Abstract
Summer annual weed species in northern Australian summer fallows are frequently present at low densities and, increasingly, are glyphosate-resistant, creating the need for alternative herbicides for site-specific weed control. Alternative non-selective herbicide treatments are effective on problematic summer fallow weeds; however, many are yet to be evaluated as site-specific (spot spraying) treatments. This study aimed to identify herbicides that could be used in place of glyphosate to control larger/mature Chloris virgata and Sonchus oleraceus plants. The response of these weed species to 12 herbicide treatments was evaluated in pot experiments conducted over summer/autumn 2022. Despite herbicide treatments not being consistently effective across both species, there were instances where control was achieved by some herbicide treatments. S. oleraceus was controlled (i.e., ≤10% plant survival) by glufosinate-ammonium, paraquat and also with protoporphyrinogen-oxidase (PPO)-inhibiting herbicides saflufenacil, tiafenacil and trifludimoxazin. However, these results were not consistent in repeated studies or for C. virgata. Glyphosate was the only herbicide that controlled C. virgata. A glyphosate replacement as a spot-spraying treatment was not identified, and until further studies are more successful, alternative approaches are needed to preserve the ongoing effectiveness of this herbicide.
1. Introduction
In dryland winter cropping regions across Australia, summer fallow weed control is required to optimise the yield potential of subsequent grain crops [1]. The primary agronomic benefits of good weed control during the summer fallow period (i.e., the period between harvest of one winter crop and planting of the next) are reducing losses of soil moisture and available nutrients [2]. Maintaining weed-free summer fallows has been shown to increase the performance of subsequent winter crops [3,4]. The adoption of conservation cropping based on reduced tillage and crop residue retention by Australian grain growers [5] has resulted in a reliance on herbicides for summer fallow weed control [6]. More specifically, glyphosate, a non-selective herbicide that inhibits 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) [7], has been relied on since its introduction in the 1980s for weed control in Australia’s summer rainfall dominant northern grains region [8]. In the ensuing decades, the repeated application of glyphosate has provided an intense selection pressure that has resulted in the widespread evolution of resistant genotypes [9]. Very many cases of glyphosate-resistant weeds have now been reported in the northern grains region [10,11,12]. A recent herbicide resistance survey in the northern region found that, of the sampled populations, 14% of sowthistle (Sonchus oleraceus) and 68% of feathertop Rhodes grass (Chloris virgata) were resistant to glyphosate [13]. Cases of glyphosate resistance in C. virgata [14] have also been reported in the southern grains region. As a result, there is an increasing and ongoing need for alternative options to control problematic summer fallow weeds.
Until recently, summer fallow spraying frequently involved whole-field herbicide applications for the control of often low weed densities (i.e., less than 1.0 plant m−2). The control of low densities of the commonly occurring weeds, C. virgata and S. oleraceus, is essential as these species have the potential to establish large persistent seedbanks from small populations [15,16]. The ability to site-specifically apply herbicides to weed plant targets significantly reduces herbicide use with substantial cost savings [17]. These savings can allow for the cost-effective application of frequently more expensive alternative herbicide treatments to reduce the reliance on glyphosate [18]. With summer fallow site-specific spraying frequently delayed due to uncertainties around additional cohort emergence and unfavourable spraying conditions, the size of targeted weeds is often larger than advised in label recommendations. Thus, higher application rates of herbicide treatments are required to control large and frequently glyphosate-resistant weeds [19,20].
Site-specific weed control (SSWC) treatments for fallow weed control are applied using a boom-spray fitted with sensor-based detection systems (e.g., WEED-IT® (Rometron, Steenderen, The Netherlands) and WeedSeeker® (Trimble, Sunnyvale, CA, USA)). These sensors detect living (green) plant material via the near-infrared to red reflectance ratio relative to the non-living background (soil and plant residues) [21]. Sensor-based spot-spraying systems have been in use by Australian growers since they were first evaluated in the 1990s [22].
Whilst not conducted in the context of SSWC, but driven by escalating glyphosate resistance, recent Australian research has focused on identifying alternative herbicide treatments for summer weeds [10,11,19]. A pot-based investigation by Desai et al. [11] demonstrated complete weed control of four to five-leaf C. virgata using either 600 g a.i. ha−1 of paraquat or 750 g a.i. ha−1 of glufosinate-ammonium. Similarly, Chauhan et al. [19] controlled (i.e., ≤10% plant survival) eight to ten-leaf glyphosate-resistant C. virgata after applying greater than or equal to 750 g a.i. ha−1 of glufosinate-ammonium. The effectiveness of both paraquat (600 g a.i. ha−1) and glufosinate-ammonium (750 g a.i. ha−1) does, however, decrease for larger C. virgata plants [19]. This indicates that higher site-specific relevant application rates are required for the control of larger C. virgata plants. Regardless, in most instances, when glufosinate-ammonium and paraquat were increased to 1140 g a.i. ha−1 [11] or 740 g a.i. ha−1 [19], respectively, they were found to be comparable to glyphosate in controlling four to ten-leaf C. virgata. Control of six-leaf S. oleraceus using paraquat (400 g a.i. ha−1) and glufosinate-ammonium (500 g a.i. ha−1) has also been demonstrated for a glyphosate resistant biotype that was not controlled by 741 g a.i. ha−1 of glyphosate [10]. In the same study, the protoporphyrinogen oxidase (PPO) inhibitor saflufenacil controlled four-leaf S. oleraceus (≥15.9 g a.i. ha−1 application rates), but this rate did not control six-leaf plants [10]. Once again, highlighting the need to apply higher site-specific relevant rates. These studies indicate that there are herbicide treatments that can be used in place of glyphosate as site-specific treatments. The objective of this study was to evaluate herbicide treatments for their potential to be used instead of glyphosate to control Chloris virgata and Sonchus oleraceus populations when applied as site-specific treatments to older and difficult-to-control plants.
2. Materials and Methods
2.1. Weed Seed Source
To screen site-specific relevant herbicide treatments for their efficacy on C. virgata and S. oleraceus, a series of pot experiments were conducted in the outdoor growth facility on the Charles Sturt University (CSU) campus, Wagga Wagga (35.03° S, 147.20° E). Weed seed samples used in the pot experiments were sourced from field locations in the Wagga Wagga area (Table 1). Prior to use, seed germinability was assessed in a germination test, where 20–40 seeds were placed on Petri dishes filled with 0.6% (w/v) amyl media agar (n = 4) that were then stored at a constant 20 °C. Germinated seeds were counted and removed daily until there was no further germination seven days after the last germinated seed was recorded, and then seed germinability (percent germination) was calculated (Table 1).

Table 1.
Species name, collection location, date of collection and germinability for seed samples of C. virgata and S. oleraceus that were subsequently used in pot experiments. Planting, spraying and harvest dates for pot experiments examining the efficacy of alternative herbicide treatments on these two summer annual weed species conducted at Charles Sturt University, Wagga Wagga, NSW in 2022.
2.2. Experimental Design and Weed Propagation
Pot experiments were conducted from January to May 2022, with experiments repeated for each species (Table 1). Over this period, average minimum and maximum temperatures were 14 °C and 27 °C, respectively, and average daily solar exposure was 18 MJ m−2 (Figure 1). Prior to planting, 20 cm diameter pots (185 mm deep) were filled with garden loam (140 mm) over approximately 30 mm of gravel. A 12-well planting template was used to create evenly spaced 8 mm diameter holes to a consistent depth of 5 mm for weed seed planting. The weed seeds were covered with a 10 mm layer of coarse sand. Sixty pots were planted for each weed species. After planting, pots were placed in a hoop-house (70% UV block shade cloth) where they were regularly watered to maintain the pots near field capacity and were consistently fertilised with Thrive® (25% N, 5% P and 8.8% K w/w).
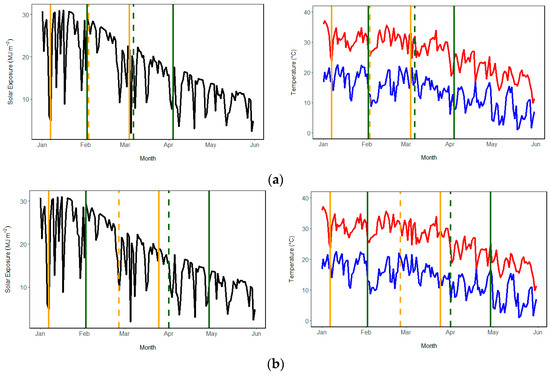
Figure 1.
Daily solar radiation and temperature data (from Wagga Wagga Airport) over the duration of the pot experiments for (a) Chloris virgata and (b) S. oleraceus. Left-hand side panels represent daily solar exposure (MJ m−2), and right-hand side panels represent daily minimum (blue) and maximum (red) temperatures [23,24]. Solid orange lines denote the start (planting) and finish (harvest) of Experiment 1, and solid green lines denote the start and finish of Experiment 2. Dashed lines denote the day of spray application for Experiment 1 (orange) and Experiment 2 (green).
For each weed species experiment, once weed seedlings were established (1 to 5 true leaves), pots were moved into the grow-out area where seedling density was thinned to 12 plants per pot. The grow-out area was covered by a retractable shade-cloth roof which remained over the seedlings for 3–6 days; after this period the plants received full sunlight for the remainder of the experiment.
2.3. Application of Herbicide Treatments
C. virgata plants were sprayed once stem elongation had commenced (Z-31) [25], and all S. oleraceus plants were sprayed at the commencement of flowering (BBCH-55) [26]. Environmental conditions on the day of spray application varied between experiments (Table 2).

Table 2.
Relative humidity at 3 pm (%), solar exposure (MJ m−2) and minimum and maximum temperatures (°C) at Wagga Wagga Airport [23,24] on the day of spray application for each weed species and experiment.
Herbicides and rates selected for testing were largely chosen based on the current label and potential future registrations for use on at least one of the weed species being screened (Table 3). Herbicide treatments were applied in a spray cabinet using a single even-fan nozzle (Teejet® DG95015EVS; Newton, Vic., Australia) at a water delivery rate of 98 L ha−1 (210 kPa, 5.9 km h−1). It should be noted that an application error for two treatments in C. virgata-R1 resulted in saflufenacil + trifludimoxazin being applied at 18 and 9 g a.i. ha−1 (Table 3).

Table 3.
Herbicides, application rates and adjuvants used for each treatment applied.
To avoid exposing plants to rain post-herbicide application, C. virgata-R2 and both experiments of S. oleraceus were placed undercover in a hoop-house for at least 6 h before being moved back to the grow-out area. With no threat of rain, treated pots in the C. virgata-R1 experiment were immediately returned to the grow-out area.
2.4. Data Collection and Analysis
Visual plant injury was assessed weekly up until 28 days after herbicide application. Plant injury was defined as the proportion of desiccated/necrotic tissue across all plants in a pot relative to the untreated control and was recorded as a percentage (i.e., 0% = no visible injury to 100% = no living plants). Plant survival in each pot was assessed 28 days after treatment where plants with chlorotic growing points were identified as controlled, while growing points that were unaffected or with new growth were identified as survived. Surviving plants were then harvested by cutting off at ground level. The harvested plants were placed into paper bags, one for each pot, which were then dried in a dehydrator for approximately 72 h at 70 °C. Dried samples were weighed and dry biomass per surviving plant was calculated for each pot. These methods were repeated across all weed species and experiments.
In the grow-out area, pots were arranged in a randomised complete block design created in RStudio, Version 4.1.2 [27] using the “design.rcbd” function in the agricolae package, Version 1.3.5 [28]. This design accounted for 13 herbicide treatments (including a control) in four blocks. A blocking design was used to account for any spatial variation such as shading. Statistical analysis was conducted in RStudio [27], using ggplot, Version 3.3.5 [29] and SigmaPlot (SigmaPlot 14.0; Systat Software Inc., San Jose, CA, USA) for data presentation. Paired t-tests were performed on the plant biomass data between experiments for both C. virgata and S. oleraceus to determine whether the datasets could be combined. To conduct the same analysis on the proportional datasets, separate generalised linear mixed models with logit link-functions considering either visual plant injury or plant survival as a function of the experiment were built [30]. In all generalised linear mixed models, experimental blocks were considered random effects. Applications containing saflufenacil + trifludimoxazin were removed for these analyses in the case of C. virgata due to an inconsistency in application rates between experiments (as noted in Table 3). For C. virgata, significant differences between experiments were observed in the plant biomass, injury and survival data (p < 0.05). For S. oleraceus, the plant injury and survival data exhibited significant differences between experiments (p < 0.05), whilst plant biomass data were not significantly different between experiments (p > 0.05). Since differences between experiments existed in a majority of instances, all experiments for C. virgata and S. oleraceus were analysed separately.
One-way analyses of variance (ANOVA) with blocking terms were constructed to analyse pot biomass data. Plant survival data underwent an arcsine square-root transformation before being analysed using generalised least square (GLS) regression through the nlme package [31], summarised as:
arcsine(Survival(1/2)) = μ + (Herbicide Treatment effect) + (Block effect) + ε
Notably, model assumptions were not met for plant survival in C. virgata-R1 and so no analysis took place in this instance. Visual plant injury data also underwent an arcsine square-root transformation prior to being analysed using a GLS regression which incorporated weighted variance structures and compound symmetry correlation structures, using the nlme package, Version 3.1.153 [31]. These structures ensured that the model residuals maintained heterogeneity and were independent (which was necessary with the repeated measures design). The model built to analyse the plant injury data can be summarised as:
arcsine(Injury(1/2)) = μ + (Herbicide Treatment effect) + (Time effect) +
(Herbicide Treatment × Time effect) + (Block effect) + ε
Separate models were built for both weed species and experiments. All GLS regression models underwent an analysis of deviance to consider the significance of each model effect. Post hoc analyses were conducted using either the HSD.test function (for ANOVA) from the agricolae package [28] or the cld function (for GLS) from the multcomp package, Version 1.4.19 [32], both of which adjust for family-wise error using the Tukey method.
3. Results
3.1. Plant Survival
None of the herbicides evaluated, including glyphosate, consistently controlled (i.e., ≤10% plant survival) both summer weed species (C. virgata and S. oleraceus) across both experimental runs (Table 4). In general, herbicide treatments failed to control the large established plants (i.e., late tillering for grass weeds and flowering for broadleaf weeds) at growth stages typical of when site-specific spot-spray treatments are applied. Overall, effective control (<10% survival) was only achieved in eight instances, seven of which were in S. oleraceus-R1 (glufosinate-ammonium, paraquat, saflufenical, saflufenical + trifludimoxazin and paraquat + PPO inhibitor mixtures), and also glyphosate in C. virgata-R2 (Table 4). Differences in herbicide efficacy between experiments for C. virgata and S. oleraceus were likely due in part to contrasting humidity and cloud cover conditions between respective herbicide application timings that likely lead to variations in herbicide uptake and translocation [33,34,35]. Clearly, though, the evaluated herbicides and application rates would be not suited for routine use as spot-spraying treatments on older/larger weeds.

Table 4.
Plant survival at 28 days after application of post-emergence herbicide treatments applied to Chloris virgata at stem elongation and Sonchus oleraceus at flowering. Presented means are back transformed. Significant differences (p < 0.05) between treatments within the same column are indicated by differing letters. Model root mean square errors are also presented for each experiment.
3.2. Chloris virgata Growth
Glyphosate was the most effective herbicide in reducing growth and causing damage to C. virgata plants. In both C. virgata-R1 and C. virgata-R2, glyphosate resulted in the largest biomass reductions, 91% and 98%, and the highest plant injuries of 78% and 99%, respectively (Table 5). Glufosinate-ammonium was the next most effective herbicide; however, its efficacy was inconsistent with greater levels of growth suppression and plant damage during the generally warmer, more humid conditions on the day of spray application in C. virgata-R2 (Table 2). In C. virgata-R2, the only other herbicides to produce substantial reductions (72 to 81%) in plant biomass were paraquat alone or in combination with PPO inhibitors (i.e., saflufenacil, tiafenacil and saflufenacil + trifludimoxazin) (Table 5). However, as the plant injury ratings indicate, C. virgata plants did recover from these treatments (Figure 2).

Table 5.
Biomass of surviving Chloris virgata plants and visual plant injury at 28 days after the application (DAA) of post-emergence herbicide treatments to plants at stem elongation in two experiments conducted over the summer/autumn. Presented means for visual plant injury are back transformed. Significant differences (p < 0.05) between treatments within the same column are indicated by differing letters. Model root mean square errors are also presented for each experiment.
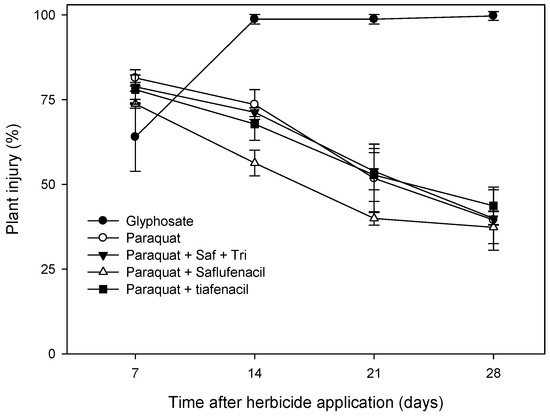
Figure 2.
Visual plant injury assessments conducted every 7 days for 28 days after applications of paraquat, glyphosate and mixtures of paraquat and protoporphyrinogen oxidase inhibitors on stem-elongating Chloris virgata plants grown in pots in the outdoor growth facility at Charles Sturt University, Wagga Wagga, NSW in 2022. To highlight the differences in speed of activity and to identify recovery from specific herbicide treatment effects, the data presented are from the second (C. virgata-R2) of the repeated studies evaluating herbicides for potential use as spot-spray fallow weed control treatments. Presented means are back transformed. Error bars represent the standard error around the mean of three or four replicates. ‘Saf + tri’ refers to ‘Saflufenacil + Trifludimoxazin’.
3.3. Sonchus oleraceus Growth
Herbicide treatment effects on S. oleraceus plant growth and resulting plant injury levels were inconsistent between S. oleraceus-R1 and S. oleraceus-R2, indicating a strong environmental influence on efficacy. Paraquat and PPO inhibitor treatments (except tiafenacil) and their combinations were markedly more damaging to plant growth in the first experimental run than in the second, where the maximum biomass reduction from an alternative herbicide was only 76% (Table 6). In contrast, glyphosate was more effective in the second experiment than in the first. Saflufenacil and paraquat were more rapid-acting than glyphosate on S. oleraceus plants in S. oleraceus-R1, reaching maximum visual plant injury at 14 days after application (Figure 3). The addition of paraquat to PPO inhibitors generally led to greater suppression of plant growth, particularly for tiafenacil (from 38% biomass reduction to 82%) and saflufenacil + trifludimoxazin (from 86% biomass reduction to 100%) in S. oleraceus-R1.

Table 6.
Biomass of surviving Sonchus oleraceus plants and visual plant injury at 28 days after application (DAA) of post-emergence herbicide treatments to plants at flowering in two experiments conducted over the summer/autumn. Presented means for visual plant injury are back transformed. Within a column, treatments with the same letters are not significantly different (p > 0.05). Model root mean square errors are also presented for each experiment.
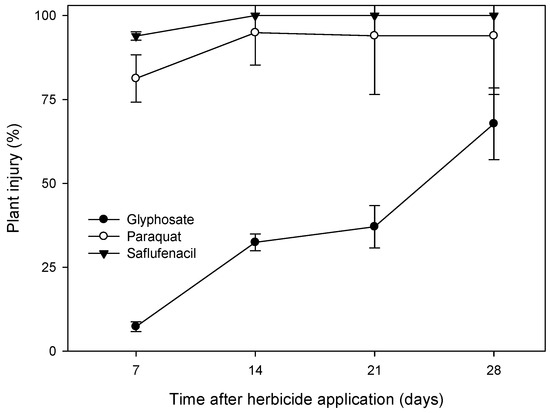
Figure 3.
Visual plant injury assessments conducted every 7 days for 28 days after applications of saflufenacil, paraquat and glyphosate on flowering Sonchus oleraceus plants grown in pots in the outdoor growth facility at Charles Sturt University, Wagga Wagga, NSW in 2022. To highlight the differences in speed of activity and to identify recovery from specific herbicide treatment effects, the data presented are from the first (S. oleraceus-R1) of the repeated studies evaluating herbicides for potential use as spot-spray fallow weed control treatments. Presented means are back transformed. Error bars represent the standard error around the mean of four replicates.
4. Discussion
The reduced herbicide efficacy on generally larger and older weed plants resulted in generally poor and inconsistent weed control by all evaluated treatments; consequently, a suitable alternative to glyphosate was not identified. All herbicides failed to consistently control generally larger and older C. virgata and S. oleraceus plants. This lack of efficacy was more evident when environmental conditions were less conducive for herbicide uptake and activity. For both paraquat and PPO inhibitors, the lower light conditions experienced in S. oleraceus-R1 (Table 2) potentially decreased the immediate destruction of leaf and vascular tissue, improving herbicide mobility [34,35] and, ultimately, efficacy (Table 4). Conversely, the higher light conditions in S. oleraceus-R2 likely limited the efficacy of both paraquat and PPO inhibitor herbicides. This is less likely for glufosinate-ammonium, which has been shown to be more effective when exposed to light after spray application [36] and is not limited by its own fast activity [37]. Instead, lower relative humidity on the day of spray application for S. oleraceus-R2 potentially limited the translocation and efficacy of glufosinate-ammonium [33] relative to S. oleraceus-R1. The substantial size of C. virgata [19] at the time of spray application is potentially responsible for failed control of these species (Table 4) by treatments that were effective on plants in S. oleraceus-R1. These fast-acting ‘contact’ herbicides [36,38,39] were potentially ‘diluted’ amongst the large C. virgata plants [40]. As a result, these large plants were able to withstand and outgrow applications of contact herbicides (Figure 2). It should also be noted that there are likely several metabolic [41] and physiochemical [42] differences between the weed species used in the present study that may have also contributed to the inconsistent response of alternate herbicides. More broadly, whilst reasons can be speculated for many of the inconsistencies in herbicide efficacy observed in the present study, these reasons do not excuse inconsistent weed control. The lack of consistency shown by alternate treatments in the present study highlights their inadequacy for completely replacing glyphosate in site-specific summer fallow weed control.
Glyphosate was generally more efficient than any of the individual herbicides assessed in these studies, with the implication that this herbicide will continue to be relied on for SSWC despite a proliferation of glyphosate resistance throughout northern Australian cropping regions [13]. In this study, glyphosate was the only treatment to control C. virgata (Table 4) and has previously been at least comparable to a variety of alternate treatments on a susceptible C. virgata biotype [11]. This also somewhat contradicts research by Desai et al. [11], who found that paraquat and glufosinate-ammonium were more effective than glyphosate on smaller C. virgata plants, highlighting the need for higher application rates of paraquat and glufosinate-ammonium than those used in the present study when controlling larger C. virgata plants. Despite not providing control, glyphosate was as or more effective than all alternate herbicide treatments in S. oleraceus-R2 (Table 4), similar to the results of Chauhan and Jha [10] on susceptible S. oleraceus seedlings. It should, however, be acknowledged that the same level of effectiveness was not consistent between experiments for C. virgata and S. oleraceus, where seasonal temperature differences (Figure 1) likely influenced the efficacy of glyphosate [10,43]. The results from this present study suggest that glyphosate will, concerningly, remain the primary option for site-specific fallow weed control.
The ever-present threat of glyphosate resistance [13] dictates the need for further research aimed at identifying alternatives. This should include investigating the use of increased application rates, above those used in this investigation (Table 3), to control large summer fallow weeds. Previous research indicates that contact herbicides, such as glufosinate-ammonium and saflufenacil, are rate responsive in controlling C. virgata [19] and S. oleraceus [10], respectively, suggesting that efficacy would improve at elevated rates. Further research should also include the evaluation of a broader range of alternate non-selective herbicide options, such as glufosinate + PPO inhibitors [44] and paraquat + isoxaflutole [19], as well as sequential applications of alternative herbicides [45], in the context of SSWC.
5. Conclusions
The outcomes of the research presented here highlight the challenge of identifying alternative herbicide options that can be used in place of glyphosate for site-specific fallow weed control. No specific replacement for glyphosate was identified, and indeed, no alternative herbicide treatments for specific summer weed species were found. It is evident that a direct replacement of glyphosate is highly unlikely. Therefore, there is a need for a concerted industry-wide effort towards identifying alternatives, with consideration of physical as well as chemical options for summer fallow weed control. Without effective alternative weed control options, the occurrence and frequency of glyphosate resistance will continue to grow such that this herbicide will soon no longer be an option for weed control in Australian cropping systems.
Author Contributions
Conceptualization, A.M. and J.C.B.; Data curation, A.M.; Formal analysis, A.M.; Investigation, A.M.; Methodology, A.M., J.C.B. and M.J.W.; Resources, A.M. and J.C.B.; Supervision, J.C.B. and M.J.W.; Writing—original draft, A.M.; Writing—review and editing, J.C.B. and M.J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grains Research and Development Corporation, grant number US00084. The GRDC had no involvement in the study design; collection, analysis or interpretation of data; or in writing the manuscript.
Data Availability Statement
The data that support this study are available in the article and upon request to the corresponding author.
Acknowledgments
The authors would like to acknowledge Allison Chambers for their technical assistance with the maintenance of the pot trials at Charles Sturt University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hunt, J.R.; Kirkegaard, J.A. Re-evaluating the contribution of summer fallow rain to wheat yield in Southern Australia. Crop Pasture Sci. 2011, 62, 915–929. [Google Scholar] [CrossRef]
- Zeleke, K.T. Fallow management increases soil water and nitrogen storage. Agric. Water Manag. 2017, 186, 12–20. [Google Scholar] [CrossRef]
- Hunt, J.R.; Browne, C.; McBeath, T.M.; Verburg, K.; Craig, S.; Whitbread, A.M. Summer fallow weed control and residue management impacts on winter crop yield though soil water and N accumulation in a winter-dominant, low rainfall region of southern Australia. Crop Pasture Sci. 2013, 64, 922–934. [Google Scholar] [CrossRef]
- Zeleke, K.T. Effect of summer fallow management on crop yield: Field experiment and simulation analysis. Agric. Water Manag. 2018, 203, 405–410. [Google Scholar] [CrossRef]
- Llewellyn, R.S.; D’Emden, F.H.; Kuehne, G. Extensive use of no-tillage in grain growing regions of Australia. Field Crops Res. 2012, 132, 204–212. [Google Scholar] [CrossRef]
- Osten, V.A.; Walker, S.R.; Storrie, A.; Widderick, M.; Moylan, P.; Robinson, G.R.; Galea, K. Survey of weed flora and management relative to cropping practices in the north-eastern grain region of Australia. Aust. J. Exp. Agric. 2007, 47, 57–70. [Google Scholar] [CrossRef]
- Steinrucken, H.C.; Amrhein, N. The Herbicide Glyphosate is a Potent Inhibitor of 5-Enolpyruvyl-Shikimic-Acid 3-Phosphate Synthase. Biochem. Biophys. Res. Commun. 1980, 94, 1207–1212. [Google Scholar] [CrossRef]
- Charles, G. A grower survey of weeds and herbicide use in the New South Wales cotton industry. Aust. J. Exp. Agric. 1991, 31, 387–392. [Google Scholar] [CrossRef]
- Heap, I.; Duke, S.O. Overview of glyphosate-resistant weeds worldwide. Pest Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Jha, P. Glyphosate Resistance in Sonchus oleraceus and Alternative Herbicide Options for Its Control in Southeast Australia. Sustainability 2020, 12, 8311. [Google Scholar] [CrossRef]
- Desai, H.S.; Thompson, M.; Chauhan, B.S. Target-Site Resistance to Glyphosate in Chloris virgata Biotypes and Alternative Herbicide Options for its Control. Agronomy 2020, 10, 1266. [Google Scholar] [CrossRef]
- Ndirangu Wangari, T.; Mahajan, G.; Chauhan, B.S. Glyphosate resistance in junglerice (Echinochloa colona) and alternative herbicide options for its effective control. Weed Technol. 2022, 36, 38–47. [Google Scholar] [CrossRef]
- Broster, J.C.; Jalaludin, A.; Widderick, M.J.; Chambers, A.J.; Walsh, M.J. Herbicide Resistance in Summer Annual Weeds of Australia’s Northern Grains Region. Agronomy 2023, 13, 1862. [Google Scholar] [CrossRef]
- Ngo, T.D.; Krishnan, M.; Boutsalis, P.; Gill, G.; Preston, C. Target-site mutations conferring resistance to glyphosate in feathertop Rhodes grass (Chloris virgata) populations in Australia. Pest Manag. Sci. 2018, 74, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, I.; Colosi, J.; Lewin, R.A. The Biology of Canadian Weeds.: 63. Sonchus asper (L.) Hill and S. oleraceus L. Can. J. Plant Sci. 1984, 64, 731–744. [Google Scholar] [CrossRef]
- Ngo, T.D.; Boutsalis, P.; Preston, C.; Gill, G. Growth, Development, and Seed Biology of Feather Fingergrass (Chloris virgata) in Southern Australia. Weed Sci. 2017, 65, 413–425. [Google Scholar] [CrossRef]
- Timmermann, C.; Gerhards, R.; Kühbauch, W. The Economic Impact of Site-Specific Weed Control. Precis. Agric. 2003, 4, 249–260. [Google Scholar] [CrossRef]
- Fischer, J.W.; Thorne, M.E.; Lyon, D.J. Weed-sensing technology modifies fallow control of rush skeletonweed (Chondrilla juncea). Weed Technol. 2020, 34, 857–862. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Congreve, M.; Mahajan, G. Management options for large plants of glyphosate-resistant feather fingergrass (Chloris virgata) in Australian fallow conditions. PLoS ONE 2021, 16, e0261788. [Google Scholar] [CrossRef]
- Davidson, B.; Cook, T.; Chauhan, B.S. Alternative Options to Glyphosate for Control of Large Echinochloa colona and Chloris virgata Plants in Cropping Fallows. Plants 2019, 8, 245. [Google Scholar] [CrossRef]
- Peteinatos, G.G.; Weis, M.; Andújar, D.; Rueda Ayala, V.; Gerhards, R. Potential use of ground-based sensor technologies for weed detection. Pest Manag. Sci. 2014, 70, 190–199. [Google Scholar] [CrossRef]
- Biller, R.H. Reduced Input of Herbicides by Use of Optoelectronic Sensors. J. Agric. Eng. Res. 1998, 71, 357–362. [Google Scholar] [CrossRef]
- Bureau of Meteorology. Daily Weather Observations for New South Wales. Available online: http://www.bom.gov.au/climate/dwo/IDCJDW0200.shtml (accessed on 8 August 2022).
- Bureau of Meteorology. Climate Data Online. Available online: http://www.bom.gov.au/climate/data/index.shtml?bookmark=193 (accessed on 15 August 2022).
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 10 October 2022).
- de Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 10 October 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. Available online: https://CRAN.R-project.org/package=nlme (accessed on 10 October 2022).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Coetzer, E.; Al-Khatib, K.; Loughin, T.M. Glufosinate efficacy, absorption, and translocation in amaranth as affected by relative humidity and temperature. Weed Sci. 2001, 49, 8–13. [Google Scholar] [CrossRef]
- Harre, N.T.; Duncan, G.W.; Young, J.M.; Young, B.G. Relating initial paraquat injury to final efficacy in selected weed species influenced by environmental conditions. Weed Technol. 2021, 35, 279–288. [Google Scholar] [CrossRef]
- Mellendorf, T.G.; Young, J.M.; Matthews, J.L.; Young, B.G. Influence of Application Variables on the Foliar Efficacy of Saflufenacil on Horseweed (Conyza canadensis). Weed Sci. 2015, 63, 578–586. [Google Scholar] [CrossRef]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. Physiological Factors Affecting Uptake and Translocation of Glufosinate. J. Agric. Food Chem. 2020, 68, 3026–3032. [Google Scholar] [CrossRef]
- Grossmann, K.; Schiffer, H. Protoporphyrinogen oxidase-inhibiting activity of the new, wheat-selective isoindoldione herbicide, cinidon-ethyl. Pestic. Sci. 1999, 55, 687–695. [Google Scholar] [CrossRef]
- Slade, P.; Bell, E.G. The Movement of Paraquat in Plants. Weed Res. 1966, 6, 267–274. [Google Scholar] [CrossRef]
- Ziska, L.H.; Faulkner, S.; Lydon, J. Changes in biomass and root:shoot ratio of field-grown Canada thistle (Cirsium arvense), a noxious, invasive weed, with elevated CO2: Implications for control with glyphosate. Weed Sci. 2004, 52, 584–588. [Google Scholar] [CrossRef]
- Van Eerd, L.L.; Hoagland, R.E.; Zablotowicz, R.M.; Hall, J.C. Pesticide metabolism in plants and microorganisms. Weed Sci. 2003, 51, 472–495. [Google Scholar] [CrossRef]
- Kirkwood, R.C. Recent developments in our understanding of the plant cuticle as a barrier to the foliar uptake of pesticides. Pestic. Sci. 1999, 55, 69–77. [Google Scholar] [CrossRef]
- Peerzada, A.M.; Williams, A.; O’Donnell, C.; Adkins, S.W. Effect of elevated temperature on growth and glyphosate susceptibility of Chloris truncata R.Br., Sonchus oleraceus L., and Conyza bonariensis (L.) Cronq. Arch. Agron. Soil Sci. 2021, 69, 358–373. [Google Scholar] [CrossRef]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. Glufosinate enhances the activity of protoporphyrinogen oxidase inhibitors. Weed Sci. 2020, 68, 324–332. [Google Scholar] [CrossRef]
- Widderick, M.; McLean, A. Optimal intervals differ for double knock application of paraquat after glyphosate or haloxyfop for improved control of Echinochloa colona, Chloris virgata and Chloris truncata. Crop Prot. 2018, 113, 1–5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).