Effect of Processing Solid Organic Municipal Wastes on Their Phosphorus Fertilizer Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Organic Soil Amendments Derived from Municipal Waste
2.3. Experimental Procedure
2.3.1. Incubation Experiment
2.3.2. Pot Experiment
2.4. Measurements and Chemical Analyses
2.5. Calculation of Indicators for the P Fertilization Effect
2.6. Statistical Analysis
3. Results
3.1. Effect of Processing on Ptotal, PCAL and pH of the Amendments
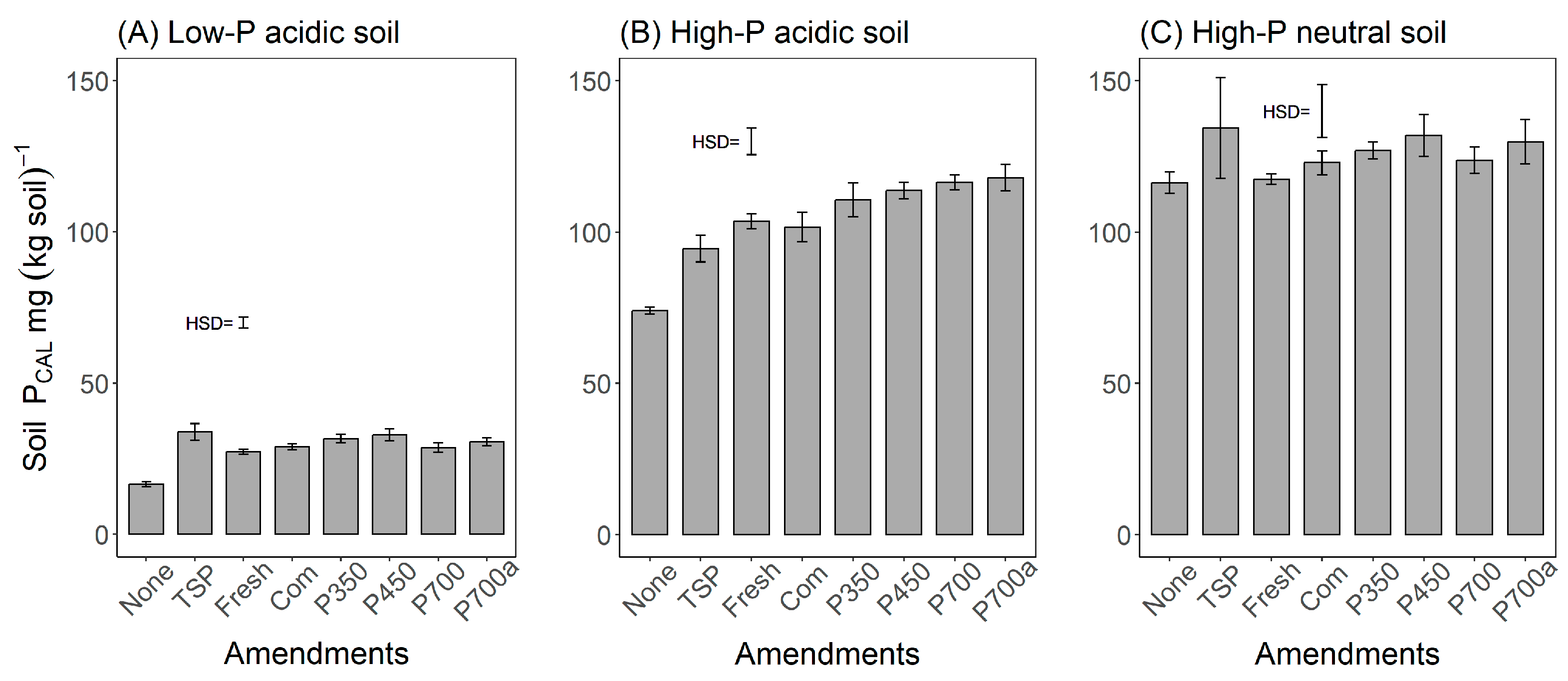
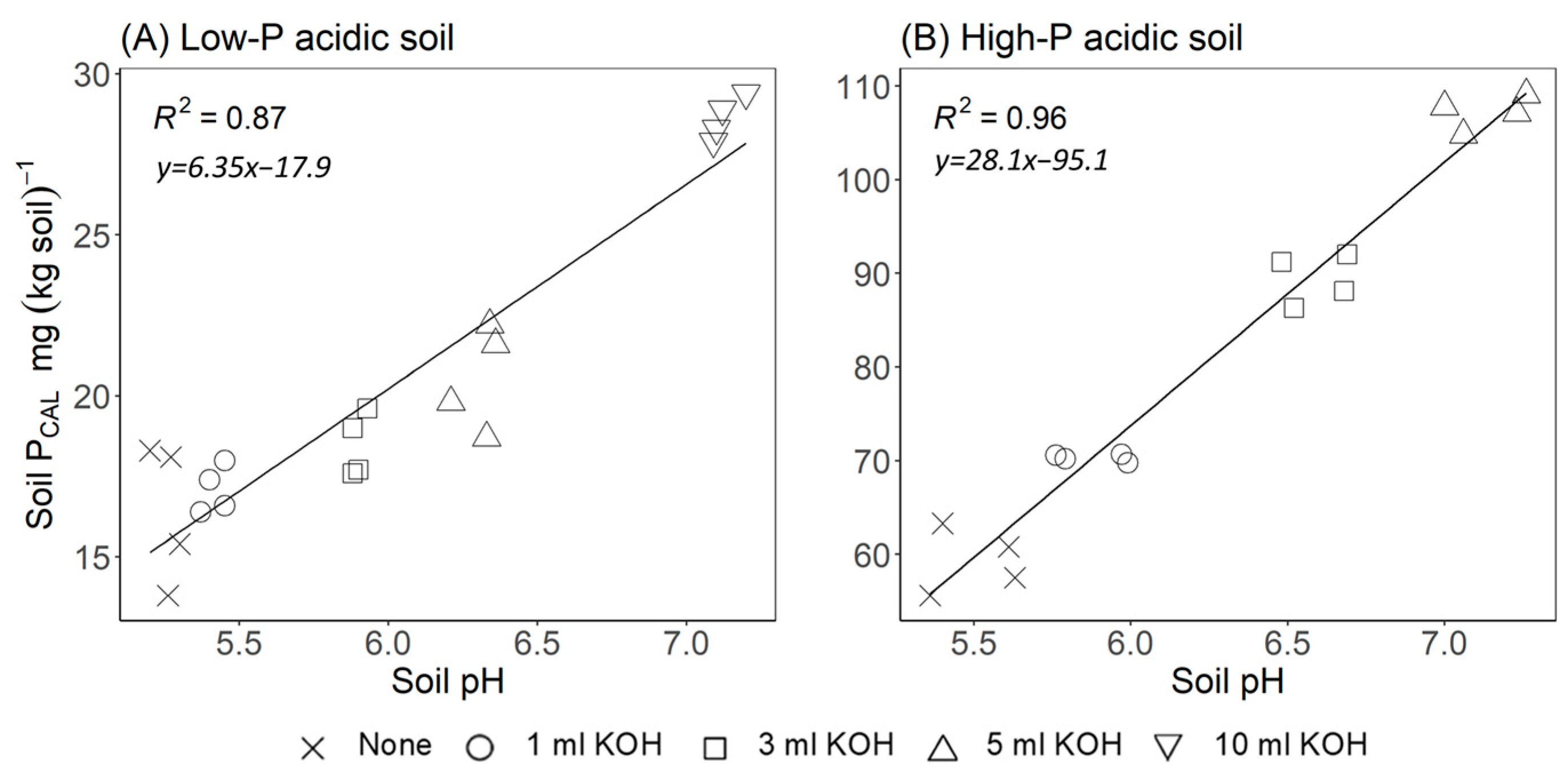
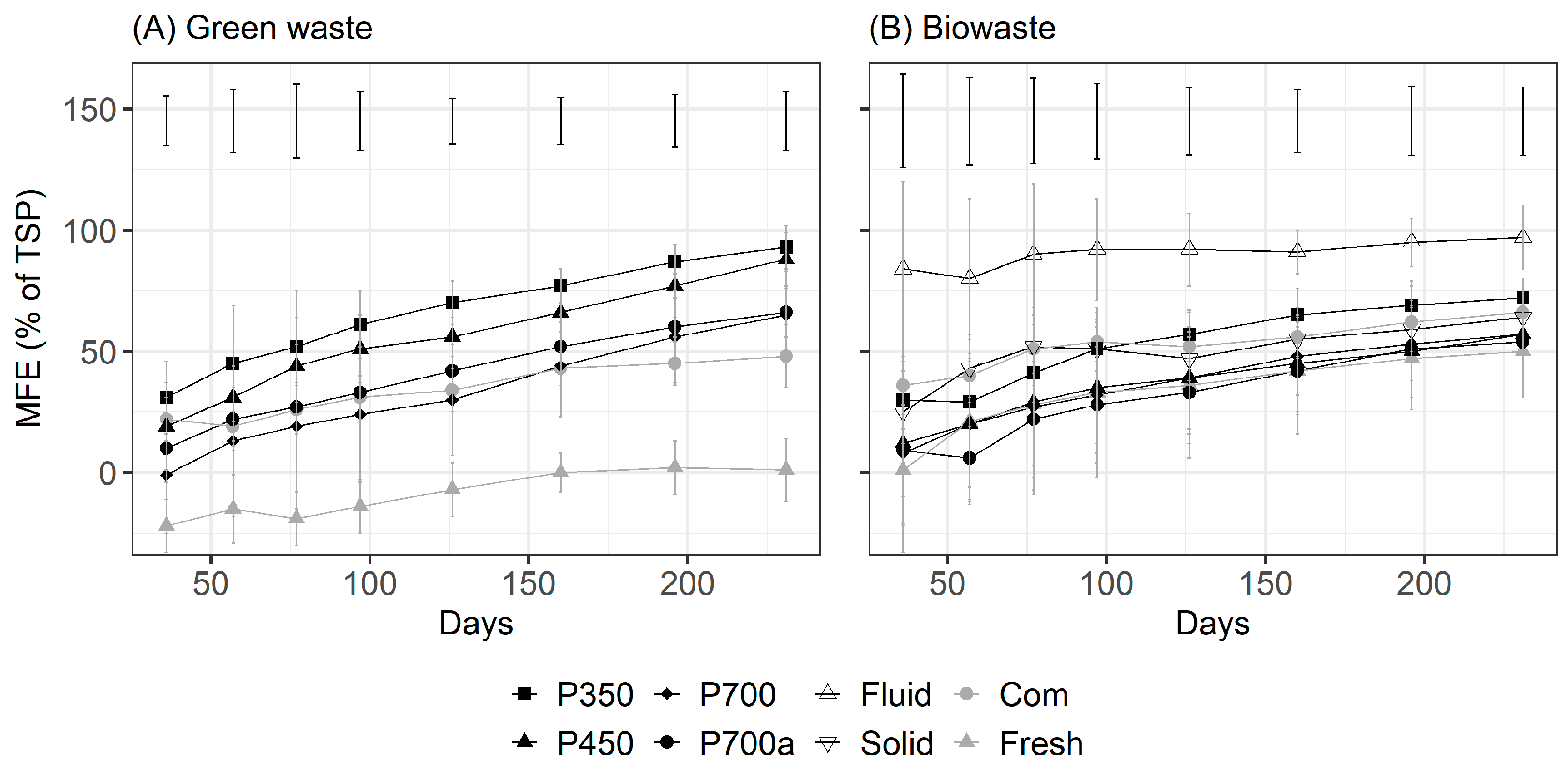
3.2. Effect of Green Waste Processing on the Soil PCAL Concentration
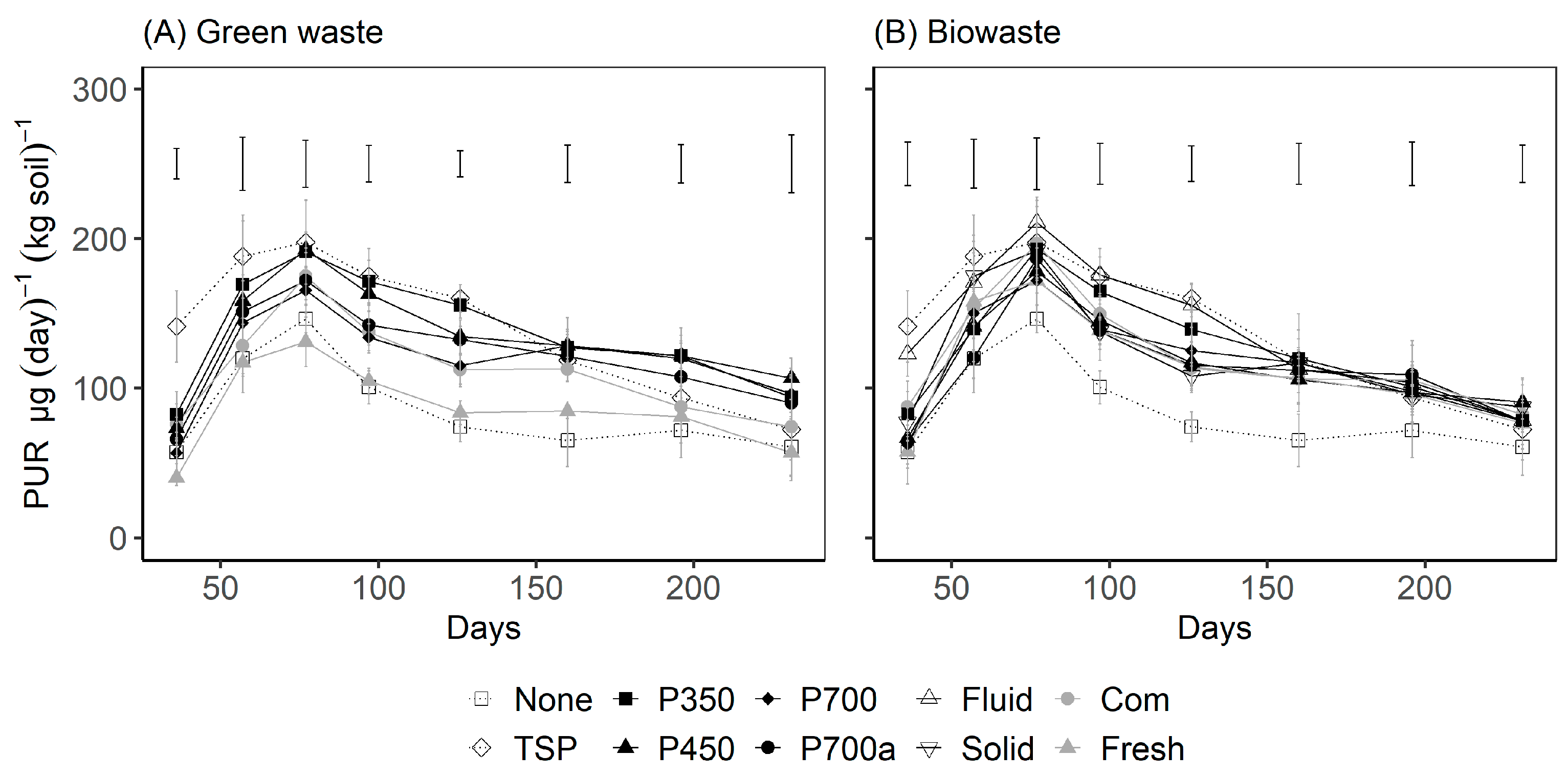
3.3. Effect of Amendments on Shoot Growth of Ryegrass
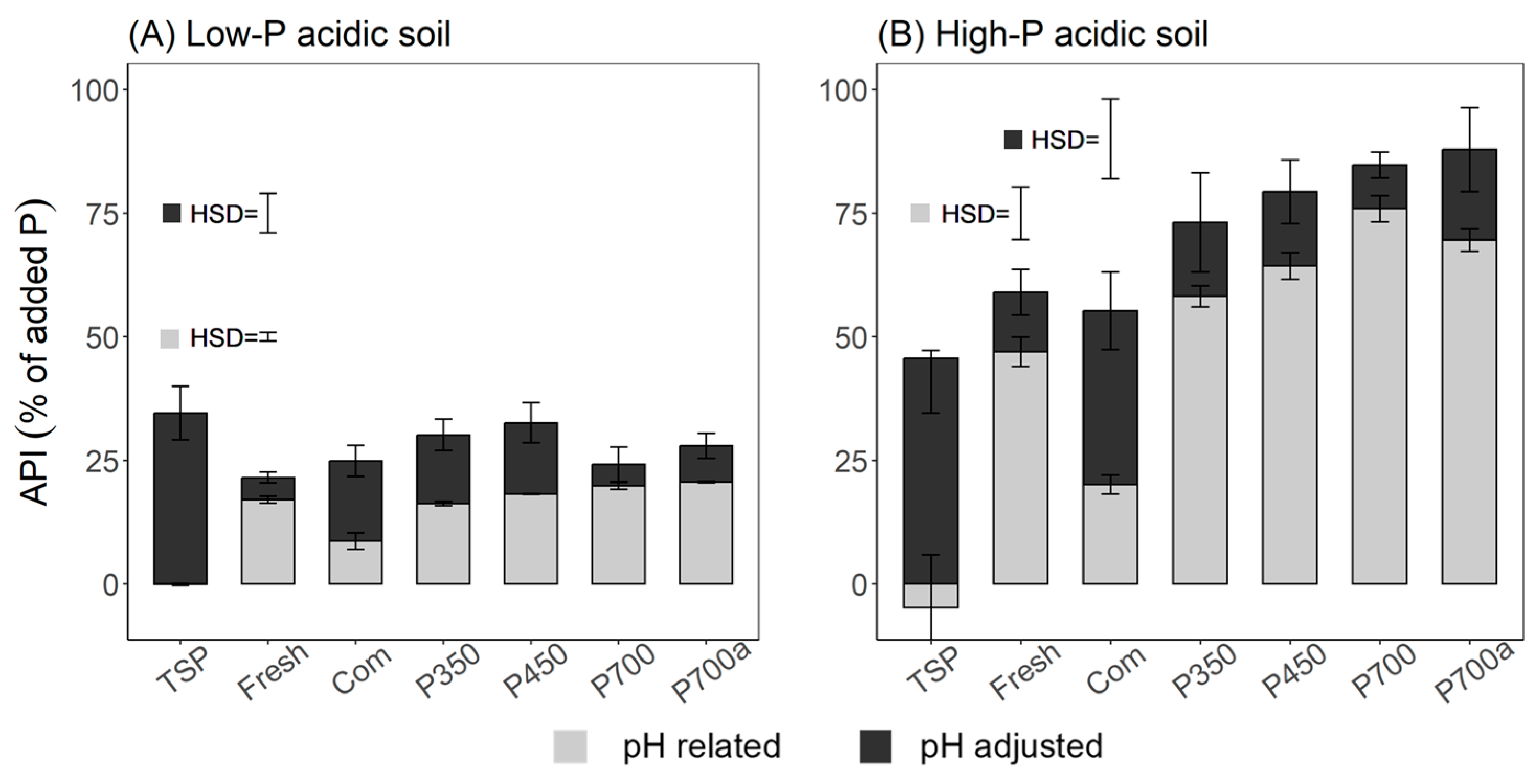
3.4. Effect of the Amendments on P Uptake
4. Discussion
4.1. Effects of the Mode of Treatment on the P Concentrations in the Amendments
4.2. Effects of the Mode of Treatment on the Soil P Concentration After Application of the Amendments
4.3. Effect of the Mode of Treatment on P Uptake
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; pp. 271–283. [Google Scholar]
- Mew, M.C.; Steiner, G.; Geissler, B. Phosphorus Supply Chain—Scientific, Technical, and Economic Foundations: A Transdisciplinary. Sustainability 2018, 10, 1087. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Peak Phosphorus: Clarifying the Key Issues of a Vigorous Debate about Long-Term Phosphorus Security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van der Bruggen, B.; Verstraete, W.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 45, 336–384. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission: On the Review of the List of Critical Raw Materials for the EU and the Implementation of the Raw Materials Initiative; European Commission: Brussels, Belgium, 2014; COM no. 297. [Google Scholar]
- Schoumans, O.F.; Bouraoui, F.; Kabbe, C.; Oenema, O.; van Dijk, K.C. Phosphorus management in Europe in a changing world. Ambio 2015, 44, 180–192. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Doody, D.G.; Sylvester-Bradley, R. Achieving Sustainable Phosphorus Use in Food Systems through Circularisation. Sustainability 2018, 10, 1804. [Google Scholar] [CrossRef]
- Hollas, C.E.; Bolsan, A.C.; Venturin, B.; Bonassa, G.; Tápparo, D.C.; Cândido, D.; Antes, F.G.; Vanotti, M.B.; Szögi, A.A.; Kunz, A. Second-Generation Phosphorus: Recovery from Wastes towards the Sustainability of Production Chains. Sustainability 2021, 13, 5919. [Google Scholar] [CrossRef]
- Schneider, F.; Haderlein, S.B. Potential effects of biochar on the availability of phosphorus—Mechanistic insights. Geoderma 2016, 277, 83–90. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Global Waste Management Outlook 2024: Beyond an Age of Waste—Turning Rubbish into a Resource. Nairobi. 2024. Available online: https://wedocs.unep.org/20.500.11822/44939 (accessed on 26 January 2024).
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.W.; Ramadass, K.; Vinu, A.; Kirkham, M.B.; Bolan, N.S. A review on the valorisation of food waste as a nutrient source and soil amendment. Environ. Pollut. 2021, 272, 115985. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, E.K.; Reimer, M.; Smolders, E.; Smith, S.; Bigalge, M.; Palmqvist, A.; Brandt, K.K.; Möller, K.; Harder, R.; Hermann, L.; et al. Do contaminants compromise the use of recycled nutrients in organic agriculture? A review and synthesis of current knowledge on contaminant oncentrations, fate in the environment and risk assessment. Sci. Total Environ. 2024, 912, 168901. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Oberson, A.; Bünemann, E.K.; Cooper, J.; Friedel, J.K.; Glæsnerk, N.; Hörtenhuber, S.; Løes, A.K.; Mäder, P.; Meyer, G.; et al. Improved Phosphorus Recycling in Organic Farming: Navigating Between Constraints. Adv. Agron. 2018, 147, 159–237. [Google Scholar] [CrossRef]
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenat, A.; Zurbrügg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and middle-income settings. Rev. Environ. Sci. Biotechnol. 2017, 16, 81–130. [Google Scholar] [CrossRef]
- Cao, X.; Williams, P.N.; Zhan, Y.; Scott, A.; Coughlin, S.A.; McGrath, J.W.; Chin, J.P.; Xu, Y. Municipal solid waste compost: Global trends and biogeochemical cycling. Soil Environ. Health 2023, 1, 100038. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015. [Google Scholar] [CrossRef]
- Chen, C.R.; Phillips, I.R.; Condron, L.M.; Goloran, J.; Xu, Z.H.; Chan, K.Y. Impacts of greenwaste biochar on ammonia volatilization from bauxite processing residue sand. Plant Soil 2013, 367, 301–312. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Kumar, V.K.; Panwar, N.L. Pyrolysis technologies for biochar production in waste management: A review. Clean Energy 2024, 4, 61–78. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.C.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; James, A.; Ippolito, J.A.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Reeve, J.R.; Endelman, J.B.; Miller, B.E.; Hole, D.J. Residual Effects of Compost on Soil Quality and Dryland Wheat Yield Sixteen Years after Compost Application. Soil Sci. Soc. Am. 2011, 76, 278–285. [Google Scholar] [CrossRef]
- Adugna, G. A review on impact of compost on soil properties, water use and crop productivity. Acad. Res. J. Agric. Sci. Res. 2016, 4, 93–104. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of Organic Wastes through Composting: Process Performance and Compost Application in Agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Kranz, C.N.; McLaughlin, R.A.; Johnson, A.; Miller, M.; Heitman, J.H. The effects of compost incorporation on soil physical properties in urban soils—A concise review. J. Environ. Manag. 2020, 261, 110209. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural Benefits and Environmental Risks of Soil Fertilization with Anaerobic Digestates: A Review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota e A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Muirhead, B.; Wright, G.; Bird, M.I. Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 2015, 213, 72–85. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Pandit, B.H.; Cornelissen, G.; Kammann, C.I. Biochar-Based Fertilization with Liquid Nutrient Enrichment: 21 Field Trials Covering 13 Crop Species in Nepal. Land Degrad. Dev. 2017, 28, 2324–2342. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and green waste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element contaminated soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Hazratqulov, S.; von Ahlefeldt, G.; Liu, R.; Bessler, H.; Almuina-Villar, H.; Dieguez-Alonso, A.; Engels, C. Processing Municipal Waste for Phytostabilization of Heavy Metal Contaminated Soils. Soil Syst. 2024, 8, 109. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, F.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar onsoil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Kratz, S.; Vogel, C.; Adam, C. Agronomic performance of P recycling fertilizers and methods to predict it: A review. Nutr. Cycl. Agroecosyst. 2019, 115, 1–39. [Google Scholar] [CrossRef]
- Christiansen, N.H.; Sørensen, P.; Labouriau, R.; Christensen, B.T.; Rubæk, G.H. Characterizing phosphorus availability in waste products by chemical extractions and plant uptake. J. Plant Nutr. Soil Sci. 2020, 183, 416–428. [Google Scholar] [CrossRef]
- Duboc, O.; Hernandez-Mora, A.; Wenzel, W.W.; Santner, J. Improving the prediction of fertilizer phosphorus availability to plants with simple, but non-standardized extraction techniques. Sci. Total Environ. 2022, 806, 150486. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Steingrobe, B.; Römer, W.; Claassen, N. Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr. Cycl. Agroecosyst. 2011, 91, 173–184. [Google Scholar] [CrossRef]
- Hamilton, H.A.; Brod, E.; Hanserud, O.; Müller, D.B.; Brattebø, H.; Haraldsen, T.K. Recycling potential of secondary phosphorus resources as assessed by integrating substance flow analysis and plant-availability. Sci. Total Environ. 2016, 575, 1546–1555. [Google Scholar] [CrossRef]
- Sinaj, S.; Traore, O.; Frossard, E. Effect of compost and soil properties on the availability of compost phosphate for white clover (Trifolium repens L.). Nutr. Cycl. Agroecosyst. 2002, 62, 89–102. [Google Scholar] [CrossRef]
- Debicka, M.; Jamroz, E.; Bekier, J.; Cwielag-Piasecka, I.; Kocowicz, A. The Influence of Municipal Solid Waste Compost on the Tranformations of Phosphorus Forms in Soil. Agronomy 2023, 13, 1234. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 3, 242–257. [Google Scholar] [CrossRef]
- Bachmann, S.; Uptmoor, R.; Eichler-Löbermann, B. Phosphorus distribution and availability in untreated and mechanically separated biogas digestates. Sci. Agric. 2016, 73, 9–17. [Google Scholar] [CrossRef]
- Biederman, L.; Harpole, S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338. [Google Scholar] [CrossRef]
- Tesfaye, F.; Liu, X.; Zheng, J.; Cheng, K.; Bian, R.; Zhang, X.; Li, L.; Drosos, M.; Joseph, S.; Pan, G. Could biochar amendment be a tool to improve soil availability and plant uptake of phosphorus? A meta-analysis of published experiments. Environ. Sci. Pollut. Res. Int. 2021, 28, 34108–34120. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A Critical Review on Soil Chemical Processes that Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Barrow, N.J.; Campbell, N.A. Methods of measuring residual value of fertilizers. Aust. J. Exp. Agric. 1972, 12, 502–510. [Google Scholar] [CrossRef]
- VDLUFA (Ed.) Methode A 5.1.1. Bestimmung des pH-Wertes. In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. I Die Untersuchung von Böden; VDLUFA: Paderborn, Germany, 2016. [Google Scholar]
- Schüller, H. Die CAL-Methode, eine neue Methode zur Bestimmung des pflanzenverfügbaren Phosphates in Böden. Z. Pflanzenernähr. Bodenkd. 1969, 123, 48–63. [Google Scholar] [CrossRef]
- VDLUFA (Ed.) Methode A 6.2.1.1 Bestimmung von Phosphor und Kalium im Calcium-Acetat-Lactat-Auszug. In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. I Die Untersuchung von Böden; VDLUFA: Paderborn, Germany, 2012. [Google Scholar]
- Hanson, W.C. The photometric determination of phosphorus in fertilizers using the phosphovanado–molybdate complex. J. Sci. Food. Agric. 1950, 1, 172–173. [Google Scholar] [CrossRef]
- Jones, E.; Harden, S.; Crawley, M.J. The R Book; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Smith, G.S.; Cornforth, I.C.; Henderson, H.V. Critical Leaf Concentrations for Deficiencies of Nitrogen, Potassium, Phosphorus, Sulphur, and Magnesium in Perennial Ryegrass. New Phytol. 1985, 101, 393–409. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Xi, B.; Wei, Z.; Li, X.; Cao, Z. Changes in phosphorus fractions during organic wastes composting from different sources. Bioresour. Technol. 2015, 189, 349–356. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Yang, C.; Lu, S. Pyrolysis temperature affects phosphorus availability of rice straw and canola stalk biochars and biochar-amended soils. J. Soils Sediments 2021, 21, 2817–2830. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Gao, S.; Jones, D.L. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management: Science, Technology and Implementation, 3rd ed.; Earthscan Publication: London, UK; New York, NY, USA, 2024; pp. 401–440. [Google Scholar]
- Xie, S.; Tran, H.T.; Pu, M.; Zhang, T. Transformation characteristics of organic matter and phosphorus in composting processes of agricultural organic waste: Research trends. Mater. Sci. Energy Technol. 2023, 6, 331–342. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, S.; Song, C.; Zhao, R.; Jia, L.; Wei, Z. Response of phosphorus fractions transformation and microbial community to carbon-to-phosphorus ratios during sludge composting. J. Environ. Manag. 2024, 360, 121145. [Google Scholar] [CrossRef] [PubMed]
- Bruun, S.; Harmer, S.L.; Bekiaris, G.; Christel, W.; Zuin, L.; Hu, Y.; Jensen, L.S.; Lombi, E. The effect of different pyrolysis temperatures on the speciation and availability in soil of P in biochar produced from the solid fraction of manure. Chemosphere 2017, 169, 377–386. [Google Scholar] [CrossRef]
- Knijnenburg, J.T.N.; Suwanree, S.; Macquarrie, D.; Kasemsiri, P.; Jetsrisuparb, K. Phosphorus recovery from animal manures through pyrolysis: Phosphorus transformations, release mechanisms, and applications of manure biochars in agriculture. RSC Sustain. 2024, 3, 1084–1101. [Google Scholar] [CrossRef]
- Buss, W.; Bogush, A.; Ignatyev, K.; Mašek, O. Unlocking the Fertilizer Potential of Waste-Derived Biochar ACS Sustain. Chem. Eng. 2020, 8, 12295–12303. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M.; Bishop, P. Predicting phosphorus bioavailability from high-ash Biochars. Plant Soil 2012, 357, 173–187. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Shao, H.; Sun, J. Pyrolysis temperature affects phosphorus transformation in biochar: Chemical fractionation and 31P NMR analysis. Sci. Total Environ. 2016, 569–570, 65–72. [Google Scholar] [CrossRef]
- Buss, W.; Wurzer, C.; Manning, D.A.C.; Rohling, E.J.; Borevitz, J.; Mašek, O. Mineral-enriched biochar delivers enhanced nutrient recovery and carbon dioxide removal. Commun. Earth Environ. 2022, 3, 67. [Google Scholar] [CrossRef]
- Liu, T.; Shao, T.; Jiang, J.; Ma, W.; Feng, R.; Dong, D.; Wang, Y.; Bai, T.; Xu, Y. Influence of potassium addition on phosphorus availability and heavy metals immobility of biochar derived from swine manure. Sci. Rep. 2024, 14, 21069. [Google Scholar] [CrossRef]
- Brod, E.; Øgaard, A.F.; Hansen, E.; Wragg, D.; Haraldsen, T.K.; Krogstad, T. Waste products as alternative phosphorus fertilisers Part I: Inorganic P species affect fertilisation effects depending on soil pH. Nutr. Cycl. Agroecosyst. 2015, 103, 167–185. [Google Scholar] [CrossRef]
- Tuszynska, A.; Czerwionka, K.; Obarska-Pempkowiak, H. Phosphorus concentration and availability in raw organic waste and post fermentation products. J. Environ. Manag. 2021, 278, 111468. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, S.; Borgonova, G.; Scaglioni, L.; Bedussi, F.; D’Imparzano, G.; Tambone, F.; Adani, F. Phosphorus speciation during anaerobic digestion and subsequent solid/liquid separation. Sci. Total Environ. 2020, 734, 139784. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, C.; Jönsson, H. Higher pH and faster decomposition in biowaste composting by increased aeration. Waste Manag. 2008, 28, 518–526. [Google Scholar] [CrossRef]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting parameters and compost quality: A literature review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.; Yuan, K.N.; Zhu, Z.X. Desorption and plant-availability of phosphate sorbed by some important minerals. Plant Soil 1994, 162, 89–97. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Zhang, H.; Stutter, M.; Giles, C.D.; Darch, T.; George, T.S.; Shand, C.; Lumsdon, D.; Blackwell, M.; Wearing, C.; et al. A holistic approach to understanding the desorption of phosphorus in soils. Environ. Sci. Technol. 2016, 50, 3371–3381. [Google Scholar] [CrossRef]
- Shiralipour, A.; McConnell, D.B.; Smith, W.H. Physical and chemical properties of soils as affected by municipal solid waste compost application. Biomass Bioenergy 1992, 3, 261–266. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Janda, J.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Phosphorus Use Efficiency of Bio-Based Fertilizers: Bioavailability and Fractionation. Pedosphere 2016, 26, 310–325. [Google Scholar] [CrossRef]
- Sarvi, M.; Hagner, M.; Velmala, S.; Soinne, H.; Uusitalo, R.; Keskinen, R.; Ylivainio, K.; Kaseva, J.; Rasa, K. Bioavailability of phosphorus in granulated and pyrolyzed broiler manure. Environ. Technol. Innov. 2021, 23, 101584. [Google Scholar] [CrossRef]
- Shi, W.; Healy, M.G.; Ashekuzzaman, S.M.; Daly, K.; Fenton, O. Mineral fertiliser equivalent value of dairy processing sludge and derived biochar using ryegrass (Lolium perenne L.) and spring wheat (Triticum aestivum). J. Environ. Manag. 2022, 321, 116012. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Murphy, D.V.; George, S.J.; Lapis-Gaza, H.; Xu, M.; Gleeson, D.B. Increasing the Size of the Microbial Biomass Altered Bacterial Community Structure which Enhances Plant Phosphorus Uptake. PLoS ONE 2016, 11, e0166062. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Xiang, Y.; Deng, Q.; Duan, H.; Guo, Y. Effects of biochar application on root traits: A meta-analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Gagnon, B.; Isabelle, D.; Noura, Z.; Martin, H.C.; Léon-Étienne, P.; Tom, A.F.; Francis, J.F.; Katherine, E.B. Forms of phosphorus in composts and in compost-amended soils following incubation. Can. J. Soil Sci. 2012, 92, 711–721. [Google Scholar] [CrossRef]
- Honvault, N.; Faucon, M.P.; McLaren, T.; Houben, D.; Frossard, E.; Oberson, A. Influence of cover crop residue traits on phosphorus availability and subsequent uptake by plants. Nutr. Cycl. Agroecosyst. 2024, 128, 131–148. [Google Scholar] [CrossRef]
- Brod, E.; Øgaard, A.F.; Krogstad, T.; Haraldsen, T.K.; Frossard, E.; Oberson, A. Drivers of Phosphorus Uptake by Barley Following Secondary Resource Application. Front. Nutr. 2016, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Grigatti, M.; Boanini, E.; Mancarella, S.; Simoni, A.; Centemero, M.; Veeken, A.H.M. Phosphorous extractability and ryegrass availability from bio-waste composts in a calcareous soil. Chemosphere 2017, 174, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Grigatti, M.; Boanini, E.; Cavani, L.; Ciavatta, C.; Marzadori, C. Phosphorus in Digestate-Based Compost: Chemical Speciation and Plant-Availability. Waste Biomass Valorization 2015, 6, 481–493. [Google Scholar] [CrossRef]
- Brod, E.; Øgaard, A.F.; Haraldsen, T.K.; Krogstad, T. Waste products as alternative phosphorus fertilisers part II: Predicting P fertilisation effects by chemical extraction. Nutr. Cycl. Agroecosyst. 2015, 103, 187–199. [Google Scholar] [CrossRef]
- Delin, S. Fertilizer value of phosphorus in different residues. Soil Use Manag. 2016, 32, 17–26. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R.; Fixen, P.E.; Curtin, D. Phosphorus: Its efficient use in agriculture. Adv. Agron. 2014, 123, 177–228. [Google Scholar] [CrossRef]
- Achat, D.L.; Sperandio, M.; Daumer, M.L.; Santellani, A.C.; Prud’Homme, L.; Akhtar, M.; Morel, M. Plant-availability of phosphorus recycled from pig manures and dairy effluents as assessed by isotopic labeling techniques. Geoderma 2014, 232–234, 24–33. [Google Scholar] [CrossRef]
- Oberson, A.; Tagmann, H.U.; Langmeier, M.; Dubois, D.; Mäder, P.; Frossard, E. Fresh and residual phosphorus uptake by ryegrass from soils with different fertilization histories. Plant Soil 2010, 334, 391–407. [Google Scholar] [CrossRef]
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis—Model and research methods. Soil Biol. Biochem. 2011, 43, 883–894. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]

| Soils | Ptotal, g (kg Soil)−1 | PCAL, g (kg Soil)−1 | pH |
|---|---|---|---|
| Low-P acidic soil | 0.56 ± 0.002 | 0.018 ± 0.001 | 5.26 ± 0.04 |
| High-P acidic soil | 0.91 ± 0.004 | 0.079 ± 0.004 | 5.64 ± 0.07 |
| High-P neutral soil | 1.90 ± 0.016 | 0.113 ± 0.010 | 7.32 ± 0.09 |
| Treatment of Municipal Waste | Abbreviation | |
|---|---|---|
| No further treatment | Fresh_BW | Fresh_GW |
| Composting | Com_BW | Com_GW |
| Pyrolysis at 350 °C | P350_BW | P350_GW |
| Pyrolysis at 450 °C | P450_BW | P450_GW |
| Pyrolysis at 700 °C | P700_BW | P700_GW |
| Pyrolysis at 700 °C, modified atmospheric conditions | P700a_BW | P700a_GW |
| Anaerobic digestion for biogas production, solid residue | FRsolid_BW | |
| Anaerobic digestion for biogas production, fluid residue | FRfluid_BW | |
| Processing | Ptotal | PCAL | PCAL | pH |
|---|---|---|---|---|
| g (kg DM)−1 | g (kg DM)−1 | % of Total P | ||
| (A) Green waste-derived amendments | ||||
| Fresh | 1.0 ± 0.1 c | 0.43 ± 0.01 c | 41 ± 3 a | 5.4 ± 0.02 f |
| Com | 2.1 ± 0.3 b | 0.57 ± 0.02 b | 27 ± 3 b | 7.1 ± 0.01 e |
| P350 | 2.1 ± 0.1 b | 0.86 ± 0.02 a | 42 ± 3 a | 7.7 ± 0.04 d |
| P450 | 2.0 ± 0.1 b | 0.88 ± 0.01 a | 44 ± 2 a | 8.8 ± 0.06 c |
| P700 | 2.7 ± 0.1 a | 0.46 ± 0.03 c | 17 ± 1 c | 12.4 ± 0.01 a |
| P700a | 3.1 ± 0.2 a | 0.45 ± 0.02 c | 15 ± 1 c | 11.4 ± 0.01 b |
| (B) Biowaste-derived amendments | ||||
| Fresh | 2.2 ± 0.1 c | 1.02 ± 0.04 c | 46 ± 3 ab | 4.9 ± 0.01 h |
| Com | 3.3 ± 0.3 bc | 0. 98 ± 0.06 c | 30 ± 5 bc | 7.5 ± 0.06 e |
| P350 | 4.0 ± 0.3 abc | 1.07 ± 0.03 bc | 27 ± 2 cd | 8.6 ± 0.02 d |
| P450 | 4.9 ± 0.2 ab | 1.27 ± 0.02 b | 26 ± 2 cd | 9.1 ± 0.02 c |
| P700 | 4.5 ± 0.4 ab | 0.32 ± 0.01 d | 7 ± 1 e | 12.2 ± 0.02 a |
| P700a | 4.9 ± 0.1 ab | 0.53 ± 0.04 d | 11 ± 1 de | 11.2 ± 0.07 b |
| Fluid | 5.0 ± 0.8 a | 3.22 ± 0.17 a | 62 ± 3 a | 7.2 ± 0.11 f |
| Solid | 3.1 ± 1.4 bc | 1.17 ± 0.02 bc | 40 ± 1 bc | 7.0 ± 0.06 g |
| Source of Variance | PCAL Concentration | Amendment-Ind. PCAL Increase (API) | Soil pH | pH-Adjusted PCAL Increase (APIpHadj.) |
|---|---|---|---|---|
| Low-P acidic soil | ||||
| Fertilizer variant | <0.001 | <0.001 | <0.001 | <0.001 |
| High-P acidic soil | ||||
| Fertilizer variant | <0.001 | <0.001 | <0.001 | <0.001 |
| High-P neutral soil | ||||
| Fertilizer variant | 0.079 | 0.046 | <0.001 | |
| Source | Growth Periods | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| ||||||||
| Processing | <0.001 | 0.018 | 0.019 | 0.259 | <0.001 | <0.001 | <0.001 | 0.016 |
| ||||||||
| Processing | <0.001 | 0.042 | 0.075 | 0.013 | 0.006 | 0.030 | 0.734 | 0.778 |
| ||||||||
| Feedstock (FS) | 0.156 | 0.838 | 0.642 | 0.520 | 0.072 | 0.009 | 0.009 | 0.265 |
| Processing | <0.001 | 0.076 | 0.013 | 0.020 | <0.001 | <0.001 | <0.001 | 0.007 |
| FS × Processing | 0.939 | 0.068 | 0.326 | 0.360 | <0.001 | 0.0149 | 0.002 | 0.085 |
| Source | Growth Period | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean | |
| |||||||||
| Processing | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.013 | <0.001 |
| |||||||||
| Processing | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | 0.589 | 0.777 | 0.513 | <0.001 |
| |||||||||
| Feedstock | 0.101 | 0.842 | 0.006 | 0.227 | 0.574 | 0.093 | 0.124 | 0.188 | 0.647 |
| Processing | <0.001 | 0.112 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | 0.004 | <0.001 |
| FS × Processing | 0.121 | <0.001 | 0.008 | <0.001 | <0.001 | 0.018 | 0.001 | 0.066 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazratqulov, S.; Bessler, H.; Adam, A.; Radelhof, T.; Engels, C. Effect of Processing Solid Organic Municipal Wastes on Their Phosphorus Fertilizer Value. Agronomy 2025, 15, 2296. https://doi.org/10.3390/agronomy15102296
Hazratqulov S, Bessler H, Adam A, Radelhof T, Engels C. Effect of Processing Solid Organic Municipal Wastes on Their Phosphorus Fertilizer Value. Agronomy. 2025; 15(10):2296. https://doi.org/10.3390/agronomy15102296
Chicago/Turabian StyleHazratqulov, Shohnazar, Holger Bessler, Anna Adam, Theodor Radelhof, and Christof Engels. 2025. "Effect of Processing Solid Organic Municipal Wastes on Their Phosphorus Fertilizer Value" Agronomy 15, no. 10: 2296. https://doi.org/10.3390/agronomy15102296
APA StyleHazratqulov, S., Bessler, H., Adam, A., Radelhof, T., & Engels, C. (2025). Effect of Processing Solid Organic Municipal Wastes on Their Phosphorus Fertilizer Value. Agronomy, 15(10), 2296. https://doi.org/10.3390/agronomy15102296





