Archaeobotanical Insights into Kañawa (Chenopodium pallidicaule Aellen) Domestication: A Rustic Seed Crop of the Andean Altiplano
Abstract
1. Introduction
2. Background
2.1. Kañawa Cultivation and Culinary Characteristics
2.2. Kañawa Domestication: Genetic and Archaeological Antecedents
2.3. Chronology and Climate
3. Materials and Methods
4. Results
4.1. The Appearance of Kañawa
4.2. Climate and Kañawa
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazile, D.; Bertero, H.D.; Nieto, C. State of the Art Report on Quinoa around the World in 2013; FAO and CIRAD: Rome, Italy, 2015. [Google Scholar]
- Simmonds, N.W. The Grain Chenopods of the Tropical American Highlands. Econ. Bot. 1965, 19, 223–235. [Google Scholar] [CrossRef]
- Hunziker, A. Las Especies Alimenticias de Amarantus y Chenopodium Cultivadas por Los Indios de América. Rev. Argent. Agron. 1943, 10, 146–154. [Google Scholar]
- National Research Council (U.S.) and Advisory Committee on Technology Innovation. Lost Crops of the INCAS: Little-Known Plants of the Andes with Promise for Worldwide Cultivation; National Academy Press: Washington, DC, USA, 1989. [Google Scholar]
- Rodriguez, J.P.; Jacobsen, S.-E.; Andreasen, C.; Sørensen, M. Cañahua (Chenopodium pallidicaule): A Promising New Crop for Arid Areas. In Emerging Research in Alternative Crops; Hirich, A., Choukr-Allah, R., Ragab, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 221–243. [Google Scholar] [CrossRef]
- Vargas, C.C. Nota Etnobotánica Sobre La Cañihua (Chenopodium pallidicaule). Rev. Argent. De Agron. 1938, 5, 224–230. [Google Scholar]
- Sauer, J.D. The Grain Amaranths and Their Relatives: A Revised Taxanomic and Geographic Survey. Ann. Mo. Bot. Gard. 1967, 54, 103–137. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The global expansion of quinoa: Trends and limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef] [PubMed]
- Planella, M.T.; López, M.L.; Bruno, M.C. La domesticación y distribución prehistórica. In Estado del Arte de la Quinua en el Mundo en 2013; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO: Santiago, Chile; CIRAD: Montpellier, France, 2014; pp. 33–48. [Google Scholar]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.-E. Nutritional Value and Use of the Andean Crops Quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Quezada, R.S. Distribución de la Diversidad Genética y Etnobotanica de Cañahua (Chenopodium pallidicaule Aellen) en las Comunidades del Altiplano Norte. Licenciatura Thesis, Faculty of Agronomy, Universidad Mayor de San Andrés, La Paz, Bolivia, 2012. Available online: http://repositorio.umsa.bo/xmlui/handle/123456789/8002 (accessed on 18 May 2023).
- Condori, R.F. Evaluacion Preliminar Agronomica y Morfologica del Germoplasma de Cañahua (Chenopodium pallidicaule Aellen) en la Estacion Experimental Belen. Licenciatura Thesis, Faculty of Agronomy, Universidad Mayor de San Andrés, La Paz, Bolivia, 2006. Available online: http://repositorio.umsa.bo/xmlui/handle/123456789/11606 (accessed on 18 May 2023).
- Rojas, W.; Soto, J.L.; Pinto, M.; Jäger, M.; Padulosi, S.; Bioversity International. Granos Andinos: Avances, Logros y Experiencias Desarrolladas en Quinua, Cañahua y Amaranto en Bolivia. 2010. Available online: https://cgspace.cgiar.org/handle/10568/104701 (accessed on 10 February 2022).
- Gade, D.W. Ethnobotany of Cañihua (Chenopodium pallidicaule), Rustic Seed Crop of the Altiplano. Econ. Bot. 1970, 24, 55–61. [Google Scholar] [CrossRef]
- Bruno, M.C.; Pinto, M.; Rojas, W. Identifying Domesticated and Wild Kañawa (Chenopodium pallidicaule) in the Archeobotanical Record of the Lake Titicaca Basin of the Andes. Econ. Bot. 2018, 72, 137–149. [Google Scholar] [CrossRef]
- Bruno, M.C. A morphological approach to documenting the domestication of Chenopodium in the Andes. In Documenting Domestication: New Genetic and Archaeological Paradigms; Zeder, M., Bradley, D., Emshwiller, E., Smith, B.D., Eds.; University of California Press: Berkeley, CA, USA, 2006; pp. 32–45. [Google Scholar]
- IPGRI, PROINPA and IFAD. Descriptores Para Cañahua (Chenopodium pallidicaule Aellen); Fundación PROINPA: La Paz, Bolivia, 2005. [Google Scholar]
- Porcel, M.P.; Rojas, W. Variabilidad genética de la colección del germoplasma de cañahua (Chenopodium pallidicaule Aellen) de Bolivia. Rev. Investig. Innov. Agropecu. Recur. Nat. 2016, 3, 125–133. [Google Scholar]
- Goland, C. Cultivating Diversity: Field Scattering as Agricultural Risk Management in Cuyo Cuyo, Department of Puno, Peru, Volume 1 & 2; University of North Carolina: Chapel Hill, NC, USA, 1991. [Google Scholar]
- Fonesca, M.; Mayer, E. Comunidad y Produccion en la Agricultura Andina; FOMCIENIAS: Lima, Peru, 1988. [Google Scholar]
- Orlove, B.S.; Godoy, R. Sectoral Fallowing Systems in the Central Andes. J. Ethnobiol. 1986, 6, 169–204. [Google Scholar]
- Rodriguez, J.P.; Jacobsen, S.-E.; Sørensen, M.; Andreasen, C. Germination Responses of Cañahua (Chenopodium pallidicaule Aellen) to Temperature and Sowing Depth: A Crop Growing Under Extreme Conditions. J. Agron. Crop Sci. 2016, 202, 542–553. [Google Scholar] [CrossRef]
- Morales, M.M.R. Evaluación de las Pérdidas de Grano y Grado de Impurezas en Cuatro Métodos de Cosecha de Cañahua (Chenopodium pallidicaule Aellen) en la Comunidad de Quipaquipani, Viacha. Licenciatura Thesis, Faculty of Agronomy, Universidad Mayor de San Andrés, La Paz, Bolivia, 2014. Available online: http://repositorio.umsa.bo/xmlui/handle/123456789/4270 (accessed on 18 May 2023).
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.-M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.; et al. The genome of Chenopodium quinoa. Nature 2017, 542, 307. [Google Scholar] [CrossRef] [PubMed]
- Mangelson, H.; Jarvis, D.E.; Mollinedo, P.; Rollano-Penaloza, O.M.; Palma-Encinas, V.D.; Gomez-Pando, L.R.; Jellen, E.N.; Maughan, P.J. The genome of Chenopodium pallidicaule: An emerging Andean super grain. Appl. Plant Sci. 2019, 7, e11300. [Google Scholar] [CrossRef]
- Ruas, P.M.; Bonifacio, A.; Ruas, C.F.; Fairbanks, D.J.; Andersen, W.R. Genetic Relationship among 19 Accessions of Six Species of Chenopodium L., by Random Amplified Polymorphic DNA Fragments (RAPD). Euphytica 1999, 105, 25–32. [Google Scholar] [CrossRef]
- Vargas, A.; Elzinga, D.B.; Rojas-Beltran, J.A.; Bonifacio, A.; Geary, B.; Stevens, M.R.; Jellen, E.N.; Maughan, P.J. Development and use of microsatellite markers for genetic diversity analysis of cañahua (Chenopodium pallidicaule Aellen. Genet. Resour. Crop Evol. 2011, 58, 727–739. [Google Scholar] [CrossRef]
- Browman, D.L. Chenopod Cultivation, Lacustrine Resources, and Fuel Use at Chiripa, Bolivia. In New World Paleoethnobotany: Collected Papers in Honor of Leonard W. Blake; Voigt, E.E., Pearsall, D.M., Eds.; Missouri Archaeological Society: Springfield, MO, USA, 1989; pp. 137–172. [Google Scholar]
- Erickson, C.L. Chiripa Ethnobotanical Report: Flotation Recovered Archaeological Remains from an Early Settled Village on the Altiplano of Bolivia. Bachelor’s Thesis, Washington University: St. Louis, MO, USA, 1976. [Google Scholar]
- Pearsall, D.M. Ethnobotanical report: Plant utilization at a hunting base camp. In Prehistoric Hunters of the High Andes; Rick, J., Ed.; Academic Press: New York, NY, USA, 1980; pp. 191–231. [Google Scholar]
- Pearsall, D.M. Adaptation of prehistoric hunter-gatherers in the high Andes: The changing role of plant resources. In Foraging and Farming; Harris, D., Hillman, G., Eds.; Unwin Hyman: London, UK, 1989; pp. 318–332. [Google Scholar]
- Fritz, G.J.; Smith, B.D. Old collections and new technology: Documenting the domestication of Chenopodium in eastern North America. Midcont. J. Archaeol. 1988, 13, 3–27. [Google Scholar]
- Fritz, G.J.; Bruno, M.C.; Langlie, B.S.; Smith, B.D.; Kistler, L. Cultigen Chenopods in the Americas: A Hemispherical Perspective. In Social Perspectives on Ancient Lives from Paleoethnobotanical Data; Sayre, M.P., Bruno, M.C., Eds.; Springer: Cham, Switzerland, 2017; pp. 55–75. [Google Scholar]
- Bruno, M.C.; Capriles, J.M.; Hastorf, C.A.; Fritz, S.C.; Weide, D.M.; Domic, A.I.; Baker, P.A. The Rise and Fall of Wiñaymarka: Rethinking Cultural and Environmental Interactions in the Southern Basin of Lake Titicaca. Hum. Ecol. 2021, 49, 131–145. [Google Scholar] [CrossRef]
- Hastorf, C.A. The Formative Period in the Titicaca Basin. In The Handbook of South American Archaeology; Silverman, H., Isbell, W.H., Eds.; Springer Science + Business Media, LLC: New York, NY, USA, 2008; pp. 545–561. [Google Scholar]
- Janusek, J.W. Vessels, Time, and Society: Toward a Ceramic Chronology in the Tiwanaku Heartland. In Tiwanaku and its Hinterland: Archaeological and Paleoecological Investigations of an Andean Civilization; Kolata, A.L., Ed.; Smithsonian Institution Press: Washington, DC, USA, 2003; pp. 30–89. [Google Scholar]
- Marsh, E.J.; Roddick, A.P.; Bruno, M.C.; Smith, S.C.; Janusek, J.W.; Hastorf, C.A. Temporal Inflection Points in Decorated Pottery: A Bayesian Refinement of the Late Formative Chronology in the Southern Lake Titicaca Basin, Bolivia. Lat. Am. Antiq. 2019, 30, 798–817. [Google Scholar] [CrossRef]
- Abbott, M.B.; Binford, M.; Brenner, M.; Kelts, K. A 3500 14C yr High-Resolution Record of Water-Level Changes in Lake Titicaca, Bolivia-Peru. Quat. Res. 1997, 47, 169–180. [Google Scholar] [CrossRef]
- Baker, P.A.; Fritz, S.C.; Garland, J.; Ekdahl, E. Holocene hydrologic variation at Lake Titicaca, Bolivia/Peru, and its relationship to North Atlantic climate variation. J. Quat. Sci. Publ. Quat. Res. Assoc. 2005, 20, 655–662. [Google Scholar] [CrossRef]
- Guédron, S.; Delaere, C.; Fritz, S.C.; Tolu, J.; Sabatier, P.; Devel, A.L.; Heredia, S.; Vérin, C.; Alves, E.Q.; Baker, P.A. Holocene variations in Lake Titicaca water level and their implications for sociopolitical developments in the central Andes. Proc. Natl. Acad. Sci. USA 2023, 120, e2215882120. [Google Scholar] [CrossRef]
- Weide, D.M.; Fritz, S.C.; Hastorf, C.A.; Bruno, M.C.; Baker, P.A.; Guédron, S.; Salenbien, W. A~ 6000 yr diatom record of mid-to late Holocene fluctuations in the level of Lago Wiñaymarca, Lake Titicaca (Peru/Bolivia). Quat. Res. 2017, 88, 179–192. [Google Scholar] [CrossRef]
- Aldenderfer, M.S.; Blanco, L.F. Reflexiones para avanzar en los estudios del período arcaico en los Andes centro-sur. Chungará Rev. Antropol. Chil. 2011, 43, 531–550. [Google Scholar] [CrossRef][Green Version]
- Bandy, M.S. Early Village Society in the Formative Period in the Southern Lake Titicaca Basin. In Andean Archaeology III: North and South; Isbell, W., Silverman, H., Eds.; Springer Science + Business Media, LLC: New York, NY, USA, 2006; pp. 210–236. [Google Scholar]
- Bruno, M.C.; Whitehead, W.T. Chenopodium cultivation and Formative period agriculture at Chiripa, Bolivia. Lat. Am. Antiq. 2003, 14, 339–355. [Google Scholar] [CrossRef]
- Bruno, M.C. Beyond raised fields: Exploring farming practices and processes of agricultural change in the ancient Lake Titicaca Basin of the Andes. Am. Anthropol. 2014, 116, 130–145. [Google Scholar] [CrossRef]
- Whitehead, W.T. Redefining Plant Use at the Formative Site of Chiripa in the Southern Titicaca Basin. In Andean Archaeology III: North and South; Isbell, W., Silverman, H., Eds.; Springer Science + Business Media, LLC: New York, NY, USA, 2006; pp. 258–278. [Google Scholar]
- Hastorf, C.A.; Bandy, M.S. Multi-Community Polity Formation in the Lake Titicaca Basin, Bolivia; Proposal to the National Science Foundation: Washington, DC, USA, 2002. [Google Scholar]
- Janusek, J.W. Tiwanaku and Its Precursors: Recent Research and Emerging Perspectives. J. Archaeol. Res. 2004, 12, 121–183. [Google Scholar] [CrossRef]
- Janusek, J.W. Ancient Tiwanaku; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2008. [Google Scholar]
- Kolata, A.L. The Tiwanaku: Portrait of an Andean Civilization; Blackwell: Oxford, UK, 1993. [Google Scholar]
- Janusek, J.W.; Kolata, A.L. Pre-Hispanic Rural History in the Katari Valley. In Tiwanaku and its Hinterland: Archaeological and Paleoecological Investigations of an Andean Civilization; Smithsonian Institution Press: Washington, DC, USA, 2003; pp. 129–174. [Google Scholar]
- Stanish, C. Prehispanic Agricultural Strategies of Intensification in the Titicaca Basin of Peru and Bolivia. In Agricultural Strategies; Cotsen Institute of Archaeology, University of California: Los Angeles, CA, USA, 2006; pp. 364–397. [Google Scholar]
- Wright, M.F.; Hastorf, C.A.; Lennstrom, H.A. Pre-Hispanic Agriculture and Plant Use at Tiwanaku: Social and Political Implications. In Tiwanaku and its Hinterland: Archaeological and Paleoecological Investigations of an Andean Civilization; Kolata, A.L., Ed.; Smithsonian Institution Press: Washington, DC, USA, 2003; pp. 384–403. [Google Scholar]
- Bandy, M.S.; Hastorf, C.A. (Eds.) Kala Uyuni: An Early Political Center in the Southern Lake Titicaca Basin; Contributions of the Archaeological Research Facility, No. 64; Regents of the University of California: Berkeley, CA, USA, 2007. [Google Scholar]
- Bruno, M.C. Waranq Waranqa: Ethnobotanial Perspectives on Agricultural Intensification in the Lake Titicaca Basin (Taraco Peninsula, Bolivia). Ph.D. Dissertation, Washington University: St. Louis, MO, USA, 2008. [Google Scholar]
- Hastorf, C.A. Early Settlement at Chiripa, Bolivia: Research of the Taraco Archaeological Project; Archaeological Research Facility Publication No. 57; University of California, Berkeley Archaeological Research Facility: Berkeley, CA, USA, 1999. [Google Scholar]
- Hastorf, C.A.; Bandy, M.; Whitehead, W.; Steadman, L.; Moore, K.; Paz Soria, J.L.; Roddick, A.; Bruno, M.; Fernandez, C.; Killackey, K.; et al. Proyecto Arqueológico Taraco Informe de las Excavaciones de la Temporada del 2004 en los Sitios de Kumi Kipa, Sonaji y Chiripa; Unidad Nacional de Arqueología de Bolivia: La Paz, Bolivia, 2005. [Google Scholar]
- Couture, N.C.; Blom, D.; Bruno, M.; Rodas, D.; Arratia, E.; Augustine, J.; Paye, S.; Ramos, M.; Vallières, C. Proyecto Arqueológico Jach’a Marka: Diversidad Urbana en Tiwanaku: Arqueolgía Funeraíia y Residencial en Mollo Kontu; Unidad Nacional de Arqueología de Bolivia: La Paz, Bolivia, 2007. [Google Scholar]
- Couture, N.C.; Blom, D.; Bruno, M. Proyecto Arqueológico Jach’a Marka: Informe de Investigaciones Realizadas en 2007; Unidad Nacional de Arquología de Bolivia: La Paz, Bolivia, 2008. [Google Scholar]
- Couture, N.C.; Blom, D.; Bruno, M. Proyecto Arqueologico Jacha Marka: Informe de Investigaciones Realizadas en 2008; Unidad Nacional de Arquología de Bolivia: La Paz, Bolivia, 2010. [Google Scholar]
- Mattox, C.W. Materializing Value: A Comparative Analysis of Status and Distinction in Urban Tiwanaku, Bolivia. Master’s Thesis, McGill University, Montreal, QC, Canada, 2011. [Google Scholar]
- Hastorf, C.A. A Manual for Building a Mechanized Flotation Machine, Modified from the SMAP Machine First Described by Patty Jo Watson in 1976; University of California Berkeley The McCown Archaeobotanical Laboratory: Berkeley, CA, USA, 1999. [Google Scholar]
- Popper, V. Selecting Quantitative Measurements in Paleoethnobotany. In Current Paleoethnobotany: Analytical Methods and Cultural Interpretations of Archaeological Plant Remains; Popper, C.A.H.V., Ed.; University of Chicago: Chicago, IL, USA, 1988; pp. 1–16. [Google Scholar]
- Bruno, M.C.; Hastorf, C.A. Gifts from the camelids: Archaeobotanical insights into camelid pastoralism through the study of dung. In The Archaeology of Andean Pastoralism; Capriles, J.M., Tripcevich, N., Eds.; University of New Mexico Press: Albuquerque, NM, USA, 2016; pp. 55–65. [Google Scholar]
- Cohen, A.; Roddick, A. Excavations in the AC (Achachi Coa Kkollu) Sector. In Taraco Archaeological Project: Report on 2003 Excavations at Kala Uyuni; Unidad Nacional de Arqueología de Bolivia: La Paz, Bolivia, 2004; pp. 38–59. [Google Scholar]
- Jacobsen, S.-E. The Situation for Quinoa and Its Production in Southern Bolivia: From Economic Success to Environmental Disaster. J. Agron. Crop Sci. 2011, 197, 390–399. [Google Scholar] [CrossRef]
- Kerssen, T.M. Food sovereignty and the quinoa boom: Challenges to sustainable re-peasantisation in the southern Altiplano of Bolivia. Third World Q. 2015, 36, 489–507. [Google Scholar] [CrossRef]
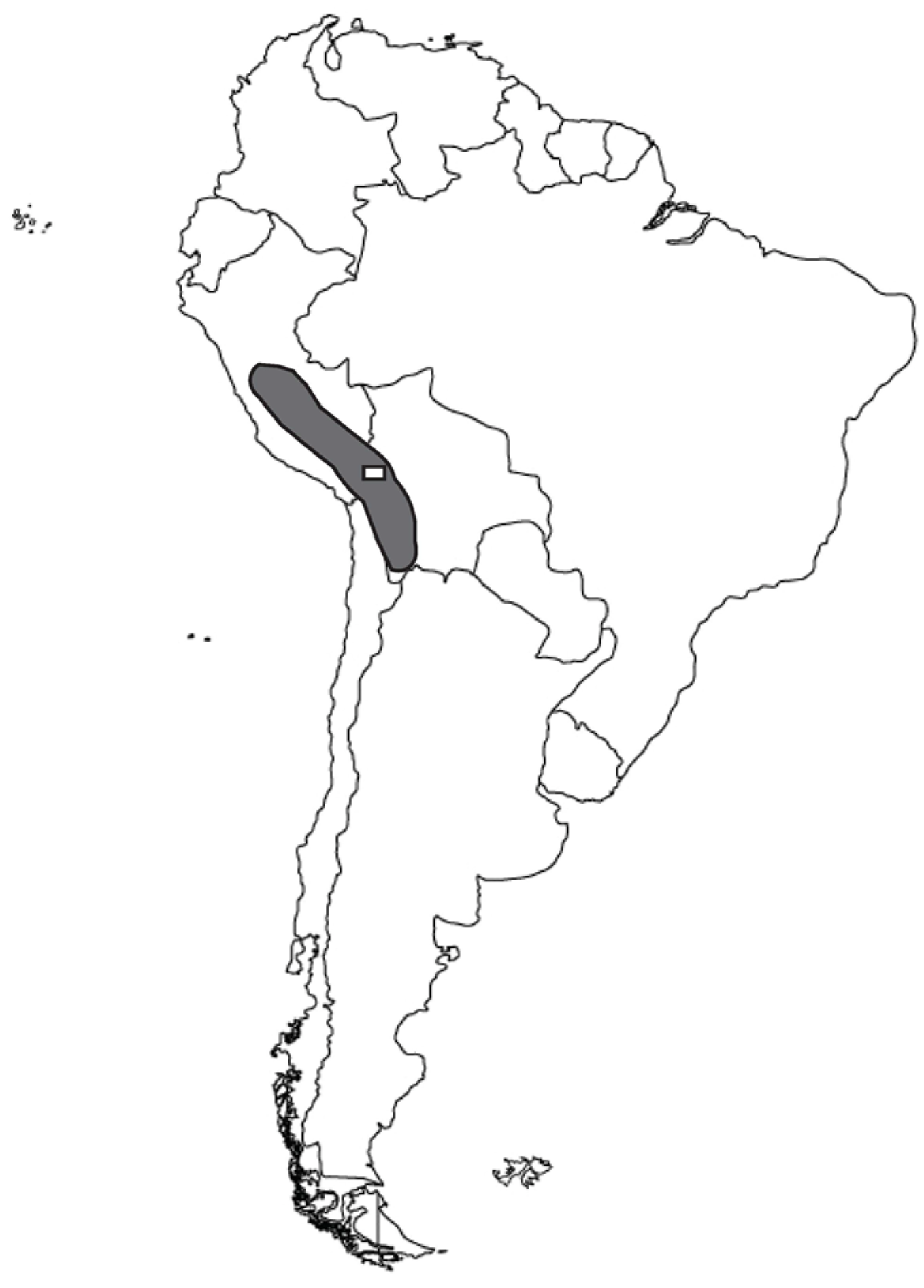

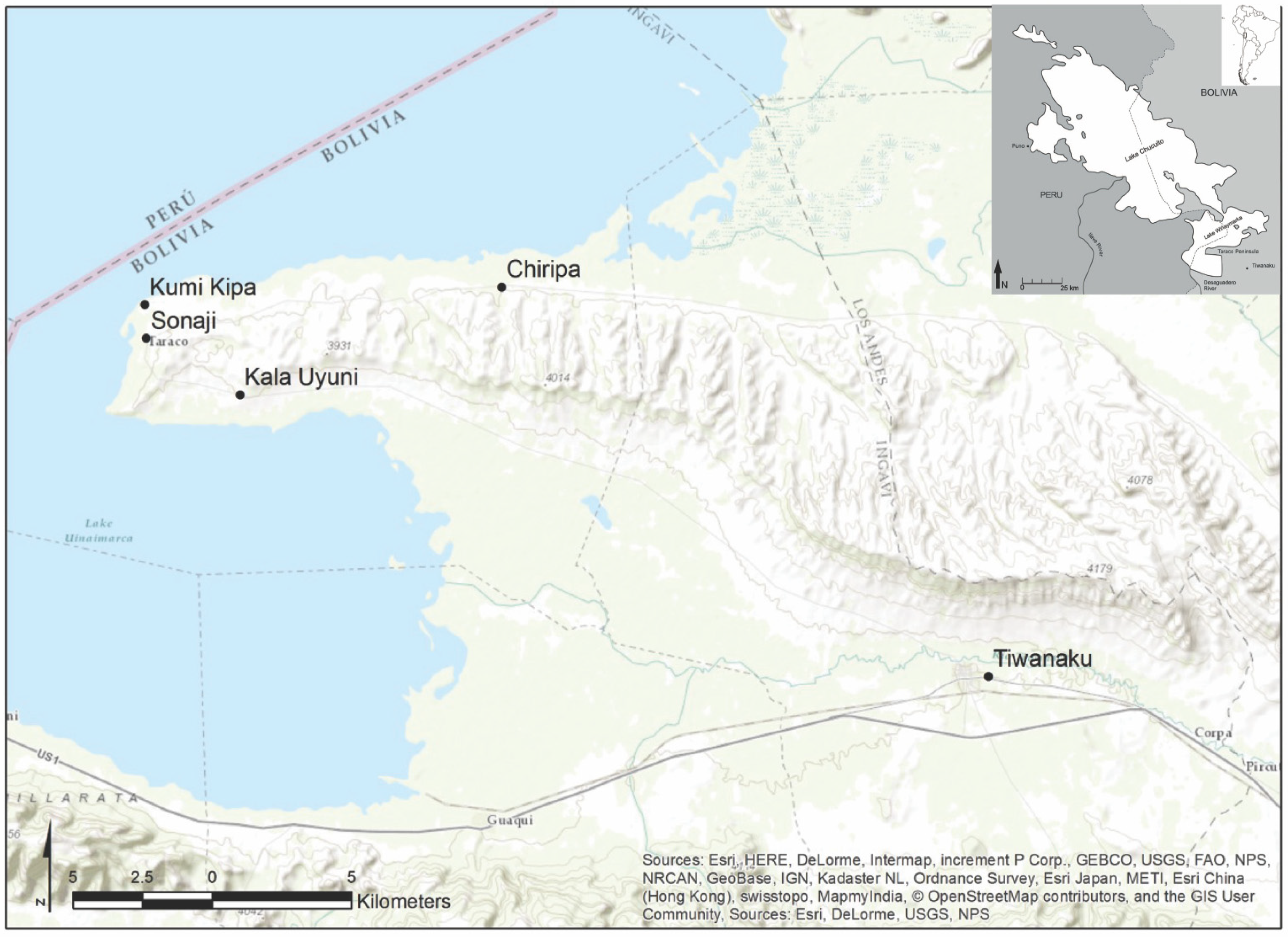
| Taxon | Seed Diameter | Testa Thickness | Seed Coat Texture | Margin Configuration | Beak Prominence |
|---|---|---|---|---|---|
| Modern Kañawa Domesticated | 0.90–1.40 mm | 4.25–10.60 microns | Canaliculate Smooth center/ Canaliculate at beak | Rounded–Truncate | Prominent to Weak |
| Modern Illama Wild | 0.70–1.50 mm | 5.10–23.75 microns | Smooth center/ Canaliculate at beak | Rounded to Rounded–Truncate | Prominent |
| Modern Quinoa Domesticated | 1.5–2.9 mm | 1.25–3.75 microns | Smooth | Truncate | Weak to very weak |
| Modern Quinoa negra Wild | 1.06–2.95 mm | 22–55 microns | Reticulate-Alveolate | Biconvex to equatorially banded | Weak |
| Archaeological Period | Human Subsistence and Cultural Trends |
|---|---|
| Inca 1450–1532 CE | Centralized state from Central Andes. Intensive camelid herding for state; localized agriculture. |
| Pacajes 1100–1450 CE | Decentralized and autonomous polities; intensive camelid herding; expansive agriculture. |
| Tiwanaku 500–1100 CE | Centralized state across basin; intensive farming, herding, and fishing for state. Expansion of raised-field agriculture. |
| Late Formative 200 BCE–500 CE | Regional multi-community polities; intensive dry-land quinoa and tuber farming and camelid herding. |
| Middle Formative 800–200 BCE | Autonomous villages; extensive quinoa and tuber farming, camelid herding. |
| Early Formative 1500–800 BCE | Earliest settlements along lake shore, small hamlets; cultivation of early crops, quinoa, and tubers; camelid herding. |
| Late Archaic 3000–1500 BCE | Mobile hunter–gatherers; intensification of wild chenopod and tuber gathering; camelid hunting and herding. |
| Quinoa | Quinoa Negra | Kañawa | Illama | |
|---|---|---|---|---|
| Early Formative (n = 71) | 93% | 79% | 0% | 39% |
| Middle Formative (n = 87) | 93% | 77% | 0% | 79% |
| Late Formative (n = 149) | 98% | 85% | 2% | 93% |
| Tiwanaku (n = 70) | 97% | 83% | 6% | 83% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, M.C. Archaeobotanical Insights into Kañawa (Chenopodium pallidicaule Aellen) Domestication: A Rustic Seed Crop of the Andean Altiplano. Agronomy 2023, 13, 2085. https://doi.org/10.3390/agronomy13082085
Bruno MC. Archaeobotanical Insights into Kañawa (Chenopodium pallidicaule Aellen) Domestication: A Rustic Seed Crop of the Andean Altiplano. Agronomy. 2023; 13(8):2085. https://doi.org/10.3390/agronomy13082085
Chicago/Turabian StyleBruno, Maria C. 2023. "Archaeobotanical Insights into Kañawa (Chenopodium pallidicaule Aellen) Domestication: A Rustic Seed Crop of the Andean Altiplano" Agronomy 13, no. 8: 2085. https://doi.org/10.3390/agronomy13082085
APA StyleBruno, M. C. (2023). Archaeobotanical Insights into Kañawa (Chenopodium pallidicaule Aellen) Domestication: A Rustic Seed Crop of the Andean Altiplano. Agronomy, 13(8), 2085. https://doi.org/10.3390/agronomy13082085




