Weed Response to ALS-Inhibitor Herbicide (Sulfosulfuron + Metsulfuron Methyl) under Increased Temperature and Carbon Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Treatments
2.2. Herbicide Efficiency
2.3. Morphological Variable Measurement
2.4. Enzyme Assay
2.5. Statistical Analysis
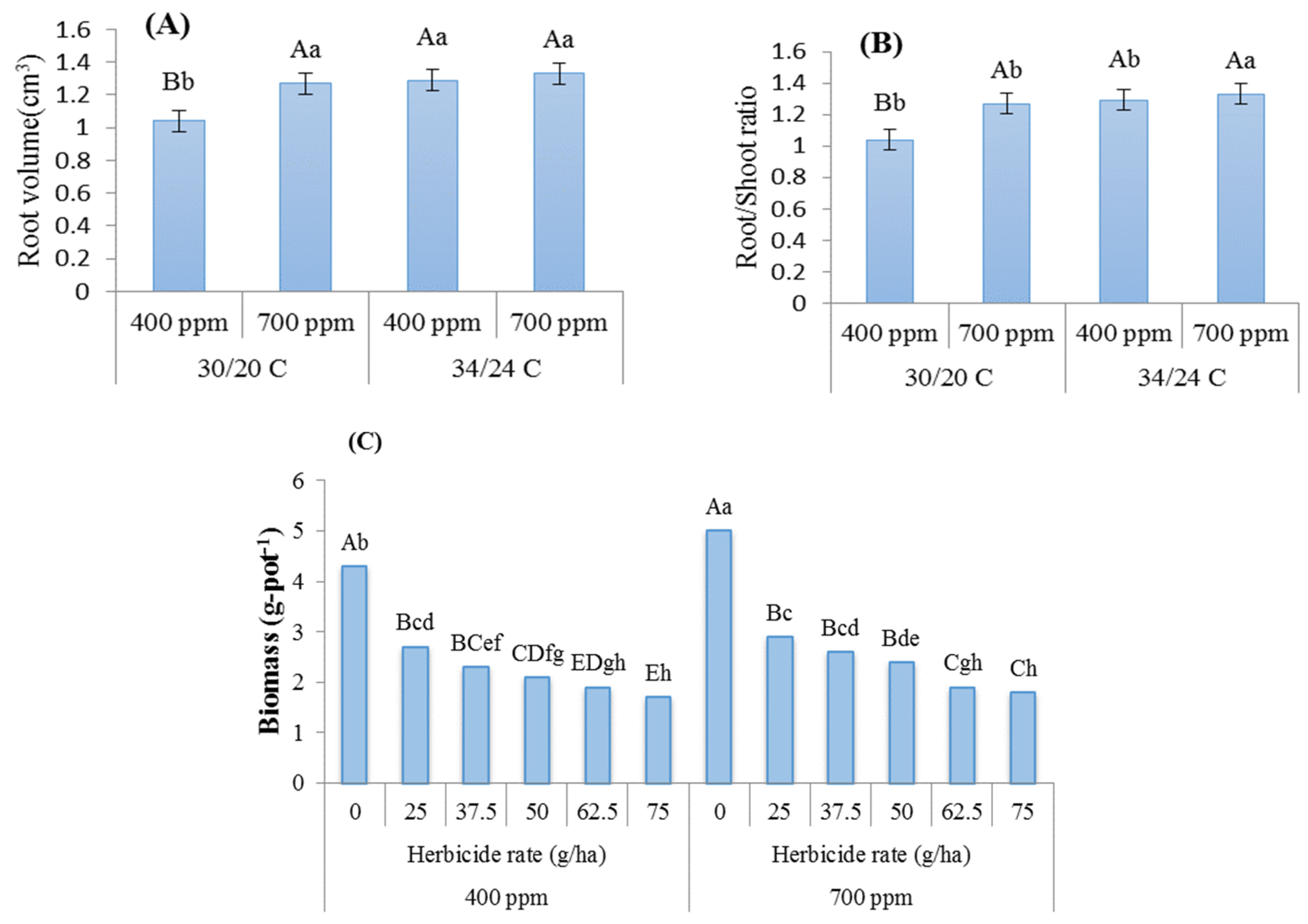
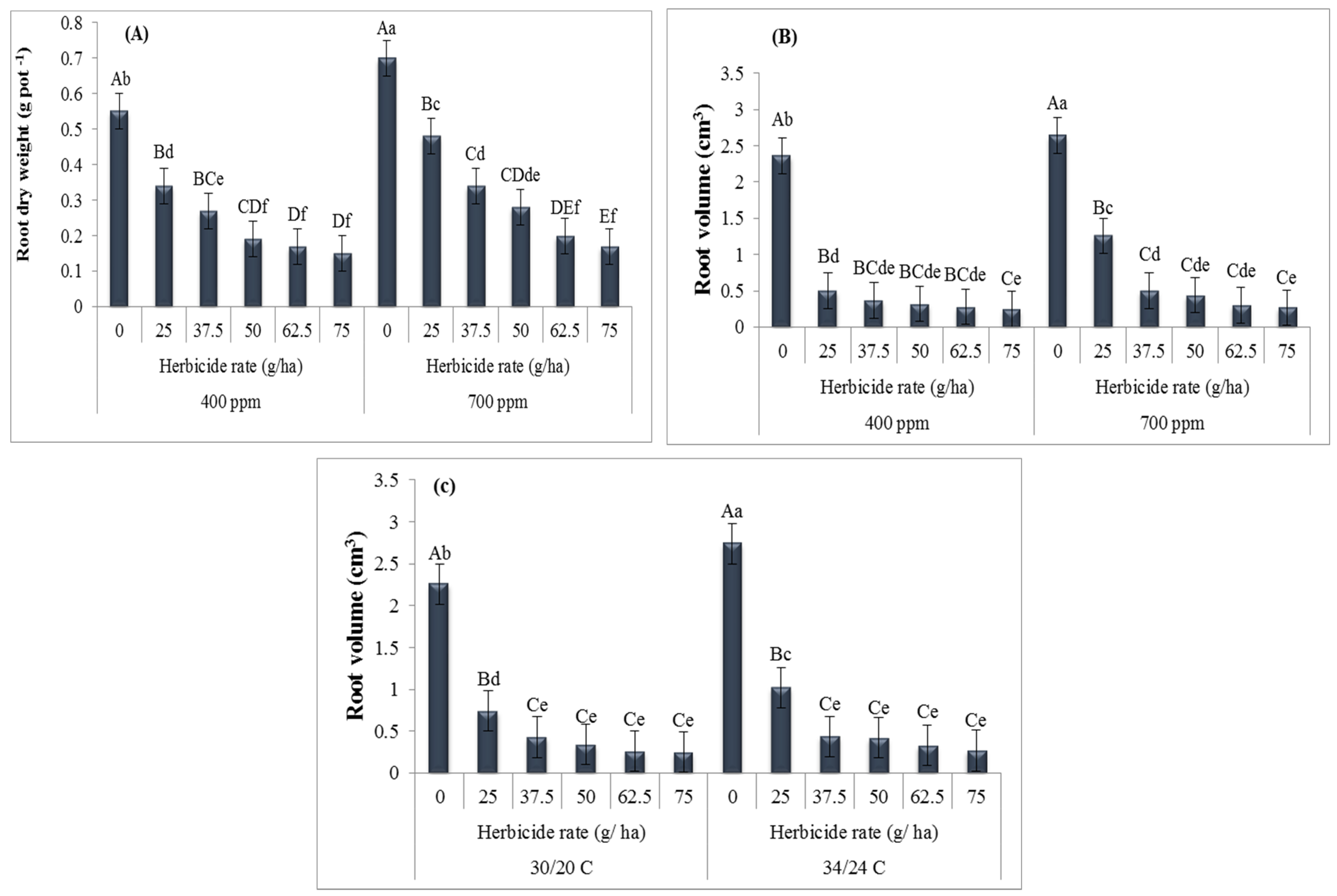
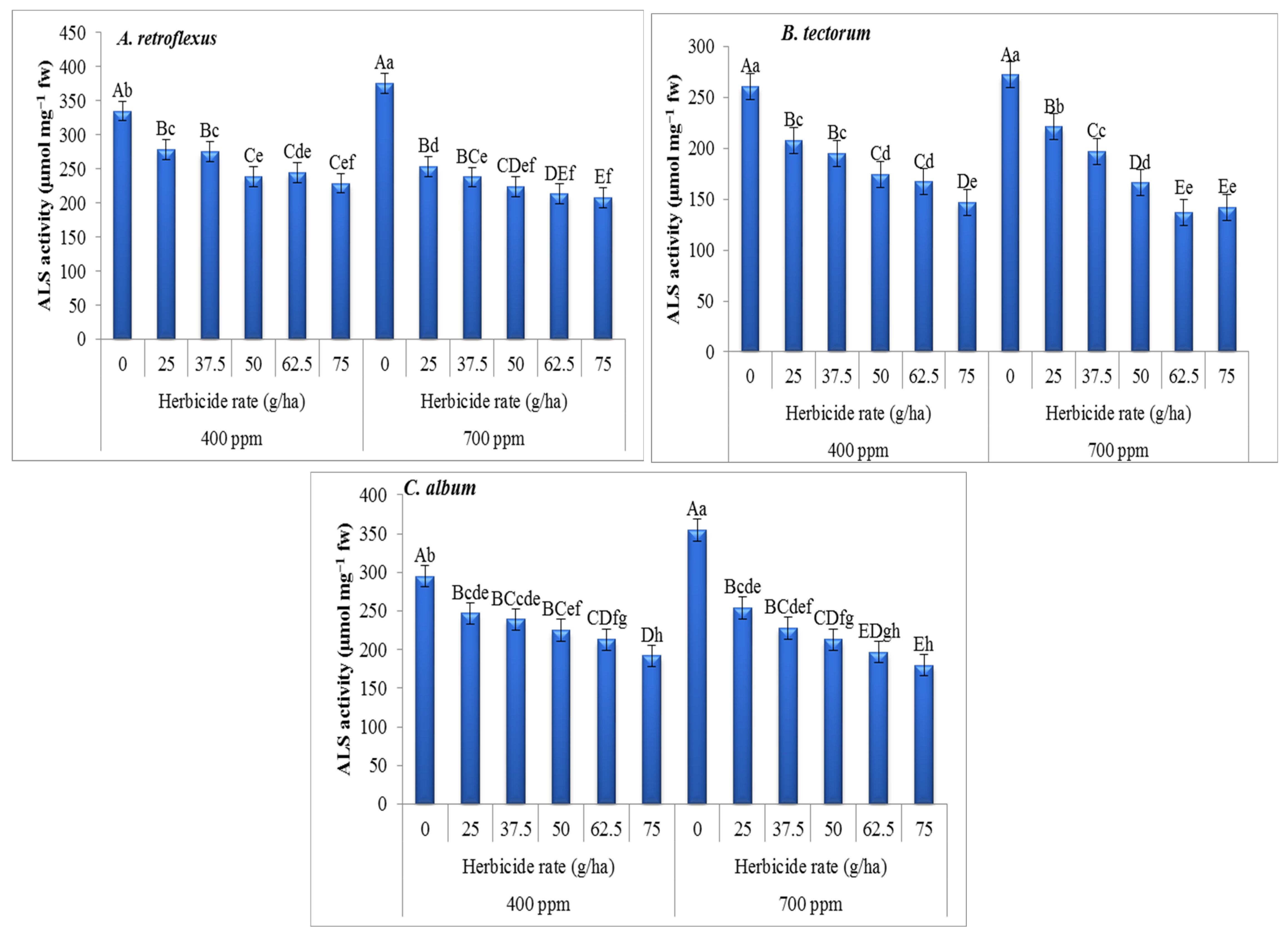
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chehabeddine, M.; Tvaronavičienė, M. Securing regional development. Insights Into Reg. Dev. 2020, 2, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Andujar, J.L. Modelling the effects of climate change and climatic variability on crops at the site scale: Effects on cereal weeds. In Climate Change and Agriculture in Europe: Assessment of Impacts and Adaptations; Harrison, P.A., Butterfield, R.E., Downing, T.E., Eds.; Environmental Change Unit, University of Oxford: Oxford, UK, 1995. [Google Scholar]
- Ziska, L.H. The impact of elevated CO2 on yield loss from a C3 and C4 weed in field-grown soybean. Glob. Change Biol. 2000, 6, 899–905. [Google Scholar] [CrossRef]
- Anwar, M.P.; Islam, A.M.; Yeasmin, S.; Rashid, M.H.; Juraimi, A.S.; Ahmed, S.; Shrestha, A. Weeds and Their Responses to Management Efforts in A Changing Climate. Agronomy 2021, 11, 1921. [Google Scholar] [CrossRef]
- Field, C.B.; Barros, V.R.; Dokken, D.J.; Mach, K.J.; Mastrandrea, M.D.; Bilir, T.E.; Chatterjee, M.; Ebi, K.L.; Estrada, Y.O.; Genova, R.C.; et al. (Eds.) Summary for policymakers. In Climate Change 2014: Impact, Adaptation, and Vulnerability, Part A: Global and Sectoral Aspects, Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 1–32. [Google Scholar]
- Benedetti, L.; Rangani, G.; Ebeling Viana, V.; Carvalho-Moore, P.; Merotto, A., Jr.; Rabaioli Camargo, E.; Antonio de Avila, L.; Roma-Burgos, N. Rapid reduction of herbicide susceptibility in junglerice by recurrent selection with sublethal rate of herbicides and heat stress. Agronomy 2020, 10, 1761. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M. Climate change’s impacts on weeds and herbicide efficacy: A review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2846–2853. [Google Scholar] [CrossRef]
- Kumar, V.; Kumari, A.; Price, A.J.; Bana, R.S.; Singh, V.; Bamboriya, S.D. Impact of Futuristic Climate Variables on Weed Biology and Herbicidal Efficacy: A Review. Agronomy 2023, 13, 559. [Google Scholar] [CrossRef]
- Ziska, L.H.; Bunce, J.A. Plant responses to rising carbon dioxide. In Plant Growth and Climate Change; Morison, J.I.L., Morecroft, M.D., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 17–47. [Google Scholar]
- Bernards, M.L. AMS-What Is It Doing in My Tank? CropWatch. 2007. Available online: https://cropwatch.unl.edu/ams-what-it-doing-my-tank (accessed on 20 December 2022).
- Bhowmik, P. Introduction to the symposium: History of sulfonylurea herbicide use in turfgrass environments. Weed Technol. 2012, 26, 575–578. [Google Scholar] [CrossRef]
- Shaner, D.L. Physiological effects of the imidazolinone herbicides. In The Imidazolinone Herbicides; Shaner, D.L., O’Connor, S.L., Eds.; Lewis: Ann Arbor, MI, USA, 1991; pp. 129–138. [Google Scholar]
- European Weed Research Council—EWRC. Report of the 3th and 4th meetings of EWRC—Committee of methods in weed research. Weed Res. 1964, 4, 88. [CrossRef]
- Miflin, B.J. Cooperative feedback control of barley acetohydroxy acid synthetase by leucine, isoleucine and valine. Arch. Biochem. Biophys. 1971, 146, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, J.Y.; Ziska, L.H. Plant responses to elevated CO2. In Encyclopedia of Life Sciences (eLS); Wiley International: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Ziska, L.H. Elevated carbon dioxide alters chemical management of Canada thistle in no-till soybean. Field Crops Res. 2010, 119, 299–303. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Yadav, S.; Mishra, A. Introgression of C4 Pathway Gene(s) in C3 Plants to Improve Photosynthetic Carbon Assimilation for Crop Improvement: A Biotechnological Approach. In Photosynthesis, Productivity and Environmental Stress; Wiley: Hoboken, NJ, USA, 2019; Chapter 13. [Google Scholar] [CrossRef]
- Walker, B.J.; VanLoocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Blätke, M.A.; Bräutigam, A. Evolution of C4 photosynthesis predicted by constraint-based modelling. eLife 2019, 8, e49305. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.D. C4 photosynthesis: A unique blend of modified biochemistry, anatomy, and ultrastructure. Biochemical. Biophys. Acta 1987, 895, 81–106. [Google Scholar] [CrossRef]
- Ainsworth, N. Integration of Herbicides with Arthropod Biocontrol Agents for Weed Control. Biocontrol Sci. Technol. 2003, 13, 547–570. [Google Scholar] [CrossRef]
- Clements, D.R.; Jones, V.L. Rapid Evolution of Invasive Weeds Under Climate Change: Present Evidence and Future Research Needs. Front. Agron. 2021, 3, 664304. [Google Scholar] [CrossRef]
- Clements, D.R.; DiTommaso, A. Climate Change and the Persistence of Weeds. In Persistence Strategies of Weeds; Wiley: Hoboken, NJ, USA, 2022; pp. 219–243. [Google Scholar]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob. Change Biol. 2008, 14, 565–575. [Google Scholar] [CrossRef]
- Palmieri, V.E.; Alvarez, C.E.; Permingeat, H.R.; Perotti, V.E. A122S, A205V, D376E, W574L and S653N substitutions in acetolactate synthase (ALS) from Amaranthus palmeri show different functional impacts on herbicide resistance. Pest Manag. Sci. 2022, 78, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Gómez de Barreda, D.; Pardo, G.; Osca, J.M.; Catala-Forner, M.; Consola, S.; Garnica, I.; López-Martínez, N.; Palmerín, J.A.; Osuna, M.D. An Overview of Rice Cultivation in Spain and the Management of Herbicide-Resistant Weeds. Agronomy 2021, 11, 1095. [Google Scholar] [CrossRef]
- Yang, C.; Wang, H.; Duan, Y.; Bei, F.; Jia, S.; Wang, J.; Wang, H.; Liu, W. Enhanced Herbicide Metabolism and Target-Site Mutations Confer Multiple Resistance to Fomesafen and Nicosulfuron in Amaranthus retroflexus L. Biology 2023, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Ziska, L.H.; Gealy, D.R.; Tomecek, M.B.; Jackson, A.K.; Black, H.L. Recent and projected increases in atmospheric CO2 concentration can enhance gene flow between wild and genetically altered rice (Oryza sativa). PLoS ONE 2012, 7, e37522. [Google Scholar] [CrossRef]
- Siddiqui, A.O.; Yazlik, A.; Jabran, K. Weed Management and Climate Change. In Building Climate Resilience in Agriculture; Springer: Cham, Switzerland, 2022; pp. 211–223. [Google Scholar]
- Upadhyaya, M.K.; Clements, D.R.; Shrestha, A. Persistence Strategies of Weeds: Synopsis and the Future. In Persistence Strategies of Weeds; Wiley: Hoboken, NJ, USA, 2022; pp. 325–351. [Google Scholar]
- Johnson, B.C.; Young, B.G. Influence of temperature and relative humidity on the foliar activity of mesotrione. Weed Sci. 2002, 50, 157161. [Google Scholar] [CrossRef]
- Ziska, L.H.; Goins, E.W. Elevated atmospheric carbon dioxide and weed populations in glyphosate treated soybean. Crop Sci. 2006, 46, 1354–1359. [Google Scholar] [CrossRef]
- Kells, J.J.; Meggitt, W.F.; Penner, D. Absorption, translocation, and activity of fluazifop-butyl as influenced by plant growth stage and environment. Weed Sci. 1984, 32, 143–149. [Google Scholar] [CrossRef]

| Category Number | Herbicide Effectiveness on Weeds | Weed Control (%) |
|---|---|---|
| 1 | None | 0–29.9 |
| 2 | Very bad | 30–54.9 |
| 3 | Bad | 55–69.9 |
| 4 | Weak | 70–81.9 |
| 5 | Moderate | 82–89.9 |
| 6 | Good to acceptable | 90–94.9 |
| 7 | Very good | 95–97.9 |
| 8 | Excellent | 98–99.9 |
| 9 | Total plant death | 100 |
| Mean Square | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | df | Height (cm) | SdW (g pot−1) | RdW (g pot−1) | Biomass (g pot−1) | R/S | RV (cm3) | ASL Activity | HE (%) | |
| A. retroflexus | Temperature (T) | 1 | 394.07 ** | 0.49 ns | 83.40 ns | 0.02 ns | 13.70 ** | 1.66 ** | 5536 ** | 1.5 ns |
| CO2 | 1 | 195.51 ** | 0.48ns | 65.24 ns | 1.45 ** | 0.24 ns | 0.002 ns | 5236 ** | 13.5 ** | |
| Herbicide rates (H) | 5 | 355.04 ** | 8.62 ** | 75.24 ns | 15.98 ** | 0.95 ns | 2.67 ** | 40,269 ** | 142.1 ** | |
| T × CO2 | 1 | 21.57 ns | 0.0001 ns | 72.48 ns | 0.003 ns | 0.03 ns | 0.13 ns | 4 ns | 0.37 ns | |
| T × H | 5 | 2.7 ns | 0.038 ns | 74.00 ns | 0.022 ns | 0.20 ns | 0.01 ns | 214 ns | 0.12 ns | |
| CO2 × H | 5 | 0.82 ns | 0.047 ns | 74.3 ns | 0.07 ns | 0.35 ns | 0.002 ns | 3220 ** | 1.92 ** | |
| T × CO2 × H | 5 | 1.63 ns | 0.041 ns | 73.59 ns | 0.043 ns | 0.12 ns | 0.001 ns | 101 ns | 0.3 ns | |
| Error | 72 | 9.33 | 0.175 | 74.28 | 0.18 | 0.58 | 0.083 | 407 | 0.51 | |
| B. tectorum | Temperature (T) | 1 | 20.18 ns | 0.069 ns | 0.43 ** | 0.15 ns | 2.25 ** | 0.53 ** | 1953 ** | 2.04 * |
| CO2 | 1 | 0.35 ns | 0.53 ** | 0.43 ** | 1.92 ** | 0.11 ns | 0.44 ** | 244 ns | 3.37 ** | |
| Herbicide rates (H) | 5 | 557.38 ** | 5.25 ** | 3.82 ** | 17.99 ** | 0.48 ns | 4.37 ** | 33,342 ** | 202 ** | |
| T × CO2 | 1 | 6.42 ns | 0.13 ns | 0.083 ns | 0.005 ns | 1.17 * | 0.21 * | 667 ns | 0.04 ns | |
| T × H | 5 | 9.77 ns | 0.0068 ns | 0.019 ns | 0.04 ns | 0.05 ns | 0.04 ns | 163 ns | 0.64 ns | |
| CO2 × H | 5 | 13 ns | 0.079 ns | 0.042 ns | 0.21 ** | 0.04 ns | 0.08 ns | 998 ** | 2.57 ** | |
| T × CO2 × H | 5 | 4.54 ns | 0.046 ns | 0.003 ns | 0.03 ns | 0.12 ns | 0.01 ns | 292 ns | 0.24 ns | |
| Error | 72 | 6.52 | 0.067 | 0.048 | 0.07 | 0.26 | 0.05 | 266 | 0.38 | |
| C. album | Temperature (T) | 1 | 14.4 * | 0.49 ns | 0.15 * | 0.001 ns | 156 ns | 6600 ns | 1365 ns | 0.042 ns |
| CO2 | 1 | 61.3 ** | 0.48 ns | 0.42 ** | 0.63 ** | 146.37 ns | 6384 ns | 155 ns | 9.375 ** | |
| Herbicide rates (H) | 5 | 320.1 ** | 8.62 ** | 3.47 ** | 22.86 ** | 122.58 ns | 6810 ns | 38,040 ** | 194.4 ** | |
| T × CO2 | 1 | 1.2 ns | 0.0001 ns | 0.0008 ns | 0.04 ns | 116.99 ns | 6828 ns | 45 ns | 1.5 ns | |
| T × H | 5 | 3.52 ns | 0.038 ns | 0.0138 ns | 0.002 ns | 121.42 ns | 6725 ns | 207 ns | 0.34 ns | |
| CO2 × H | 5 | 4.92 ns | 0.047 ns | 0.0334 ns | 0.07 ns | 119.07 ns | 6688 ns | 3386 ** | 2.07 ** | |
| T × CO2 × H | 5 | 1.19 ns | 0.041 ns | 0.0011 ns | 0.005 ns | 119.47 ns | 6709 ns | 591 ns | 0.25 ns | |
| Error | 72 | 2.38 | 0.14 | 0.0274 | 0.03 | 119.01 | 6736 | 349 | 0.51 | |
| E. crus-galli | Temperature (T) | 1 | 38.76 ** | 0.012 ns | 0.026 * | 0.002 ns | 1.13 ** | 0.57 ** | 661 ns | 3.37 ** |
| CO2 | 1 | 34.41 ** | 0.136 ** | 0.161 ** | 0.59 ** | 0.29 ** | 1.16 ** | 2185 ns | 15.04 ** | |
| Herbicide rates (H) | 5 | 281.71 ** | 0.686 ** | 0.494 ** | 2.34 ** | 0.11 ** | 12.02 ** | 54,925 ** | 124.2 ** | |
| T × CO2 | 1 | 0.019 ns | 0.0054 ns | 0.0030 ns | 0.06 ns | 0.02 ns | 0.032 ns | 1218 ns | 0 ns | |
| T × H | 5 | 1.105 ns | 0.0017 ns | 0.0028 ns | 0.004 ns | 0.023 ns | 0.137 * | 1181 ns | 0.87 * | |
| CO2 × H | 5 | 1.961 ns | 0.0058 ns | 0.011 ** | 0.030 ns | 0.015 ns | 0.308 ** | 1010 ns | 1.99 ** | |
| T × CO2 × H | 5 | 0.414 ns | 0.0014 ns | 0.0031 ns | 0.008 ns | 0.017 ns | 0.091 ns | 702 ns | 0.8 ns | |
| Error | 72 | 1.411 | 0.0058 | 0.0041 | 0.015 | 0.030 | 0.042 | 635 | 0.35 | |
| Species | Height (cm) | SdW (g pot−1) | RdW (g pot−1) | Biomass (g pot−1) | R/S | RV (cm3) | ASL Activity | HE (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Temperature °C | |||||||||
| A. retroflexus | 30/20 | 22.07 ± 2.14 a | ns | ns | ns | 2.70 ± 0.28 a | 1.04 ± 0.01 b | 252.20 ± 33.5 b | ns |
| 34/24 | 18.02 ± 1.45 b | ns | ns | ns | 1.94 ± 0.36 b | 1.30 ± 0.02 a | 267.39 ± 14.54 a | ns | |
| B. tectorum | 30/20 | ns | ns | 0.97 ± 0.07 b | ns | ns | ns | 186.58 ± 17.7 b | 1.16 ± 0.24 b |
| 34/24 | ns | ns | 1.10 ± 0.01 a | ns | ns | ns | 195.60 ± 31 a | 1.31 ± 0.11 a | |
| C. album | 30/20 | 15.27 ± 2.19 a | ns | 0.50 ± 0.08 b | ns | ns | ns | ns | ns |
| 34/24 | 14.49 ± 2.01 b | ns | 0.58 ± 0.05 a | ns | ns | ns | ns | ns | |
| E. crus-galli | 30/20 | 17.06 ± 0.84 a | ns | 0.30 ± 0.02 b | ns | 1.48 ± 0.29 a | ns | ns | ns |
| 34/24 | 15.79 ± 1.28 b | ns | 0.34 ± 0.02 a | ns | 1.26 ± 0.2 b | ns | ns | ns | |
| CO2 (ppm) | |||||||||
| A. retroflexus | 400 | 18.62 ± 2.02 b | ns | ns | 1.89 ± 0.12 b | ns | ns | ns | ns |
| 700 | 21.47 ± 1.6 a | ns | ns | 2.14 ± 0.08 a | ns | ns | ns | ns | |
| B. tectorum | 400 | ns | 1.46 ± 0.18 b | 0.97 ± 0.02 b | ns | ns | ns | ns | ns |
| 700 | ns | 1.61 ± 0.32 a | 1.10 ± 0.07 a | ns | ns | ns | ns | ns | |
| C. album | 400 | 14.08 ± 1.33 b | ns | 0.47 ± 0.05 b | 1.09 ± 0.13 b | ns | ns | ns | ns |
| 700 | 15.68 ± 0.61 a | ns | 0.60 ± 0.03 a | 1.25 ± 0.33 a | ns | ns | ns | ns | |
| E. crus-galli | 400 | 15.82 ± 1.11 b | 0.39 ± 0.04 b | ns | 0.67 ± 0.03 b | 1.43 ± 0.17 a | ns | ns | ns |
| 700 | 17.02 ± 2.82 a | 0.46 ± 0.04 a | ns | 0.83 ± 0.05 a | 1.32 ± 0.12 b | ns | ns | ns | |
| Herbicide rates (g ha−1) | |||||||||
| A. retroflexus | 25 | 19.75 ± 2.57 c | 1.40 ± 0.11 b | ns | 1.96 ± 0.17 b | ns | 1.10 ± 0.08 c | ns | ns |
| 37.5 | 20.96 ± 0.79 bc | 1.41 ± 0.08 b | ns | 2.04 ± 0.11 b | ns | 1.31 ± 0.03 b | ns | ns | |
| 50 | 15.73 ± 1.43 d | 0.72 ± 0.02 c | ns | 1.11 ± 0.20 c | ns | 0.79 ± 0.07 d | ns | ns | |
| 62.5 | 22.98 ± 1.55 b | 1.60 ± 0.06 b | ns | 2.35 ± 0.23 b | ns | 1.43 ± 0.1 b | ns | ns | |
| 75 | 14.01 ± 1.09 d | 0.61 ± 0.01 c | ns | 0.93 ± 0.04 c | ns | 0.64 ± 0.02 d | ns | ns | |
| 0 | 26.85 ± 2.2 a | 2.66 ± 0.12 a | ns | 3.71 ± 0.16 a | ns | 1.73 ± 0.03 a | ns | ns | |
| B. tectorum | 25 | 19.89 ± 3.2 c | 1.36 ± 0.08 c | 0.87 ± 0.02 cd | ns | ns | 1.11 ± 0.08 c | ns | ns |
| 37.5 | 21.44 ± 0.29 bc | 1.48 ± 0.27 bc | 0.92 ± 0.04 c | ns | ns | 1.16 ± 0.04 bc | ns | ns | |
| 50 | 17.18 ± 1.21 d | 1.10 ± 0.26 d | 0.75 ± 0.06 de | ns | ns | 0.89 ± 0.03 d | ns | ns | |
| 62.5 | 22.63 ± 2.87 b | 1.6 ± 0.07 b | 1.08 ± 0.02 b | ns | ns | 1.31 ± 0.03 b | ns | ns | |
| 75 | 15.41 ± 1 d | 1.06 ± 0.08 d | 0.60 ± 0.03 e | ns | ns | 0.72 ± 0.07 | ns | ns | |
| 0 | 32.20 ± 0.9 a | 2.63 ± 0.04 a | 1.97 ± 0.04 a | ns | ns | 2.21 ± 0.22 a | ns | ns | |
| C. album | 25 | 14.51 ± 1.66 c | ns | 0.32 ± 0.01 cd | 0.59 ± 0.01 c | ns | ns | ns | ns |
| 37.5 | 15.12 ± 0.79 bc | ns | 0.43 ± 0.02 bc | 0.90 ± 0.03 b | ns | ns | ns | ns | |
| 50 | 11.64 ± 1.22 d | ns | 0.28 ± 0.03 d | 0.51 ± 0.03 c | ns | ns | ns | ns | |
| 62.5 | 16.15 ± 0.85 b | ns | 0.52 ± 0.06 b | 1.07 ± 0.01 b | ns | ns | ns | ns | |
| 75 | 9.390 ± 0.79 e | ns | 0.21 ± 0.02 d | 0.41 ± 0.03 c | ns | ns | ns | ns | |
| 0 | 22.46 ± 1.88 a | ns | 1.46 ± 0.09 a | 3.56 ± 0.35 a | ns | ns | ns | ns | |
| E. crus-galli | 25 | 15.06 ± 1.05 d | 0.34 ± 0.02 d | ns | 0.59 ± 0.04 d | 1.45 ± 0.18a | ns | 195.8 ± 13.79 c | ns |
| 37.5 | 16.37 ± 1.33 c | 0.42 ± 0.01 c | ns | 0.72 ± 0.03 c | 1.38 ± 0.07ab | ns | 247.8 ± 24.45 b | ns | |
| 50 | 13.21 ± 0.69 e | 0.27 ± 0.04 e | ns | 0.46 ± 0.05 e | 1.46 ± 0.11a | ns | 185.8 ± 9.8 d | ns | |
| 62.5 | 17.76 ± 2.17 b | 0.52 ± 0.03 b | ns | 0.94 ± 0.06 b | 1.28 ± 0.24b | ns | 264.7 ± 30 b | ns | |
| 75 | 12.22 ± 1.4 f | 0.22 ± 0.09 e | ns | 0.38 ± 0.07 e | 1.39 ± 0.05ab | ns | 174.5 ± 15.5 d | ns | |
| 0 | 23.93 ± 1.96 a | 0.79 ± 0.03 a | ns | 1.42 ± 0.02 a | 1.26 ± 0.15b | ns | 327.4 ± 28.8 a | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazikhanlou Sani, Y.; Yousefi, A.R.; Jamshidi, K.; Shekari, F.; Gonzalez-Andujar, J.L.; Korres, N.E. Weed Response to ALS-Inhibitor Herbicide (Sulfosulfuron + Metsulfuron Methyl) under Increased Temperature and Carbon Dioxide. Agronomy 2023, 13, 2084. https://doi.org/10.3390/agronomy13082084
Ghazikhanlou Sani Y, Yousefi AR, Jamshidi K, Shekari F, Gonzalez-Andujar JL, Korres NE. Weed Response to ALS-Inhibitor Herbicide (Sulfosulfuron + Metsulfuron Methyl) under Increased Temperature and Carbon Dioxide. Agronomy. 2023; 13(8):2084. https://doi.org/10.3390/agronomy13082084
Chicago/Turabian StyleGhazikhanlou Sani, Yousef, Ali Reza Yousefi, Khalil Jamshidi, Farid Shekari, Jose L. Gonzalez-Andujar, and Nicholas E. Korres. 2023. "Weed Response to ALS-Inhibitor Herbicide (Sulfosulfuron + Metsulfuron Methyl) under Increased Temperature and Carbon Dioxide" Agronomy 13, no. 8: 2084. https://doi.org/10.3390/agronomy13082084
APA StyleGhazikhanlou Sani, Y., Yousefi, A. R., Jamshidi, K., Shekari, F., Gonzalez-Andujar, J. L., & Korres, N. E. (2023). Weed Response to ALS-Inhibitor Herbicide (Sulfosulfuron + Metsulfuron Methyl) under Increased Temperature and Carbon Dioxide. Agronomy, 13(8), 2084. https://doi.org/10.3390/agronomy13082084









