Siberian Wildrye (Elymus sibiricus) Seed Vigor Estimation for the Prediction of Emergence Performance under Diverse Environmental Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Materials
2.2. Standard Germination Experiment
2.3. Early Individual Count of Radicle Emergence
2.4. Field Emergence Experiment
2.5. Pot Emergence under Different Environmental Conditions
2.6. Data Analyses
3. Results
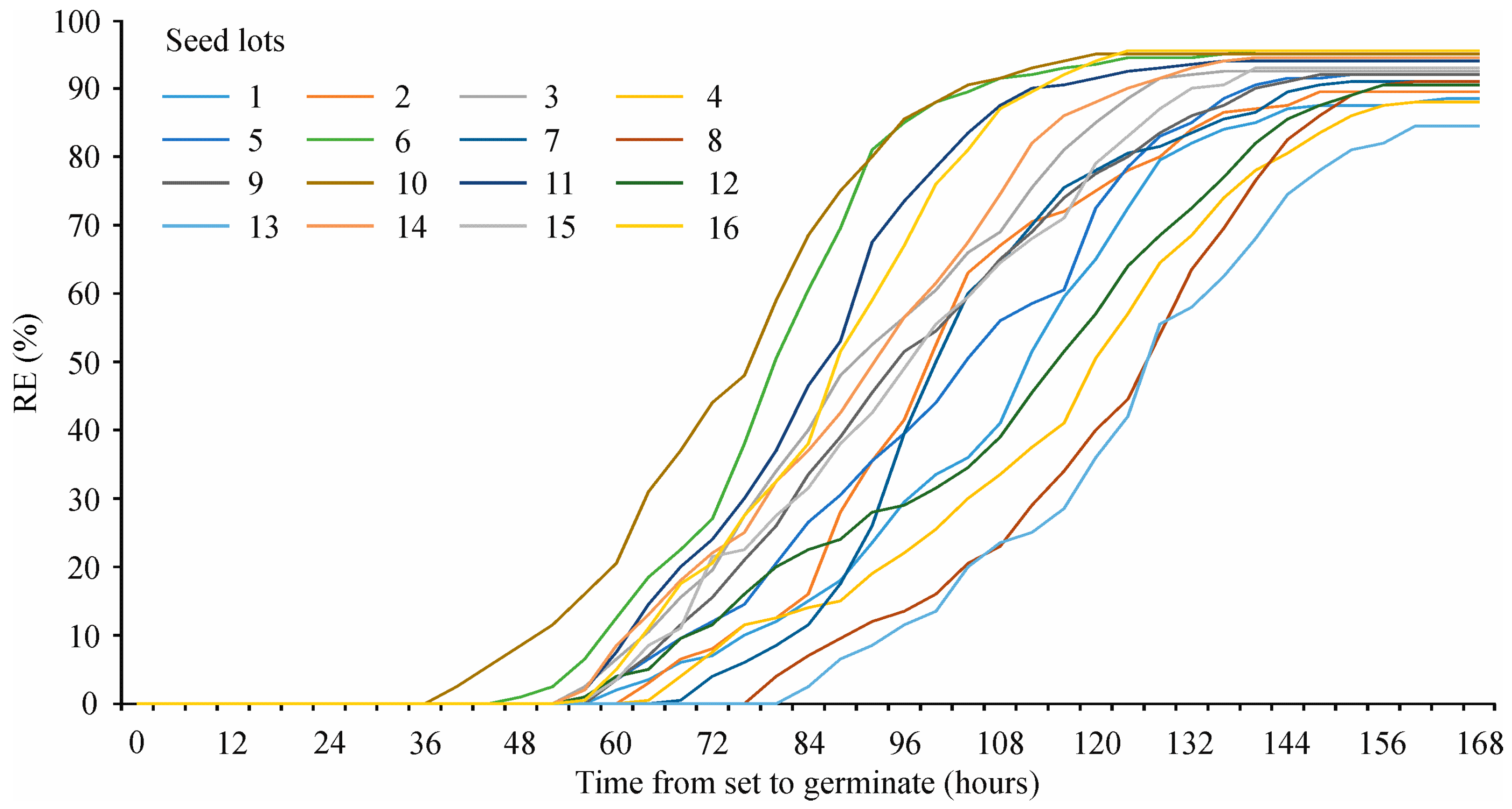
3.1. Standard Germination
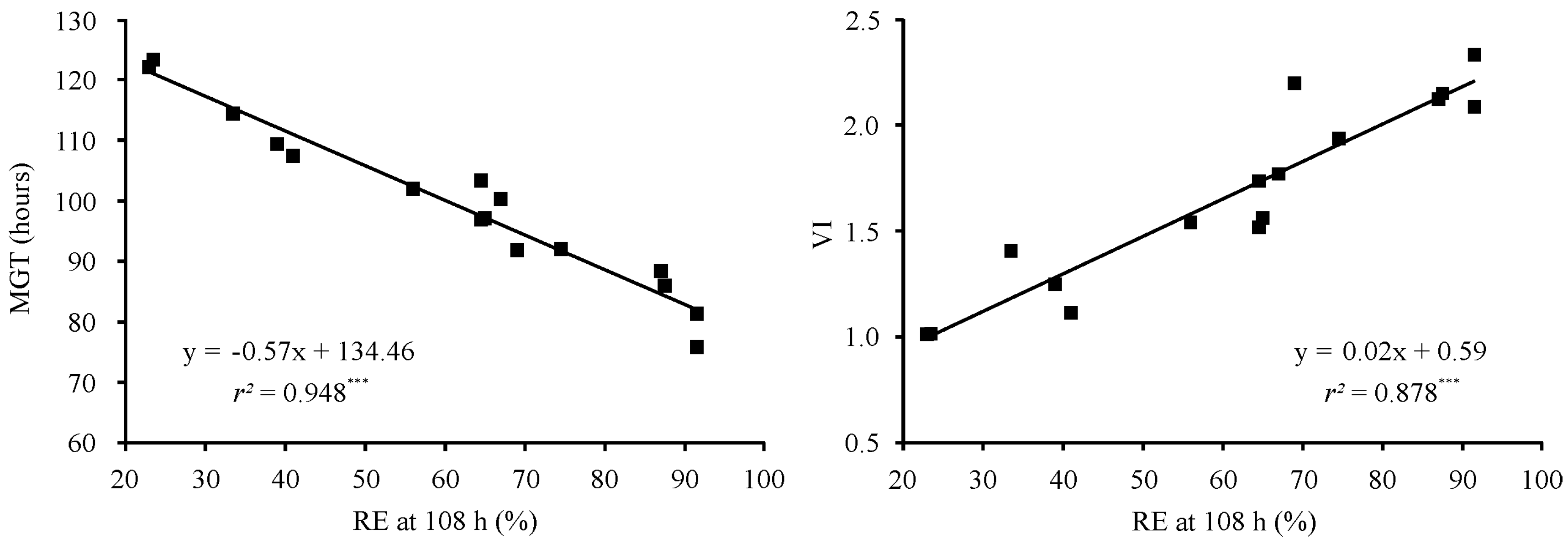
3.2. Radicle Emergence, Mean Germination Time, and Vigor Index
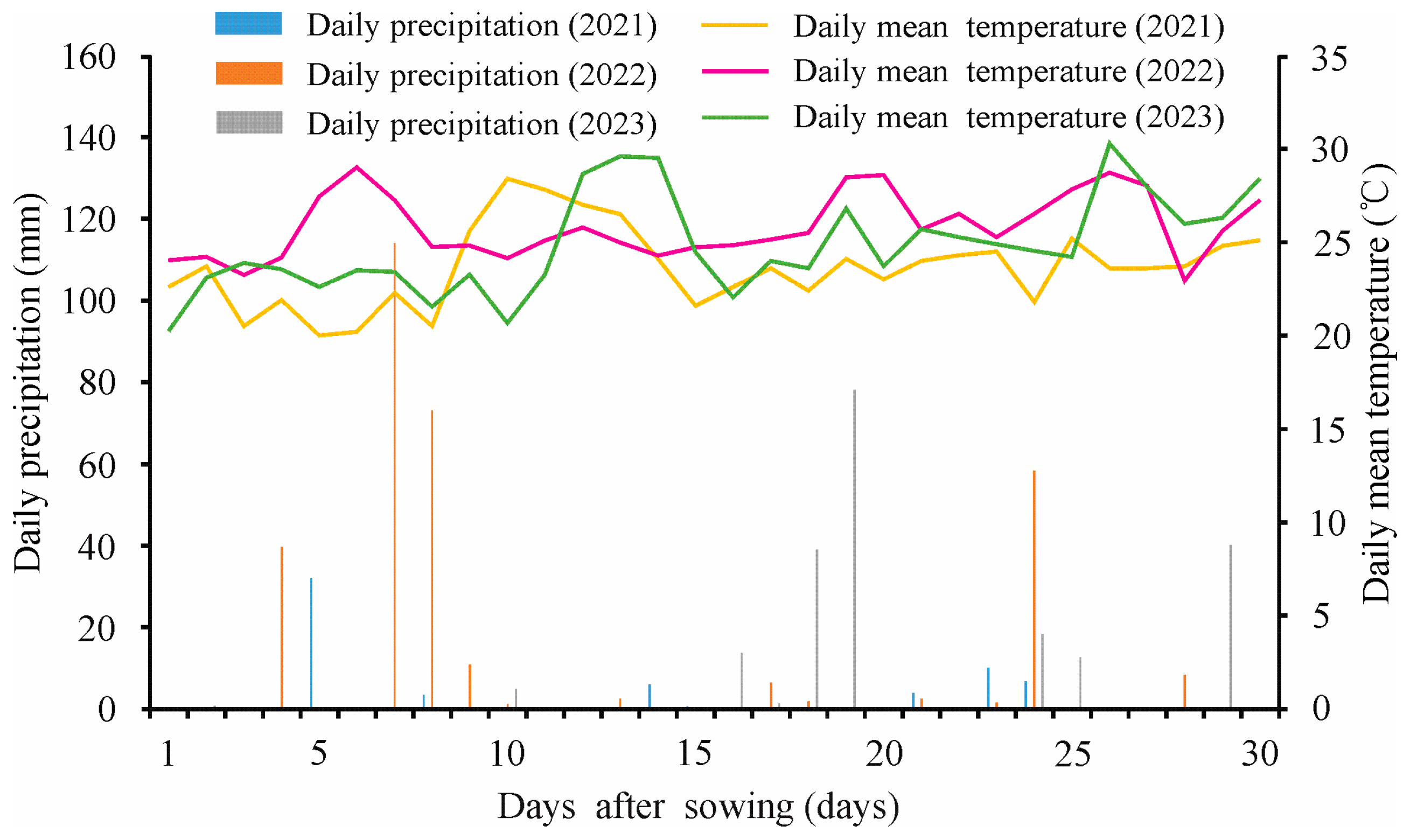
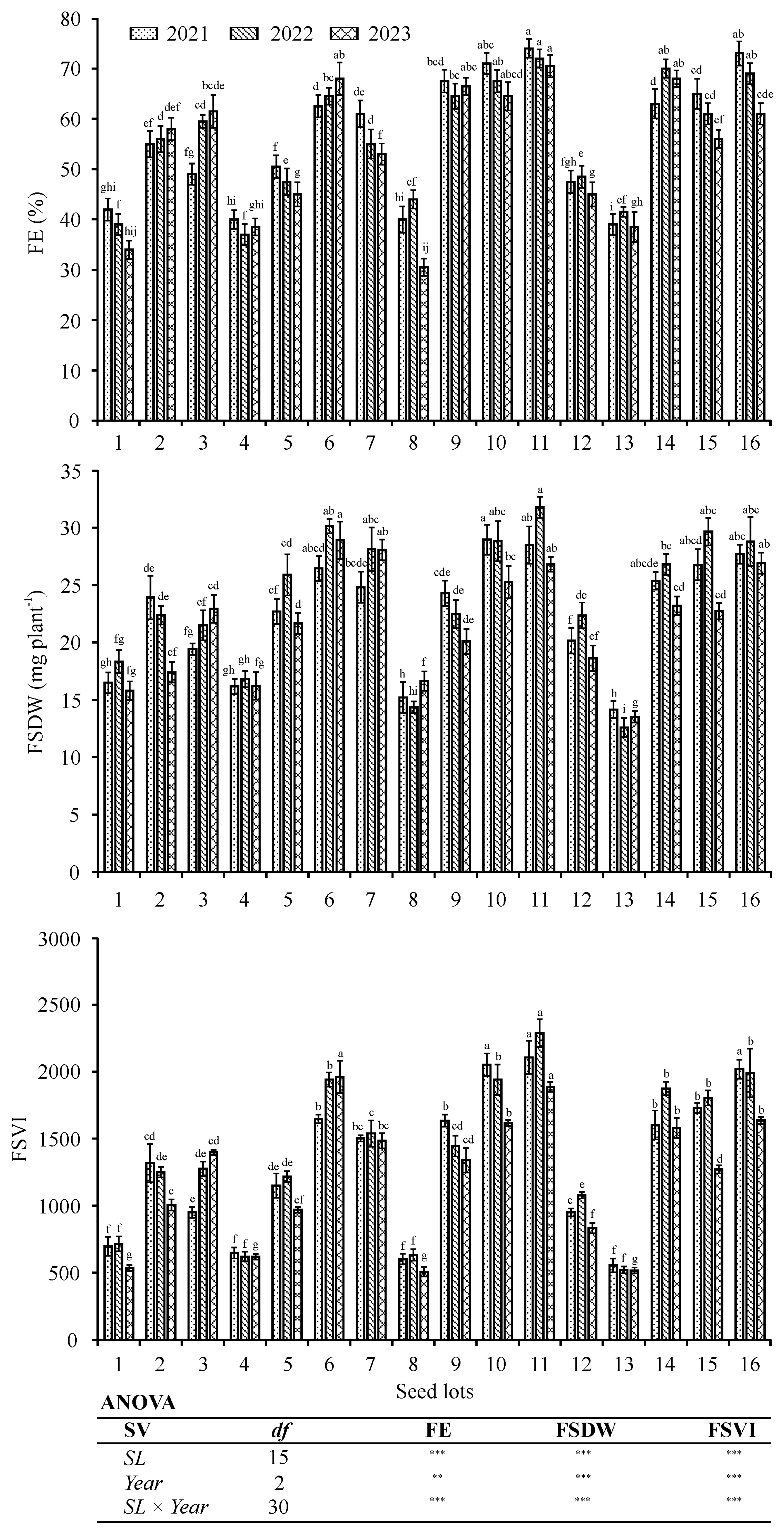
3.3. Three-Year Field Experiments
3.4. Pot Experiments
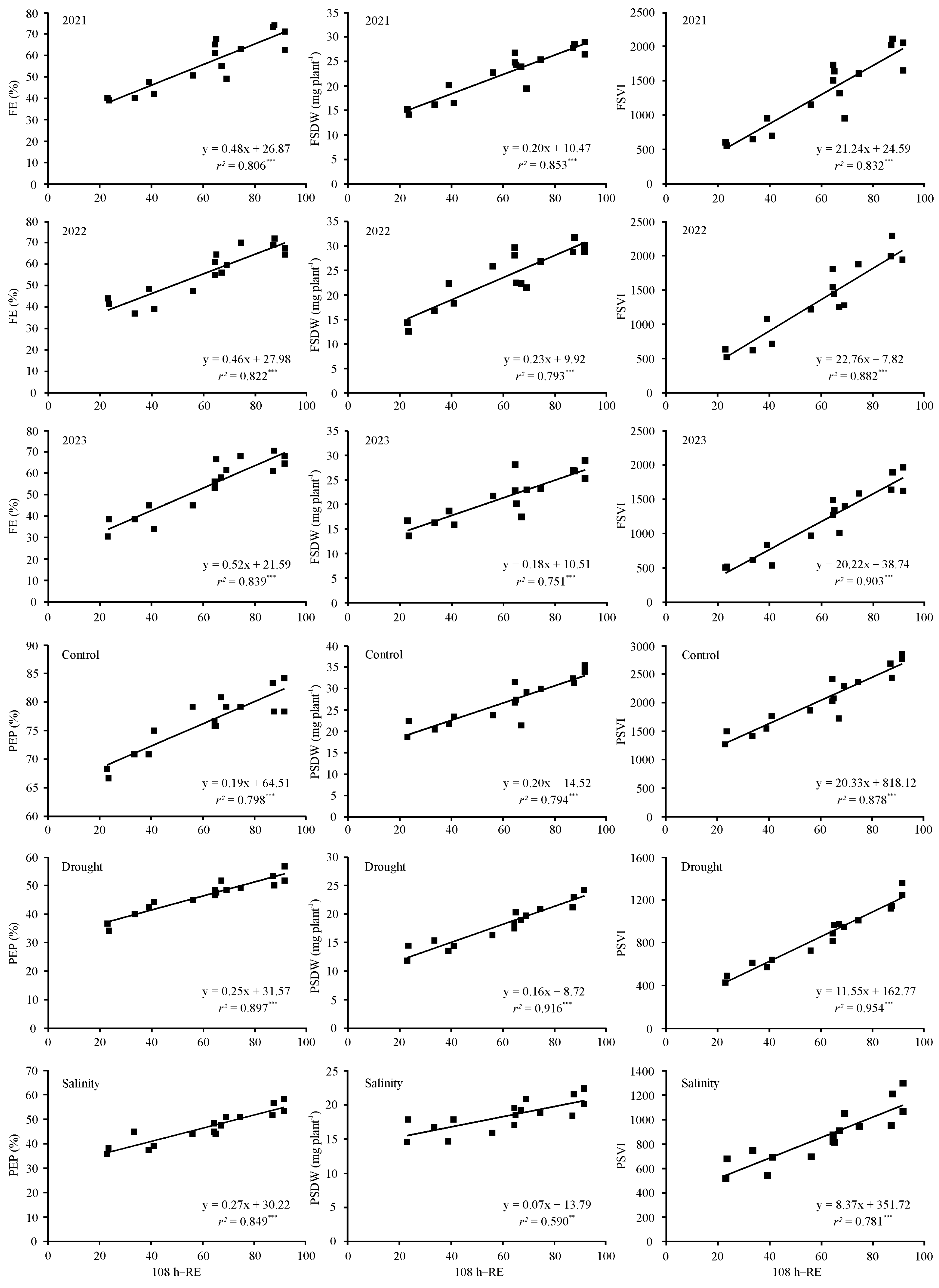
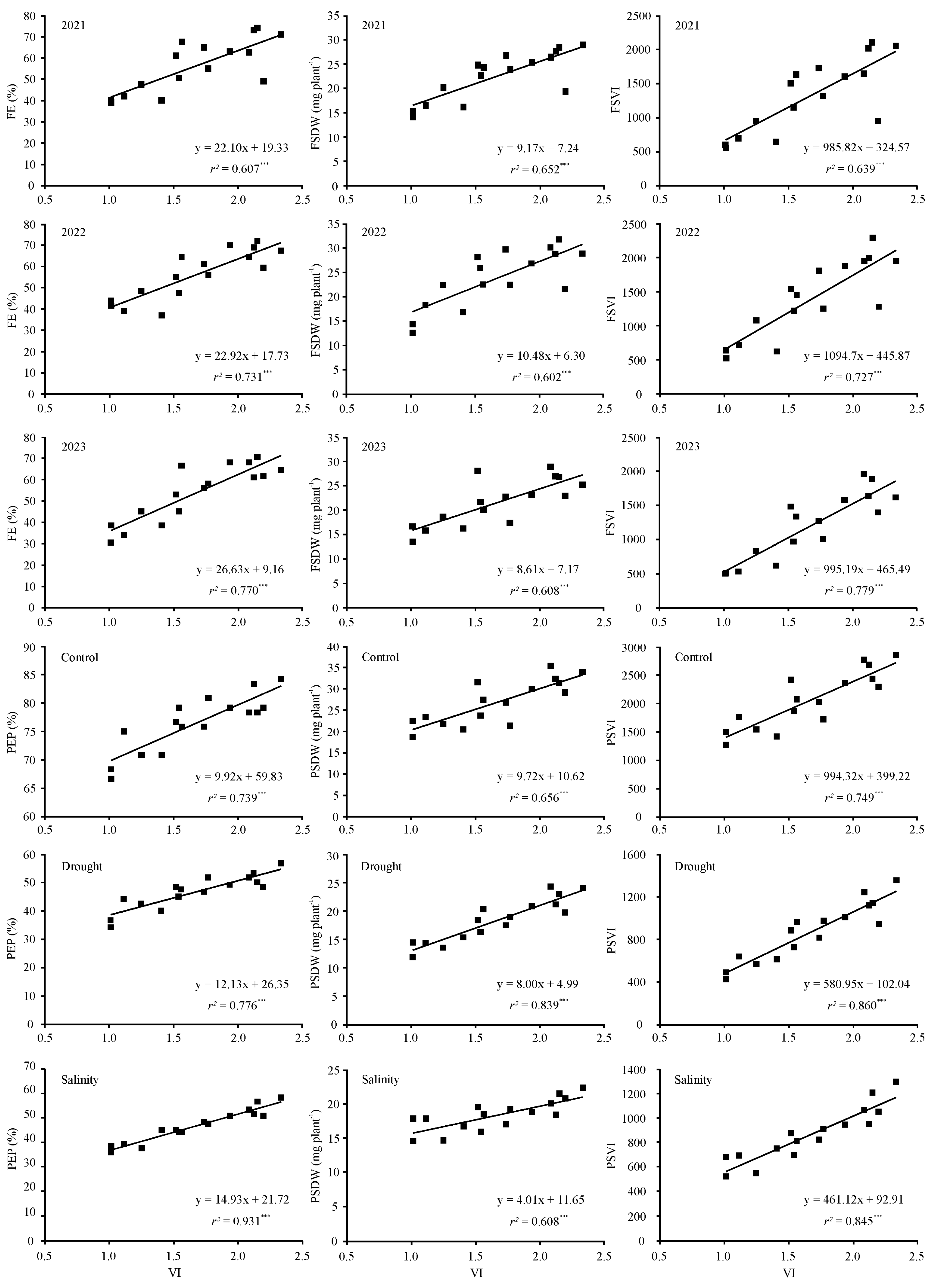
3.5. Relationships between Standard Germination, Radicle Emergence, Mean Germination Time, and Vigor Index with Seedling Emergence Performance under Diverse Environmental Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ntakirutimana, F.; Xiao, B.W.; Xie, W.G.; Zhang, J.C.; Zhang, Z.Y.; Wang, N.; Yan, J.J. Potential effects of awn length variation on seed yield and components, seed dispersal and germination performance in Siberian wildrye (Elymus sibiricus L.). Plants 2019, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Hou, L.Y.; Zhang, Q.; Yu, X.N.; Zhao, L.; Lu, J.X.; Mao, P.S.; Hannaway, D.B. Influence of row spacing and P and N applications on seed yield components and seed yield of Siberian wildrye (Elymus sibiricus L.). Crop Sci. 2017, 57, 2205–2212. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Zhang, Z.Y.; Wan, Y.Y.; Tian, J.Y.; Xie, W.G. Development of EST-SSR markers linked to flowering candidate genes in Elymus sibiricus L. based on RNA sequencing. Plants 2020, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Xie, W.G.; Yu, X.X.; Zhang, Z.Y.; Zhao, Y.Q.; Wang, N.; Wang, Y.R. Selection of suitable reference genes for RT-qPCR gene expression analysis in Siberian Wild Rye (Elymus sibiricus) under different experimental conditions. Genes 2019, 10, 451. [Google Scholar] [CrossRef]
- Shang, Z.H.; Cao, J.J.; Guo, R.Y.; Long, R.J.; Deng, B. The response of soil organic carbon and nitrogen 10 years after returning cultivated alpine steppe to grassland by abandonment or reseeding. Catena 2014, 119, 28–35. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xie, W.G.; Zhang, J.C.; Wang, N.; Zhao, Y.Q.; Wang, Y.R.; Bai, S.Q. Construction of the first high-density genetic linkage map and identification of seed yield-related QTLs and candidate genes in Elymus sibiricus, an important forage grass in Qinghai-Tibet Plateau. BMC Genom. 2019, 20, 861. [Google Scholar] [CrossRef]
- Wang, Y.R.; Yu, L.; Nan, Z.B.; Liu, Y.L. Vigor tests used to rank seed lot quality and predict field emergence in four forage species. Crop Sci. 2004, 44, 535–541. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Tao, Q.B.; Zhang, R.Z.; Ma, Y.L.; Xing, J.Y.; Zhou, S.H.; Liu, Y.H.; Meng, F.S.; Sun, J. A seed vigour test based on radicle emergence during germination at 5 ℃ for four forage species. Seed Sci. Technol. 2023, 51, 361–369. [Google Scholar] [CrossRef]
- Tao, Q.B.; Chen, D.L.; Bai, M.J.; Zhang, Y.Q.; Zhang, R.Z.; Chen, X.F.; Sun, X.T.; Niu, T.X.; Nie, Y.T.; Zhong, S.Z.; et al. Hydrotime model parameters estimate seed vigor and predict seedling emergence performance of Astragalus sinicus under various environmental conditions. Plants 2023, 12, 1876. [Google Scholar] [CrossRef]
- Chen, X.L.; Wei, Z.C.; Chen, D.L.; Hu, X.W. Base water potential but not hydrotime predicts seedling emergence of Medicago sativa under water stress conditions. PeerJ 2022, 10, e13206. [Google Scholar] [CrossRef]
- Stanisavljević, R.; Velijević, N.; Štrbanović, R.; Postić, D.; Aleksić, G.; Trkulja, N.; Knežević, J.; Dodig, D. Seed quality of vetch (Vicia sativa L.) affected by different seed colors and sizes after various storage periods. Int. J. Agric. Biol. 2018, 20, 2655–2660. [Google Scholar]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Soltani, E.; Adeli, R.; Akbari, G.A.; Ramshini, H. Application of hydrotime model to predict early vigour of rapeseed (Brassica napus L.) under abiotic stresses. Acta Physiol. Plant. 2017, 39, 252. [Google Scholar] [CrossRef]
- Mavi, K.; Demir, I. Controlled deterioration for vigour assessment and predicting seedling growth of winter squash (Cucurbita maxima) seed lots under salt stress. N. Z. J. Crop Hortic. Sci. 2005, 33, 193–197. [Google Scholar] [CrossRef]
- Mavi, K.; Demir, I.; Matthews, S. Mean germination time estimates the relative emergence of seed lots of three cucurbit crops under stress conditions. Seed Sci. Technol. 2010, 38, 14–25. [Google Scholar] [CrossRef]
- Araújo, J.E.O.; Dias, D.C.F.S.; de Miranda, R.M.; Nascimento, W.M. Adjustment of the electrical conductivity test to evaluate the seed vigor of chickpea (Cicer arietinum L.). J. Seed Sci. 2022, 44, e202244003. [Google Scholar] [CrossRef]
- Cheshmi, M.; Khajeh-Hosseini, M. Single count of radicle emergence, DNA replication during seed germination and vigour in alfalfa seed lots. Seed Sci. Technol. 2020, 48, 367–380. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Mo, Q.; Powell, A.A.; Wang, Y.R. DNA replication during seed germination, deterioration, and its relation to vigor in alfalfa and white clover. Crop Sci. 2018, 58, 1393–1401. [Google Scholar] [CrossRef]
- Venuste, M.; Chen, D.L.; Hu, X.W. Detection of seed vigour differences in Festuca sinensis seed lots. Seed Sci. Technol. 2022, 50, 61–75. [Google Scholar]
- Forti, V.A.; de Carvalho, C.; Sliwinska, E.; Cicero, S.M. Flow cytometry as a tool for analyses of soya bean seed vigour. Seed Sci. Technol. 2018, 46, 217–224. [Google Scholar] [CrossRef]
- Ermis, S.; Ozden, E.; Demir, I. Early radicle emergence count after controlled deterioration (CD) predicts emergence potential better than final standard germination after CD in tomato varieties. Seed Sci. Technol. 2015, 43, 278–283. [Google Scholar] [CrossRef]
- Wang, X.M.; Wu, B.K.; Zhang, Q.X.; Jia, L.Q.; Qi, H.N.; Zhao, G.W. Evidence for seed vigor associated with fatty acids and its composition in rice. Agron. J. 2021, 113, 2618–2628. [Google Scholar] [CrossRef]
- Alahakoon, A.A.C.B.; Abeysiriwardena, D.S.Z.; Damunupola, J.W.; Hay, F.R.; Gama-Arachchige, N.S. Accelerated aging test of seed vigour for predicting field emergence of wet direct-seeded rice. Crop Pasture Sci. 2021, 72, 773–781. [Google Scholar] [CrossRef]
- Tao, Q.B.; Sun, J.P.; Zhang, Y.Q.; Sun, X.T.; Li, Z.Y.; Zhong, S.Z.; Sun, J. Single count of radicle emergence and mean germination time estimate seed vigour of Chinese milk vetch (Astragalus sinicus). Seed Sci. Technol. 2022, 50, 47–59. [Google Scholar] [CrossRef]
- TeKrony, D.M.; Egli, D.B. Relationship between laboratory indices of soybean seed vigor and field emergence. Crop Sci. 1977, 17, 573–577. [Google Scholar] [CrossRef]
- Matthews, S.; Hosseini, M.K. Mean germination time as an indicator of emergence performance in soil of seed lots of maize (Zea mays). Seed Sci. Technol. 2006, 34, 339–347. [Google Scholar] [CrossRef]
- Wang, Y.R.; Yu, L.; Nan, Z.B. Use of seed vigour tests to predict field emergence of lucerne (Medicago sativa). N. Z. J. Agric. Res. 1996, 39, 255–262. [Google Scholar] [CrossRef]
- Leão-Araújo, E.F.; dos Santos, J.F.; da Silva, C.B.; Filho, J.M.; Vieira, R.D. Controlled deterioration test and use of the Seed Vigor Imaging System (SVIS®) to evaluate the physiological potential of crambe seeds. J. Seed Sci. 2017, 39, 393–400. [Google Scholar] [CrossRef][Green Version]
- Sanches, M.F.G.; dos Santos, J.F.; Martins, C.C.; Vieira, R.D.; de Souza, F.H.D. Accelerated ageing test for vigour assessment of pigeon pea ‘Cajanus cajan’ (L.) Millsp. seeds. Aust. J. Crop Sci. 2014, 8, 1298–1303. [Google Scholar]
- Sliwinska, E. Nuclear DNA replication and seed quality. Seed Sci. Res. 2009, 19, 15–25. [Google Scholar] [CrossRef]
- Xie, J.; Li, Y.; Jiang, G.Q.; Sun, H.Y.; Liu, X.J.; Han, L.P. Seed color represents salt resistance of alfalfa seeds (Medicago sativa L.): Based on the analysis of germination characteristics, seedling growth and seed traits. Front. Plant Sci. 2023, 14, 1104948. [Google Scholar] [CrossRef] [PubMed]
- Ambika, S.; Manonmani, V.; Somasundaram, G. Review on effects of seed size on seedling vigour and seed yield. Res. J. Seed Sci. 2014, 7, 31–38. [Google Scholar] [CrossRef]
- Atak, M.; Kaya, M.D.; Kaya, G.; Kaya, M.; Khawar, K.M. Dark green colored seeds increase the seed vigor and germination ability in dry green pea (Pisum sativum L.). Pak. J. Bot. 2008, 40, 2345–2354. [Google Scholar]
- Luo, Y.; Lin, C.; Fu, Y.Y.; Huang, Y.T.; He, F.; Guan, Y.J.; Hu, J. Single counts of radicle emergence can be used as a fast method to test seed vigour of indica rice. Seed Sci. Technol. 2017, 45, 222–229. [Google Scholar] [CrossRef]
- Luo, Y.; Guan, Y.J.; Huang, Y.T.; Li, J.; Li, Z.; Hu, J. Single counts of radicle emergence provides an alternative method to test seed vigour in sweet corn. Seed Sci. Technol. 2015, 43, 519–525. [Google Scholar] [CrossRef]
- Shinohara, T.; Ducournau, S.; Matthews, S.; Wagner, M.; Powell, A.A. Early counts of radicle emergence, counted manually and by image analysis, can reveal differences in the production of normal seedlings and the vigour of seed lots of cauliflower. Seed Sci. Technol. 2021, 49, 219–235. [Google Scholar] [CrossRef]
- Demir, I.; Ermis, S.; Mavi, K.; Matthews, S. Mean germination time of pepper seed lots (Capsicum annuum L.) predicts size and uniformity of seedlings in germination tests and transplant modules. Seed Sci. Technol. 2008, 36, 21–30. [Google Scholar] [CrossRef]
- Bhuker, A.; Pandey, V.; Singh, S.; Dalal, P.K.; Raj, P. Radicle emergence test-A quick germination test in different field crops. Seed Res. 2020, 48, 80–82. [Google Scholar]
- Khajeh-Hosseini, M.; Lomholt, A.; Matthews, S. Mean germination time in the laboratory estimates the relative vigour and field performance of commercial seed lots of maize (Zea mays L.). Seed Sci. Technol. 2009, 37, 446–456. [Google Scholar] [CrossRef]
- Amirmoradi, S.; Feizi, H. Can mean germination time predict seed vigor of canola (Brassica napus L.) seed lots? Acta Agrobot. 2017, 70, 1729. [Google Scholar] [CrossRef][Green Version]
- Matthews, S.; Khajeh-Hosseini, M. Length of the lag period of germination and metabolic repair explain vigour differences in seed lots of maize (Zea mays). Seed Sci. Technol. 2007, 35, 200–212. [Google Scholar] [CrossRef]
- Yan, H.F.; Yu, X.D.; Jia, W.; Mao, P.S. Length of the lag period of germination predicts the vigour differences and field emergence potential in Italian ryegrass (Lolium multiflorum) seed lots. Seed Sci. Technol. 2017, 45, 238–242. [Google Scholar] [CrossRef]
- Cheshmi, M.; Khajeh-Hosseini, M. Effect of temperature on length of the lag period and its relationship with field performance of alfalfa (Medicago sativa) seeds. Seed Sci. Technol. 2018, 46, 317–326. [Google Scholar] [CrossRef]
- GB/T 2930.4; Rules of Seed Testing for Forage, Turfgrass and Other Herbaceous Plant—The Germination Test. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2017. Available online: https://main.spc.net.cn/bwonline/9a1bb00a4a3e07c8dd60ac169f359862.html (accessed on 1 November 2017).
- Tao, Q.B.; Lv, Y.Y.; Mo, Q.; Bai, M.J.; Han, Y.H.; Wang, Y.R. Impacts of priming on seed germination and seedling emergence of Cleistogenes songorica under drought stress. Seed Sci. Technol. 2018, 46, 239–258. [Google Scholar] [CrossRef]
- Hu, X.W.; Huang, X.H.; Wang, Y.R. Hormonal and temperature regulation of seed dormancy and germination in Leymus chinensis. Plant Growth Regul. 2012, 67, 199–207. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination—Aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Zeng, Z.K.; Guo, C.; Yan, X.F.; Song, J.J.; Wang, C.P.; Xu, X.T.; Hao, Y.F. QTL mapping and KASP marker development for seed vigor related traits in common wheat. Front. Plant Sci. 2022, 13, 994973. [Google Scholar] [CrossRef]
- Zhao, H.J.; Liu, Q.L.; Fu, H.W.; Hu, X.H.; Wu, D.X.; Shu, Q.Y. Effect of non-lethal low phytic acid mutations on grain yield and seed viability in rice. Field Crops Res. 2008, 108, 206–211. [Google Scholar] [CrossRef]
- Yang, X.J.; Baskin, C.C.; Baskin, J.M.; Liu, G.Z.; Huang, Z.Y. Seed mucilage improves seedling emergence of a sand desert shrub. PLoS ONE 2012, 7, e34597. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Yang, X.J.; Baskin, C.C.; Baskin, J.M.; Dong, M.; Huang, Z.Y. Effects of amount and frequency of precipitation and sand burial on seed germination, seedling emergence and survival of the dune grass Leymus secalinus in semiarid China. Plant Soil 2014, 374, 399–409. [Google Scholar] [CrossRef]
- Vandelook, F.; Assche, J.A.V. Temperature requirements for seed germination and seedling development determine timing of seedling emergence of three monocotyledonous temperature forest spring geophytes. Ann. Bot. 2008, 102, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment—A shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Mitchell, R.; Vogel, K.P.; Sarath, G. Managing and enhancing switchgrass as a bioenergy feedstock. Biofuels Bioprod. Biorefin. 2008, 2, 487–606. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat, and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.L.; Zhang, X.K.; Lv, Y.; Xu, J.S. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Zheng, M.M.; Tao, Y.; Hussain, S.; Jiang, Q.W.; Peng, S.B.; Huang, J.L.; Cui, K.H.; Nie, L.X. Seed priming in dry direct-seeded rice: Consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul. 2016, 78, 167–178. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Ghorbani, R.; Seel, W.; Leifert, C. Effect of environmental factors on germination and emergence of Amaranthus retroflexus. Weed Sci. 1999, 47, 505–510. [Google Scholar] [CrossRef]
- Hall, R.D.; Wiesner, L.E. Relationship between seed vigor tests and field performance of ‘Regar’ Meadow Bromegrass. Crop Sci. 1990, 30, 967–970. [Google Scholar] [CrossRef]
- Matthews, S.; Demir, I.; Celikkol, T.; Kenanoglu, B.B.; Mavi, K. Vigour tests for cabbage seeds using electrical conductivity and controlled deterioration to estimate relative emergence in transplant modules. Seed Sci. Technol. 2009, 37, 736–746. [Google Scholar] [CrossRef]
- Ilbi, H.; Kavak, S.; Eser, B. Cool germination test can be an alternative vigour test for maize. Seed Sci. Technol. 2009, 37, 516–519. [Google Scholar] [CrossRef]
- Matthews, S.; Noli, E.; Demir, I.; Khajeh-Hosseini, M.; Wagner, M.H. Evaluation of seed quality: From physiology to international standardization. Seed Sci. Res. 2012, 22, S69–S73. [Google Scholar] [CrossRef]
- Guan, Y.J.; Yin, M.Q.; Jia, X.W.; An, J.Y.; Wang, C.; Pan, R.H.; Song, W.J.; Hu, J. Single counts of radicle emergence can be used as a vigour test to predict seedling emergence potential of wheat. Seed Sci. Technol. 2018, 46, 349–357. [Google Scholar] [CrossRef]
- Ilbi, H.; Powell, A.A.; Alan, O. Single radicle emergence count for predicting vigour of marigold (Tagetes spp.) seed lots. Seed Sci. Technol. 2020, 48, 381–389. [Google Scholar] [CrossRef]
- Ermis, S.; Oktem, G.; Mavi, K.; Hay, F.R.; Demir, I. The radicle emergence test and storage longevity of cucurbit rootstock seed lots. Seed Sci. Technol. 2022, 50, 1–10. [Google Scholar] [CrossRef]
- Marin, M.; Laverack, G.; Powell, A.A.; Matthews, S. Potential of the electrical conductivity of seed soak water and early counts of radicle emergence to assess seed quality in some native species. Seed Sci. Technol. 2018, 46, 71–86. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Wang, Y.R.; Powell, A.A. Frequent individual counts of radicle emergence and mean just germination time predict seed vigour of Avena sativa and Elymus nutans. Seed Sci. Technol. 2016, 44, 189–198. [Google Scholar]
- Munyaneza, V.; Li, D.M.; Jia, P.; Hu, X.W. Various vigour test methods to rank seed lot quality and predict field emergence in two forage grasses. Seed Sci. Technol. 2022, 50, 345–356. [Google Scholar] [CrossRef]
- Cheah, K.S.; Osborne, D.J. DNA lesions occur with loss of viability in embryos of ageing rye seed. Nature 1978, 272, 593–599. [Google Scholar] [CrossRef]
- Osborne, D.J. Biochemical control systems operating in the early hours of germination. Can. J. Bot. 1983, 61, 3568–3577. [Google Scholar] [CrossRef]
- Powell, A.A.; Matthews, S. Seed ageing/repair hypothesis leads to new testing methods. Seed Technol. 2012, 34, 15–25. [Google Scholar]


| Seed Lot | Production Year | Storage Period (Years) | Seed Moisture Content (%) | TGW (g) | SG (%) | RE at 108 h (%) | MGT (Hours) | GI | SLL (cm) | VI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2017 | 4 | 8.4 | 3.62 | 91.5 cd | 41.0 f | 107.3 c | 0.43 i | 2.6 g | 1.11 hi |
| 2 | 2017 | 4 | 8.9 | 3.89 | 92.5 bcd | 67.0 d | 100.2 e | 0.46 gh | 3.8 ab | 1.77 d |
| 3 | 2018 | 3 | 8.4 | 3.50 | 93.0 abcd | 69.0 cd | 91.8 g | 0.53 e | 4.1 a | 2.20 ab |
| 4 | 2017 | 4 | 8.7 | 3.67 | 94.0 abc | 33.5 f | 114.4 b | 0.40 j | 3.5 bcd | 1.41 fg |
| 5 | 2016 | 5 | 9.0 | 3.56 | 94.5 abc | 56.0 e | 102.0 de | 0.48 g | 3.2 cde | 1.54 ef |
| 6 | 2019 | 2 | 8.5 | 3.58 | 95.5 ab | 91.5 a | 81.3 i | 0.61 b | 3.4 bcd | 2.08 bc |
| 7 | 2020 | 1 | 8.4 | 3.24 | 91.0 cd | 64.5 d | 103.3 d | 0.45 hi | 3.3 cd | 1.52 f |
| 8 | 2016 | 5 | 8.6 | 3.73 | 95.5 ab | 23.0 g | 122.0 a | 0.38 j | 2.6 g | 1.01 i |
| 9 | 2018 | 3 | 8.5 | 3.44 | 92.5 bcd | 65.0 d | 97.1 f | 0.50 f | 3.1 def | 1.56 ef |
| 10 | 2020 | 1 | 8.8 | 3.58 | 96.0 ab | 91.5 a | 75.8 j | 0.67 a | 3.5 bcd | 2.33 a |
| 11 | 2020 | 1 | 8.8 | 3.55 | 95.0 abc | 87.5 ab | 85.9 h | 0.57 c | 3.8 ab | 2.15 ab |
| 12 | 2019 | 2 | 9.2 | 3.81 | 93.0 bcd | 39.0 f | 109.3 c | 0.44 hi | 2.8 fg | 1.25 gh |
| 13 | 2016 | 5 | 8.4 | 3.67 | 89.5 d | 23.5 g | 123.2 a | 0.35 k | 2.9 efg | 1.01 i |
| 14 | 2018 | 3 | 8.6 | 3.35 | 94.5 abc | 74.5 d | 92.0 g | 0.54 de | 3.6 bc | 1.94 cd |
| 15 | 2020 | 1 | 8.7 | 3.48 | 96.5 a | 64.5 d | 96.8 f | 0.51 f | 3.4 bcd | 1.74 de |
| 16 | 2019 | 2 | 8.9 | 3.76 | 96.0 ab | 87.0 b | 88.4 h | 0.56 cd | 3.8 ab | 2.12 abc |
| Seed Lot | Control Condition | Drought Stress | Salinity Stress | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PEP (%) | PSDW (mg Plant−1) | PSVI | PEP (%) | PSDW (mg Plant−1) | PSVI | PEP (%) | PSDW (mg Plant−1) | PSVI | |
| 1 | 75.0 abcd | 23.5 fg | 1762.0 de | 44.2 bcde | 14.4 gh | 638.8 gh | 39.2 def | 17.8 cdef | 691.4 fg |
| 2 | 80.8 abc | 21.4 gh | 1720.1 def | 51.7 ab | 18.9 cde | 973.1 d | 47.5 bcd | 19.2 abcde | 909.1 cd |
| 3 | 79.2 abcd | 29.2 cd | 2295.2 b | 48.3 abc | 19.7 cd | 945.0 de | 50.8 abc | 20.8 abc | 1050.6 b |
| 4 | 70.8 bcd | 20.5 gh | 1415.1 gh | 40.0 cde | 15.4 fg | 611.1 ghi | 45.0 bcde | 16.7 efg | 747.8 efg |
| 5 | 79.2 abcd | 23.8 efg | 1862.3 cd | 45.0 bcde | 16.3 efg | 724.8 fg | 44.2 cdef | 15.9 fg | 695.6 fg |
| 6 | 78.3 abcd | 35.4 a | 2766.3 a | 51.7 ab | 24.3 a | 1242.4 ab | 53.3 ab | 20.1 abcd | 1065.1 b |
| 7 | 76.7 abcd | 31.5 bc | 2415.8 b | 48.3 abc | 18.4 cde | 883.5 de | 45.0 bcde | 19.5 abcde | 875.5 cd |
| 8 | 68.3 cd | 18.7 h | 1268.3 h | 36.7 de | 11.8 h | 425.8 j | 35.8 f | 14.6 g | 519.9 h |
| 9 | 75.8 abcd | 27.4 de | 2072.7 c | 47.5 abcd | 20.3 bcd | 962.2 d | 44.2 cdef | 18.5 bcdef | 812.9 def |
| 10 | 84.2 a | 33.9 ab | 2853.1 a | 56.7 a | 24.1 a | 1354.7 a | 58.3 a | 22.3 a | 1294.5 a |
| 11 | 78.3 abcd | 31.3 bc | 2434.4 b | 50.0 abc | 22.9 ab | 1139.7 bc | 56.7 a | 21.5 ab | 1208.8 a |
| 12 | 70.8 cd | 21.8 gh | 1543.8 efg | 42.5 bcde | 13.5 gh | 568.6 hi | 37.5 ef | 14.6 g | 546.7 h |
| 13 | 66.7 d | 22.5 gh | 1495.9 fgh | 34.2 e | 14.4 gh | 489.4 ij | 38.3 ef | 17.8 cdef | 679.9 g |
| 14 | 79.2 abcd | 29.9 cd | 2359.6 b | 49.2 abc | 20.8 bc | 1006.6 cd | 50.8 abc | 18.8 bcdef | 945.2 bc |
| 15 | 75.8 abcd | 26.8 def | 2022.2 c | 46.7 abcd | 17.5 def | 814.8 ef | 48.3 bc | 17.0 defg | 822.5 cde |
| 16 | 83.3 ab | 32.4 abc | 2682.8 a | 53.3 ab | 21.1 bc | 1117.8 bc | 51.7 abc | 18.4 bcdef | 948.9 bc |
| Means of 16 seed lots | 76.4 | 26.9 | 2060.6 | 46.6 | 18.4 | 868.6 | 46.7 | 18.3 | 863.4 |
| ANOVA | |||||||||
| SV | df | PEP | PSDW | PSVI | |||||
| SL | 15 | *** | *** | *** | |||||
| EC | 2 | *** | *** | *** | |||||
| SL × EC | 30 | ns | *** | *** | |||||
| FE | FSDW | FSVI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2023 | 2021 | 2022 | 2023 | 2021 | 2022 | 2023 | |
| SG | 0.499 * | 0.497 | 0.354 | 0.550 * | 0.554 * | 0.489 | 0.541 * | 0.572 * | 0.458 |
| MGT | −0.843 *** | −0.866 *** | −0.881 *** | −0.877 *** | −0.853 *** | −0.815 *** | −0.863 *** | −0.897 *** | −0.907 *** |
| VI | 0.779 *** | 0.855 *** | 0.878 *** | 0.808 *** | 0.776 *** | 0.780 *** | 0.799 *** | 0.853 *** | 0.883 *** |
| RE at 64 h | 0.672 ** | 0.716 ** | 0.693 ** | 0.700 ** | 0.652 ** | 0.622 * | 0.709 ** | 0.730 ** | 0.721 ** |
| RE at 68 h | 0.698 ** | 0.745 ** | 0.730 ** | 0.720 ** | 0.668 ** | 0.623 * | 0.729 ** | 0.751 ** | 0.738 ** |
| RE at 72 h | 0.737 ** | 0.763 ** | 0.751 ** | 0.766 ** | 0.724 ** | 0.655 ** | 0.768 ** | 0.788 *** | 0.760 ** |
| RE at 76 h | 0.730 ** | 0.772 *** | 0.788 *** | 0.755 ** | 0.728 ** | 0.693 ** | 0.755 ** | 0.793 *** | 0.802 *** |
| RE at 80 h | 0.717 ** | 0.771 *** | 0.779 *** | 0.748 ** | 0.732 ** | 0.714 ** | 0.743 ** | 0.793 *** | 0.811 *** |
| RE at 84 h | 0.729 ** | 0.780 *** | 0.789 *** | 0.754 ** | 0.735 ** | 0.722 ** | 0.753 ** | 0.800 *** | 0.823 *** |
| RE at 88 h | 0.773 *** | 0.827 *** | 0.832 *** | 0.797 *** | 0.762 ** | 0.753 ** | 0.796 *** | 0.836 *** | 0.859 *** |
| RE at 92 h | 0.804 *** | 0.849 *** | 0.860 *** | 0.826 *** | 0.801 *** | 0.791 *** | 0.826 *** | 0.871 *** | 0.897 *** |
| RE at 96 h | 0.849 *** | 0.874 *** | 0.883 *** | 0.868 *** | 0.843 *** | 0.837 *** | 0.867 *** | 0.905 *** | 0.929 *** |
| RE at 100 h | 0.874 *** | 0.887 *** | 0.893 *** | 0.899 *** | 0.872 *** | 0.859 *** | 0.893 *** | 0.925 *** | 0.941 *** |
| RE at 104 h | 0.884 *** | 0.896 *** | 0.908 *** | 0.913 *** | 0.878 *** | 0.865 *** | 0.902 *** | 0.929 *** | 0.948 *** |
| RE at 108 h | 0.898 *** | 0.907 *** | 0.916 *** | 0.924 *** | 0.891 *** | 0.867 *** | 0.912 *** | 0.939 *** | 0.950 *** |
| RE at 112 h | 0.886 *** | 0.904 *** | 0.909 *** | 0.910 *** | 0.886 *** | 0.861 *** | 0.897 *** | 0.932 *** | 0.942 *** |
| RE at 116 h | 0.874 *** | 0.896 *** | 0.899 *** | 0.893 *** | 0.875 *** | 0.857 *** | 0.879 *** | 0.919 *** | 0.933 *** |
| RE at 120 h | 0.866 *** | 0.875 *** | 0.884 *** | 0.898 *** | 0.894 *** | 0.856 *** | 0.875 *** | 0.915 *** | 0.919 *** |
| RE at 124 h | 0.839 *** | 0.843 *** | 0.859 *** | 0.880 *** | 0.891 *** | 0.838 *** | 0.850 *** | 0.895 *** | 0.893 *** |
| RE at 128 h | 0.820 *** | 0.830 *** | 0.847 *** | 0.853 *** | 0.867 *** | 0.811 *** | 0.829 *** | 0.877 *** | 0.857 *** |
| RE at 132 h | 0.824 *** | 0.837 *** | 0.838 *** | 0.862 *** | 0.872 *** | 0.806 *** | 0.833 *** | 0.880 *** | 0.865 *** |
| RE at 136 h | 0.810 *** | 0.822 *** | 0.824 *** | 0.859 *** | 0.874 *** | 0.804 *** | 0.821 *** | 0.871 *** | 0.854 *** |
| RE at 140 h | 0.818 *** | 0.832 *** | 0.817 *** | 0.868 *** | 0.883 *** | 0.808 *** | 0.829 *** | 0.880 *** | 0.852 *** |
| RE at 144 h | 0.827 *** | 0.842 *** | 0.801 *** | 0.871 *** | 0.892 *** | 0.843 *** | 0.836 *** | 0.890 *** | 0.863 *** |
| RE at 148 h | 0.839 *** | 0.858 *** | 0.809 *** | 0.880 *** | 0.886 *** | 0.845 *** | 0.846 *** | 0.895 *** | 0.869 *** |
| RE at 152 h | 0.826 *** | 0.854 *** | 0.783 *** | 0.864 *** | 0.870 *** | 0.858 *** | 0.835 *** | 0.889 *** | 0.865 *** |
| RE at 156 h | 0.792 *** | 0.828 *** | 0.744 ** | 0.831 *** | 0.841 *** | 0.840 *** | 0.803 *** | 0.862 *** | 0.836 *** |
| RE at 160 h | 0.805 *** | 0.850 *** | 0.755 ** | 0.831 *** | 0.834 *** | 0.851 *** | 0.816 *** | 0.877 *** | 0.855 *** |
| RE at 164 h | 0.802 *** | 0.845 *** | 0.748 ** | 0.828 *** | 0.834 *** | 0.848 *** | 0.813 *** | 0.874 *** | 0.851 *** |
| RE at 168 h | 0.802 *** | 0.845 *** | 0.748 ** | 0.828 *** | 0.834 *** | 0.848 *** | 0.813 *** | 0.874 *** | 0.851 *** |
| PEP | PSDW | PSVI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Drought | Salinity | Control | Drought | Salinity | Control | Drought | Salinity | |
| SG | 0.437 | 0.441 | 0.539 * | 0.341 | 0.396 | 0.054 | 0.394 | 0.440 | 0.356 |
| MGT | −0.881 *** | −0.936 *** | −0.920 *** | −0.874 *** | −0.938 *** | −0.761 ** | −0.923 *** | −0.965 *** | −0.887 *** |
| VI | 0.860 *** | 0.881 *** | 0.965 *** | 0.810 *** | 0.916 *** | 0.779 *** | 0.865 *** | 0.927 *** | 0.919 *** |
| RE at 64 h | 0.689 ** | 0.746 ** | 0.824 *** | 0.755 ** | 0.808 *** | 0.673 ** | 0.796 *** | 0.834 *** | 0.813 *** |
| RE at 68 h | 0.729 ** | 0.781 *** | 0.843 *** | 0.748 ** | 0.824 *** | 0.666 ** | 0.798 *** | 0.852 *** | 0.818 *** |
| RE at 72 h | 0.724 ** | 0.788 *** | 0.863 *** | 0.761 ** | 0.824 *** | 0.653 ** | 0.805 *** | 0.850 *** | 0.819 *** |
| RE at 76 h | 0.736 ** | 0.810 *** | 0.874 *** | 0.794 *** | 0.862 *** | 0.674 ** | 0.832 *** | 0.880 *** | 0.832 *** |
| RE at 80 h | 0.717 ** | 0.790 *** | 0.856 *** | 0.802 *** | 0.855 *** | 0.649 ** | 0.834 *** | 0.869 *** | 0.813 *** |
| RE at 84 h | 0.716 ** | 0.788 *** | 0.858 *** | 0.811 *** | 0.869 *** | 0.666 ** | 0.840 *** | 0.878 *** | 0.822 *** |
| RE at 88 h | 0.780 *** | 0.844 *** | 0.889 *** | 0.837 *** | 0.908 *** | 0.703 ** | 0.876 *** | 0.922 *** | 0.852 *** |
| RE at 92 h | 0.787 *** | 0.860 *** | 0.905 *** | 0.858 *** | 0.933 *** | 0.728 ** | 0.891 *** | 0.942 *** | 0.871 *** |
| RE at 96 h | 0.824 *** | 0.898 *** | 0.920 *** | 0.899 *** | 0.955 *** | 0.762 ** | 0.932 *** | 0.967 *** | 0.891 *** |
| RE at 100 h | 0.864 *** | 0.932 *** | 0.927 *** | 0.900 *** | 0.958 *** | 0.769 *** | 0.941 *** | 0.978 *** | 0.894 *** |
| RE at 104 h | 0.885 *** | 0.945 *** | 0.930 *** | 0.894 *** | 0.963 *** | 0.786 *** | 0.939 *** | 0.983 *** | 0.901 *** |
| RE at 108 h | 0.893 *** | 0.947 *** | 0.921 *** | 0.891 *** | 0.957 *** | 0.768 ** | 0.937 *** | 0.977 *** | 0.884 *** |
| RE at 112 h | 0.903 *** | 0.956 *** | 0.904 *** | 0.886 *** | 0.939 *** | 0.763 ** | 0.835 *** | 0.965 *** | 0.871 *** |
| RE at 116 h | 0.899 *** | 0.955 *** | 0.877 *** | 0.887 *** | 0.920 *** | 0.756 ** | 0.935 *** | 0.951 *** | 0.851 *** |
| RE at 120 h | 0.917 *** | 0.951 *** | 0.879 *** | 0.870 *** | 0.904 *** | 0.723 ** | 0.923 *** | 0.935 *** | 0.833 *** |
| RE at 124 h | 0.917 *** | 0.942 *** | 0.857 *** | 0.848 *** | 0.875 *** | 0.694 ** | 0.903 *** | 0.910 *** | 0.805 *** |
| RE at 128 h | 0.909 *** | 0.919 *** | 0.854 *** | 0.847 *** | 0.868 *** | 0.702 ** | 0.900 *** | 0.896 *** | 0.804 *** |
| RE at 132 h | 0.913 *** | 0.925 *** | 0.838 *** | 0.814 *** | 0.846 *** | 0.663 ** | 0.874 *** | 0.884 *** | 0.778 *** |
| RE at 136 h | 0.917 *** | 0.923 *** | 0.829 *** | 0.790 *** | 0.831 *** | 0.630 ** | 0.855 *** | 0.872 *** | 0.759 ** |
| RE at 140 h | 0.887 *** | 0.899 *** | 0.805 *** | 0.771 *** | 0.806 *** | 0.562 * | 0.834 *** | 0.848 *** | 0.716 ** |
| RE at 144 h | 0.865 *** | 0.882 *** | 0.774 *** | 0.793 *** | 0.790 *** | 0.537 * | 0.849 *** | 0.834 *** | 0.691 ** |
| RE at 148 h | 0.857 *** | 0.887 *** | 0.772 *** | 0.780 *** | 0.793 *** | 0.521 * | 0.838 *** | 0.840 *** | 0.685 ** |
| RE at 152 h | 0.811 *** | 0.843 *** | 0.761 ** | 0.768 ** | 0.771 *** | 0.469 | 0.821 *** | 0.814 *** | 0.660 ** |
| RE at 156 h | 0.752 ** | 0.797 ** | 0.727 ** | 0.728 ** | 0.727 ** | 0.400 | 0.777 *** | 0.771 *** | 0.615 * |
| RE at 160 h | 0.738 ** | 0.780 *** | 0.746 ** | 0.763 ** | 0.751 ** | 0.423 | 0.804 *** | 0.784 *** | 0.638 ** |
| RE at 164 h | 0.744 ** | 0.785 *** | 0.743 ** | 0.766 ** | 0.748 ** | 0.426 | 0.808 *** | 0.784 *** | 0.637 ** |
| RE at 168 h | 0.744 ** | 0.785 *** | 0.743 ** | 0.766 ** | 0.748 ** | 0.426 | 0.808 *** | 0.784 *** | 0.637 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Q.; Xing, J.; Meng, F.; Zhang, Y.; Liu, X.; Guo, S.; Shan, Y.; Zhong, S.; Sun, J.; Zhao, Y. Siberian Wildrye (Elymus sibiricus) Seed Vigor Estimation for the Prediction of Emergence Performance under Diverse Environmental Conditions. Agronomy 2024, 14, 173. https://doi.org/10.3390/agronomy14010173
Tao Q, Xing J, Meng F, Zhang Y, Liu X, Guo S, Shan Y, Zhong S, Sun J, Zhao Y. Siberian Wildrye (Elymus sibiricus) Seed Vigor Estimation for the Prediction of Emergence Performance under Diverse Environmental Conditions. Agronomy. 2024; 14(1):173. https://doi.org/10.3390/agronomy14010173
Chicago/Turabian StyleTao, Qibo, Jiayi Xing, Fansheng Meng, Yaqi Zhang, Xinyu Liu, Shuo Guo, Ye Shan, Shangzhi Zhong, Juan Sun, and Yanhua Zhao. 2024. "Siberian Wildrye (Elymus sibiricus) Seed Vigor Estimation for the Prediction of Emergence Performance under Diverse Environmental Conditions" Agronomy 14, no. 1: 173. https://doi.org/10.3390/agronomy14010173
APA StyleTao, Q., Xing, J., Meng, F., Zhang, Y., Liu, X., Guo, S., Shan, Y., Zhong, S., Sun, J., & Zhao, Y. (2024). Siberian Wildrye (Elymus sibiricus) Seed Vigor Estimation for the Prediction of Emergence Performance under Diverse Environmental Conditions. Agronomy, 14(1), 173. https://doi.org/10.3390/agronomy14010173





