Raffinose Priming Improves Seed Vigor by ROS Scavenging, RAFS, and α-GAL Activity in Aged Waxy Corn
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Treatments
- Non-aged seeds + distilled water (CK);
- Non-aged seeds + raffinose solution (CK + RAF);
- Artificially aged seeds + distilled water (AA);
- Artificially aged seeds + raffinose solution (AA + RAF).
2.2. Determination of Seed Germination Indexes
2.3. Seed Vigor Test
2.4. Determination of Antioxidant Enzyme Activities
2.5. Histochemical Detection and Quantification of Superoxide Anion and Hydrogen Peroxide
2.6. Determination of Raffinose Synthase Activity
2.7. Determination of α-Galactosidase Activity
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Promotive Effect of Raffinose on Germination of Seeds
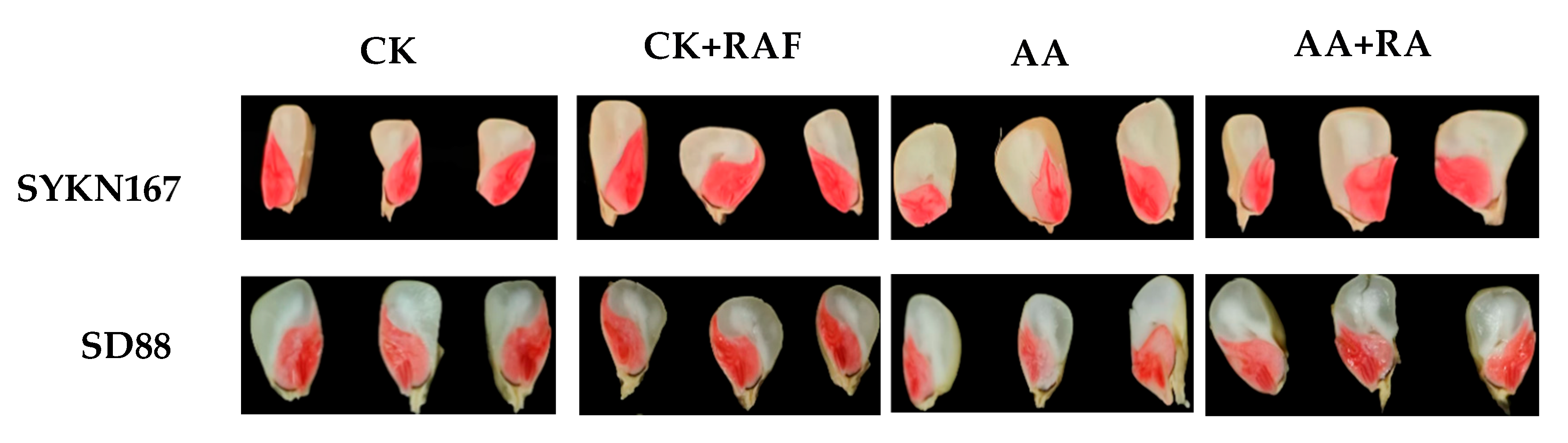
3.2. Effect of Raffinose Priming on Seed Vigor
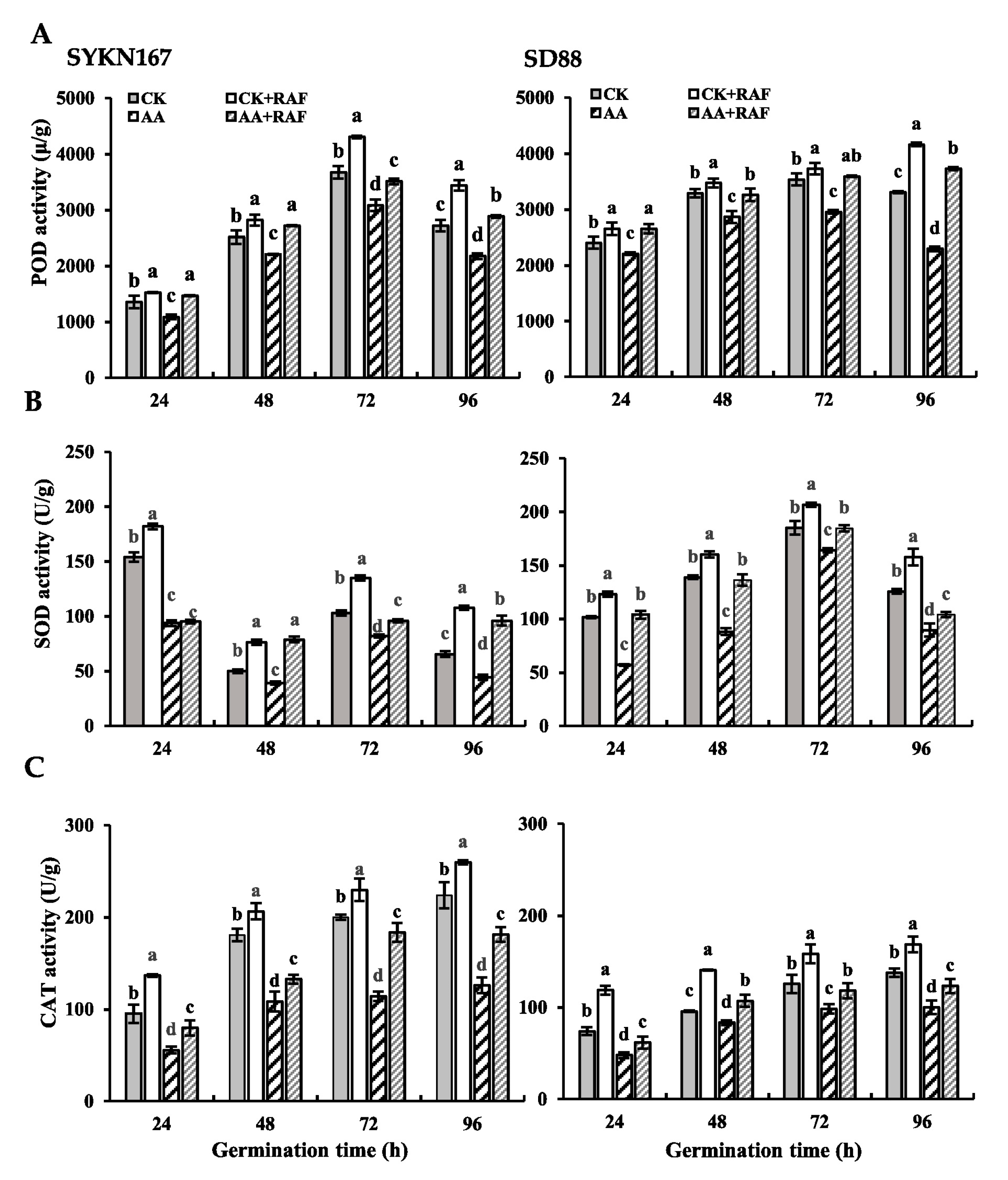
3.3. Effect of Raffinose on Antioxidant Enzyme Activity
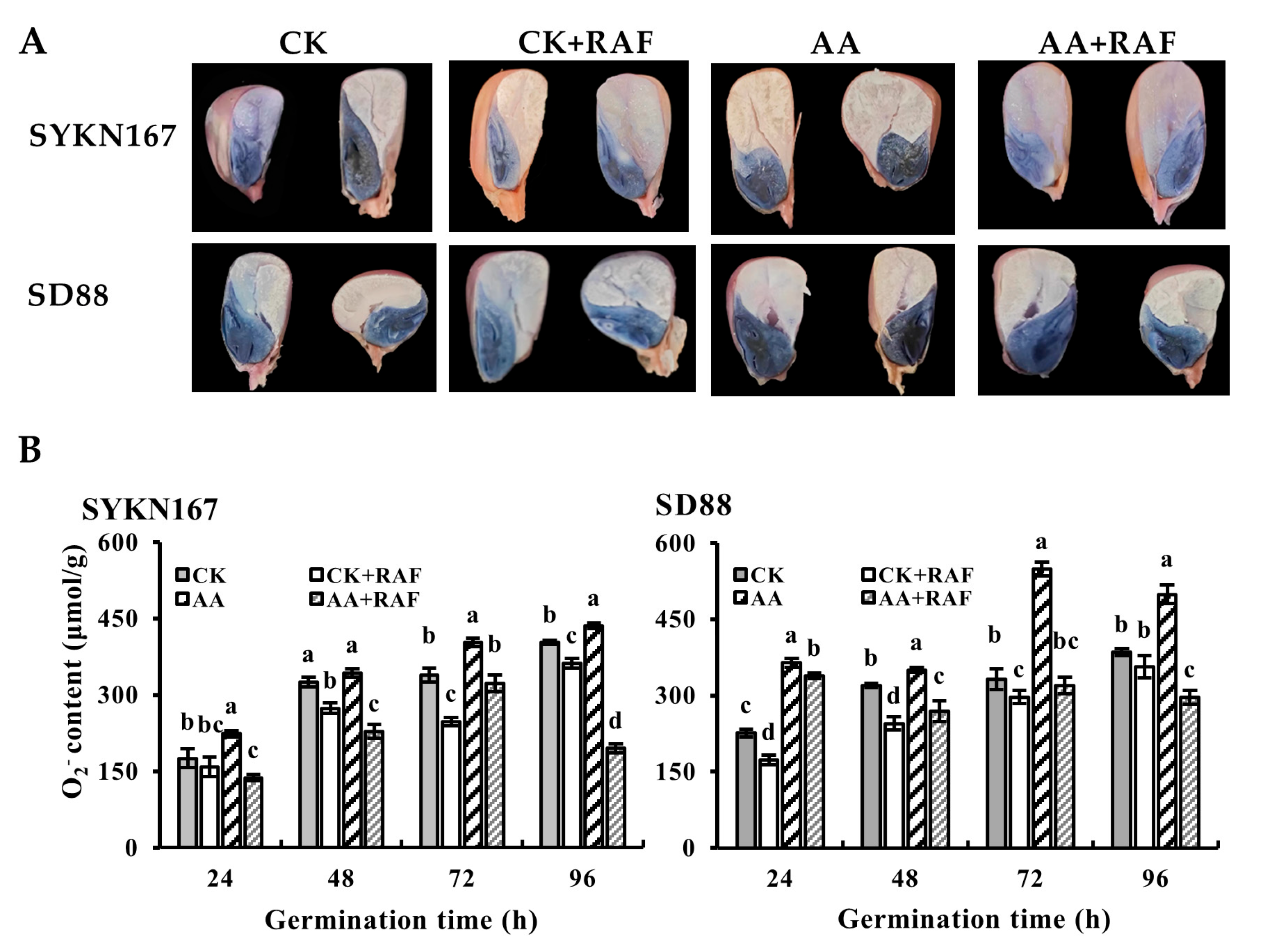
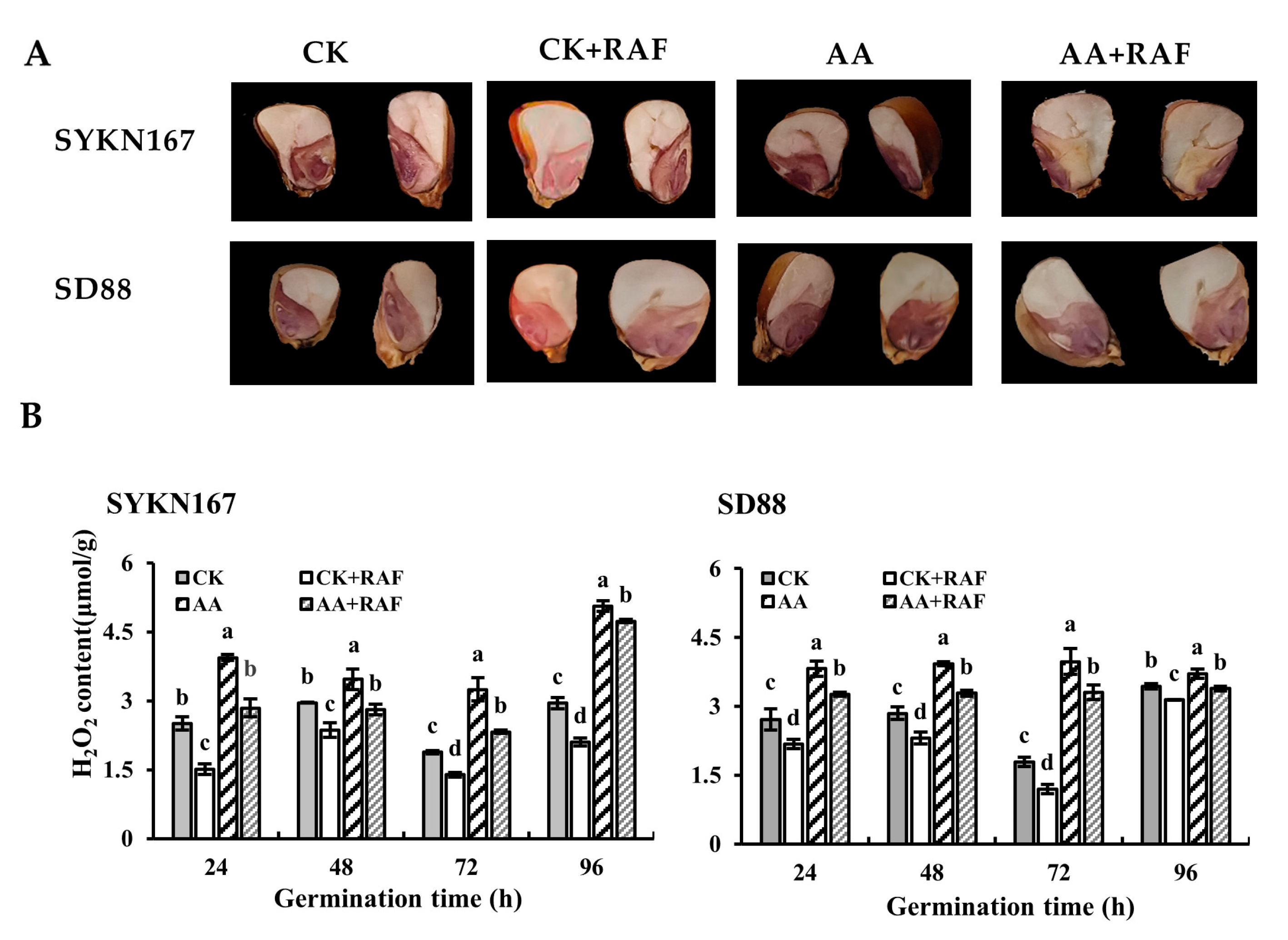
3.4. Effect of Raffinose on Reactive Oxygen Species Levels During Waxy Corn Seed Germination
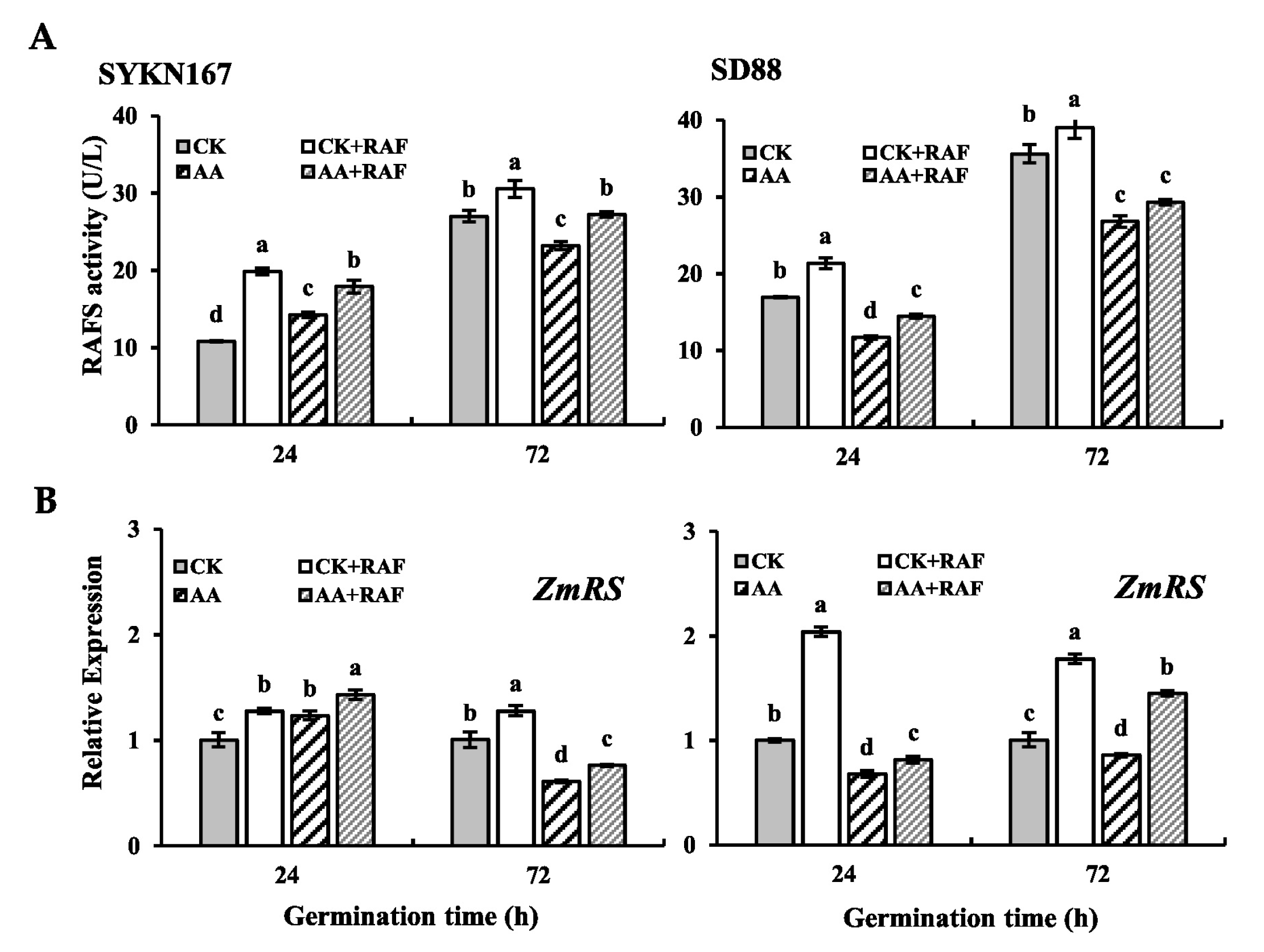
3.5. Changes in Raffinose Synthase Activity During Germination
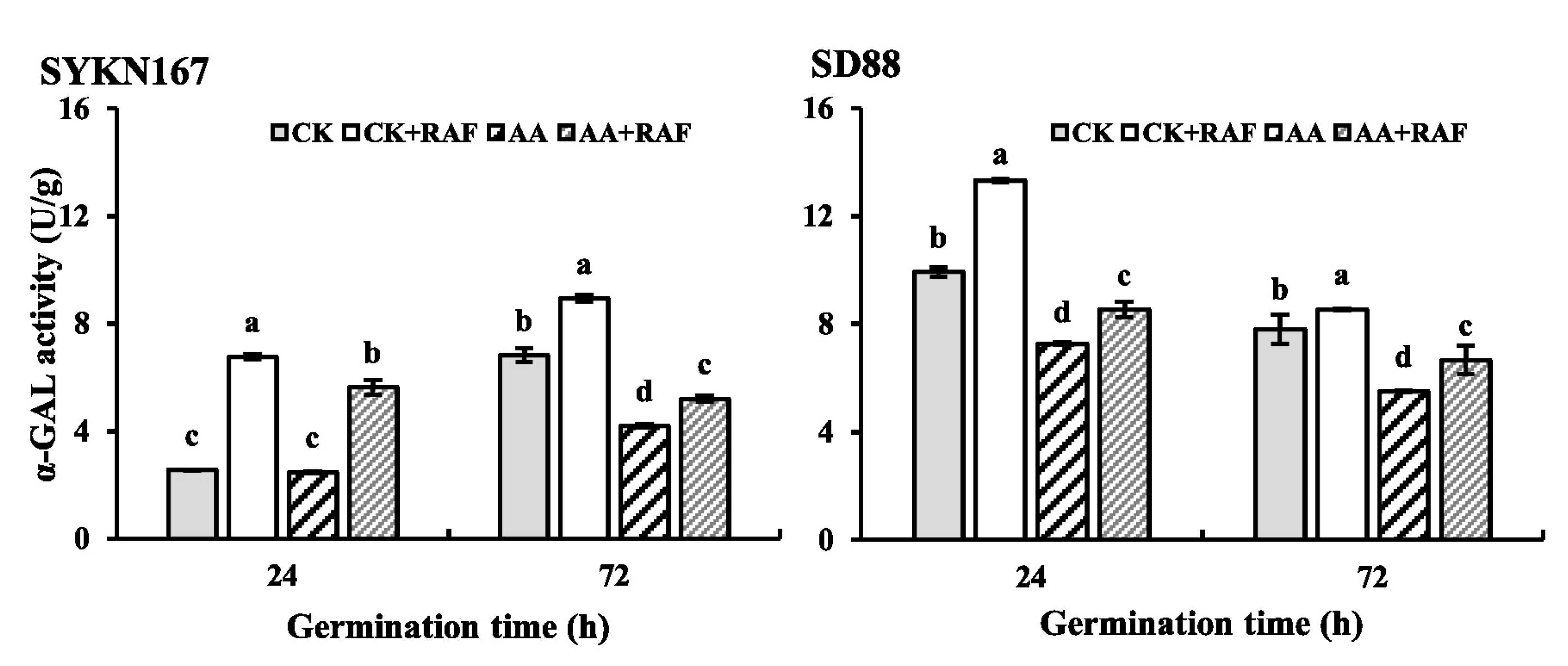
3.6. Changes in α-Galactosidase Activity During Germination
4. Discussion
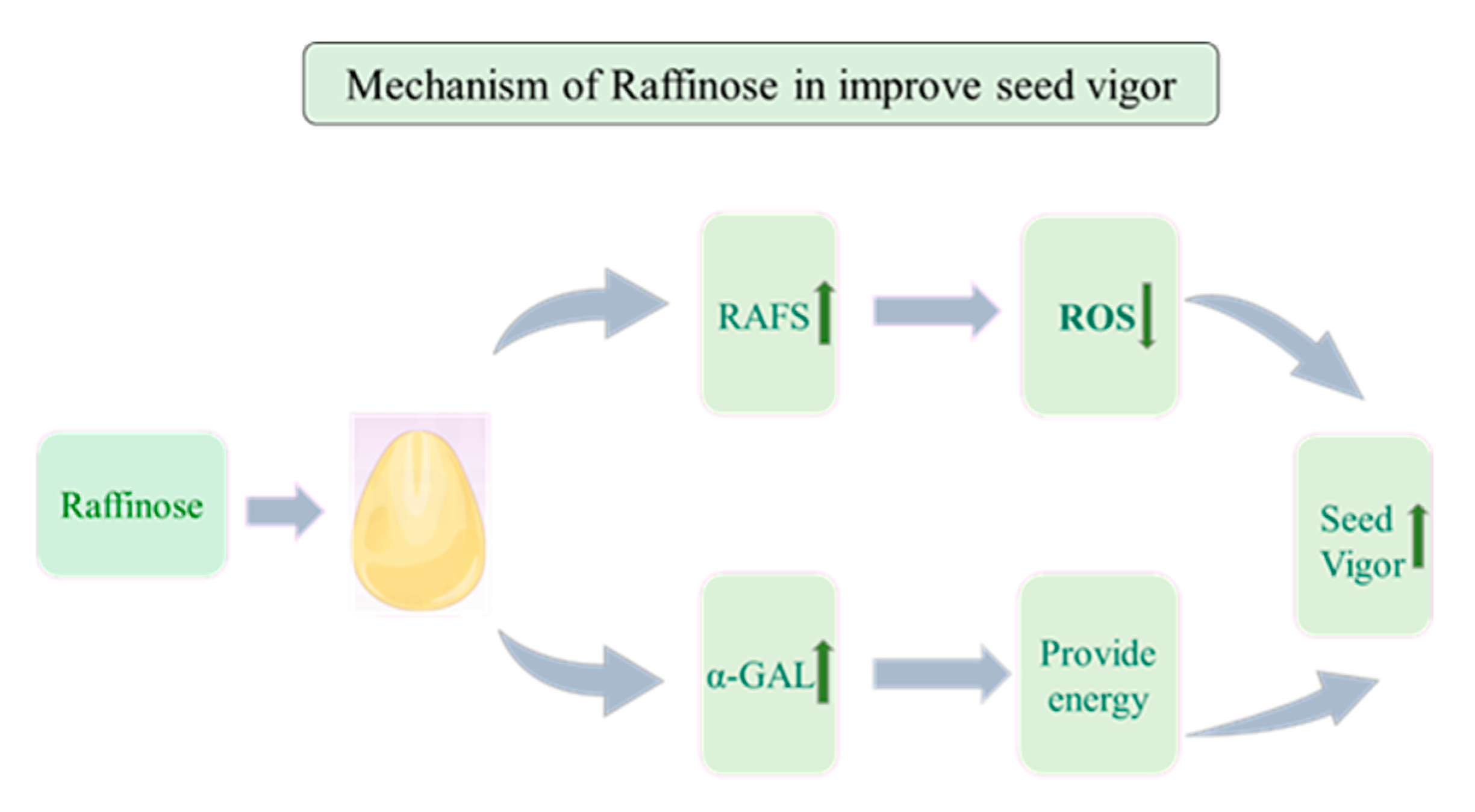
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Michalak, M.; Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P. DNA Methylation as an Early Indicator of Aging in Stored Seeds of “Exceptional” Species Populus nigra L. Cells 2022, 11, 2080. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Xu, H.J.; Yin, G.K.; Zhou, Y.C.; Lu, X.X.; Xin, X. Dynamic Changes in Membrane Lipid Metabolism and Antioxidant Defense During Soybean (Glycine max L. Merr.) Seed Aging. Front. Plant Sci. 2022, 13, 908949. [Google Scholar] [CrossRef]
- Wang, B.; Yang, R.C.; Ji, Z.Q.; Zhang, H.X.; Zheng, W.B.; Zhang, H.H.; Feng, F.Q. Evaluation of Biochemical and Physiological Changes in Sweet Corn Seeds under Natural Aging and Artificial Accelerated Aging. Agronomy 2022, 12, 1028. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhou, E.Q.; Yao, M.N.; Xue, D.; Zhao, N.; Zhou, Y.; Li, B.; Wang, K.H.; Miao, Y.M.; Gu, C.Y.; et al. PEG-6000 Priming Improves Aged Soybean Seed Vigor via Carbon Metabolism, ROS Scavenging, Hormone Signaling, and Lignin Synthesis Regulation. Agronomy 2023, 13, 3021. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Mishra, S.N.; Kumari, N.; Mishra, N.N.; Mishra, K. Estimation of Hormonal Seed Treatments on Enzyme Activities After Accelerated Ageing (Artificial Ageing) in Chickpea (Cicer arietinum L.). Legume Res. 2023, 46, 421–427. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.M.; Wang, D.; Liu, Y.; Dirk, L.M.A.; Goodman, J.; Downie, A.B.; Wang, J.M.; Wang, G.Y.; Zhao, T.Y. Regulation of Seed Vigor by Manipulation of Raffinose Family Oligosaccharides in Maize and Arabidopsis thaliana. Mol. Plant. 2017, 10, 1540–1555. [Google Scholar] [CrossRef]
- Salvi, P.; Varshney, V.; Majee, M. Raffinose family oligosaccharides (RFOs): Role in seed vigor and longevity. Biosci. Rep. 2022, 42, BSR20220198. [Google Scholar] [CrossRef]
- Yan, S.J.; Liu, Q.; Li, W.Y.; Yan, J.B.; Fernie, A.R. Raffinose Family Oligosaccharides: Crucial Regulators of Plant Development and Stress Responses. Crit. Rev. Plant Sci. 2022, 41, 286–303. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.M.; Liu, Y.; Li, X.D.; Hao, G.L.; Han, Q.H.; Dirk, L.M.A.; Downie, A.B.; Ruan, Y.L.; Wang, J.M.; et al. Raffinose synthase enhances drought tolerance through raffinose synthesis or galactinol hydrolysis in maize and Arabidopsis plants. J. Biol. Chem. 2020, 295, 8064–8077. [Google Scholar] [CrossRef]
- Gangl, R.; Tenhaken, R. Raffinose family oligosaccharides act as galactose stores in seeds and are required for rapid germination of Arabidopsis in the dark. Front. Plant Sci. 2016, 7, 71115. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Puthur, J.T. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Zhang, M.Y.; Zhang, J.J.; Dai, H.B.; Zhang, Z.P.; Miao, M.M. CsAGA1 and CsAGA2 Mediate RFO Hydrolysis in Partially Distinct Manner in Cucumber Fruits. Int. J. Mol. Sci. 2021, 22, 13285. [Google Scholar] [CrossRef] [PubMed]
- Neha, T.; Mohapatra, S.; Sirhindi, G.; Dogra, V. Seed priming with brassinolides improves growth and reinforces antioxidative defenses under normal and heat stress conditions in seedlings of Brassica juncea. Physiol. Plant. 2022, 174, e13814. [Google Scholar] [CrossRef]
- Maxime, V.; Ebru, T.Ö.; Wim, E.D.V. Fructan oligosaccharide priming alters apoplastic sugar dynamics and improves resistance against Botrytis cinerea in chicory. J. Exp. Bot. 2022, 73, 4214–4235. [Google Scholar] [CrossRef]
- Nie, L.X.; Song, S.K.; Yin, Q.; Zhao, T.C.; Liu, H.Y.; He, A.B.; Wang, W.Q. Enhancement in Seed Priming-Induced Starch Degradation of Rice Seed Under Chilling Stress via GA-Mediated α-Amylase Expression. Rice 2022, 15, 19. [Google Scholar] [CrossRef]
- Nandhini, D.U.; Somasundaram, E.; Amanullah, M.M. Effect of rhizobial nod factors (lipo-chitooligosaccharide) on seedling growth of blackgram under salt stress. Legume Res. 2017, 41, 159–162. [Google Scholar] [CrossRef]
- Nandhini, D.U.; Somasundaram, E. Effects of seed priming on salt tolerance of maize (Zea mays L.) seedlings by lipo-chitooligosaccharide. Bangladesh J. Bot. 2022, 51, 381–385. [Google Scholar] [CrossRef]
- Cao, Q.J.; Li, G.; Cui, Z.G.; Yang, F.T.; Jiang, X.L.; Diallo, L.; KONG, F.L. Seed Priming with Melatonin Improves the Seed Germination of Waxy Maize under Chilling Stress via Promoting the Antioxidant System and Starch Metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Hazra, A.K.; Mandi, S.S. Accelerated aging of seeds in hot water germination characteristics of aged wheat seeds. Seed Sci. Technol. 1985, 13, 683–690. [Google Scholar] [CrossRef]
- Guo, Z.T.; Zhao, J.J.; Wang, M.P.; Song, S.; Xia, Z.L. Sulfur dioxide promotes seed germination by modulating reactive oxygen species production in maize. Plant Sci. 2021, 312, 111027. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: II. Purification and Quantitative Relationship with Water-soluble Protein in Seedlings. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.; Contreras, L.M.; Kurz, L.; Wilkesman, J. Detection of Guaiacol Peroxidase on Electrophoretic Gels. Methods Mol. Biol. 2017, 1626, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Feurtado, J.A.; Banik, M.; Bewley, J.D. The cloning and characterization of α-galactosidase present during and following germination of tomato (Lycopersicon esculentum Mill.) seed. J. Exp. Bot. 2001, 52, 1239–1249. [Google Scholar] [CrossRef][Green Version]
- Zheng, Q.; Teng, Z.; Zhang, J.; Ye, N. ABA Inhibits Rice Seed Aging by Reducing H2O2 Accumulation in the Radicle of Seeds. Plants 2024, 13, 809. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Yan, H.F.; Mao, P.S. Comparative Time-Course Physiological Responses and Proteomic Analysis of Melatonin Priming on Promoting Germination in Aged Oat (Avena sativa L.) Seeds. Int. J. Mol. Sci 2021, 22, 811. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2017, 80, 3–12. [Google Scholar] [CrossRef]
- Abdullah, Y.L.H.A.; Al-Hazmi, N.E.; Naguib, D.M. Rhizobacteria-priming improves common bean seeds germination under different abiotic stresses through improving hydrolysis and antioxidant enzymes kinetics parameters. Rhizosphere 2024, 29, 100842. [Google Scholar] [CrossRef]
- Liu, A.L.; Wei, M.Y.; Li, D.H.; Zhou, R.; Zhang, X.R.; You, J. Cloning and Function Analysis of Sesame Galactinol Synthase Gene SiGolS6 in Arabidopsis. Sci. Agric. Sin. 2020, 53, 3432–3442. [Google Scholar] [CrossRef]
- Xia, F.S.; Cheng, H.; Chen, L.L.; Zhu, H.S.; Mao, P.S.; Wang, M.Y. Influence of exogenous ascorbic acid and glutathione priming on mitochondrial structural and functional systems to alleviate aging damage in oat seeds. BMC Plant Biol. 2020, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.L.; Zhang, Y.; Sun, J.; Meng, J.S.; Tao, J. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, K.L.; Dukowic-Schulze, S.; Miller, M.E.; Chen, C.; Kianian, S.F. The role of mitochondria in plant development and stress tolerance. Free Radic. Biol. Med. 2016, 100, 238–256. [Google Scholar] [CrossRef]
- Ratajczak, E.; Małecka, A.; Ciereszko, I.; Staszak, A.M. Mitochondria Are Important Determinants of the Aging of Seeds. Int. J. Mol. Sci. 2019, 20, 1568. [Google Scholar] [CrossRef]
- Ratajczak, E.; Maecka, A.; Bagniewska-Zadworna, A.; Kalemba, E.M. The production, localization and spreading of reactive oxygen species contributes to the low vitality of long-termstored common beech (Fagus sylvatica L.) seeds. J. Plant Physiol. 2015, 174, 147–156. [Google Scholar] [CrossRef]
- Kamal, K.Y.; Khodaeiaminjan, M.; Yahya, G.; El-Tantawy, A.A.; Moneim, D.A.; El-Esawi, M.A.; Abd-Elaziz, M.A.A.; Nassrallah, A.A. Modulation of cell cycle progression and chromatin dynamic as tolerance mechanisms to salinity and drought stress in maize. Physiol. Plant. 2021, 172, 684–695. [Google Scholar] [CrossRef]
- Sasi, M.; Awana, M.; Samota, M.K.; Tyagi, A.; Kumar, S.; Sathee, L.; Krishnan, V.; Praveen, S.; Singh, A. Plant growth regulator induced mitigation of oxidative burst helps in the management of drought stress in rice (Oryza sativa L.). Environ. Exp. Bot. 2021, 185, 104413. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Lü, J.G.; Sui, X.L.; Ma, S.; Li, X.; Liu, H.; Zhang, Z.X. Suppression of cucumber stachyose synthase gene (CsSTS) inhibits phloem loading and reduces low temperature stress tolerance. Plant Mol. Biol. 2017, 95, 1–15. [Google Scholar] [CrossRef]
- Nishizawa-Yokoi, A.; Yabuta, Y.; Shigeoka, S. The contribution of carbohydrates including raffinose family oligosaccharides and sugar alcohols to protection of plant cells from oxidative damage. Plant Signal. Behav. 2008, 3, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Lang, S.R.; Wang, D.M.; Xue, H.; Wang, X.F. Functional characterization of galactinol synthase and raffinose synthase in desiccation tolerance acquisition in developing Arabidopsis seeds. J. Plant Physiol. 2018, 230, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, A.; Peterbauer, T.; Richter, A. Inhibition of raffinose oligosaccharide breakdown delays germination of pea seeds. J. Plant Physiol. 2007, 164, 1093–1096. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Li, D.; Dirk, L.M.A.; Downie, A.B.; Zhao, T.Y. ZmAGA1 Hydrolyzes RFOs Late during the Lag Phase of Seed Germination, Shifting Sugar Metabolism toward Seed Germination Over Seed Aging Tolerance. J. Agric. Food Chem. 2021, 69, 11606–11615. [Google Scholar] [CrossRef]
- Arunraj, R.; Skori, L.; Kumar, A.; Hickerson, N.M.N.; Shoma, N.; Vairamani, M.; Samuel, M.A. Spatial regulation of alpha-galactosidase activity and its influence on raffinose family oligosaccharides during seed maturation and germination in Cicer arietinum. Plant Signal. Behav. 2020, 15, 1709707. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Goszczynska, J. Inhibition of raffinose family oligosaccharides and galactosyl pinitols breakdown delays germination of winter vetch (Vicia villosa Roth.) seed. Acta Soc. Bot. Pol. 2009, 78, 203–208. [Google Scholar] [CrossRef]

| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| β-actin | CATGGAGAACTGGCATCACACCTT | CTGCGTCATTTTCTCTCTGTTGGC |
| ZmRS | GAGCTCTACGATGGTTTGCACTCCC | CCATCCACAGCGAGTTGTAGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Xiao, R.; Yu, T.; Guo, T.; Zhong, X.; Qu, J.; Du, W.; Xue, W. Raffinose Priming Improves Seed Vigor by ROS Scavenging, RAFS, and α-GAL Activity in Aged Waxy Corn. Agronomy 2024, 14, 2843. https://doi.org/10.3390/agronomy14122843
Zhu M, Xiao R, Yu T, Guo T, Zhong X, Qu J, Du W, Xue W. Raffinose Priming Improves Seed Vigor by ROS Scavenging, RAFS, and α-GAL Activity in Aged Waxy Corn. Agronomy. 2024; 14(12):2843. https://doi.org/10.3390/agronomy14122843
Chicago/Turabian StyleZhu, Min, Ru Xiao, Tong Yu, Tao Guo, Xuemei Zhong, Jianzhou Qu, Wanli Du, and Wei Xue. 2024. "Raffinose Priming Improves Seed Vigor by ROS Scavenging, RAFS, and α-GAL Activity in Aged Waxy Corn" Agronomy 14, no. 12: 2843. https://doi.org/10.3390/agronomy14122843
APA StyleZhu, M., Xiao, R., Yu, T., Guo, T., Zhong, X., Qu, J., Du, W., & Xue, W. (2024). Raffinose Priming Improves Seed Vigor by ROS Scavenging, RAFS, and α-GAL Activity in Aged Waxy Corn. Agronomy, 14(12), 2843. https://doi.org/10.3390/agronomy14122843






