Abstract
Prickly sida is a troublesome annual weed native to the USA. It was introduced in Greece over two decades ago and has since been infesting cotton crops, where it proved difficult to control. A two-year field study (2021–2022) was conducted in a cotton field (Karditsa, central Greece) heavily infested with prickly sida to evaluate the efficacy of nine treatments against this weed, using pre-emergence (PRE) and early post-emergence (EPOST) herbicides applied at the field rate. At 8 weeks after treatment, prickly sida control was over 90% with pyrithiobac PRE (68.9 g ai ha−1) alone or in mixture with s-metolachlor (960 g ai ha−1) or with isoxaben (150 g ai ha−1) and with the application of pyrithiobac + s-metolachlor PRE (34.5 + 960 g ai ha−1) followed by pyrithiobac EPOST (34.5 g ai ha−1). Flurochloridone + fluometuron PRE (375 + 2000 g ai ha−1), flumioxazin + s-metolachlor PRE (50 + 960 g ai ha−1), or pendimethalin + terbuthylazine PRE (1500 + 750 g ai ha−1) controlled prickly sida by 89–93% in 2021 and 82–90% in 2022. Pyrithiobac EPOST alone or in mixture with trifloxysulfuron did not adequately control prickly sida. Yield was significantly lower in the weedy check and EPOST compared with the PRE treatments. None of the herbicides caused any visual injury to the crop or any reduction in cotton density. Our results can help in the successful management of prickly sida in non-transgenic cotton to maximize cotton yield and quality.
1. Introduction
Prickly sida (Sida spinosa L.) is a dicotyledonous C3 plant native to the United States. It is a member of the Malvaceae family, behaving in the temperate zone as a summer annual with an upright growth habit or in tropical areas as a shrubby perennial [1]. It is a troublesome weed in annual summer crops like cotton, soybean, and maize [2], with a high adaptation to the conditions associated with them, such as partial sun early in the season followed by full sun later on [1]. Thus, it can tolerate the shade of the crop canopy, reaching flowering (Figure S1a) and seed maturity after crop senescence or harvest [1,3]. It has a prolonged emergence period lasting from April to September, as verified by previous research [4,5] and personal observation, which peaks in May and July–August [5].
Prickly sida has long been known as a weed of upland cotton (Gossypium hirsutum L., family Malvaceae) in the USA. This weed was ranked as the most troublesome weed in 1974 and the third most troublesome in 1995 [6]. However, later surveys ranked it as one but last among the 15 most dominant weed species present in cotton fields [7], although it remained among the common weed species observed along roadsides [8,9]. The advent of transgenic cotton with tolerance to glyphosate or bromoxynil is probably behind this in-crop population shift because prickly sida is susceptible to both herbicides [10,11,12].
In Greece, prickly sida was first recorded about two decades ago in the valley of the Louros River, near Preveza (SW Greece), and in Palamas, near Karditsa (central Greece), infesting cotton fields at low weed densities [13]. Large-scale surveys of the cotton field weed flora held at a similar time around these areas [14] did not record this species, probably because of its low initial abundance. However, prickly sida gradually acclimatized and proved to be difficult to control with usual weed management methods. Today it is an established problem in the major cotton-producing areas of the country, while it continues to infest new fields.
Cotton planted at the standard row spacing (0.96–1 m) requires a lengthy critical period free from weed competition starting 2–3 weeks after emergence (WAE) and lasting up to 9–11 WAE to avoid growth reduction and eliminate potential yield losses [15,16]. Where transgenic cotton is not an option, chemical control of weeds relies mostly on residual herbicides applied pre-plant incorporated (PPI) or pre-emergence (PRE) to reduce early season weed interference, which is further assisted with post-emergence (POST) selective herbicides. Fluometuron is the most common PRE herbicide used for the control of annual dicotyledonous weeds in cotton fields in several states within the USA [17] and also in Greece. However, cotton growers observed that the usual PRE application of fluometuron had poor results on prickly sida. The inconsistent efficacy of fluometuron against this weed is confirmed by previous research [17,18,19,20] and probably contributed to the increase in prickly sida infestation in cotton fields in Greece. An increase in the abundance of Malvaceae weeds, such as prickly sida and Venice mallow (Hibiscus trionum L.) was also reported in the USA during the pre-transgenic cotton era because of the wide reliance on selective herbicides in cotton that were not effective against these species [21]. A widely adopted practice by cotton growers in Greece is the mixture of fluometuron + s-metolachlor PRE, which increases the spectrum of weeds controlled, including some common troublesome grasses. Other PRE herbicides available for cotton that control both broadleaf weeds and grasses include flumioxazin, flurochloridone, and the formulated mixture pendimethalin + terbuthylazine. Previous studies reported that flumioxazin PRE at 71 g ai/ha was able to control prickly sida in peanut (Arachis hypogaea L.), while pendimethalin PPI plus fluometuron PRE controlled prickly sida only by 57% [22,23,24]. There is currently no information available on the control of prickly sida using flurochloridone in cotton or any other crop.
Pyrithiobac and trifloxysulfuron are registered as POST herbicides for use in cotton in Greece (emergency authorization for 120 days). Both are used as selective POST over-the-top herbicides for broadleaf weed control in non-transgenic cotton [12,17,22,25]. They are both HRAC Group 2 herbicides and act by inhibiting the activity of the acetolactate synthase (ALS) enzyme in susceptible plants. Corbett et al. [26] reported that pyrithiobac POST provided adequate control only at a very early growth stage of prickly sida (2–5 cm) and at the dose of 72 g ai ha−1. Pyrithiobac is also reported to provide sufficient control of prickly sida when used as PRE herbicide in cotton [17,18].
This study was prompted by the limited information on herbicide control of prickly sida in Greece and the need to address the growing problem of its infestation in cotton fields. The specific objective was to compare the efficacy and selectivity of herbicide treatments applied PRE or early POST (EPOST) on prickly sida using nine combinations of the available herbicides and assess their impact on both the quantity and quality of the cotton yield.
2. Materials and Methods
2.1. Experimentation Site
A two-year field experiment was performed during the growing seasons of 2021 and 2022 in a cotton field heavily infested with prickly sida, located in the agricultural area of Paleoklissi, Karditsa, Thessaly region, Greece (39°23′49.9″ N 21°50′51.0″ E) (Figure 1). A lower infestation of other weed species included barnyard grass (Echinochloa crus-galli L.), green foxtail (Setaria viridis (L.) P. Beauv.), and Venice mallow (Hibiscus trionum L.). The soil type of the field was sandy loam with pH 5.88, 1.8% organic matter content, and 154.7 μS/cm electric conductivity. The total rainfall and mean temperature in the area from April to September (cotton sowing season) for each year are presented in Figure 2.

Figure 1.
Location of the prickly sida-infested cotton field in Paleoklissi, Karditsa (Thessaly region, Greece) and overview of the experiment. Map created with mapchart.net. Photograph by V. Kati.
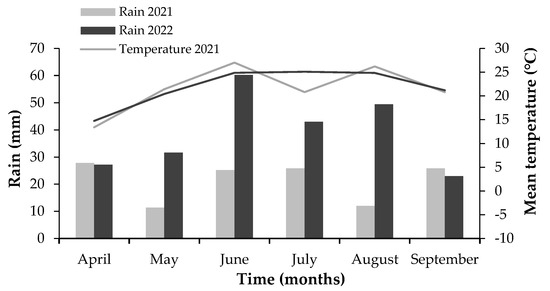
Figure 2.
Climatic conditions (mean monthly air temperature and total monthly rainfall) during the two growing seasons of 2021 and 2022.
The crop was sown with a tractor-mounted seed-drill on 6 May 2021 and 20 April 2022, with the cotton hybrid ST 402 (Corteva Agriscience) at the seed rate of 32 kg ha−1 in rows spaced at 0.96 m. The experimental part of the field received irrigation, fertilizer, and pesticide applications (except for herbicides, which were applied as follows) according to the local common farming practice, throughout the two growing seasons.
2.2. Herbicide Treatments
Ten treatments were evaluated, which included nine herbicide treatments with eight herbicide formulations (Table 1) applied PRE, EPOST, or PRE followed by (fb) EPOST (split application), and an untreated weedy check. All herbicides were applied at the recommended field rates. Herbicide treatments (g ai ha−1) were pyrithiobac–sodium (hereafter pyrithiobac for simplicity) PRE (68.9), pyrithiobac + s-metolachlor PRE (68.9 + 960), pyrithiobac + isoxaben PRE (68.9 + 150), flurochloridone + fluometuron PRE (375 + 2000), pendimethalin + terbuthylazine PRE (1500 + 750) and flumioxazin + s-metolachlor PRE (50 + 960), pyrithiobac EPOST (68.9), pyrithiobac + trifloxysulfuron EPOST (68.9 + 7.5), and pyrithiobac + s-metolachlor PRE (34.5 + 960) fb pyrithiobac EPOST (34.5).

Table 1.
Herbicide active ingredients (ai) grouped by HRAC (Herbicide Resistance Action Committee), mode of action (MOA), and information on the products used (trade name, type of formulation, and manufacturer).
The experiments were established in different parts of the field each year following a randomized complete block design (RCBD) with four replications per treatment. Each experimental plot had a 16 m2 surface area and contained four cotton rows, 5 m long. The width of the plot corresponded to the width of the boom sprayer, which was 3.2 m (3.2 m × 5 m = 16 m2). The average number of cotton plants per plot was 53 and 60 in 2021 and 2022, respectively).
All herbicide treatments were applied with an AZO hand-held air-compressed boom sprayer (Figure S1b), equipped with six flat-spray nozzles (11002 twinjet). The sprayer was calibrated to deliver 300 L/ha spray volume at 210 kPa. PRE herbicides were applied within 24 h after sowing, on 7 May 2021 and on 21 April 2022. In 2021, 24.2 mm of rain preceded the PRE treatment within a period of two weeks. That year, soil incorporation and activation of the PRE herbicides relied mainly on the rainfall (9.6 mm) that followed after the herbicide treatments. In 2022, 23.6 mm rain preceded the PRE treatment within a period of two weeks. Since the weather forecast did not predict any rainfall after the herbicide treatments, the field was irrigated with 8 mm the following day to incorporate and activate the herbicides. The pattern of rainfall that followed was 0 mm one week after the treatments and 4.4 mm within two weeks after the treatments. All the EPOST applications were performed one month after sowing, with the addition of the non-ionic surfactant Supersonic (fatty alcohol ethoxylated 20% w/v, SIPCAM SpA, Pero, Italy) at 0.25% (v/v). At the time of the EPOST treatments, the cotton was in the cotyledons to two leaf-stage (Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie (BBCH) 10–12 code) and prickly sida was in cotyledons to four leaf-stage (BBCH 10–14 code).
2.3. Assessments
Weed control was assessed at 4 and 8 weeks after treatment (WAT) for the PRE herbicides and at 4 WAT for the EPOST herbicides. Assessments at 8 WAT PRE and 4 WAT EPOST were held on the same day. Herbicide efficacy (% control) on prickly sida was based on the number of alive prickly sida plants/m2 in the area between and including the two central cotton rows of each plot. It was calculated according to Equation 1, adapted from Abbot’s formula [27]:
where PSUC = prickly sida density in the untreated weedy check and PST = prickly sida density in the treated plots.
% control = [(PSUC − PST)/PSUC] × 100,
The assessment of possible injury to the crop was based on crop emergence (number of cotton plants per meter of crop row in one of the two central cotton rows) and on any visible symptoms of phytotoxicity visible on the leaves and shoots of the crop plants. Cotton plant density was also recorded at 4 WAT after PRE herbicide applications. The observations for phytotoxicity symptoms took place at 4 and 8 WAT for PRE and at 4 WAT for EPOST and PRE fb EPOST applications. In 2022, all the measurements were confined to three out of four initial blocks because of a heavier-than-usual rainfall that occurred three weeks after PRE applications, causing flooding in one part of the farmer’s field that affected weed density at the fourth block (Figure 2). This effect was attributed to the possible movement of pyrithiobac applied by the farmer to the rest of the field into the adjacent fourth block of our experiment. Studies on pyrithiobac runoff after rainfall are in line with this attribution [28].
Yield and fiber quality properties were recorded only for the 2022 growing season because, in 2021, a heavy infestation of the crop by the cotton bollworm (Helicoverpa armigera (Hübner, [1808])) caused variable levels of injury to the cotton bolls due to inefficient insecticide control; therefore, the yield was not recorded. Harvest was performed with a spindle-type two-row cotton harvester (John Deere), that allowed the separate collection of each plot. Yield parameters included the number of open cotton bolls just before harvest in the two central rows of each plot, the weight of seeded cotton collected from the same rows, and fiber quality properties. Subsamples of 400–500 g from the harvested seed cotton of each plot were collected and ginned to determine the lint percentage. Fiber quality properties included spinning consistency index, moisture, micronaire, maturity, upper half mean length, length uniformity index, short fiber index, strength, elongation, color grade as determined by the degree of reflectance and yellowness, trash content, trash area, and fiber amount. Fiber quality analyses were performed at the accredited laboratory of the Hellenic Agricultural Organization—Dimitra, National Center for Quality Control, Classification & Standardization of Cotton, 43100 Karditsa, Greece.
2.4. Statistical Analysis
The data on prickly sida control and cotton yield quantity and quality traits were subjected to analysis of variance (ANOVA) using the statistical program IBM SPSS Statistics, version 28. The prickly sida control data were analyzed separately for each year because the experimental errors in the two experiments in the combined analysis of variance over years were statistically significantly different. Means were separated with the Fisher’s protected least-significant difference (LSD) test at the significance level α = 0.05 (p ≤ 0.05).
3. Results
3.1. Prickly Sida Control
In 2021, the untreated weedy check had an average of 127 prickly sida plants/m2 at 4 WAT PRE and 202 plants/m2 four weeks later, i.e., at 8 WAT PRE/4 WAT EPOST (Figure S1c). At 4 WAT PRE, there were statistically significant differences in prickly sida control among treatments (Table 2). These ranged from 77% with pendimethalin + terbuthylazine to a significantly greater 98% with pyrithiobac + isoxaben. There was no significant difference between pyrithiobac + isoxaben and the pyrithiobac + s-metolachlor PRE, flumioxazin + s-metolachlor PRE, flurochloridone + fluometuron PRE and the split application pyrithiobac + s-metolachlor PRE fb pyrithiobac EPOST. Pendimethalin + terbuthylazine control of prickly sida was similar only to flurochloridone + fluometuron PRE (89%). At 8 WAT PRE/4 WAT EPOST, all the treatments with pyrithiobac PRE applied either alone or in mixture with isoxaben or s-metolachlor and in the split application, gave excellent control of prickly sida (99–100%). Slightly lower control was achieved with flurochloridone + fluometuron and flumioxazin + s-metolachlor (93 and 93%, respectively), although the differences were not significant. Pendimethalin + terbuthylazine control was similar to flurochloridone + fluometuron and to flumioxazin + s-metolachlor from the PRE treatments and also to pyrithiobac + trifloxysulfuron POST. The mixture pyrithiobac + trifloxysulfuron POST had better control of prickly sida (86%) than pyrithiobac alone and was comparable to PRE treatments with flurochloridone + fluometuron, pendimethalin + terbuthylazine, and flumioxazin + s-metolachlor. Pyrithiobac EPOST had significantly lower level of prickly sida control (71%) than any of the other treatments.

Table 2.
Prickly sida control (means) at 4 and 8 WAT with the PRE and at 4 WAT with the EPOST herbicides 1,2.
In 2022, prickly sida infestation in the untreated weedy check was lower compared with the previous year, with 23 and 22 plants/m2 at 4 WAT PRE and at 8 WAT PRE/4 WAT EPOST, respectively (Figure S1d). Despite the lower infestation, and contrary to the corresponding results in the previous year, the level of prickly sida control at 4 WAT PRE was surprisingly very low for all the treatments that contained pyrithiobac, applied either alone or in mixture with isoxaben or s-metolachlor or in the split application, and ranged from 35 to 63% (Table 2). On the other hand, flurochloridone + fluometuron, pendimethalin + terbuthylazine, and flumioxazin + s-metolachlor, resulted in the highest level of control (96, 90, and 97%, respectively) at 4 WAT PRE. However, during the later assessment held at 8 WAT PRE/4 WAT EPOST, the level of prickly sida control was greater than 92% for all treatments of pyrithiobac PRE (alone or in mixtures). This was slightly higher than with flurochloridone + fluometuron, pendimethalin + terbuthylazine, and flumioxazin + s-metolachlor (90, 82, 83%, respectively), although the differences were not significant. That year, the EPOST applications with pyrithiobac and pyrithiobac + trifloxysulfuron provided partial control of prickly sida (32 and 52% control, respectively).
3.2. Crop Injury Evaluation
No visual injury symptoms caused by the herbicide treatments on the cotton plants were observed. Cotton emergence (number of plants/m of row) was similar in all herbicide treatments and the untreated weedy check (Table 3). On average, cotton density per linear meter (Lm) was 13.3 and 15.1 plants Lm−1 in 2021 and 2022, respectively, which is within the usual expected for this hybrid in the area and for the seed-rate used.

Table 3.
Cotton density (means) recorded 4 WAT with PRE herbicides, which coincided with the EPOST applications timing 1.
3.3. Yield
The treatments with flurochloridone + fluometuron PRE, pyrithiobac + s-metolachlor PRE, pyrithiobac + isoxaben PRE, pyrithiobac + s-metolachlor PRE fb pyrithiobac EPOST, and pendimethalin + terbuthylazine PRE had similar numbers of cotton bolls, ranging from 23 to 27 cotton bolls/m2 (Table 4). The untreated weedy check and the EPOST application with pyrithiobac alone or in mixture with trifloxysulfuron had the lowest numbers (14, 17, and 18 cotton bolls m−2, respectively), which were statistically similar. Flumioxazin + s-metolachlor PRE provided a similar number of cotton balls/m2 to those of pyrithiobac PRE and pyrithiobac + isoxaben PRE.

Table 4.
Mean values for cotton boll number, seeded cotton yield, and lint yield (% of seeded cotton) recorded in the herbicide treatments against prickly sida and the untreated weedy check 1,2.
Seeded cotton yield varied significantly across treatments. All the PRE herbicide treatments and the split PRE fb EPOST resulted in similarly high yield of seeded cotton that ranged from 2552 to 3652 Kg ha−1 (Table 4). However, the pyrithiobac + s-metolachlor PRE, the pyrithiobac EPOST, and the pyrithiobac + trifloxysulfuron EPOST treatments provided similar seeded cotton yield to that of the untreated weedy check. Lint yield (% of seeded cotton) was similar across all treatments.
Regarding the cotton fiber properties, micronaire values were significantly different among the herbicide treatments, while the other fiber quality traits were similar (Table 5). A slightly higher micronaire value was recorded for the treatment with pendimethalin + terbuthylazine PRE (5.3). This was similar to the mean values obtained in the treatments with pyrithiobac PRE, pyrithiobac + s-metolachlor PRE fb pyrithiobac EPOST, and pyrithiobac sodium + isoxaben PRE. On the other hand, pyrithiobac EPOST and flurochloridone + fluometuron provided similar micronaire values to that of the untreated weedy check.

Table 5.
Cotton fiber properties (means) recorded in the herbicide treatments against prickly sida and the untreated weedy check 1,2.
4. Discussion
The different efficacy of the PRE treatments containing pyrithiobac either alone or in mixture with isoxaben or s-metolachlor at 4 WAT in 2021 and 2022 was probably due to the pattern of rainfall that preceded the measurement and the high mobility of pyrithiobac in the soil [29]. More specifically, it is likely that a heavy rainfall recorded in 2022 at 3 WAT with the PRE herbicides moved pyrithiobac to lower soil levels, making it unavailable to germinating prickly sida seeds. This lowered the efficacy of these treatments as reflected in our results one week later (4 WAT). Our observation is in line with previous studies on the relatively high leaching potential and persistence of pyrithiobac in the soil [30,31]. Pyrithiobac is a weak acid; therefore, the slightly acidic soil (pH 5.88) of our experimental field is expected to have aggravated its leaching capacity. The other PRE herbicides that were likely to have an effect on prickly sida control (flurochloridone, flumioxazin, and terbuthylazine, further discussed below) are characterized by low water solubility and mobility in the soil [29] and would therefore have been unaffected by that heavy rainfall. Nonetheless, during the final assessment held 8 WAT, the efficacy of the PRE treatments with pyrithiobac alone or in mixture with s-metolachlor or with isoxaben was more than 92% in both years. The increase in efficacy using these treatments between 4 and 8 WAT in 2022 was probably due to subsequent capillary movement upward of pyrithiobac in the weeks after the heavy rainfall that year. The potential of pyrithiobac capillary upward movement is also mentioned in previous studies [30]. As the season progressed, the rise in temperature and subsequent evaporation of the surface soil water probably increased the capillary upward flow of deeper-level soil water containing pyrithiobac. The upward flow toward drier soil of water containing dissolved fertilizer was demonstrated in an early study by Gardner [32]. As also reported in that study, a coarse texture like that in sandy loam soil, which we had in our experimental field, facilitated the upward flow of the soil water (in our case containing pyrithiobac).
The high efficacy of pyrithiobac PRE against prickly sida that we observed agrees with previous studies. Branson et al. [10] reported that pyrithiobac PRE at a similar rate (70 g ai ha−1) controlled prickly sida by 83–87%. It is absorbed both by the foliage and the roots of emerged weeds [33], as we also observed after the PRE treatment with this herbicide (Figure S2). It should be noted here that pyrithiobac may persist in the soil and be harmful to rotational crops [30]. Furthermore, pyrithiobac belongs to ALS inhibitors, which is the herbicide group most prone to resistance development [34] and should be applied in mixture with other modes of action to reduce this risk. In our study, isoxaben (inhibitor of cellulose synthesis) and s-metolachlor (inhibitor of very long-chain fatty acid synthesis) added as tank-mix companions to pyrithiobac PRE were safe for the crop and could be used to extend the range of weeds controlled. However, neither isoxaben nor s-metolachlor added to pyrithiobac PRE significantly increased its efficacy against prickly sida. Previous studies reported that isoxaben PRE at 840 g ai ha−1 did not control prickly sida in woody landscape crops [35]. Also, s-metolachlor controls mainly grasses and small-seeded broad-leaf species [36,37] but does not control prickly sida [23,38].
The high efficacy on prickly sida of the mixture of flurochloridone + fluometuron PRE was most probably due to flurochloridone activity. This assumption is based on previous studies reporting that fluometuron PRE applied alone [19] or in mixture with trifluralin [12] failed to control this weed. To our knowledge, there is currently no information available on the efficacy of flurochloridone on this weed. Our results suggest that flurochloridone can be included in a soil-applied herbicide mixture together with fluometuron PRE for the control of prickly sida in cotton and for expanding the range of controlled weed species.
The efficacy of pendimethalin + terbuthylazine PRE was probably due mainly to terbuthylazine activity. This assumption is based on previous studies where pendimethalin PPI did not control prickly sida even when supplemented with fomesafen PRE, while pendimethalin PPI plus fluometuron PRE controlled prickly sida only by 57% [22,39,40].
Our results for flumioxazin + s-metolachlor PRE efficacy agree with Askew et al. [23]. In their study, flumioxazin + metolachlor PRE applied at 70 + 2240 or at 110 + 2240 g ai ha−1 provided excellent control of prickly sida (95–100%) at 12–13 WAT, while metolachlor alone failed to control the weed. Flumioxazin rates applied in that study were 1.5 to 2 times higher than in our study and may explain the longer residual activity obtained compared with our results, where the efficacy dropped to 93 and 83% for 2021 and 2022, respectively, at 8 WAT.
The timing of pyrithiobac POST is crucial in controlling prickly sida. Several studies reported that pyrithiobac is most efficient on prickly sida when applied EPOST to small plants at the three-leaf stage or younger [17,19,41]. However, Branson et al. [10] argued that the mid-postemergence application of pyrithiobac provided 93–97% control of prickly sida, as opposed to 47–81% with EPOST application, which fluctuated greatly. In our study, the EPOST herbicide application, chosen to avoid anticipated cotton injury with later application based on previous experience and other studies [42], did not result in adequate control of prickly sida. It is likely that pyrithiobac EPOST applied when there is minimum weed ground cover will also act as a PRE herbicide, since a large herbicide amount may reach the soil surface and thus be effective against later-emerging prickly sida. This subsequent residual activity will depend on the available soil moisture. A study on the efficacy of velvetleaf from pyrithiobac reaching the soil following POST application with the weed foliage covered showed that injury occurred only when moisture levels were near field capacity [43]. The erratic results in our study with the EPOST pyrithiobac treatments on prickly sida may be due to inadequate and non-consistent soil moisture after the application, which could extend the control period of later-germinating seeds.
The addition of trifloxysulfuron to pyrithiobac EPOST increased the control of prickly sida compared with pyrithiobac EPOST alone only in the first year. It did not make any difference in the second year, with both treatments failing to control the weed. Our results agree in part with previous studies where trifloxysulfuron applied EPOST was not effective against this weed [12,17,25]. Trifloxysulfuron applied EPOST at 5 g ai ha−1 provided 48% control of prickly sida in greenhouse experiments [44]. Branson et al. [10] also reported poor efficacy on prickly sida with trifloxysulfuron EPOST at rates of up to 10 g ai/ha compared with pyrithiobac.
The split application of pyrithiobac + s-metolachlor PRE fb pyrithiobac EPOST provided season-long control of prickly sida by exploiting the capacity of pyrithiobac to act both as a soil and foliage herbicide. The lower efficacy at 4 WAT in 2022 compared with 2021 was probably a result of the different rainfall patterns discussed earlier. However, this type of application is more expensive since it requires two field sprayings. Also, the application of half the recommended pyrithiobac field dose as PRE and the other half as POST may increase the risk of herbicide resistance development, especially when dry weather reduces the PRE activity of this herbicide, thus exposing weeds to lower herbicide rates. Given the genetic variation among individuals in a weed population, low herbicide rates could allow the survival of those that carry non-target-site resistance alleles, which could accumulate after several generations and endow resistance to the full herbicide dose [45].
As stated, all herbicide treatments appeared safe for the crop. Similar results were reported in previous studies where POST applications of pyrithiobac or trifloxysulfuron in cotton caused no injury or transient symptoms to the crop and did not affect the final yield [25,43,44,46].
The significant reduction in the number of cotton bolls per square meter and seeded cotton yield in the untreated weedy check compared with the most efficacious herbicide treatments verify the detrimental effect that prickly sida competition can have on cotton production and justifies the need for its early control. This observation agrees with previous studies where prickly sida populations of 130 plants per square meter when allowed to compete with cotton during all seasons, caused a 37–41% reduction in yield [3], while a critical period free from prickly sida competition in cotton was reported to be at least 5 weeks to avoid any reduction in yield [47].
In our study, the yield was significantly higher in all the PRE treatments compared with the untreated weedy check, suggesting that these treatments reduced weed competition successfully (Table 5). The highest yields were obtained with the treatments of pyrithiobac PRE alone or in mixture with s-metolachlor or with isoxaben, the split PRE fb EPOST treatment, and the flumioxazin + s-metolachlor PRE treatment. Yields in pyrithiobac EPOST either alone or in mixture with trifloxysulfuron were the lowest among all herbicide treatments and, although higher, they were comparable to the untreated control. This was expected since these herbicide treatments did not control prickly sida. Fiber quality was significantly affected by the herbicide treatments only for micronaire (i.e., fiber fineness) with the lowest value observed in the untreated weedy check. It is likely that the reduction in weed competition obtained with herbicides and the subsequent increase in resources available to the crop led to higher micronaire values [48]. Other fiber quality characteristics, including the spinning consistency index, elongation, strength, moisture, maturity, color, and trash content, were not significantly affected by the herbicide treatments. Similar observations have been reported in previous studies [3,49].
5. Conclusions
The results of this study suggest that pyrithiobac, when applied PRE either alone or in a mixture with either s-metolachlor, isoxaben, or in a split application with s-metolachlor can provide season-long control of prickly sida. High prickly sida control was also achieved with the mixture of flurochloridone + fluometuron PRE or with flumioxazin + s-metolachlor PRE, while pendimethalin + terbuthylazine PRE control was lower. Prickly sida was not controlled with the EPOST treatments of pyrithiobac alone or in a mixture with trifloxysulfuron. The efficacy of PRE and EPOST herbicides depended on the prevailing weather conditions, especially rainfall, which followed a different pattern between the two experimental years. All herbicide treatments were safe for the crop, while all the PRE treatments and the split PRE fb EPOST treatment provided significantly higher yield than the untreated weedy check. Future research should focus on prickly sida control with herbicides applied as mixtures or separately under variable soil moisture levels since increasingly unpredictable weather conditions are a major factor that could undermine their efficacy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13102466/s1, Figure S1. Flowering prickly sida plant (a), application of PRE herbicides with an AZO boom sprayer (b), and view of the untreated weedy check in late-June 2021 (c) and 2022 (d); Figure S2: Dead prickly sida plants at the 2–3 leaf stage, 8 WAT with pyrithiobac PRE, in 2021.
Author Contributions
Conceptualization, V.K. and I.V.; methodology, T.G., V.K., I.V., C.V. and P.M.; formal analysis, G.M.; investigation, V.K., T.G., I.V., C.V. and P.M.; resources, V.K., C.V. and T.G.; data curation, V.K. and I.V.; writing—original draft preparation, V.K.; writing—review and editing, V.K., T.G., I.V., C.V., P.M. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained within this article.
Acknowledgments
We are grateful to Emeritus of Weed Science, Ilias G. Eleftherohorinos, for providing his scientific opinion on this study. We also extend our gratitude to the cotton farmer, Aris Konstantakos, for providing the experimental field, implementing the necessary crop husbandry, and assisting in the crop harvest.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
WAT: weeks after treatment; BBCH, Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie; PRE, pre-emergence; EPOST, early post-emergence.
References
- Mohler, C.L.; Teasdale, J.R.; DiTommaso, A. Manage Weeds on Your Farm: A Guide to Ecological Strategies; Sustainable Agriculture Research & Education (SARE): College Park, MD, USA, 2021; p. 416. [Google Scholar]
- Egley, G.H. Germination of developing prickly sida seeds. Weed Sci. 1976, 24, 239–243. [Google Scholar] [CrossRef]
- Ivy, H.W.; Baker, R.S. Prickly sida control and competition in cotton. Weed Sci. 1972, 20, 137–139. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Environmental conditions required for germination of prickly sida (Sida spinosa). Weed Sci. 1984, 32, 786–791. [Google Scholar] [CrossRef]
- Egley, G.H.; Williams, R.D. Emergence periodicity of six summer annual weed species. Weed Sci. 1991, 39, 595–600. [Google Scholar] [CrossRef]
- Webster, T.M.; Coble, H.D. Changes in the Weed Species Composition of the Southern United States: 1974 to 1995. Weed Technol. 1997, 11, 308–317. [Google Scholar] [CrossRef]
- Webster, T.M.; Nichols, R.L. Changes in the prevalence of weed species in the major agronomic crops of the southern united states: 1994/1995 to 2008/2009. Weed Sci. 2012, 60, 145–157. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; Bagavathiannan, M.V.; Mauromoustakos, A. Distribution of arable weed populations along eastern arkansas–mississippi delta roadsides: Factors affecting weed occurrence. Weed Technol. 2015, 29, 596–604. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; Brye, K.R.; Skinner, V.; Mauromoustakos, A.; Moonen, A.-C. Relationships between soil properties and the occurrence of the most agronomically important weed species in the field margins of eastern Arkansas—Implications for weed management in field margins. Weed Res. 2017, 57, 159–171. [Google Scholar] [CrossRef]
- Branson, J.W.; Smith, K.L.; Barrentine, J.L. Comparison of trifloxysulfuron and pyrithiobac in glyphosate-resistant and bromoxynil-resistant cotton. Weed Technol. 2005, 19, 404–410. [Google Scholar] [CrossRef]
- Reddy, K.N. Weed control and species shift in bromoxynil- and glyphosate-resistant cotton (Gossypium hirsutum) rotation systems. Weed Technol. 2004, 18, 131–139. [Google Scholar] [CrossRef]
- Porterfield, D.; Wilcut, J.W.; Wells, J.W.; Clewis, S.B. Weed management with CGA-362622 in transgenic and nontransgenic cotton. Weed Sci. 2003, 51, 1002–1009. [Google Scholar] [CrossRef]
- Lymperopoulou, S.; Giannopolitis, C.N. Galinsoga ciliata (Raf.) SF Blake and Sida spinosa L., two new weed records from Greece. Hell. Plant Prot. J. 2009, 2, 37–40. [Google Scholar]
- Economou, G.; Bilalis, D.; Avgoulas, C. Weed flora distribution in Greek cotton fields and its possible influence by herbicides. Phytoparasitica 2005, 33, 406–419. [Google Scholar] [CrossRef]
- Papamichail, D.; Eleftherohorinos, I.; Froud-Williams, R.; Gravanis, F. Critical periods of weed competition in cotton in Greece. Phytoparasitica 2002, 30, 105–111. [Google Scholar] [CrossRef]
- Bukun, B. Critical periods for weed control in cotton in Turkey. Weed Res. 2004, 44, 404–412. [Google Scholar] [CrossRef]
- Burke, I.C.; Wilcut, J.W. Weed management in cotton with CGA-362622, fluometuron, and pyrithiobac. Weed Technol. 2004, 18, 268–276. [Google Scholar] [CrossRef]
- Culpepper, S.A.; York, A.C. Weed management in no-tillage bromoxynil-tolerant cotton (Gossypium hirsutum). Weed Technol. 1997, 11, 335–345. [Google Scholar] [CrossRef]
- Paulsgrove, M.D.; Wilcut, J.W. Weed management in bromoxynil-resistant Gossypium hirsutum. Weed Sci. 1999, 47, 596–601. [Google Scholar] [CrossRef]
- Snipes, C.E.; Mueller, T.C. Cotton (Gossypium hirsutum) yield response to mechanical and chemical weed control systems. Weed Sci. 1992, 40, 249–254. [Google Scholar] [CrossRef]
- Chandler, J.M. Competition of spurred anoda, velvetleaf, prickly sida, and venice mallow in cotton. Weed Sci. 1977, 25, 151–158. [Google Scholar] [CrossRef]
- Askew, S.D.; Wilcut, J.W. Absorption, translocation, and metabolism of foliar-applied CGA 362622 in cotton, peanut, and selected weeds. Weed Sci. 2002, 50, 293–298. [Google Scholar] [CrossRef]
- Askew, S.D.; Wilcut, J.W.; Cranmer, J.R. Weed management in peanut (Arachis hypogaea) with flumioxazin preemergence. Weed Technol. 1999, 13, 594–598. [Google Scholar] [CrossRef]
- Clewis, S.B.; Askew, S.D.; Wilcut, J.W. Economic assessment of diclosulam and flumioxazin in strip- and conventional-tillage peanut. Weed Sci. 2002, 50, 378–385. [Google Scholar] [CrossRef]
- Porterfield, D.; Wilcut, J.W.; Askew, S.D. Weed management with CGA-362622, fluometuron, and prometryn in cotton. Weed Sci. 2002, 50, 642–647. [Google Scholar] [CrossRef]
- Corbett, J.L.; Askew, S.D.; Thomas, W.E.; Wilcut, J.W. Weed efficacy evaluations for bromoxynil, glufosinate, glyphosate, pyrithiobac, and sulfosate. Weed Technol. 2004, 18, 443–453. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Silburn, D.M.; Foley, J.L.; deVoil, R.C. Managing runoff of herbicides under rainfall and furrow irrigation with wheel traffic and banded spraying. Agric. Ecosyst. Environ. 2013, 180, 40–53. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Veletza, V.G.; Kaloumenos, N.S.; Papantoniou, A.N.; Kadis, S.G.; Eleftherohorinos, I.G. Activity, adsorption, mobility, and field persistence of pyrithiobac in three soils. Weed Sci. 2005, 53, 212–219. [Google Scholar] [CrossRef]
- Baskaran, S.; Kennedy, I.R. Sorption and desorption kinetics of diuron, fluometuron, prometryn and pyrithiobac sodium in soils. J. Environ. Sci. Health B 1999, 34, 943–963. [Google Scholar] [CrossRef]
- Gardner, W.H. How water moves in the soil. Crop. Soils 1979, 32, 13–18. [Google Scholar]
- Mitchell, W.H. “STAPLE”—A new cotton herbicide from DuPont. In Proceedings of the Beltwide Cotton Conference, Memphis, TN, USA, 9–12 January 1996; National Cotton Council: Memphis, TN, USA. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. Tuesday, 1 August 2023. Available online: www.weedscience.org (accessed on 20 September 2023).
- Setyowati, N.; Weston, L.A.; McNiel, R.E. Evaluation of selected preemergence herbicides in field-grown landscape crops in Kentucky. J. Environ. Hortic. 1995, 13, 196–202. [Google Scholar] [CrossRef]
- Clewis, S.B.; Wilcut, J.W.; Porterfield, D. Weed management with s-metolachlor and glyphosate mixtures in glyphosate-resistant strip- and conventional-tillage cotton (Gossypium hirsutum L.). Weed Technol. 2006, 20, 232–241. [Google Scholar] [CrossRef]
- Grichar, W.J.; Besler, B.A.; Brewer, K.D.; Minton, B.W. Using soil-applied herbicides in combination with glyphosate in a glyphosate-resistant cotton herbicide program. Crop Protect. 2004, 23, 1007–1010. [Google Scholar] [CrossRef]
- Richburg, J.S.; Wilcut, J.W.; Eastin, E.F. Weed management in peanut (Arachis hypogaea) with imazethapyr and metolachlor. Weed Technol. 1995, 9, 807–812. [Google Scholar] [CrossRef]
- Troxler, S.C.; Askew, S.D.; Wilcut, J.W.; Smith, W.D.; Paulsgrove, M.D. Clomazone, fomesafen, and bromoxynil systems for bromoxynil-resistant cotton (Gossypium hirsutum). Weed Technol. 2002, 16, 838–844. [Google Scholar] [CrossRef]
- Wilcut, J.W.; Walls, F.R., Jr.; Horton, D.N. Imazethapyr for broadleaf weed control in peanuts (Arachis hypogaea). Peanut Sci. 1991, 18, 26–30. [Google Scholar] [CrossRef]
- Bridges, D.; Grey, T.; Brecke, B. WEED SCIENCE Pyrithiobac and Bromoxynil Combinations with MSMA for Improved Weed Control in Bromoxynil-Resistant Cotton. J. Cotton Sci. 2002, 6, 91–96. [Google Scholar]
- Kaloumenos, N.S.; Veletza, V.G.; Papantoniou, A.N.; Kadis, S.G.; Eleftherohorinos, I.G. Influence of pyrithiobac application rate and timing on weed control and cotton yield in Greece. Weed Technol. 2005, 19, 207–216. [Google Scholar] [CrossRef]
- Harrison, M.A.; Hayes, R.M.; Mueller, T.C. Environment affects cotton and velvetleaf response to pyrithiobac. Weed Sci. 1996, 44, 241–247. [Google Scholar] [CrossRef]
- Koger, C.H.; Price, A.J.; Reddy, K.N. Weed control and cotton response to combinations of glyphosate and trifloxysulfuron. Weed Technol. 2005, 19, 113–121. [Google Scholar] [CrossRef]
- Délye, C.; Jasieniuk, M.; Le Corre, V. Deciphering the evolution of herbicide resistance in weeds. Trends Genet. 2013, 29, 649–658. [Google Scholar] [CrossRef]
- Allen, R.L.; Snipes, C.E.; Crowder, S.H. Fruiting response of cotton (Gossypium hirsutum) to pyrithiobac. Weed Technol. 1997, 11, 59–63. [Google Scholar] [CrossRef]
- Buchanan, G.A.; Crowley, R.H.; McLaughlin, R.D. Competition of prickly sida with cotton. Weed Sci. 1977, 25, 106–110. [Google Scholar] [CrossRef]
- Blaise, D. Effect of tillage systems on weed control, yield and fibre quality of upland (Gossypium hirsutum L.) and Asiatic tree cotton (G. arboreum L.). Soil Tillage Res. 2006, 91, 207–216. [Google Scholar] [CrossRef]
- Ma, X.; Wu, H.; Jiang, W.; Ma, Y.; Ma, Y. Interference between redroot pigweed (Amaranthus retroflexus L.) and cotton (Gossypium hirsutum L.): Growth analysis. PLoS ONE 2015, 10, e0130475. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).