Hemp Cover Cropping and Disease Suppression in Winter Wheat of the Dryland Pacific Northwest
Abstract
1. Introduction
2. Materials and Methods
2.1. Greenhouse Experiments
2.2. Cover Crop Assay
2.3. Biomass Assay
2.4. Field Experiments
2.5. qPCR Analysis
2.6. Statistical Analysis
3. Results
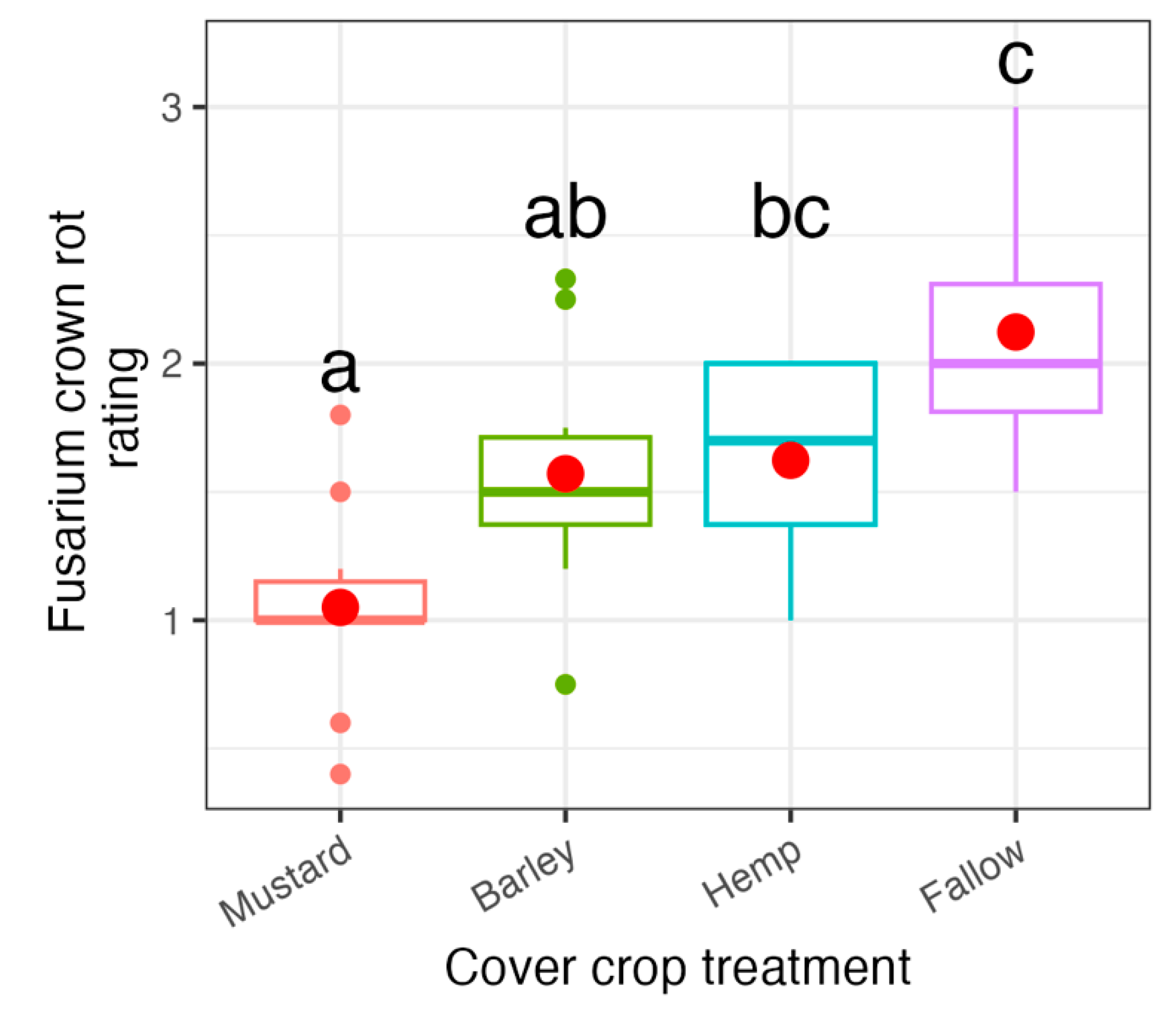
3.1. Cover Crop Assay
3.2. Biomass Assay
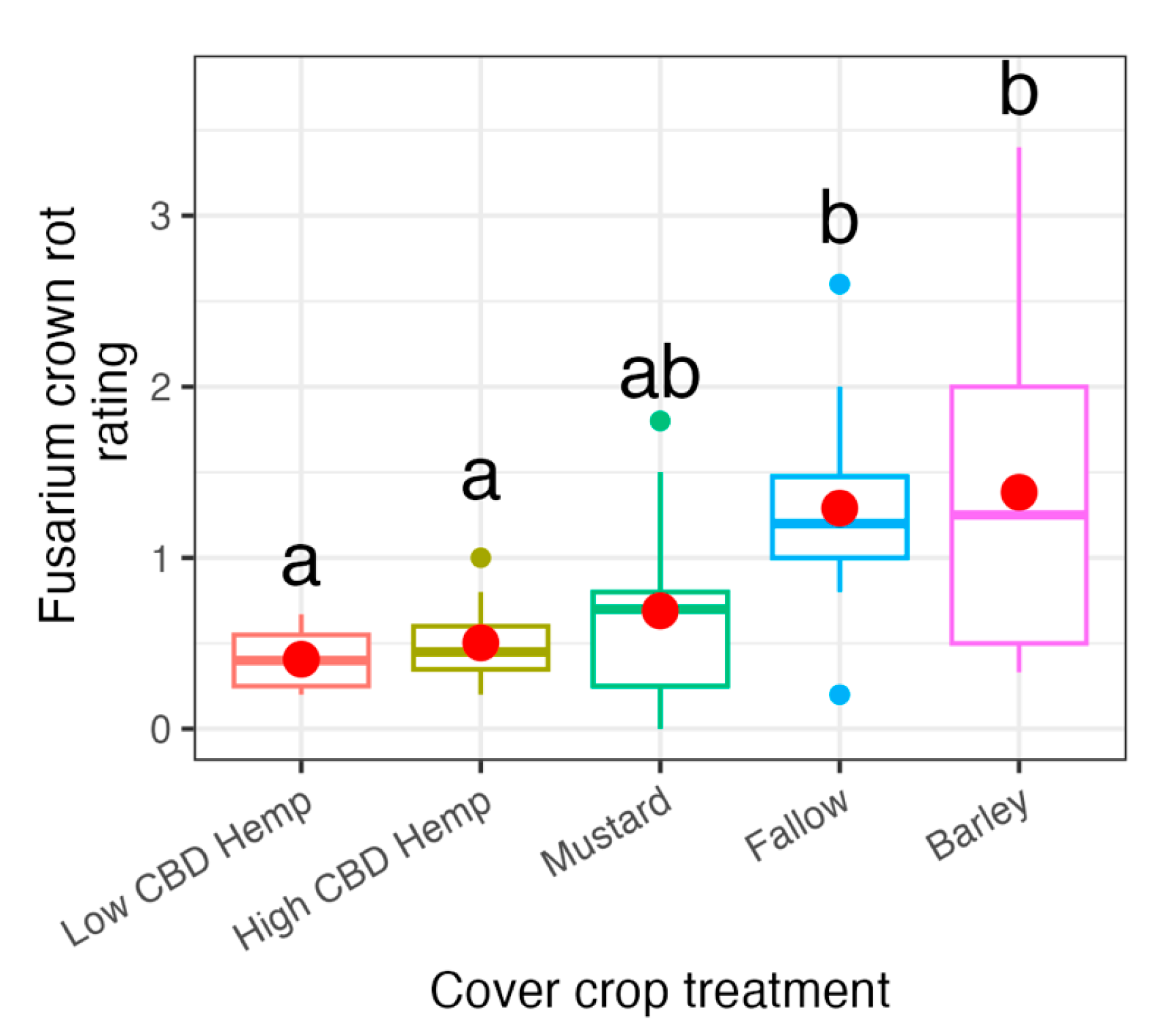
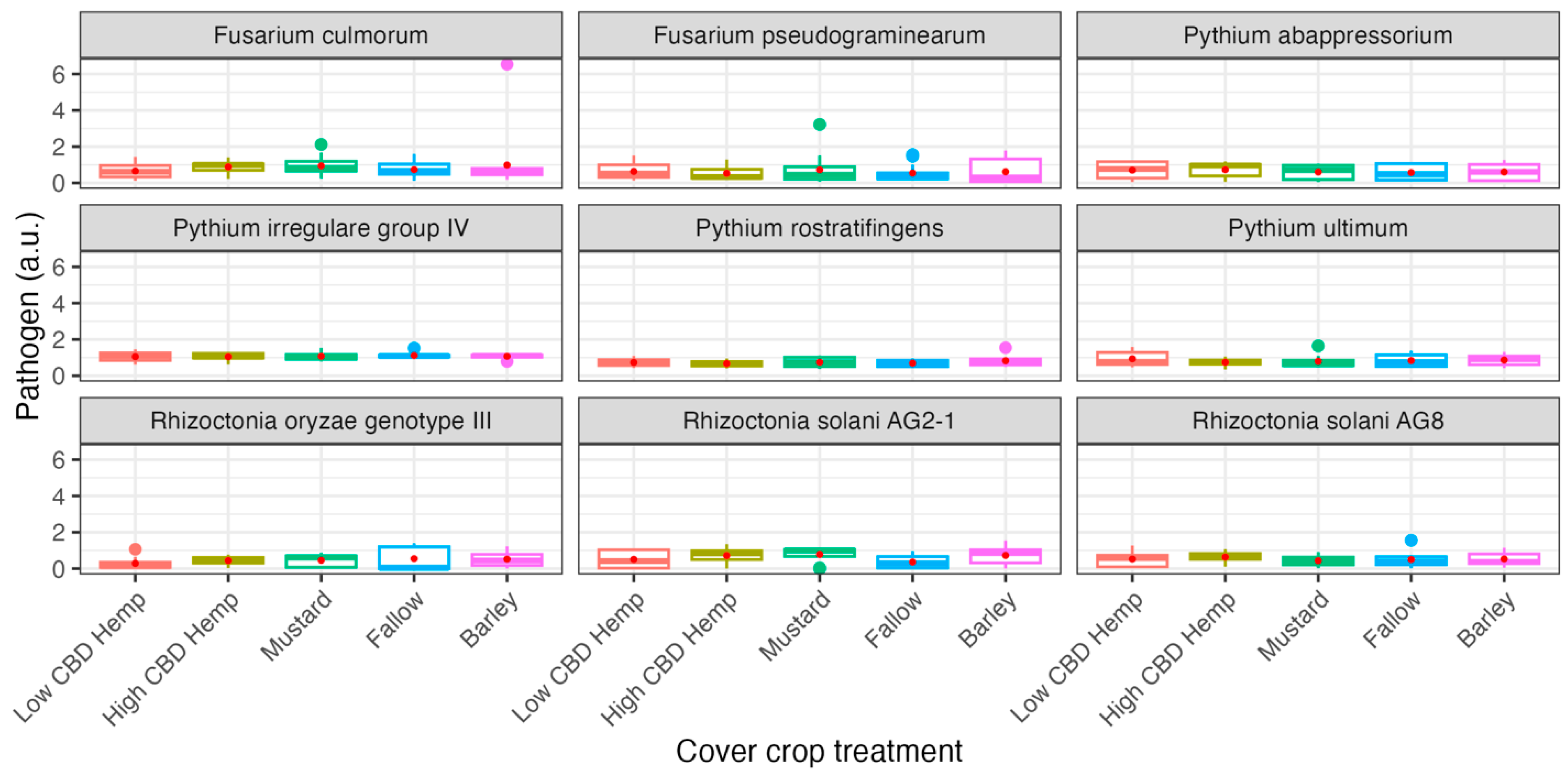
3.3. 2021 Field Experiment: qPCR Analysis
3.4. 2022 Field Experiment: qPCR Analysis
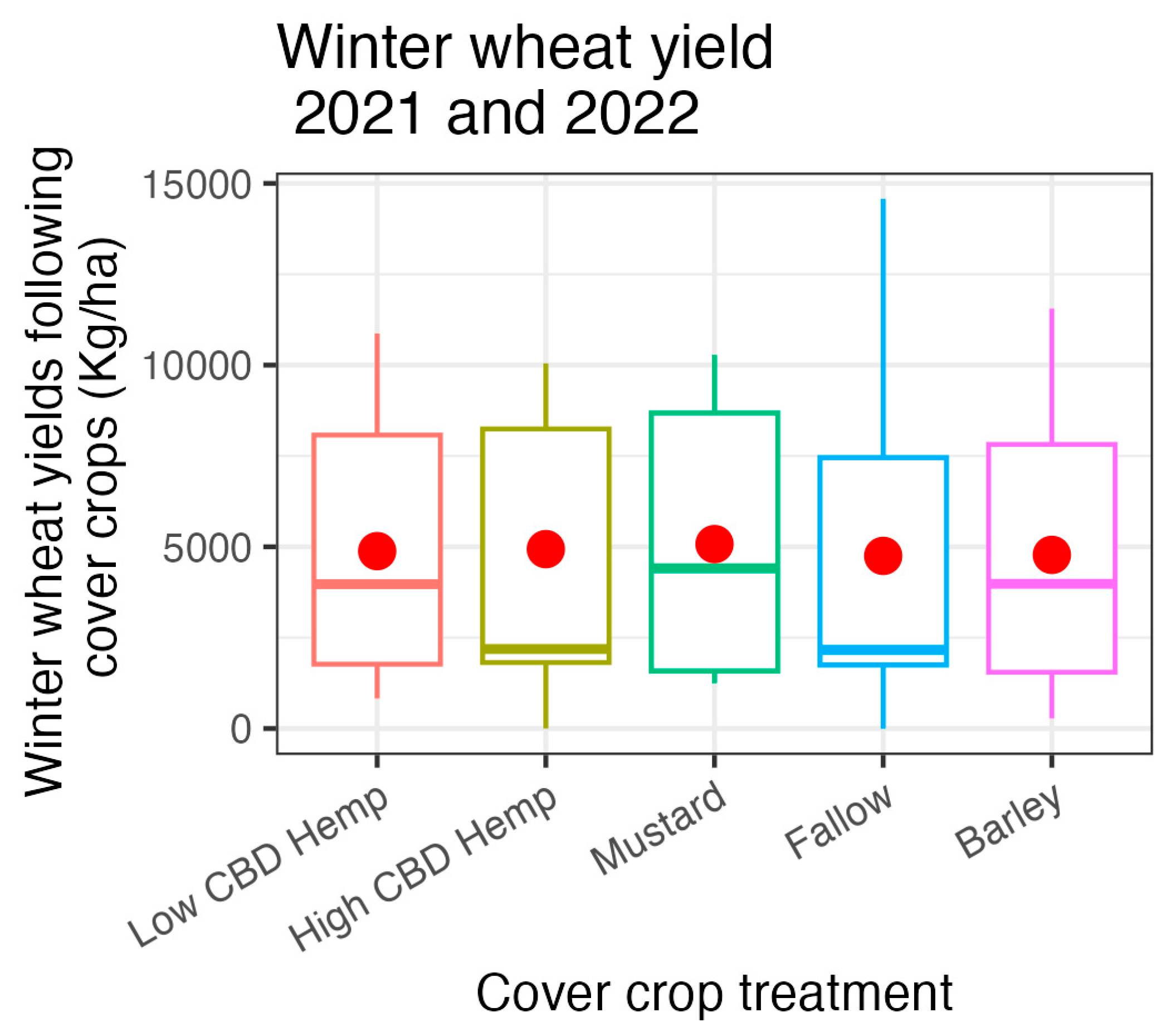
3.5. 2021 and 2022 Field Experiments: Agronomic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, M.; Fahad, S.; Du, G.; Cheng, X.; Yang, Y.; Tang, K.; Liu, L.; Liu, F.-H.; Deng, G. Evaluation of hemp (Cannabis sativa L.) as an industrial crop: A review. Environ. Sci. Pollut. Res. 2021, 28, 52832–52843. [Google Scholar] [CrossRef]
- Public Law 113-79. An Act to Provide for the Reform and Continuation of Agricultural and Other Programs of the Department of Agriculture Through Fiscal Year 2018, and for Other Purposes. 2014. Available online: https://www.govinfo.gov/content/pkg/PLAW-113publ79/pdf/PLAW-113publ79.pdf (accessed on 18 October 2024).
- Johnson, R. Hemp as an Agricultural Commodity: Congressional Research Service. 2018. Available online: https://crsreports.congress.gov/product/pdf/R/R45525 (accessed on 23 December 2022).
- Ahmed, A.F.; Islam, M.Z.; Mahmud, M.S.; Sarker, M.E.; Islam, M.R. Hemp as a potential raw material toward a sustainable world: A review. Heliyon 2022, 8, e08753. [Google Scholar] [CrossRef] [PubMed]
- USDA NASS. National Hemp Report. 2022. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/gf06h2430/xd07hw825/v692v917t/hempan22.pdf (accessed on 16 December 2022).
- USDA NASS. AGRI-FACTS. 2022. Available online: https://www.nass.usda.gov/Statistics_by_State/Regional_Office/Northwest/includes/Publications/Agri_facts/2022/AF05_1.pdf (accessed on 16 December 2022).
- Ehrensing, D.T. Feasibility of industrial hemp production in the United States Pacific Northwest. In Oregon State University Agricultural Experiment Station Bulletin SB681. 1998; Available online: https://ir.library.oregonstate.edu/concern/parent/j3860729t/file_sets/2b88qc644 (accessed on 4 December 2024).
- Steiner, J. Making Hemp a 21st Century Commodity in Oregon—and Beyond. Crops Soils 2021, 54, 24–31. [Google Scholar] [CrossRef]
- Jeliazkov, V.D.; Noller, J.S.; Angima, S.D.; Rondon, S.I.; Roseberg, R.J.; Summers, S.; Jones, G.B.; Sikora, V. What Is Industrial Hemp? Oregon State University Extension Service: Corvallis, OR, USA, 2019. [Google Scholar]
- Schillinger, W.F.; Papendick, R.I. Then and now: 125 years of dryland wheat farming in the Inland Pacific Northwest. Agron. J. 2008, 100, S-166. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Tang, L.; Fan, C.; Yuan, H.; Wu, G.; Sun, J.; Zhang, S. The Effect of Rotational Cropping of Industrial Hemp (Cannabis sativa L.) on Rhizosphere Soil Microbial Communities. Agronomy 2022, 12, 2293. [Google Scholar] [CrossRef]
- Gilchrist-Saavedra, L. Practical Guide to the Identification of Selected Diseases of Wheat and Barley; International Maize and Wheat Improvement Center (CIMMYT): El Batán, Mexico, 1997. [Google Scholar]
- Rovira, A.D. The impact of soil and crop management practices on soil-borne root diseases and wheat yields. Soil Use Manag. 1990, 6, 195–200. [Google Scholar] [CrossRef]
- Smiley, R.W.; Cook, R.J.; Paulitz, T. Controlling Root and Crown Diseases of Small Grain Cereals. Controlling Root and Crown Diseases of Small Grain Cereals; Pacific Northwest Extension Publication PNW639; Oregon State University: Corvallis, OR, USA, 2002. [Google Scholar]
- Hagerty, C.H.; Lutcher, L.; McLaughlin, K.; Hayes, P.; Garland-Campbell, K.; Paulitz, T.C.; Graebner, R.C.; Kroese, D. Reaction of winter wheat and barley cultivars to Fusarium pseudograminearum-inoculated fields in the dryland Pacific Northwest, USA. Agrosyst. Geosci. Environ. 2021, 4, e20173. [Google Scholar] [CrossRef]
- University of Idaho. Principles of Vegetation Measurement and Assessment. Estimating Biomass. 2009. Available online: https://www.webpages.uidaho.edu/veg_measure/Modules/Lessons/Module%207(Biomass&Utilization)/7_4_Estimates%20Double%20Sampling.htm (accessed on 27 February 2022).
- Yin, C.; McLaughlin, K.; Paulitz, T.C.; Kroese, D.R.; Hagerty, C.H. Population dynamics of wheat root pathogens under different tillage systems in Northeast Oregon. Plant Dis. 2020, 104, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.L.; Okubara, P.A.; Tambong, J.T.; Lévesque, C.A.; Paulitz, T.C. Identification and quantification of pathogenic Pythium spp. from soils in eastern Washington using real-time polymerase chain reaction. Phytopathology 2006, 96, 637–647. [Google Scholar] [CrossRef]
- Okubara, P.A.; Schroeder, K.L.; Paulitz, T.C. Identification and quantification of Rhizoctonia solani and R. oryzae using real-time polymerase chain reaction. Phytopathology 2008, 98, 837–847. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Zhang, Q.Y.; Li, Z.L.; Han, B.J.; Zhou, K.Q.; Hashemi, M.; Liu, X.B. Immediate responses of cyst nematode, soil-borne pathogens and soybean yield to one-season crop disturbance after continuous soybean in northeast China. Int. J. Plant Prod. 2013, 7, 341–354. [Google Scholar]
- Marquez, J.; Hajihassani, A. Successional effects of cover cropping and deep tillage on suppression of plant-parasitic nematodes and soilborne fungal pathogens. Pest Manag. Sci. 2023, 79, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Gorchs, G.; Lloveras, J.; Serrano, L.; Cela, S. Hemp yields and its rotation effects on wheat under rainfed mediterranean conditions. Agron. J. 2017, 109, 1551–1560. [Google Scholar] [CrossRef]
- Mark, T.B.; Will, S. Economic issues and perspectives for industrial hemp. In Industrial Hemp as a Modern Commodity Crop; American Society of Agronomy Crop Science Society of America Soil Science Society of America: Madison, WI, USA, 2019; pp. 107–118. [Google Scholar]
- Makkar, H.P.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites; Humana Press: Totowa, NJ, USA, 2007; Volume 393, pp. 1–122. [Google Scholar]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Anti-Inflammatory Effects of Cannabidiol (CBD) on Acne. J. Inflamm. Res. 2022, 15, 2795. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D., Jr.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a promising anti-cancer drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef] [PubMed]
- Golub, V.; Reddy, D. Cannabidiol therapy for refractory epilepsy and seizure disorders. Adv. Exp. Med. Biol. 2021, 1264, 93–110. [Google Scholar]
- Romero-Zerbo, S.Y.; García-Fernández, M.; Espinosa-Jiménez, V.; Pozo-Morales, M.; Escamilla-Sánchez, A.; Sánchez-Salido, L.; Lara, E.; Cobo-Vuilleumier, N.; Rafacho, A.; Olveira, G. The atypical cannabinoid Abn-CBD reduces inflammation and protects liver, pancreas, and adipose tissue in a mouse model of prediabetes and non-alcoholic fatty liver disease. Front. Endocrinol. 2020, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Ahmed, M.; Peiwen, Q.; Gu, Z.; Liu, Y.; Sikandar, A.; Hussain, D.; Javeed, A.; Shafi, J.; Iqbal, M.F.; An, R. Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.). Sci. Rep. 2020, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.P.; Griffin, T.S.; Honeycutt, C.W. Rotation and cover crop effects on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis. 2010, 94, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Zasada, I.; Meyer, S.; Morra, M. Brassicaceous seed meals as soil amendments to suppress the plant-parasitic nematodes Pratylenchus penetrans and Meloidogyne incognita. J. Nematol. 2009, 41, 221. [Google Scholar] [PubMed]
- Campanella, V.; Mandalà, C.; Angileri, V.; Miceli, C. Management of common root rot and Fusarium foot rot of wheat using Brassica carinata break crop green manure. Crop Prot. 2020, 130, 105073. [Google Scholar] [CrossRef]
- Kok, C.; Coenen, G.; De Heij, A. The effect of fibre hemp (Cannabis sativa L.) on selected soil-borne pathogens. J. Int. Hemp Assoc. 1994, 1, 6–9. [Google Scholar]

| Species | Primer | Sequence (5′-3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|
| Fusarium culmorum | FCKY648945_F | AGTACCACTGCATCCCAACC | 120 | [20] |
| FCKY648945_R | CTTCTCGATGGTTCGCTTGT | |||
| F. pseudograminearum | FPKY927890_F | GTGTCAATCAGTCACTAACAACC | 194 | [20] |
| FPKY927890_R | GAGGACAATAGTGACAACATACC | |||
| Pythium ultimum | ULT1F | GACACTGGAACGGGAGTCAGC | 414 | [21] |
| ULT4R | AAAGGACTCGACAGATTCTCGATC | |||
| P. irregulare group IV | IIV7F | GTATCGTCTTGGCGGAGTGG | 370 | [21] |
| ABA1R | TGCATAAACGAATATACCAACCGC | |||
| P. abappressorium | ABA1bF | GTTGTTGTGCGTCTGCGGATTTG | 397 | [21] |
| ABA1R | TGCATAAACGAATATACCAACCGC | |||
| P. rostratifingens | PROSF2 | GCGCAGATAGAGAGACTGATTTGG | 100 | [21] |
| PROSR2 | ACTACCAACACACACACACACACG | |||
| Rhizoctonia oryzae genotype III | RoGr3F | CTGTTGAAACCGGTTTACTATG | 286 | [22] |
| RoGr3R | CTTCCAAGTCCAAATACAACAATC | |||
| R. solani AG 8 | Rs8F | GGGGGAATTTATTCATTTATTGGAC | 327 | [22] |
| Rs8R | GGTGTGAAGCTGCAAAAG | |||
| R. solani AG 2–1 | AG2-1F | GTTGTAGCTGGCCCATTCATTTG | 123 | [22] |
| AG2-1R | CCTATTGCCTTTGTATTCCAAAAAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagerty, C.H.; Shrestha, G.; Wen, N.; Kroese, D.R.; Namdar, G.F.; Paulitz, T.; Wysocki, D.J. Hemp Cover Cropping and Disease Suppression in Winter Wheat of the Dryland Pacific Northwest. Agronomy 2024, 14, 2978. https://doi.org/10.3390/agronomy14122978
Hagerty CH, Shrestha G, Wen N, Kroese DR, Namdar GF, Paulitz T, Wysocki DJ. Hemp Cover Cropping and Disease Suppression in Winter Wheat of the Dryland Pacific Northwest. Agronomy. 2024; 14(12):2978. https://doi.org/10.3390/agronomy14122978
Chicago/Turabian StyleHagerty, Christina H., Govinda Shrestha, Nuan Wen, Duncan R. Kroese, Grayson F. Namdar, Tim Paulitz, and Donald J. Wysocki. 2024. "Hemp Cover Cropping and Disease Suppression in Winter Wheat of the Dryland Pacific Northwest" Agronomy 14, no. 12: 2978. https://doi.org/10.3390/agronomy14122978
APA StyleHagerty, C. H., Shrestha, G., Wen, N., Kroese, D. R., Namdar, G. F., Paulitz, T., & Wysocki, D. J. (2024). Hemp Cover Cropping and Disease Suppression in Winter Wheat of the Dryland Pacific Northwest. Agronomy, 14(12), 2978. https://doi.org/10.3390/agronomy14122978






