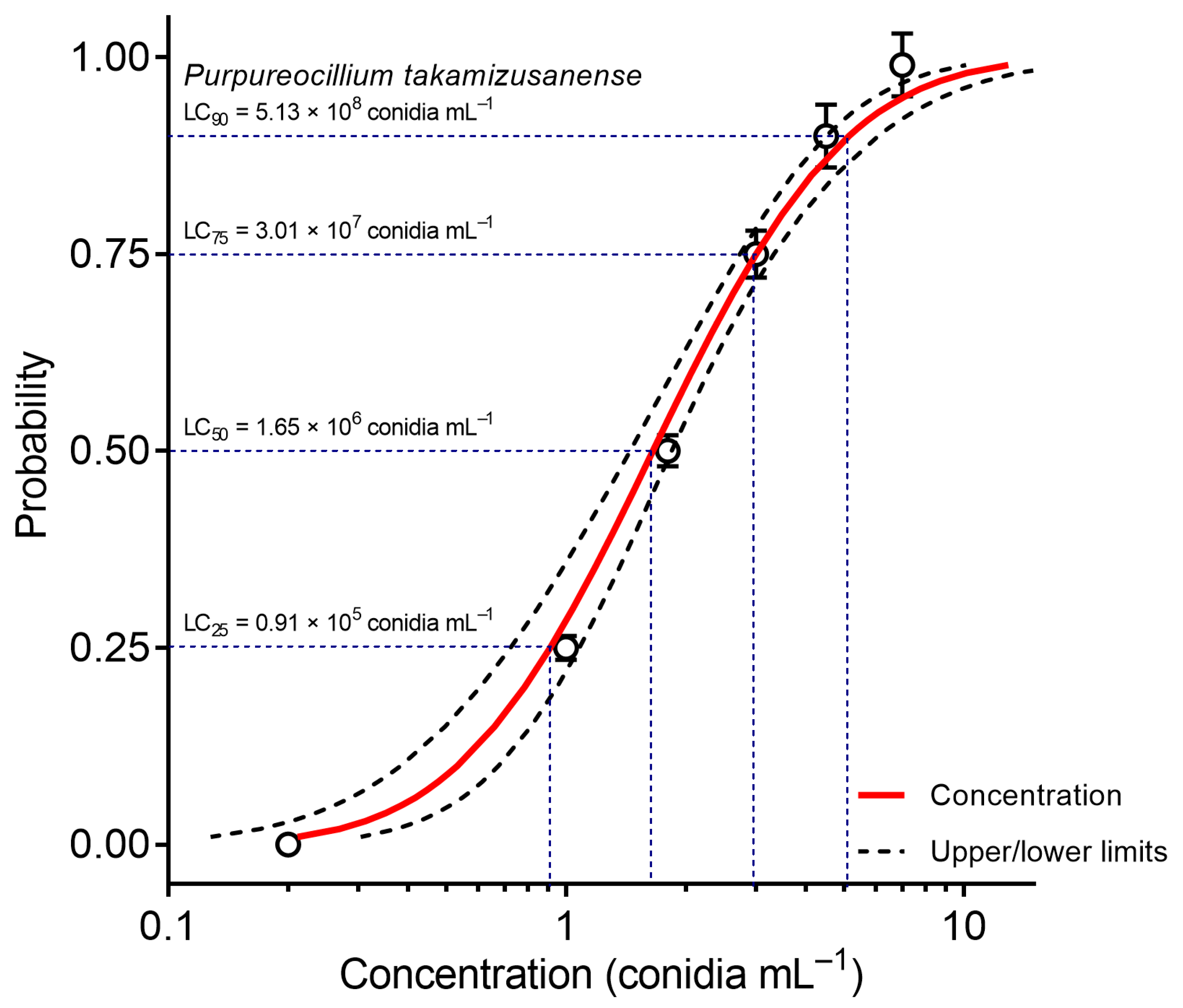
3.3. Concentration-Mortality Bioassays
3.4. Time–Mortality Bioassays
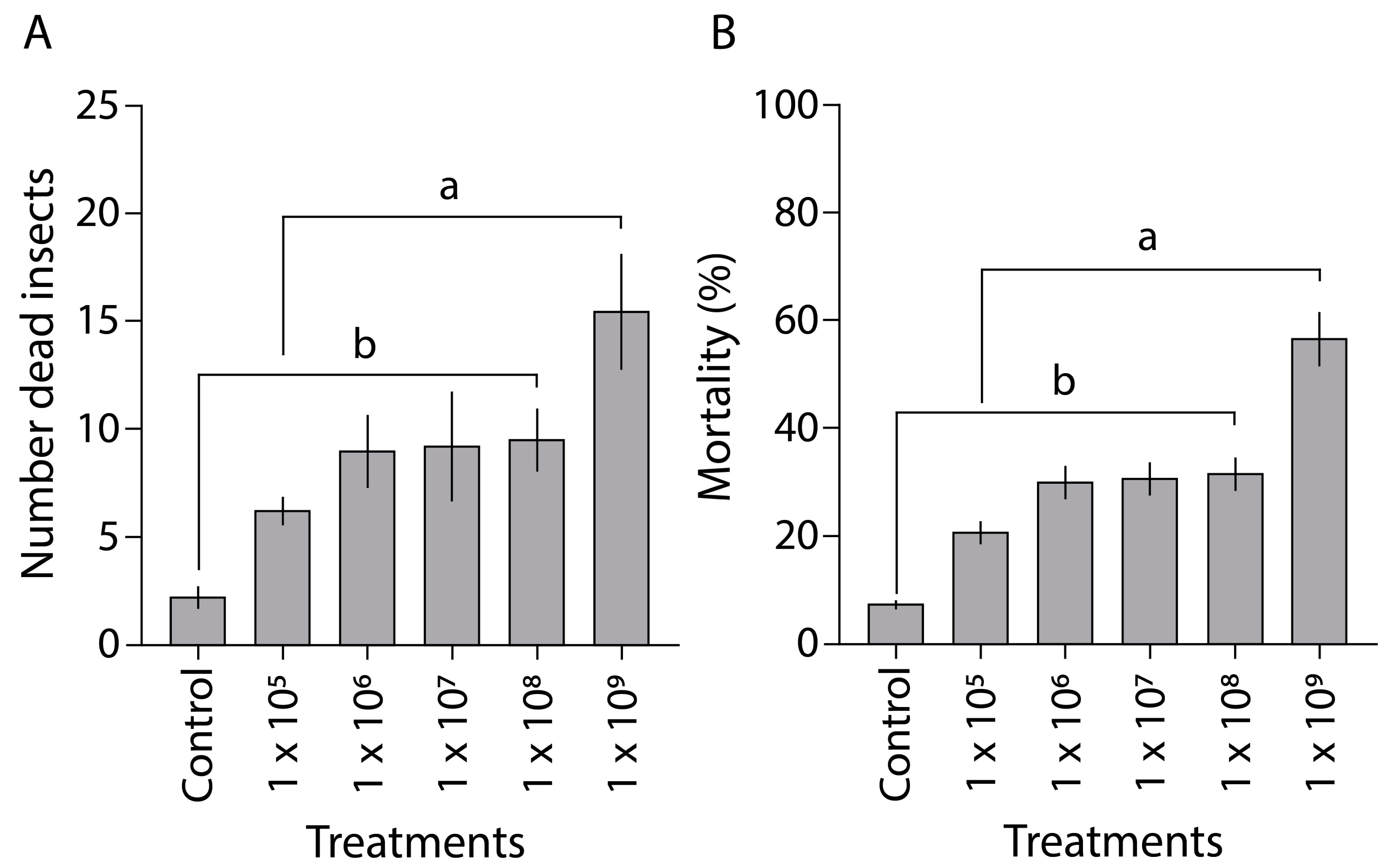
3.5. Field Conditions Pathogenicity Tests
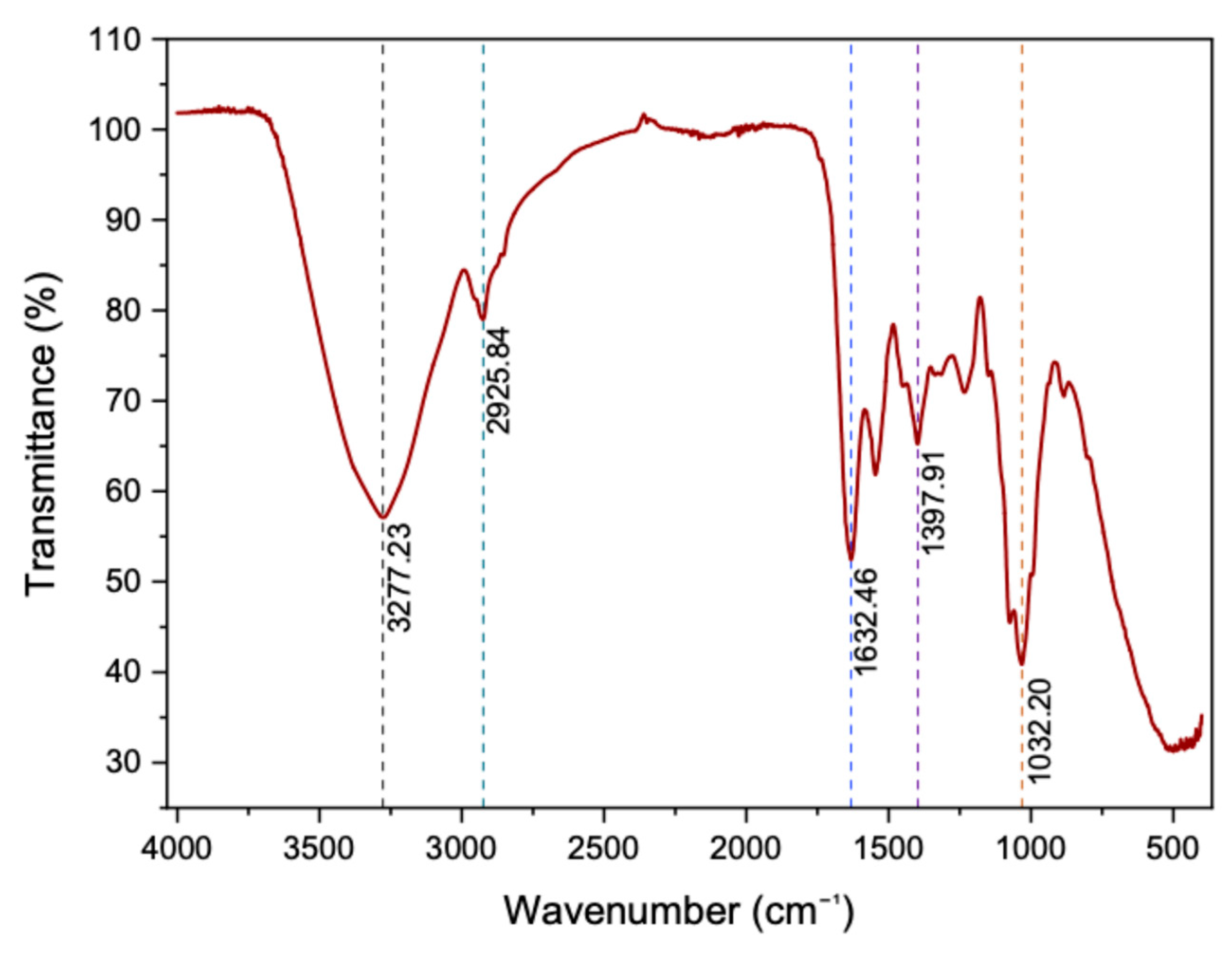
3.6. FTIR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CBI. What Is the Demand for Cocoa on the European Market? Available online: https://www.cbi.eu/market-information/cocoa/what-demand (accessed on 22 June 2025).
- Hawkins, D.; Chen, Y. Giant on a Pinhead: A Profile of the Cocoa Sector; Hardman & Co.: London, UK, 2014. [Google Scholar]
- Babin, R. Pest Management in Organic Cacao. In Handbook of Pest Management in Organic Farming; CAB International: Wallingford, UK, 2018; pp. 502–518. [Google Scholar]
- Franzen, M.; Borgerhoff Mulder, M. Ecological, Economic and Social Perspectives on Cocoa Production Worldwide. Biodivers. Conserv. 2007, 16, 3835–3849. [Google Scholar] [CrossRef]
- Abbott, P.C.; Benjamin, T.J.; Burniske, G.R.; Croft, M.C.; Fenton, M.C.; Kelly, C.R.; Lundy, M.M.; Rodriguez Camayo, F.; Wilcox, M.D., Jr. An Analysis of the Supply Chain of Cacao in Colombia; United States Agency for International Development: Washington, DC, USA, 2018. [Google Scholar]
- Mestanza, M.; David Hernández-Amasifuen, A.; Jherina Pineda-Lázaro, A.; Eriksson, D.; Carlos Guerrero-Abad, J. Genome Editing for Sustainable Agriculture in Peru: Advances, Potential Applications and Regulation. Front. Genome Ed. 2025, 7, 1611040. [Google Scholar] [CrossRef]
- Díaz-Valderrama, J.R.; Leiva-Espinoza, S.T.; Catherine Aime, M. The History of Cacao and Its Diseases in the Americas. Phytopathology 2020, 110, 1604–1619. [Google Scholar] [CrossRef]
- Schuh, R.T. On-Line Systematic Catalog of Plant Bugs (Insecta: Heteroptera: Miridae). Available online: http://research.amnh.org/pbi/catalog/ (accessed on 22 June 2025).
- Lavabre, E.M. Systematique Des Miridae Du Cacaoyer. In Les Mirides du Cacaoyer. In Les Mirides du Cacaoyer; Lavabre, E.M., Ed.; Institut Français du Cafe et du Cacao: Paris, France, 1977; pp. 47–70. [Google Scholar]
- ten Hoopen, G.M.; Deberdt, P.; Mbenoun, M.; Cilas, C. Modelling Cacao Pod Growth: Implications for Disease Control. Ann. Appl. Biol. 2012, 160, 260–272. [Google Scholar] [CrossRef]
- Arias, M.; Ninnin, P.; Ten Hoopen, M.; Alvarado, J.; Cabezas Huayllas, O.; Valderrama, B.; Alguilar, G.; Perrier, C.; Dedieu, F.; Bagny Beilhe, L. The American Cocoa Pod Borer, Carmenta Foraseminis, an Emerging Pest of Cocoa: A Review. Agric. Entomol. 2025, 27, 340–356. [Google Scholar] [CrossRef]
- ICCO. Annual Report 2012/2013; ICCO: London, UK, 2013. [Google Scholar]
- Castillo, P. Insectos Plagas y Sus Enemigos Naturales En El Cultivo de Theobroma Cacao L. (Cacao) En Los Valles de Tumbes y Zarumilla, Perú. Rev. Mangl. 2013, 10, 3–16. [Google Scholar] [CrossRef][Green Version]
- Beilhe, L.B.; Babin, R.; ten Hoopen, M. Insect Pests Affecting Cacao. In Achieving Sustainable Cultivation of Cocoa; Umaharan, P., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; pp. 303–326. [Google Scholar][Green Version]
- Castillo-Carrillo, P.S.; Sernaqué-Cortez, A.; Purizaga-Preciado, J.L. Registro Del Chinche Del Cacao Antiteuchus Tripterus (Fabricius, 1787) (Hemiptera: Pentatomidae), En Tumbes-Perú. Bol. Mus. Nac. Hist. Nat. Parag. 2020, 24, 15–20. [Google Scholar][Green Version]
- Valarezo-Cely, O.; Bermúdez, E.C.; Cedeño, B.N. Artrópodos Asociados al Cultivo de Cacao En Manabí. La. Técnica 2012, 7, 34–42. [Google Scholar] [CrossRef]
- Quintos-Coronado, C.R. Evaluación Del Daño de Antiteuchus Sp En Frutos de Cacao (Theobroma Cacao L.) En El Valle Del Bajo Mayo, Región San Martín. Bachelor’s thesis, Universidad Nacional de San Martín, Lima, Perú, 2018. [Google Scholar]
- Cañarte-Bermúdez, E.; Navarrete-Cedeño, B. Reconocimiento de Artrópodos Plaga y Controladores Biológicos Como Herramienta Para El Manejo Ecológico de Plagas En Cacao, 1st ed.; Instituto Nacional de Investigaciones Agropecuarias: Quito, Ecuador, 2021. [Google Scholar]
- Telaumbanua, M.; Haryanto, A.; Wisnu, F.K.; Lanya, B.; Wiratama, W. Design of Insect Trap Automatic Control System for Cacao Plants; EIAETM: Cluj-Napoca, Romania, 2021; Volume 8. [Google Scholar]
- Sosan, M.B.; Akingbohungbe, A.E. Occupational Insecticide Exposure and Perception of Safety Measures among Cacao Farmers in Southwestern Nigeria. Arch. Env. Occup. Health 2009, 64, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Verzeñassi, D.; Vallini, A.; Fernández, F.; Ferrazini, L.; Lasagna, M.; Sosa, A.J.; Hough, G.E. Cancer Incidence and Death Rates in Argentine Rural Towns Surrounded by Pesticide-Treated Agricultural Land. Clin. Epidemiol. Glob. Health 2023, 20, 101239. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Romero, F.; Jiao, S.; van der Heijden, M.G.A. Impact of Microbial Diversity and Pesticide Application on Plant Growth, Litter Decomposition and Carbon Substrate Use. Soil. Biol. Biochem. 2025, 208, 109866. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.-E. Major Pesticides Are More Toxic to Human Cells Than Their Declared Active Principles. Biomed. Res. Int. 2014, 2014, 179691. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in Aquatic Environments and Their Removal by Adsorption Methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- MIDAGRI. Observatorio de Commodities. Boletín Trimestral No 2021-07002; MIDAGRI: Lima, Perú, 2025. [Google Scholar]
- Samal, I.; Bhoi, T.K.; Majhi, P.K.; Murmu, S.; Pradhan, A.K.; Kumar, D.; Saini, V.; Paschapur, A.U.; Raj, M.N.; Ankur; et al. Combatting Insects Mediated Biotic Stress through Plant Associated Endophytic Entomopathogenic Fungi in Horticultural Crops. Front. Plant Sci. 2023, 13, 1098673. [Google Scholar] [CrossRef]
- Bhoi, T.K.; Mahanta, D.K.; Samreen;Samal, I.; Dash, S.; Singh, S.; Komal, J. Effect of Endophytic Entomopathogenic Fungi (EEPF) in Biological Control of Odontotermes Obesus (Rambur 1842) and Their Synergistic Impact on Plant Physiological Responses. Microbe 2025, 7, 100411. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can We Use Entomopathogenic Fungi as Endophytes for Dual Biological Control of Insect Pests and Plant Pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Mannino, M.C.; Huarte-Bonnet, C.; Davyt-Colo, B.; Pedrini, N. Is the Insect Cuticle the Only Entry Gate for Fungal Infection? Insights into Alternative Modes of Action of Entomopathogenic Fungi. J. Fungi 2019, 5, 33. [Google Scholar] [CrossRef]
- Afifah, L.; Aena, A.C.; Saputro, N.W.; Kurniati, A.; Maryana, R.; Lestari, A.; Abadi, S.; Enri, U. Maize Media Enhance the Conidia Production of Entomopathogenic Fungi Lecanicillium Lecanii Also Its Effective to Control the Weevil Cylas Formicarius (Fabricius) (Coleoptera: Brentidae). Agrivita 2022, 44, 513–525. [Google Scholar] [CrossRef]
- Pedrini, N. Molecular Interactions between Entomopathogenic Fungi (Hypocreales) and Their Insect Host: Perspectives from Stressful Cuticle and Hemolymph Battlefields and the Potential of Dual RNA Sequencing for Future Studies. Fungal Biol. 2018, 122, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Samal, I.; Bhoi, T.K.; Vyas, V.; Majhi, P.K.; Mahanta, D.K.; Komal, J.; Singh, S.; Kumar, P.V.D.; Acharya, L.K. Resistance to Fungicides in Entomopathogenic Fungi: Underlying Mechanisms, Consequences, and Opportunities for Progress. Trop. Plant Pathol. 2023, 49, 5–17. [Google Scholar] [CrossRef]
- Sani, I.; Jamian, S.; Saad, N.; Abdullah, S.; Mohd Hata, E.; Jalinas, J.; Ismail, S.I. Identification and Virulence of Entomopathogenic Fungi, Isaria Javanica and Purpureocillium Lilacinum Isolated from the Whitefly, Bemisia Tabaci (Gennadius) (Hemiptera: Aleyrodidae) in Malaysia. Egypt. J. Biol. Pest Control 2023, 33, 14. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The Production and Uses of Beauveria Bassiana as a Microbial Insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Castillo Lopez, D.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The Entomopathogenic Fungal Endophytes Purpureocillium Lilacinum (Formerly Paecilomyces Lilacinus) and Beauveria Bassiana Negatively Affect Cotton Aphid Reproduction under Both Greenhouse and Field Conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Lu, H.; Liu, S.; Liu, J.; Zhang, W.; Wu, J.; Li, J.; Bai, R.; Yan, F.; Zhao, C. Fungal Warriors: Effects of Beauveria Bassiana and Purpureocillium Lilacinum on CCYV-Carrying Whiteflies. Biomolecules 2025, 15, 593. [Google Scholar] [CrossRef]
- Dahlin, P.; Eder, R.; Consoli, E.; Krauss, J.; Kiewnick, S. Integrated Control of Meloidogyne Incognita in Tomatoes Using Fluopyram and Purpureocillium Lilacinum Strain 251. Crop Prot. 2019, 124, 104874. [Google Scholar] [CrossRef]
- Ban, S.; Azuma, Y.; Sato, H.; Suzuki, K.; Nakagiri, A. Isaria Takamizusanensis Is the Anamorph of Cordyceps Ryogamimontana, Warranting a New Combination, Purpureocillium Takamizusanense Comb. Nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 2459–2465. [Google Scholar] [CrossRef]
- Bali, G.K.; Singh, S.K.; Maurya, D.K.; Wani, F.J.; Pandit, R.S. Morphological and Molecular Identification of the Entomopathogenic Fungus Purpureocillium Lilacinum and Its Virulence against Tuta Absoluta (Meyrick) (Lepidoptera: Gelechiidae) Larvae and Pupae. Egypt. J. Biol. Pest Control 2022, 32, 86. [Google Scholar] [CrossRef]
- Lo, P.H.; Yu, Y.C.; Pai, K.F. First Report of Purpureocillium Takamizusanense as an Omopathogenic Fungus Infecting Tessaratoma Papillosa (Drury) in Taiwan. J. Plant Med. 2019, 61, 27–30. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy of Biological Applications: An Overview. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2021; pp. 1–16. [Google Scholar]
- Łopusiewicz, Ł.; Mazurkiewicz-Zapałowicz, K.; Tkaczuk, C. Chemical Changes in Spores of the Entomopathogenic Fungus Metarhizium Robertsii after Exposure to Heavy Metals, Studied through the Use of Ftir Spectroscopy. J. Elem. 2020, 25, 487–499. [Google Scholar] [CrossRef]
- Piekarczyk, J.; Ratajkiewicz, H.; Jasiewicz, J.; Sosnowska, D.; Wójtowicz, A. An Application of Reflectance Spectroscopy to Differentiate of Entomopathogenic Fungi Species. J. Photochem. Photobiol. B 2019, 190, 32–41. [Google Scholar] [CrossRef]
- Moussaid, F.Z.; Lahlali, R.; Ezrari, S.; El Barnossi, A.; Housseini, A.I. Biological Control of Alternaria Alternata MW970059, the Causal Agent of Tomato Rot, by Aspergillus Nidulans MW732187 Isolated from Green Household Waste. J. Nat. Pestic. Res. 2025, 13, 100134. [Google Scholar] [CrossRef]
- Azrag, A.G.A.; Pirk, C.W.W.; Yusuf, A.A.; Pinard, F.; Niassy, S.; Mosomtai, G.; Babin, R. Prediction of Insect Pest Distribution as Influenced by Elevation: Combining Field Observations and Temperature-Dependent Development Models for the Coffee Stink Bug, Antestiopsis Thunbergii (Gmelin). PLoS ONE 2018, 13, e0199569. [Google Scholar] [CrossRef]
- Yun, T.-S.; Park, S.-Y.; Yu, J.; Hwang, Y.; Hong, K.-J. Isolation and Identification of Fungal Species from the Insect Pest Tribolium Castaneum in Rice Processing Complexes in Korea. Plant Pathol. J. 2018, 34, 356–366. [Google Scholar] [CrossRef]
- Dummel, D.M.; Badaraco, A.; Kramer, R.; Rohatsch, P.; Agostini, J.P. Avances Sobre La Caracterización “Mal de La Tela” En plantas de Yerba Mate Ilex Paraguariensis A. St. -Hil. Rev. De. Investig. Agropecu. 2022, 48, 188–194. [Google Scholar]
- Rehner, S.A.; Minnis, A.M.; Sung, G.-H.; Luangsa-ard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and Systematics of the Anamorphic, Entomopathogenic Genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, Y.; Fan, Q.; Duan, D.E.; Zhang, G.D.; Dai, R.Q.; Dai, Y.D.; Zeng, W.B.; Chen, Z.H.; Li, D.D.; et al. Multigene Phylogeny of the Family Cordycipitaceae (Hypocreales): New Taxa and the New Systematic Position of the Chinese Cordycipitoid Fungus Paecilomyces Hepiali. Fungal Divers. 2020, 103, 1–46. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES Science Gateway: Enabling High-Impact Science for Phylogenetics Researchers with Limited Resources. In Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the eXtreme to the Campus and Beyond, Chicago, IL, USA, 16 July 2012; ACM: New York, NY, USA, 2012; pp. 1–8. [Google Scholar]
- Rambaut, A. Figtree Ver 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Bugeme, D.M.; Knapp, M.; Boga, H.I.; Wanjoya, A.K.; Maniania, N.K. Influence of Temperature on Virulence of Fungal Isolates of Metarhizium Anisopliae and Beauveria Bassiana to the Two-Spotted Spider Mite Tetranychus Urticae. Mycopathologia 2009, 167, 221–227. [Google Scholar] [CrossRef]
- Das, P.; Hazarika, L.K.; Bora, D.S.; Puzari, K.C.; Dutta, P. Effect of Temperature on Radial Growth, Sporulation, Germination, Colony Forming Unit and Biomass Production of Different Strains of Beauveria Bassiana Vuill. (Bals.). Pestology 2011, 35, 50–55. [Google Scholar]
- Riaz, M.; Chen, W.-H.; Kafle, L.; Tseng, M.-N. Morphological and Molecular Characterization of Purpureocillium Lilacinum along with Its Biopesticidal Effect against Fall Armyworm (Spodoptera Frugiperda) in Southern Taiwan. Egypt. J. Biol. Pest Control 2024, 34, 60. [Google Scholar] [CrossRef]
- Yeo, H.; Pell, J.K.; Alderson, P.G.; Clark, S.J.; Pye, B.J. Laboratory Evaluation of Temperature Effects on the Germination and Growth of Entomopathogenic Fungi and on Their Pathogenicity to Two Aphid Species. Pest Manag. Sci. 2003, 59, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; Ramírez, A.; Serrão, J.E. Susceptibility of Demotispa Neivai (Coleoptera: Chrysomelidae) to Beauveria Bassiana and Metarhizium Anisopliae Entomopathogenic Fungal Isolates. Pest Manag. Sci. 2022, 78, 126–133. [Google Scholar] [CrossRef]
- Alarcón, M.; Dalmiro, C. Descripción de Los Estadios Inmaduros de Antiteuchus Tripterus (Fabricius, 1787) (Heteroptera: Pentatomidae: Discocephalinae: Discocephalini). Rev. Nicar. De. Entomol. 2022, 261, 3–61. [Google Scholar]
- Santos, A.V.; Albuquerque, G.S. Eficiência Do Cuidado Maternal de Antiteuchus Sepulcralis (Fabricius) (Hemiptera: Pentatomidae) Contra Inimigos Naturais Do Estágio de Ovo. Neotrop. Entomol. 2001, 30, 641–646. [Google Scholar] [CrossRef]
- de Resende, R.C.; Rodrigues-Silva, A.L.; Pec, M.; Marucci, R.C.; Chagas, P.G.; Sales, F.S.; de Figueiredo, K.G.; Moino Junior, A. Doru Luteipes: Susceptibility to Entomopathogenic Fungi and the Role of Maternal Care in the Protection of Offspring Against Infection. J. Insect Behav. 2025, 38, 10. [Google Scholar] [CrossRef]
- Das, P.; Borah, B.; Saikia, P.; Hazarika, L.K.; Sharma, K.K.; Mohanasundaram, A.; Boro, R.C.; Kalita, R.; Gautom, T. Molecular Characterization of an Isaria Fumosorosea (Wize) Native Strain, and Its Pathogenicity on Eublemma Amabilis (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2022, 32, 122. [Google Scholar] [CrossRef]
- Aguirre, E.; Domínguez, J.; Villanueva, E.; Ponce-Ramirez, J.A.; de Fátima Arevalo-Oliva, M.; Siche, R.; González-Cabeza, J.; Rodríguez, G. Biodegradable Trays Based on Manihot Esculenta Crantz Starch and Zea Mays Husk Flour. Food Packag. Shelf Life 2023, 38, 101129. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shimizu, D. Monographic Studies of Cordyceps 2. Group Parasitic on Cicadidae; Bulletin of the National Science Museum: Tokyo, Japan, 1963; Volume 6. [Google Scholar]
- Nguyen, N.-H.; Tamura, T.; Shimizu, K. Draft Genome Sequence of Purpureocillium Takamizusanense, a Potential Bioinsecticide. Microbiol. Resour. Announc. 2022, 11, e0026822. [Google Scholar] [CrossRef]
- Luangsa-Ard, J.; Houbraken, J.; van Doorn, T.; Hong, S.-B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a New Genus for the Medically Important Paecilomyces Lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Dornburg, A.; Townsend, J.P.; Wang, Z. Maximizing Power in Phylogenetics and Phylogenomics: A Perspective Illuminated by Fungal Big Data. In Advances in Genetics; Townsend, J.P., Wang, Z., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017; Volume 100, pp. 1–47. [Google Scholar]
- Lo, P.-H.; El-Sayid Abdrabo, K.A.; Nai, Y.-S.; Lu, H.; Huang, Y.-T. Versatile Entomopathogenic Activity of Purpureocillium Takamizusanense against Diverse Agricultural Pests. bioRxiv 2025. [Google Scholar] [CrossRef]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Phenotypic, Molecular, and Virulence Characterization of Entomopathogenic Fungi, Beauveria Bassiana (Balsam) Vuillemin, and Metarhizium Anisopliae (Metschn.) Sorokin from Soil Samples of Ethiopia for the Development of Mycoinsecticide. Heliyon 2021, 7, e07091. [Google Scholar] [CrossRef]
- Schemmer, R.; Chládeková, P.; Medo, J.; Barta, M. Natural Prevalence of Entomopathogenic Fungi in Hibernating Pupae of Cameraria Ohridella (Lepidoptera: Gracillariidae) and Virulence of Selected Isolates. Plant Prot. Sci. 2016, 52, 199–208. [Google Scholar] [CrossRef]
- Habtegebriel, B.; Getu, E.; Dawd, M.; Seyoum, E.; Atnafu, G.; Khamis, F.; Hilbur, Y.; Ekesi, S.; Larsson, M. Molecular Characterization and Evaluation of Indigenous Entomopathogenic Fungal Isolates against Sorghum Chafer, Pachnoda Interrupta (Olivier) in Ethiopia. J. Entomol. Nematol. 2016, 8, 34–45. [Google Scholar] [CrossRef]
- Mkiga, A.M.; Mohamed, S.A.; Du Plessis, H.; Khamis, F.M.; Akutse, K.S.; Ekesi, S.; Bruck, D. Metarhizium Anisopliae and Beauveria Bassiana: Pathogenicity, Horizontal Transmission, and Their Effects on Reproductive Potential of Thaumatotibia Leucotreta (Lepidoptera: Tortricidae). J. Econ. Entomol. 2020, 113, 660–668. [Google Scholar] [CrossRef]
- Dotaona, R.; Wilson, B.A.L.; Stevens, M.M.; Holloway, J.; Ash, G.J. Screening of Tropical Isolates of Metarhizium Anisopliae (Hypocreales: Clavicipitaceae) for Virulence to the Sweet Potato Weevil, Cylas Formicarius (Coleoptera: Brentidae). Int. J. Trop. Insect Sci. 2015, 35, 153–163. [Google Scholar] [CrossRef]
- Mar, T.T.; Suwannarach, N.; Lumyong, S. Isolation of Entomopathogenic Fungi from Northern Thailand and Their Production in Cereal Grains. World J. Microbiol. Biotechnol. 2012, 28, 3281–3291. [Google Scholar] [CrossRef]
- Abdul Qayyum, M.; Bilal, H.; Naeem Ullah, U.; Ali, H.; Raza, H. Factors Affecting the Epizootics of Entomopathogenic Fungi-A Review. J. Bioresour. Manag. 2021, 8, 78–85. [Google Scholar] [CrossRef]
- Moldovan, A.; Munteanu-Molotievskiy, N.; Toderas, I. Temperature Effects on the Entomopathogenic Fungi Beauveria Bassiana Strain CNMN-FE-01: Vegetative Growth, Sporulation, Germination Rate. Curr. Trends Nat. Sci. 2022, 11, 332–338. [Google Scholar] [CrossRef]
- Chuquibala-Checan, B.; Torres-De La Cruz, M.; Leiva, S.; Hernandez-Diaz, E.; Rubio, K.; Goñas, M.; Arce-Inga, M.; Oliva-Cruz, M. In Vitro Biological Activity of Beauveria Bassiana, Beauveria Peruviensis, and Metarhizium Sp. against Hypothenemus Hampei (Coleoptera: Curculionidae). Int. J. Agron. 2023, 2023, 4982399. [Google Scholar] [CrossRef]
- Thangavel, B.; Palaniappan, K.; Manickavasagam Pillai, K.; Subbarayalu, M.; Madhaiyan, R. Pathogenicity, Ovicidal Action, and Median Lethal Concentrations (LC 50) of Entomopathogenic Fungi against Exotic Spiralling Whitefly, Aleurodicus Dispersus Russell. J. Pathog. 2013, 2013, 393787. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moraga, E.; Carrasco-Díaz, J.-A.; Santiago-Álvarez, C. Insecticidal and Antifeedant Activities of Proteins Secreted by Entomopathogenic Fungi against Spodoptera Littoralis (Lep., Noctuidae). J. Appl. Entomol. 2006, 130, 442–452. [Google Scholar] [CrossRef]
- Brown, R.; Priest, E.; Naglik, J.R.; Richardson, J.P. Fungal Toxins and Host Immune Responses. Front. Microbiol. 2021, 12, 643639. [Google Scholar] [CrossRef]
- Gençer, D.; Bayramoğlu, Z. Characterization and Pathogenicity of Beauveria Bassiana Strains Isolated from Galleria Mellonella L. (Lepidoptera: Pyralidae) in Turkey. Egypt. J. Biol. Pest Control 2022, 32, 99. [Google Scholar] [CrossRef]
- Geroh, M.; Gulati, R.; Tehri, K. Determination of Lethal Concentration and Lethal Time of Entomopathogen Beauveria Bassiana (Balsamo) Vuillemin against Tetranychus Urticae Koch. Int. J. Agric. Sci. 2015, 7, 523–528. [Google Scholar]
- Kim, J.; Baek, S.; Kim, J.S. Whitefly-Pathogenic Beauveria Bassiana JEF-507 as a Competitive Biopesticide, Chongchae-Stop. J. Appl. Entomol. 2025, 149, 165–177. [Google Scholar] [CrossRef]
- Islam, M.S.; Subbiah, V.K.; Siddiquee, S. Efficacy of Entomopathogenic Trichoderma Isolates against Sugarcane Woolly Aphid, Ceratovacuna Lanigera Zehntner (Hemiptera: Aphididae). Horticulturae 2022, 8, 2. [Google Scholar] [CrossRef]
- Eski, A.; Biryol, S.; Acici, O.; Demir, İ. Biocontrol of the Western Conifer Seed Bug, Leptoglossus Occidentalis Heidemann (Heteroptera: Coreidae) Using Indigenous Entomopathogenic Fungi. Egypt. J. Biol. Pest Control 2022, 32, 140. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, P.; Ali, A.; Zang, L.S. Molecular Identification and Virulence of Four Strains of Entomopathogenic Fungi Against the Whitefly, Bemisia Tabaci (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2022, 115, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Ashida, M.; Brey, P.T. Role of the Integument in Insect Defense: Pro-Phenol Oxidase Cascade in the Cuticular Matrix. Proc. Natl. Acad. Sci. USA 1995, 92, 10698–10702. [Google Scholar] [CrossRef]
- Brey, P.T.; Lee, W.J.; Yamakawa, M.; Koizumi, Y.; Perrot, S.; François, M.; Ashida, M. Role of the Integument in Insect Immunity: Epicuticular Abrasion and Induction of Cecropin Synthesis in Cuticular Epithelial Cells. Proc. Natl. Acad. Sci. USA 1993, 90, 6275–6279. [Google Scholar] [CrossRef]
- Strand, M.R. The Insect Cellular Immune Response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Irving, P.; Ubeda, J.-M.; Doucet, D.; Troxler, L.; Lagueux, M.; Zachary, D.; Hoffmann, J.A.; Hetru, C.; Meister, M. New Insights into Drosophila Larval Haemocyte Functions through Genome-Wide Analysis. Cell Microbiol. 2005, 7, 335–350. [Google Scholar] [CrossRef]
- Umaña, E.; Carballo, M. Biología de Antiteuchus Tripterus L. (Hemiptera: Pentatomidae) y Su Parasitoide Trissolcus Radix (Johnson) (Hymenoptera: Scelionidae) En Macadamia. Manejo Integr. De. Plagas 1995, 38, 16–19. [Google Scholar]
- Quesada-Moraga, E.; González-Mas, N.; Yousef-Yousef, M.; Garrido-Jurado, I.; Fernández-Bravo, M. Key Role of Environmental Competence in Successful Use of Entomopathogenic Fungi in Microbial Pest Control. J. Pest Sci. 2024, 97, 1–15. [Google Scholar] [CrossRef]
- De Croos, J.N.A.; Bidochka, M.J. Cold-Induced Proteins in Cold-Active Isolates of the Insectpathogenic Fungus Metarhizium Anisopliae. Mycol. Res. 2001, 105, 868–873. [Google Scholar] [CrossRef]
- Vidhate, R.P.; Dawkar, V.V.; Punekar, S.A.; Giri, A.P. Genomic Determinants of Entomopathogenic Fungi and Their Involvement in Pathogenesis. Microb. Ecol. 2023, 85, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Salzano, F.; Staropoli, A.; Marra, R.; Turrà, D.; Lorito, M.; Vinale, F. Nitrogen Source Orchestrates PH Modulation and Secondary Metabolism in Trichoderma Harzianum. Chem. Biol. Technol. Agric. 2025, 12, 19. [Google Scholar] [CrossRef]
- Nassary, E.K. Fungal Biocontrol Agents in the Management of Soil-Borne Pathogens, Insect Pests, and Nematodes: Mechanisms and Implications for Sustainable Agriculture. Microbe 2025, 7, 100391. [Google Scholar] [CrossRef]
- Rodrigues de Miranda, R.P.; Soares, T.K.d.A.; Castro, D.P.; Genta, F.A. General Aspects, Host Interaction, and Application of Metarhizium Sp. in Arthropod Pest and Vector Control. Front. Fungal Biol. 2024, 5, 1456964. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Stączek, S.; Zdybicka-Barabas, A.; Wojda, I.; Wiater, A.; Mak, P.; Suder, P.; Skrzypiec, K.; Cytryńska, M. Fungal α-1,3-Glucan as a New Pathogen-Associated Molecular Pattern in the Insect Model Host Galleria Mellonella. Molecules 2021, 26, 5097. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S. Insect Pathogenic Fungi: Genomics, Molecular Interactions, and Genetic Improvements. Annu. Rev. Entomol. 2017, 62, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, H.; Du, Y.; Keyhani, N.O.; Xia, Y.; Jin, K. Members of Chitin Synthase Family in Metarhizium Acridum Differentially Affect Fungal Growth, Stress Tolerances, Cell Wall Integrity and Virulence. PLoS Pathog. 2019, 15, e1007964. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Keyhani, N.O.; Deng, J.; Zhao, X.; Huang, S.; Luo, Z.; Jin, K.; Zhang, Y. Insect Fungal Pathogens Secrete a Cell Wall-Associated Glucanase That Acts to Help Avoid Recognition by the Host Immune System. PLoS Pathog. 2023, 19, e1011578. [Google Scholar] [CrossRef]
- Peng, Y.-J.; Ding, J.-L.; Lin, H.-Y.; Feng, M.-G.; Ying, S.-H. A Virulence-Related Lectin Traffics into Eisosome and Contributes to Functionality of Cytomembrane and Cell-Wall in the Insect-Pathogenic Fungus Beauveria Bassiana. Fungal Biol. 2021, 125, 914–922. [Google Scholar] [CrossRef]
- Cen, K.; Li, B.; Lu, Y.; Zhang, S.; Wang, C. Divergent LysM Effectors Contribute to the Virulence of Beauveria Bassiana by Evasion of Insect Immune Defenses. PLoS Pathog. 2017, 13, e1006604. [Google Scholar] [CrossRef]
- Wang, C.; St. Leger, R.J. A Collagenous Protective Coat Enables Metarhizium Anisopliae to Evade Insect Immune Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 6647–6652. [Google Scholar] [CrossRef]
- Tsoupras, A.; Kouvelis, V.N.; Pappas, K.M.; Demopoulos, C.A.; Typas, M.A. Anti-Inflammatory and Anti-Thrombotic Properties of Lipid Bioactives from the Entomopathogenic Fungus Beauveria Bassiana. Prostaglandins Other Lipid Mediat. 2022, 158, 106606. [Google Scholar] [CrossRef]
- Litwin, A.; Bernat, P.; Nowak, M.; Słaba, M.; Różalska, S. Lipidomic Response of the Entomopathogenic Fungus Beauveria Bassiana to Pyrethroids. Sci. Rep. 2021, 11, 21319. [Google Scholar] [CrossRef] [PubMed]


| Province | District | Locale | Latitude (S) | Longitude (W) | Altitude (m.a.s.l.) * | Production System |
|---|---|---|---|---|---|---|
| Bagua | Copallin | Lluhuana | 5°40′37.4″ | 78°24′33.7″ | 917 | Agroforestry |
| La Peca | La Tranquilla | 5°37′44″ | 78°25′06″ | 1060 | Agroforestry | |
| Utcubamba | Cajaruro | San José bajo | 5°42′22.8″ | 78°24′23.4″ | 663 | Monoculture |
| Cajaruro | San José bajo | 5°42′28.1″ | 78°23′38.1″ | 700 | Agroforestry | |
| Cajaruro | La Cruz | 5°41′22.8″ | 78°24′10.9″ | 835 | Agroforestry |
| Lethal Time | Estimated Time (Days) | 95% Confidence Interval (Days) | Slope ± Standard Error | χ2 (p-Value) |
|---|---|---|---|---|
| LT50 | 3.08 | 2.86–3.30 | 1.671 ± 0.13 | 1.89 (0.10) |
| LT90 | 7.29 | 6.48–8.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Diaz, E.; Martínez, L.C.; Díaz-Valderrama, J.R.; Cumpa-Velasquez, L.M.; Oliva-Cruz, S.M.; Huaman-Pilco, A.F.; Rubio, K.; León-Alcántara, E.E.; Ix-Balam, M.A. Purpureocillium takamizusanense: A New Entomopathogenic Fungus in the Americas and Its Pathogenicity Against the Cacao Black Bug, Antiteuchus tripterus (Hemiptera: Pentatomidae). Agronomy 2025, 15, 2315. https://doi.org/10.3390/agronomy15102315
Hernandez-Diaz E, Martínez LC, Díaz-Valderrama JR, Cumpa-Velasquez LM, Oliva-Cruz SM, Huaman-Pilco AF, Rubio K, León-Alcántara EE, Ix-Balam MA. Purpureocillium takamizusanense: A New Entomopathogenic Fungus in the Americas and Its Pathogenicity Against the Cacao Black Bug, Antiteuchus tripterus (Hemiptera: Pentatomidae). Agronomy. 2025; 15(10):2315. https://doi.org/10.3390/agronomy15102315
Chicago/Turabian StyleHernandez-Diaz, Elgar, Luis Carlos Martínez, Jorge Ronny Díaz-Valderrama, Liz Marjory Cumpa-Velasquez, Segundo Manuel Oliva-Cruz, Angel F. Huaman-Pilco, Karol Rubio, Eduardo Enrique León-Alcántara, and Manuel Alejandro Ix-Balam. 2025. "Purpureocillium takamizusanense: A New Entomopathogenic Fungus in the Americas and Its Pathogenicity Against the Cacao Black Bug, Antiteuchus tripterus (Hemiptera: Pentatomidae)" Agronomy 15, no. 10: 2315. https://doi.org/10.3390/agronomy15102315
APA StyleHernandez-Diaz, E., Martínez, L. C., Díaz-Valderrama, J. R., Cumpa-Velasquez, L. M., Oliva-Cruz, S. M., Huaman-Pilco, A. F., Rubio, K., León-Alcántara, E. E., & Ix-Balam, M. A. (2025). Purpureocillium takamizusanense: A New Entomopathogenic Fungus in the Americas and Its Pathogenicity Against the Cacao Black Bug, Antiteuchus tripterus (Hemiptera: Pentatomidae). Agronomy, 15(10), 2315. https://doi.org/10.3390/agronomy15102315







