Recycling Reservoir Sediments and Rice Husk for Sustainable Rice Seedling Production
Abstract
1. Introduction
2. Materials and Methods
2.1. The Preparation of Reservoir Sediments and Amendment Materials
2.2. Preliminary Experiments
2.3. Rice Seedling Cultivation
2.4. Soil and Soil Solution Analyses
2.5. Plant Analyses
2.6. Statistics
3. Results
3.1. Preliminary Experiments
3.2. Rice Seedling Cultivation
3.2.1. Rice Seedling Growth
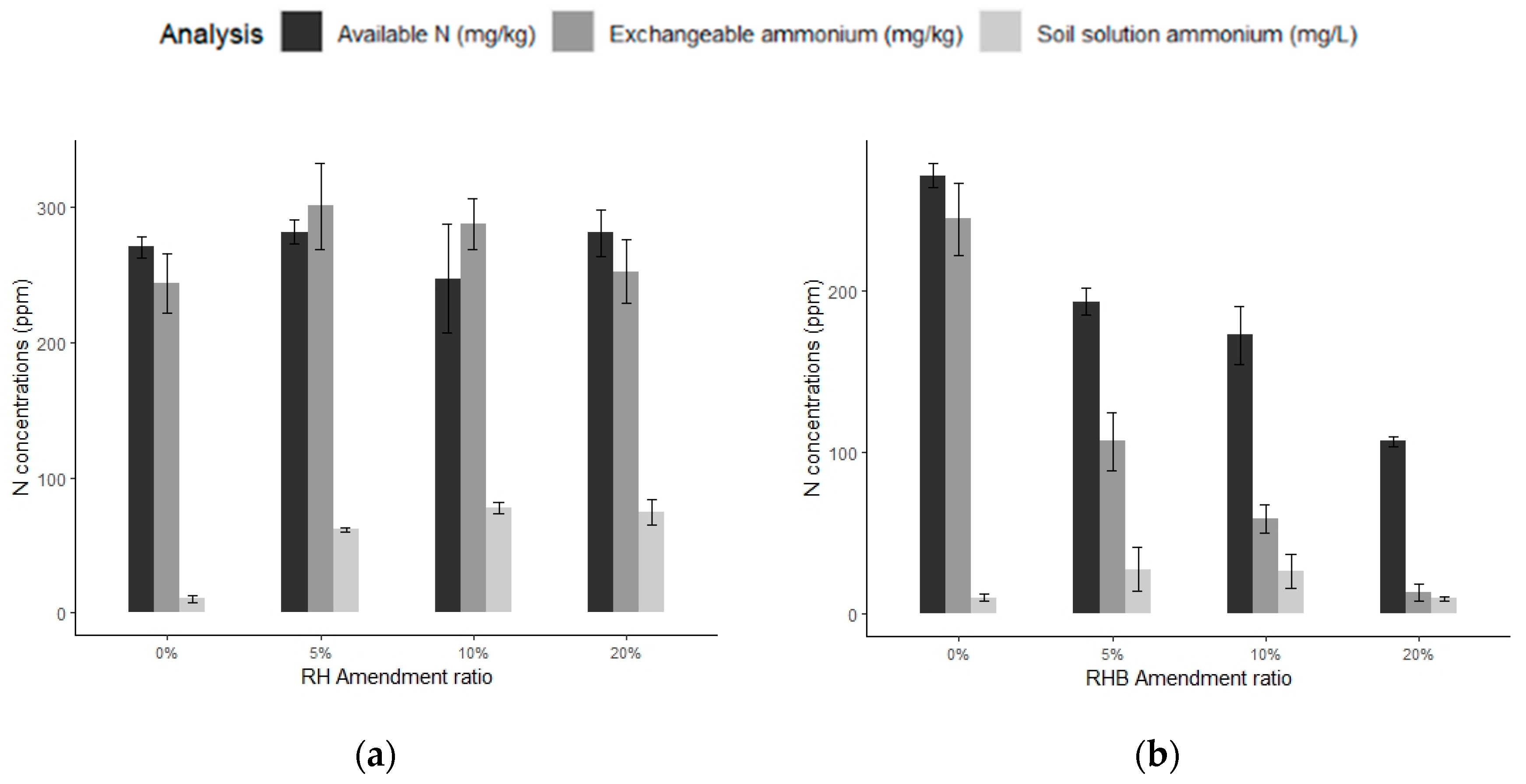
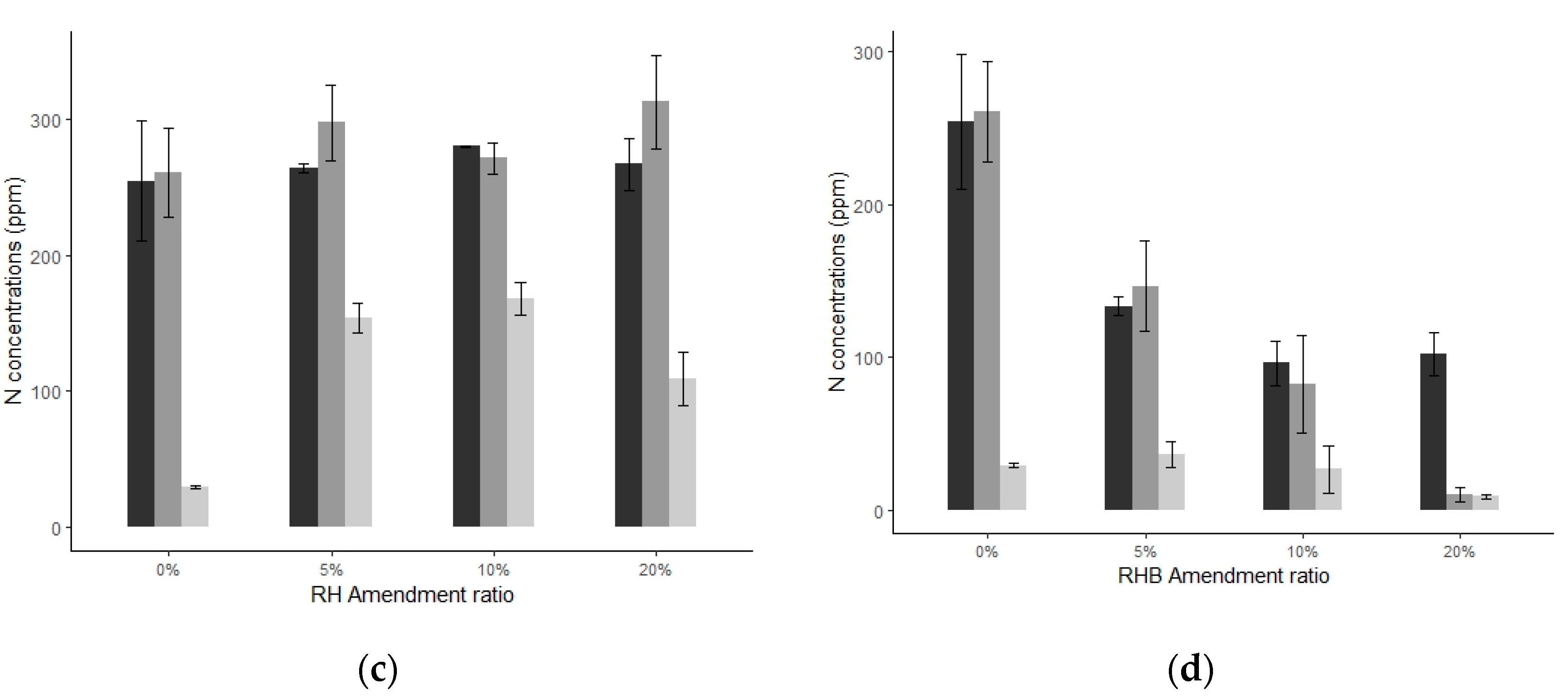
3.2.2. Soil Solution Analyses
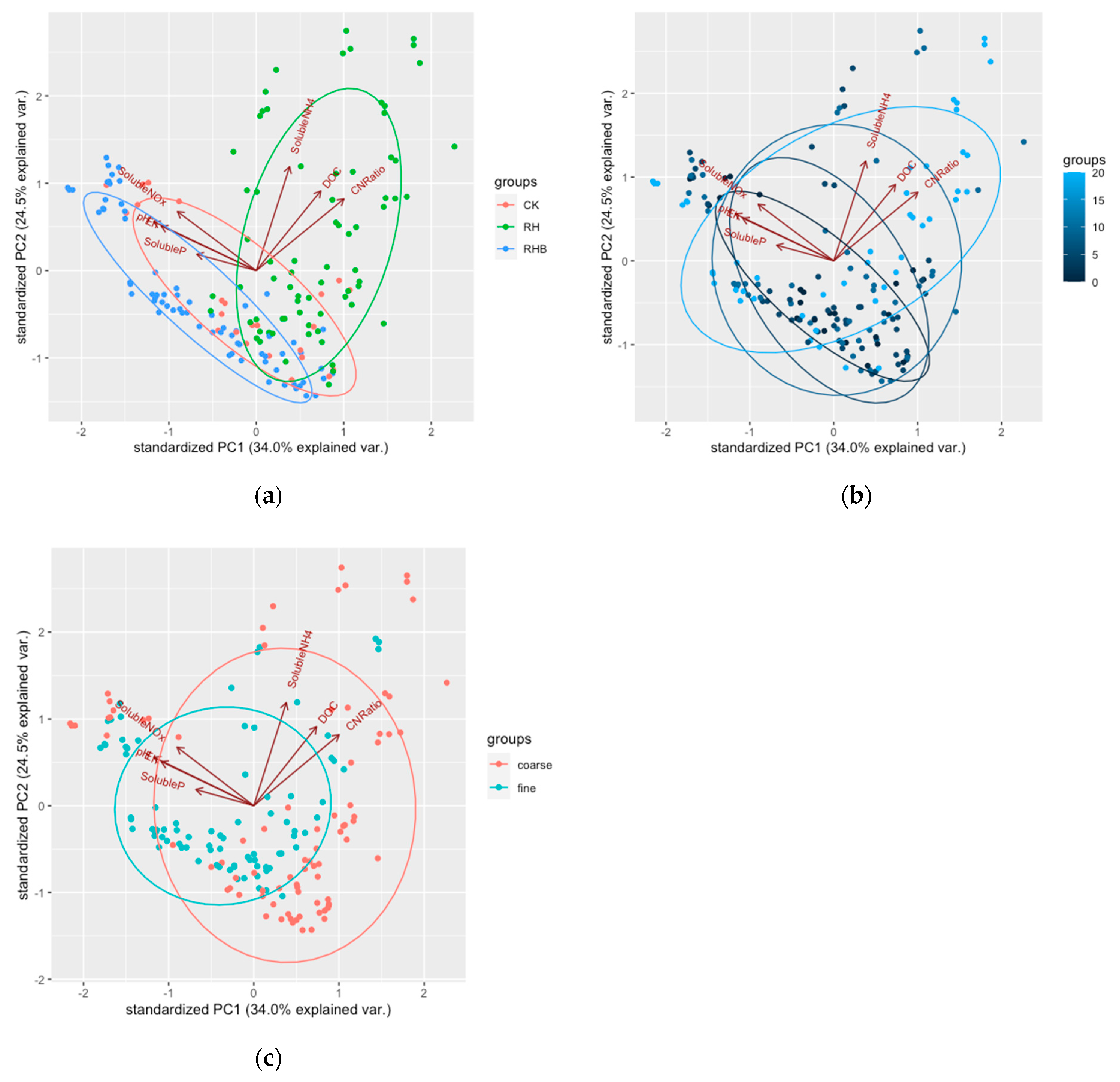
3.2.3. PCA of the Soil Solution Chemistry
4. Discussion
4.1. The Optimal Amendment Ratio and Material
4.2. The Dominant Factors That Drove the Difference Between Amending RH and RHB
4.3. Mechanisms Underlying the Different N Supply Between the RH- and RHB-Amended Sediments
4.4. Technical Limitations and Practical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RH | Rice husk |
| RHB | Rice husk biochar |
| N | Nitrogen |
| C | Carbon |
| P | Phosphorus |
| K | Potassium |
| Na | Sodium |
| Ca | Calcium |
| Mg | Magnesium |
| FS | Fine-textured sediments |
| CS | Coarse-textured sediments |
| NS | Nursery soil |
| FTIR | Fourier transform infrared spectroscopy |
| CRD | Completely randomized design |
| DAS | Days after sowing the seeds |
| Eh | Redox potential |
| EC | Electrical conductivity |
| DOC | Dissolved organic carbon |
| LSD | Least significant difference |
| PCA | Principal component analysis |
| ANOVA | Analysis of variance |
References
- Alam, M.K.; Bell, R.W.; Hasanuzzaman, M.; Salahin, N.; Rashid, M.H.; Akter, N.; Akhter, S.; Islam, M.S.; Islam, S.; Naznin, S.; et al. Rice (Oryza sativa L.) Establishment Techniques and Their Implications for Soil Properties, Global Warming Potential Mitigation and Crop Yields. Agronomy 2020, 10, 888. [Google Scholar] [CrossRef]
- Noltze, M.; Schwarze, S.; Qaim, M. Impacts of Natural Resource Management Technologies on Agricultural Yield and Household Income: The System of Rice Intensification in Timor Leste. Ecol. Econ. 2013, 85, 59–68. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Q.; Cao, X.; Lianfeng, Z.; Yali, K.; Jin, Q.; Zhang, J. Effects of Substrates with Different Properties on Chilling Tolerance of Early Rice Seedlings. Chin. J. Rice Sci. 2021, 35, 503–512. [Google Scholar]
- Wu, W.Z.; Lin, G.Q. Development of Substrates for Rice Seedling Cultivation (in Chinese). In Tainan District Agricultural Bulletin; 2001; pp. 25–59. Available online: https://book.tndais.gov.tw/Magazine/mag36-4.htm. (accessed on 12 October 2025).
- Lei, W.; Ding, Y.; Li, G.; Tang, S.; Wang, S. Effects of Soilless Substrates on Seedling Quality and the Growth of Transplanted Super Japonica Rice. J. Integr. Agric. 2017, 16, 1053–1063. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, H.; Cai, X.; Xu, C.; Li, Q.; Wang, J.; He, D. Research on Matrix Formula of Substrate for Seedling in Rice Closed Stereo Seedling System. Trans. Chin. Soc. Agri Eng. 2017, 33, 204–210. [Google Scholar]
- Wang, H.-W.; Kondolf, M.; Tullos, D.; Kuo, W.-C. Sediment Management in Taiwan’s Reservoirs and Barriers to Implementation. Water 2018, 10, 1034. [Google Scholar] [CrossRef]
- Dadson, S.J.; Hovius, N.; Chen, H.; Dade, W.B.; Hsieh, M.-L.; Willett, S.D.; Hu, J.-C.; Horng, M.-J.; Chen, M.-C.; Stark, C.P.; et al. Links between Erosion, Runoff Variability and Seismicity in the Taiwan Orogen. Nature 2003, 426, 648–651. [Google Scholar] [CrossRef]
- Asthana, B.N.; Khare, D. Reservoir Sedimentation. In Recent Advances in Dam Engineering; Springer International Publishing: Cham, Switzerland, 2022; pp. 265–288. ISBN 978-3-030-32277-9. [Google Scholar]
- Garbowski, T.; Bar-Michalczyk, D.; Charazińska, S.; Grabowska-Polanowska, B.; Kowalczyk, A.; Lochyński, P. An Overview of Natural Soil Amendments in Agriculture. Soil. Tillage Res. 2023, 225, 105462. [Google Scholar] [CrossRef]
- Oades, J.M. Soil Organic Matter and Structural Stability: Mechanisms and Implications for Management. Plant Soil. 1984, 76, 319–337. [Google Scholar] [CrossRef]
- Eden, M.; Gerke, H.H.; Houot, S. Organic Waste Recycling in Agriculture and Related Effects on Soil Water Retention and Plant Available Water: A Review. Agron. Sustain. Dev. 2017, 37, 11. [Google Scholar] [CrossRef]
- Hsu, E. Cost-Benefit Analysis for Recycling of Agricultural Wastes in Taiwan. Waste Manag. 2021, 120, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural Waste Management Strategies for Environmental Sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Das, K.; Dhal, G.C. Global Status of Agricultural Waste-Based Industries, Challenges, and Future Prospects. In Agricultural Waste to Value-Added Products; Neelancherry, R., Gao, B., Wisniewski, A., Jr., Eds.; Springer Nature: Singapore, 2023; pp. 21–45. ISBN 978-981-9944-71-2. [Google Scholar]
- Hossen, A.M.; Hossain, M.M.; Haque, E.M.; Bell, R.W. Effect of Growing Media on Mat Type Seedling Raised for Mechanical Rice Transplanting. Res. Agric. Eng. 2018, 64, 157–167. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.H.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. Biochar for Soil Amendment. In Char and Carbon Materials Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–146. ISBN 978-0-12-814893-8. [Google Scholar]
- Liu, S.; Cen, B.; Yu, Z.; Qiu, R.; Gao, T.; Long, X. The Key Role of Biochar in Amending Acidic Soil: Reducing Soil Acidity and Improving Soil Acid Buffering Capacity. Biochar 2025, 7, 52. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.-J.; Novak, A.; Yang, Y.; Wang, J. Role of Biochar in Improving Sandy Soil Water Retention and Resilience to Drought. Water 2021, 13, 407. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does Biochar Improve Soil Water Retention? A Systematic Review and Meta-Analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Wu, G.; Tham, P.E.; Chew, K.W.; Munawaroh, H.S.H.; Tan, I.S.; Wan-Mohtar, W.A.A.Q.I.; Sriariyanun, M.; Show, P.L. Net Zero Emission in Circular Bioeconomy from Microalgae Biochar Production: A Renewed Possibility. Bioresour. Technol. 2023, 388, 129748. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-Size Analysis. In SSSA Book Series; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. ISBN 978-0-89118-864-3. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Particle Density. In SSSA Book Series; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 377–382. ISBN 978-0-89118-864-3. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. In SSSA Book Series; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 635–662. ISBN 978-0-89118-864-3. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil. Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Agronomy Monographs; Page, A.L., Ed.; Wiley: Hoboken, NJ, USA, 1982; Volume 9, pp. 403–430. ISBN 978-0-89118-072-2. [Google Scholar]
- Rhoades, J.D. Cation Exchange Capacity. In Agronomy Monographs; Page, A.L., Ed.; Wiley: Hoboken, NJ, USA, 1982; Volume 9, pp. 149–157. ISBN 978-0-89118-072-2. [Google Scholar]
- Kempers, A.J.; Zweers, A. Ammonium Determination in Soil Extracts by the Salicylate Method. Commun. Soil. Sci. Plant Anal. 1986, 17, 715–723. [Google Scholar] [CrossRef]
- Øien, A.; Selmer-Olsen, A.R. A Laboratory Method for Evaluation of Available Nitrogen in Soil. Acta Agric. Scand. 1980, 30, 149–156. [Google Scholar] [CrossRef]
- García-Robledo, E.; Corzo, A.; Papaspyrou, S. A Fast and Direct Spectrophotometric Method for the Sequential Determination of Nitrate and Nitrite at Low Concentrations in Small Volumes. Mar. Chem. 2014, 162, 30–36. [Google Scholar] [CrossRef]
- Schnetger, B.; Lehners, C. Determination of Nitrate plus Nitrite in Small Volume Marine Water Samples Using Vanadium(III)Chloride as a Reduction Agent. Mar. Chem. 2014, 160, 91–98. [Google Scholar] [CrossRef]
- Lv, B.; Li, X.; Ma, H.; Sun, Y.; Wei, L.; Jiang, C.; Liang, Z. Differences in Growth and Physiology of Rice in Response to Different Saline-Alkaline Stress Factors. Agron. J. 2013, 105, 1119–1128. [Google Scholar] [CrossRef]
- Singh, V.P.; Maiti, R.K. A Review on Factors Affecting Crop Growth in Rice (Oryza sativa L.). Farming Manag. 2016, 1, 101–114. [Google Scholar]
- Rhoades, J.D.; Loveday, J. Salinity in Irrigated Agriculture. In Agronomy; Stewart, B.A., Nielsen, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1990; pp. 1089–1142. ISBN 978-0-89118-102-6. [Google Scholar]
- Havlin, J.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 8th ed.; Pearson: Upper Saddle River, NJ, USA, 2013; ISBN 978-93-325-7034-4. [Google Scholar]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 135–189. ISBN 978-0-12-384905-2. [Google Scholar]
- Gilmour, J.T.; Norman, R.J.; Mauromoustakos, A.; Gale, P.M. Kinetics of Crop Residue Decomposition: Variability among Crops and Years. Soil. Sci. Soc. Am. J. 1998, 62, 750–755. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson: New York, NY, USA, 2016; ISBN 978-1-292-16223-2. [Google Scholar]
- Mikkelsen, D.S.; De Datta, S.K.; Obcemea, W.N. Ammonia Volatilization Losses from Flooded Rice Soils. Soil. Sci. Soc. Am. J. 1978, 42, 725–730. [Google Scholar] [CrossRef]
- Knowles, R. Denitrification. Microbiol. Rev. 1982, 46, 43–70. [Google Scholar] [CrossRef]
- He, Z.L.; Alva, A.K.; Calvert, D.V.; Banks, D.J. Ammonia Volatilization from Different Fertilizer Sources and Effects of Temperature and Soil pH. Soil. Sci. 1999, 164, 750–758. [Google Scholar] [CrossRef]
- Nommik, H.; Vahtras, K. Retention and Fixation of Ammonium and Ammonia in Soils. In Agronomy Monographs; Stevenson, F.J., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1982; pp. 123–171. ISBN 978-0-89118-216-0. [Google Scholar]
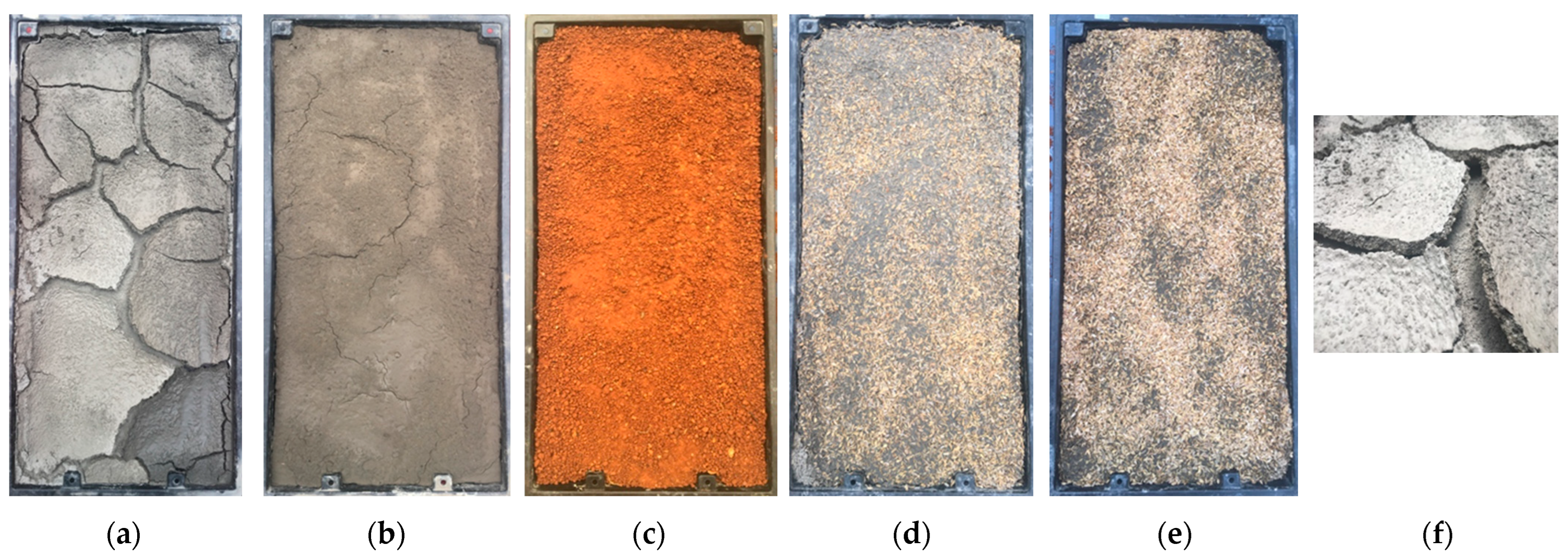
| Properties | FS | CS | NS |
|---|---|---|---|
| Soil texture | Clay | Sandy loam | Clay |
| Sand (%) | 0.0 | 73.0 | 15.5 |
| Silt (%) | 37.5 | 16.0 | 25.5 |
| Clay (%) | 62.5 | 11.0 | 59.0 |
| pH | 7.9 | 7.7 | 5.3 |
| EC (dS m−1) | 1.40 | 0.98 | 0.10 |
| CEC (cmolc kg−1) | 7.46 | 5.36 | 7.86 |
| Organic C (%) | 1.1 | 0.6 | 0.4 |
| Total N (g kg−1) | 230 | 190 | 80 |
| Total P (mg kg−1) | 607 | 533 | 410 |
| Total K (g kg−1) | 25.4 | 23.9 | 12.5 |
| Available N (mg kg−1) | 18.3 | 35.5 | 7.1 |
| Available P (mg kg−1) | 3.14 | 0.87 | 0.69 |
| Exchangeable K (mg kg−1) | 234 | 156 | 283 |
| Exchangeable Na (mg kg−1) | 16.4 | 7.1 | 14.1 |
| Exchangeable Ca (g kg−1) | 1.82 | 1.20 | 0.25 |
| Exchangeable Mg (g kg−1) | 1.62 | 1.08 | 1.08 |
| Treatments | Substrate Available Water Content (%) | Substrate Porosity (%) | ||
|---|---|---|---|---|
| Sediments | Amendment | Addition Rate (w/w) | ||
| FS | - | 0% | 14.6 ± 3.3 c | 47.5 ± 0.5 d |
| Whole RH | 7.6% | 22.6 ± 0.8 ab | 61.2 ± 2.0 b | |
| 10% | 21.7 ± 0.2 ab | 64.4 ± 3.3 b | ||
| 20% | 22.2 ± 0.2 ab | 69.1 ± 4.3 a | ||
| Ground RH | 7.6% | 14.0 ± 5.0 c | 46.7 ± 0.5 d | |
| 10% | 18.6 ± 2.8 bc | 48.0 ± 0.6 d | ||
| 20% | 24.7 ± 1.3 a | 55.6 ± 0.4 c | ||
| CS | - | 0% | 1.5 ± 2.4 E | 13.7 ± 2.3 D |
| Whole RH | 7.6% | 12.8 ± 0.1 AB | 30.8 ± 2.3 BC | |
| 10% | 12.2 ± 0.5 BC | 31.2 ± 7.3 BC | ||
| 20% | 7.4 ± 0.3 D | 69.1 ± 0.4 A | ||
| Ground RH | 7.6% | 11.7 ± 0.2 BC | 14.9 ± 6.6 D | |
| 10% | 10.9 ± 0.2 C | 27.1 ± 6.8 C | ||
| 20% | 14.2 ± 0.5 A | 36.7 ± 6.1 B | ||
| ANOVA test | Sediment texture (T) | <0.001 *** | <0.001 *** | |
| Amendment (A) | <0.001 *** | <0.001 *** | ||
| T × A | <0.001 *** | <0.001 *** | ||
| Treatments | Height of Rice Seedling (cm) | Shoot Dry Matter (g pot−1) | Leaf Chlorophyll (a + b) (g kg-dw−1) | |
|---|---|---|---|---|
| Sediments/Soils | Amendment Ratios and Materials | |||
| NS | - | 11.06 ± 0.36 | 1.25 ± 0.07 | 14.5 ± 2.44 |
| FS | +0% RH/RHB | 11.87 ± 0.32 c | 1.22 ± 0.09 bc | 16.6 ± 2.2 b |
| +5% RH | 12.30 ± 0.11 bc | 1.39 ± 0.04 ab | 15.3 ± 0.5 b | |
| +10% RH | 12.13 ± 0.30 c | 1.46 ± 0.13 a | 15.0 ± 1.7 b | |
| +20% RH | 12.06 ± 0.12 c | 1.34 ± 0.03 abc | 18.3 ± 2.0 a | |
| +5% RHB | 12.78 ± 0.60 ab | 1.33 ± 0.07 abc | 15.9 ± 1.8 c | |
| +10% RHB | 13.34 ± 0.41 a | 1.47 ± 0.05 a | 13.9 ± 0.9 c | |
| +20% RHB | 12.21 ± 0.40 bc | 1.16 ± 0.23 c | 12.3 ± 0.70 c | |
| CS | +0% RH/RHB | 10.78 ± 0.77 CD | 0.98 ± 0.09 C | 16.3 ± 2.3 B |
| +5% RH | 12.27 ± 0.48 BC | 1.26 ± 0.09 AB | 18.3 ± 2.5 A | |
| +10% RH | 11.99 ± 0.83 BCD | 1.25 ± 0.12 AB | 18.6 ± 2.1 A | |
| +20% RH | 10.69 ± 0.65 D | 1.37 ± 0.07 A | 16.8 ± 2.2 AB | |
| +5% RHB | 14.18 ± 0.17 A | 1.13 ±0.11 ABC | 15.6 ± 1.2 C | |
| +10% RHB | 13.16 ± 0.58 AB | 1.11 ± 0.16 ABC | 16.8 ± 1.3 C | |
| +20% RHB | 11.17 ± 1.85 CD | 1.06 ± 0.31 BC | 15.9 ± 2.7 C | |
| ANOVA test | Sediment texture (T) | 0.108 | <0.001 *** | 0.390 |
| Amendment (A) | <0.001 *** | 0.005 ** | <0.001 *** | |
| T × A | 0.029 * | 0.327 | 0.030 * | |
| Experimental Batch | Rate of Gas Volatilization (mg-N L−1 h−1) | |
|---|---|---|
| FS + 20% RH + NPK Fertilizer | FS + 20% RHB + NPK Fertilizer | |
| System recovery rate | 58.9% | 58.9% |
| Batch 1–3 h † | 0.0164 | 0.1026 |
| Batch 2–5 h † | 0.0408 | 0.1207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, P.-T.; Wang, S.-L. Recycling Reservoir Sediments and Rice Husk for Sustainable Rice Seedling Production. Agronomy 2025, 15, 2387. https://doi.org/10.3390/agronomy15102387
Kao P-T, Wang S-L. Recycling Reservoir Sediments and Rice Husk for Sustainable Rice Seedling Production. Agronomy. 2025; 15(10):2387. https://doi.org/10.3390/agronomy15102387
Chicago/Turabian StyleKao, Pei-Tzu, and Shan-Li Wang. 2025. "Recycling Reservoir Sediments and Rice Husk for Sustainable Rice Seedling Production" Agronomy 15, no. 10: 2387. https://doi.org/10.3390/agronomy15102387
APA StyleKao, P.-T., & Wang, S.-L. (2025). Recycling Reservoir Sediments and Rice Husk for Sustainable Rice Seedling Production. Agronomy, 15(10), 2387. https://doi.org/10.3390/agronomy15102387








