Soybean Response to N Fertilization Compared with Co-Inoculation of Bradyrhizobium japonicum and Azospirillum brasilense
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Weather Conditions
2.3. Data Statistical Analysis
3. Results and Discussion
3.1. Plant Population
3.2. Plant Height
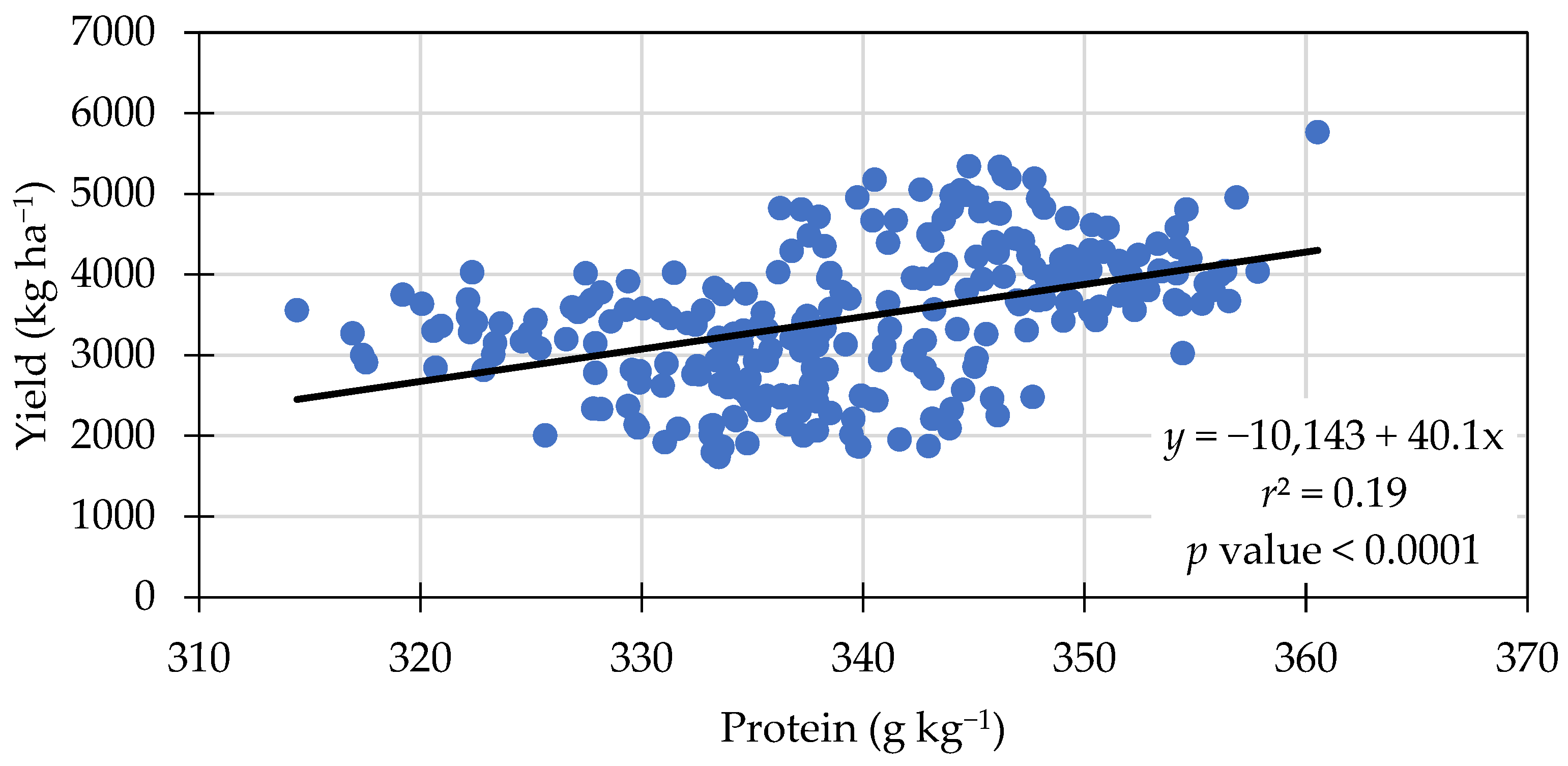
3.3. Protein Content
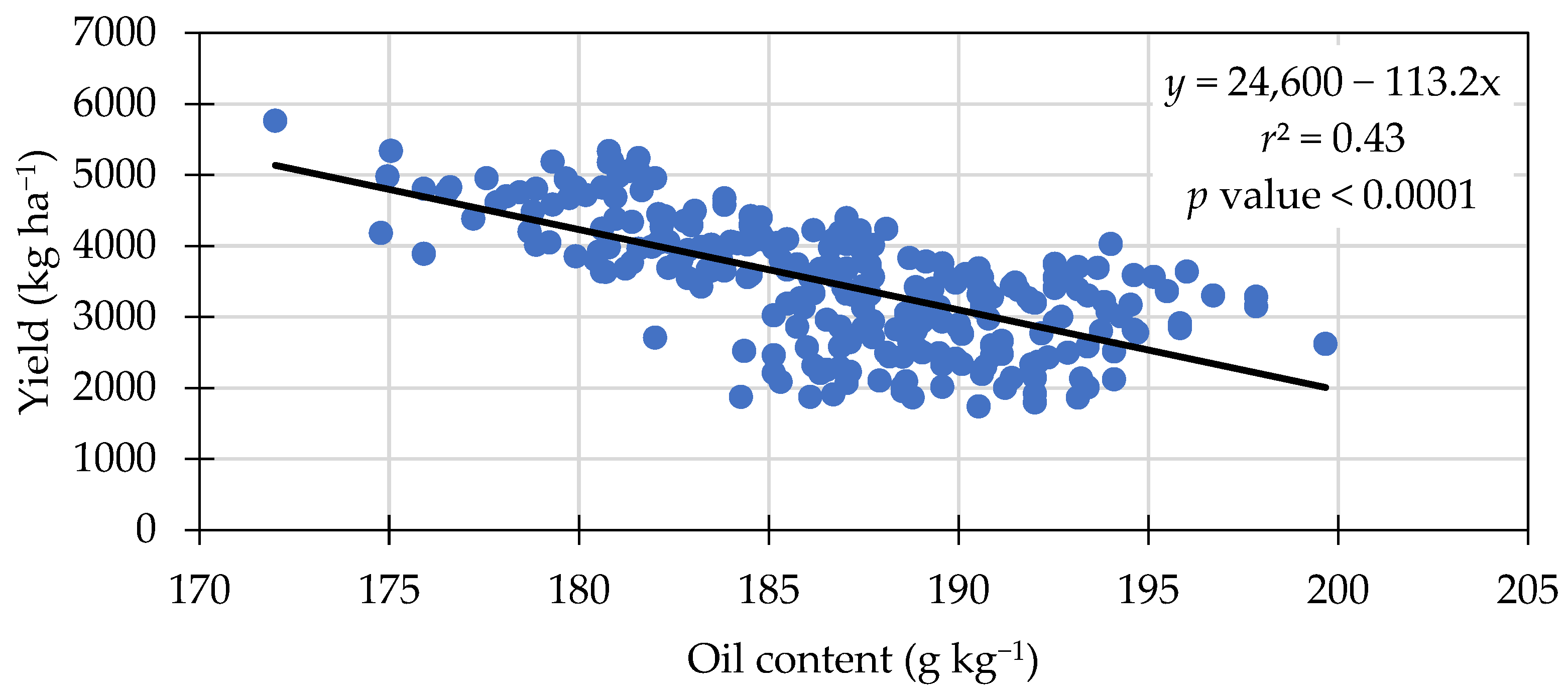
3.4. Oil Content
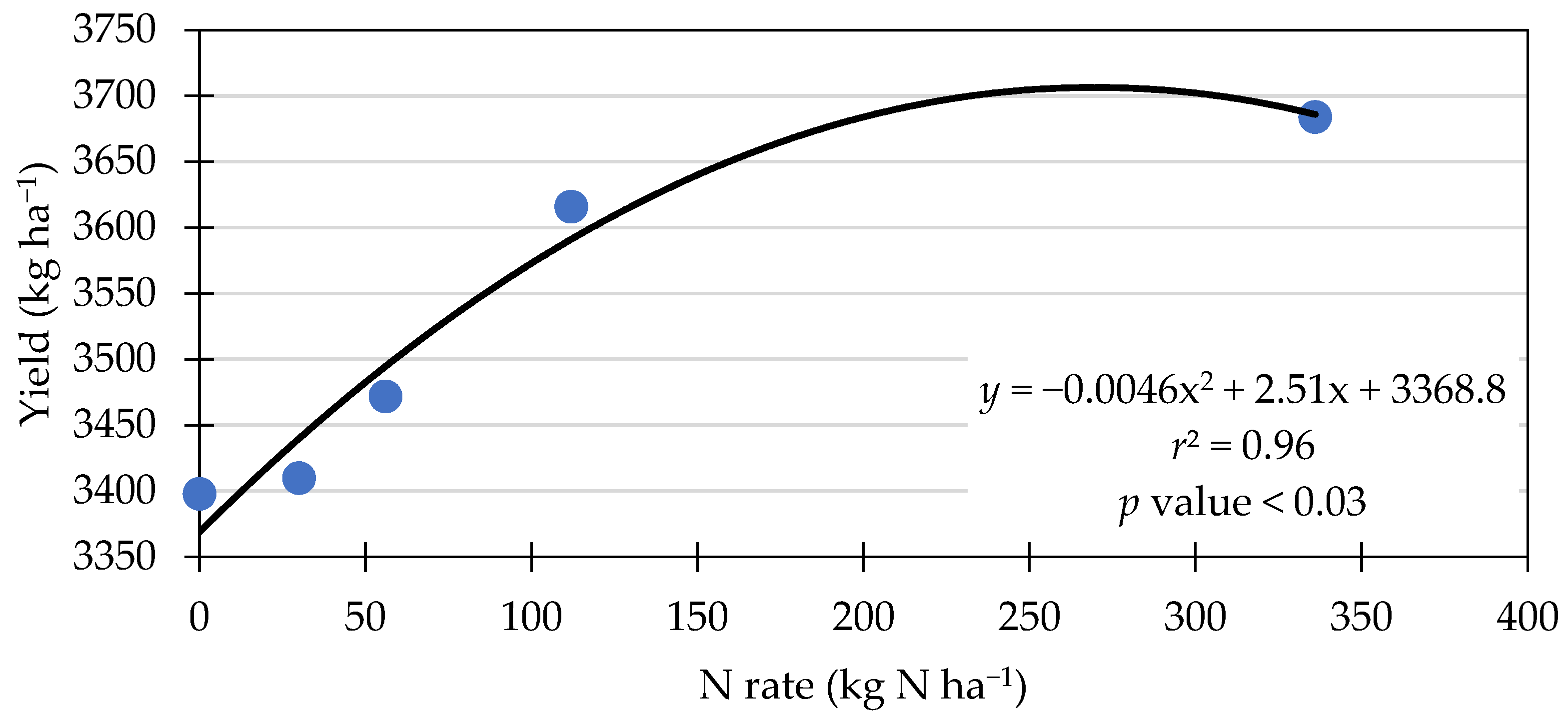
3.5. Seed Yield
3.6. Thousand-Seed Weight
3.7. Protein Yield per Hectare
3.8. Partial Profit Analysis
3.9. Co-Inoculation with Azospilillum
3.10. Nodulation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA National Agricultural Statistics Service. Quick Stats. 2023. Available online: https://quickstats.nass.usda.gov/ (accessed on 10 June 2023).
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Doberman, A. Nitrogen uptake, fixation, and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Vitti, G.; Trevisan, W. Agricultural management of grain legumes; has it led to an increase in nitrogen fixation? Field Crops Res. 2000, 65, 165–181. [Google Scholar] [CrossRef]
- Cigelske, B.D.; Kandel, H.; DeSutter, T.M. Soybean nodulation and plant response to Nitrogen and Sulfur fertilization in the northern US. Agric. Sci. 2020, 11, 592–607. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, glycine max (L.) merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Zapata, F.; Danso, S.K.A.; Hardarson, G.; Fried, M. Time course of nitrogen fixation in field-grown soybean using nitrogen-15 methodology. Agron. J. 1987, 79, 172–176. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Tamagno, S.; Sadras, V.O.; Haegele, J.W.; Armstrong, P.R.; Ciampitti, I.A. Interplay between nitrogen fertilizer and biological nitrogen fixation in soybean: Implications on seed yield and biomass allocation. Sci. Rep. 2018, 8, 17502. [Google Scholar] [CrossRef]
- Chibeba, A.M.; Guimarães, M.F.; Brito, O.R.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Co-inoculation of soybean with Bradyrhizbium and Azospirillum promotes early nodulation. Am. J. Plant Sci. 2015, 6, 1641–1649. [Google Scholar] [CrossRef]
- Bashan, Y.; Levanony, H. Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can. J. Microbiol. 1990, 36, 591–608. [Google Scholar] [CrossRef]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbial. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, D.N.; Oliver, I.M.; Contreras, M.A.; Ruiz-Sainz, J.E. Soybean interactions with soil microbes, agronomical and molecular aspects. Agron. Sustain. Dev. 2011, 31, 173–190. [Google Scholar] [CrossRef]
- Galal, Y. Dual inoculation with strains of Bradyrhizobium japonicum and Azospirillum brasilense to improve growth and biological Nitrogen fixation of soybean (Glycine max L.). Biol. Fertil. Soils 1997, 24, 317–322. [Google Scholar] [CrossRef]
- Groppa, M.D.; Zawoznik, M.S.; Tomaro, M.L. Effect of co-inoculation with Bradyrhizobium japonicum and Azospirillum brasilense on soybean plants. Eur. J. Soil Biol. 1998, 34, 75–80. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Co-inoculation of soybeans and common beans with rhizobia and azospirilla: Strategies to improve sustainability. Biol. Fertil. Soils 2013, 49, 791–801. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. Am. J. Plant Sci. 2015, 6, 811–817. [Google Scholar] [CrossRef]
- Rego, C.H.Q.; Cardoso, F.B.; Cândido, A.C.S.; Teodoro, P.E.; Alves, C.Z. Co-inoculation with Bradyrhizobium and Azospirillum increases yield and quality of soybean seeds. Agron. J. 2018, 110, 2302–2309. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Hungria, M.; da Silva Sena, J.V.; Poggere, G.; dos Reis, A.R.; Correa, R.S. Meta-analysis reveals benefits of co-inoculation of soybean with Azospirillum brasilense and Bradyrhizobium spp. in Brazil. Appl. Soil Ecol. 2021, 163, 103913. [Google Scholar] [CrossRef]
- De Borja Reis, A.F.; Moro Rosso, L.H.; Adee, E.; Davidson, D.; Kovács, P.; Purcell, L.C.; Below, F.E.; Casteel, S.N.; Knott, C.; Kandel, H.; et al. Seed inoculation with Azospirillum brasilense in the U.S. soybean systems. Field Crops Res. 2022, 283, 108537. [Google Scholar] [CrossRef]
- Hardarson, G.; Zapata, F.; Danso, K.S.A. Effect of plant genotype and nitrogen fertilizer on symbiotic fixation by soybean cultivars. Plant Soil 1984, 82, 397–405. [Google Scholar] [CrossRef]
- Buttery, B.R.; Park, S.J.; Hume, D.J. Potential for increasing nitrogen fixation in grain legumes. Can. J. Plant Sci. 1992, 72, 323–349. [Google Scholar] [CrossRef]
- Purcell, L.C.; King, C.A. Drought and nitrogen source effects on nitrogen nutrition, seed growth, and yield in soybean. J. Plant Nutr. 1996, 19, 969–993. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Westgate, M.E. Meta-analysis of environmental effects on soybean seed composition. Field Crops Res. 2009, 110, 147–156. [Google Scholar] [CrossRef]
- Kandel, H.J.; Brodshaug, J.A.; Steele, D.D.; Ransom, J.K.; DeSutter, T.M.; Sands, G.R. Subsurface drainage effects on soil penetration resistance and water table depth on a clay soil in the Red River of the North Valley, USA. Agric. Eng. Int. CIGR J. 2013, 15, 1–10. [Google Scholar]
- USDA. Web Soil Survey. 2023. Available online: http://websoilsurvey.sc.egov.usda.gov/ (accessed on 10 June 2023).
- Kandel, H.; Endres, G. Soybean Production Field Guide for North Dakota; NDSU Ext. Ser. Pub. A1172 (Revised); North Dakota State University: Fargo, ND, USA, 2023; Available online: https://www.ndsu.edu/agriculture/sites/default/files/2023-01/a1172.pdf (accessed on 28 July 2023).
- MRGA, Maple River Grain and Agronomy. Cash Bids. 2023. Available online: https://www.mrga.com/grain/cash-bids (accessed on 18 April 2023).
- Progressive Farmer. Fertilizer Markets. 2023. Available online: https://www.dtnpf.com/agriculture/web/ag/home (accessed on 18 April 2023).
- NDAWN North Dakota Agricultural Weather Network. 2023. Available online: https://ndawn.ndsu.nodak.edu/ (accessed on 10 June 2023).
- Cigelske, B.D. Soybean response to Nitrogen and Sulfur fertilization. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2016. Available online: https://library.ndsu.edu/ir/bitstream/handle/10365/28249/Cigelske_ndsu_0157N_11674.pdf (accessed on 28 July 2023).
- Ouyang, D.; MacKenzie, A.F.; Fan, M. Phytotoxicity of banded urea amended with triple superphosphate and potassium chloride. Agron. J. 1998, 90, 734–739. [Google Scholar] [CrossRef]
- Pan, W.L.; Madsen, I.J.; Bolton, R.P.; Graves, L.; Sistrunk, T. Ammonia/ammonium toxicity root symptoms induced by inorganic and organic fertilizers and placement. Agron. J. 2016, 108, 2485–2492. [Google Scholar] [CrossRef]
- Wan, X.; Wu, W.; Li, C.; Liu, Y.; Wen, X.; Liao, Y. Soil ammonia volatilization following urea application suppresses root hair formation and reduces seed germination in six wheat varieties. Environ. Exp. Bot. 2016, 132, 130–139. [Google Scholar] [CrossRef]
- Bhangu, R.; Virk, H.K. Nitrogen management in soybean: A review. Agri. Rev. 2019, 40, 129–135. [Google Scholar] [CrossRef]
- Cafaro La Menza, N.; Grassini, P.; Glewen, K.; Mamo, M.; Pryor, R.; Seymour, R.; Nygren, A.; Mueller, N.; Naeve, S. What have we learned about soybean seed constituents in irrigated and dryland producer fields in Nebraska? Cropwatch 2021—Univ. of Nebraska—Lincoln. Available online: https://cropwatch.unl.edu/2021/what-have-we-learned-about-soybean-seed-constituents-irrigated-and-dryland-producer-fields (accessed on 28 July 2022).
- Voldeng, H.D.; Cober, E.R.; Hume, D.J.; Gillard, C.; Morrison, M.J. Fifty-eight years of genetic improvement of short-season soybean cultivars in Canada. Crop Sci. 1997, 37, 428–431. [Google Scholar] [CrossRef]
- Morrison, M.J. Agronomic changes from 58 years of genetics improvement of short-season soybean cultivars in Canada. Agron. J. 2000, 92, 780–784. [Google Scholar] [CrossRef]
- Macák, M.; Candráková, E.; Hanáčková, E. The seed quality and yield of soybean varieties in dependence of growing conditions. Res. J. Agric. Sci. 2010, 42, 158–161. [Google Scholar]
- Favoretto, V.R. A Meta-Analysis of How Management Practices Affect Soybean Yield and Quality. Master’s Thesis, University of Illinois, Urbana, IL, USA, 2019. Available online: https://www.researchgate.net/publication/361264958_A_META-ANALYSIS_OF_HOW_MANAGEMENT_PRACTICES_AFFECT_SOYBEAN_YIELD_AND_QUALITY (accessed on 28 July 2023).
- Haq, M.U.; Mallarino, A.P. Response of soybean grain oil and protein concentrations to foliar and soil fertilization. Agron. J. 2005, 97, 910–918. [Google Scholar] [CrossRef]
- Brar, N.; Lawley, Y. Short-season soybean yield and protein unresponsive to starter nitrogen fertilizer. Agron. J. 2020, 112, 5012–5023. [Google Scholar] [CrossRef]
- Osborne, S.L.; Riedell, W.E. Starter nitrogen fertilizer impact on soybean yield and quality in the northern Great Plains. Agron. J. 2006, 98, 1569. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Kaur, G.; Orlowski, J.M.; Shapiro, C.A.; Lee, C.D.; Wortmann, C.; Holshouser, D.; Nafziger, E.D.; Kandel, H.; Niekamp, J.; et al. Soybean response to nitrogen application across the United States: A synthesis-analysis. Field Crops Res. 2018, 215, 74–82. [Google Scholar] [CrossRef]
- Shapiro, C.A.; Wortmann, C.S. Corn response to nitrogen rate, row spacing and plant density in Eastern Nebraska. Agron. J. 2006, 98, 529–535. [Google Scholar] [CrossRef]
- Marcos Brignoli, F.; de Oliveira Zampar, E.J.; Henrique Vieira de Almeida, J.; Cassim, B.M.A.R.; Inoue, T.T.; Batista, M.A. Effect of different methods of inoculation and co-inoculation of Bradyrhizobium spp. and Azospirillum brasilense on soybean agronomic performance in fields with a history of inoculation. Arch. Agron. Soil Sci. 2023, 69, 2925–2937. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Bobrecka-Jamro, D.; Pikuła, W.; Jańczak-Pieniążek, M. Effect of Nitrogen fertilization and inoculation with Bradyrhizobium Japonicum on nodulation and yielding of soybean. Agronomy 2023, 13, 1341. [Google Scholar] [CrossRef]
- Streeter, J.G. Inhibition of legume nodule formation and N2 fixation by nitrate. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Gibson, A.H.; Harper, J.E. Nitrate effect on nodulation of soybean by Bradyrhizobium japonicum 1. Crop Sci. 1985, 25, 497–501. [Google Scholar] [CrossRef]
- Layzell, D.B.; Moloney, A.H. Dinitrogen fixation. In Physiology and Determination of Crop Yield; Amer Society of Agronomy: Madison, WI, USA, 1994; pp. 311–335. [Google Scholar]
- Mendes, I.C.; Hungria, M.; Vargas, M.A.T. Soybean response to starter Nitrogen and Bradyrhizobium inoculation on a Cerrado Oxisol under no-tillage and conventional tillage systems. Rev. Bras. De Ciência Do Solo 2003, 27, 81–87. [Google Scholar] [CrossRef]
- Hungria, M.; Franchini, J.C.; Campo, R.J.; Crispino, C.C.; Moraes, J.Z.; Sibaldelli, R.N.R.; Mendes, I.C.; Arihara, J. Nitrogen nutrition of soybean in Brazil: Contributions of biological N2 fixation and N fertilizer to grain yield. Can. J. Plant Sci. 2006, 86, 927–939. [Google Scholar] [CrossRef]
- Ohyama, T.; Fujikake, H.; Yashima, H.; Tanabata, S.; Ishikawa, S.; Sato, T.; Nishiwaki, T.; Ohtake, N.; Sueyoshi, K.; Ishii, S.; et al. Effect of Nitrate on Nodulation and Nitrogen Fixation of Soybean. In Soybean Physiology and Biochemistry; El-Shemy, H., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 333–364. [Google Scholar] [CrossRef][Green Version]
- Xia, X.; Ma, C.; Dong, S.; Xu, Y.; Gong, Z. Effects of nitrogen concentrations on nodulation and nitrogenase activity in dual root systems of soybean plants. Soil Sci. Plant Nutr. 2017, 63, 470–482. [Google Scholar] [CrossRef]
- Bacanamwo, M.; Harper, J.E. The feedback mechanism of nitrate inhibition of nitrogenase activity in soybean may involve asparagines and/or products of its metabolism. Physiol. Plant 1997, 100, 371–377. [Google Scholar] [CrossRef]
- Gordon, A.J.; Skøt, L.; James, C.L.; Minchin, F.R. Short-term metabolic responses of soybean root nodules to nitrate. J. Exp. Bot. 2002, 53, 423–428. [Google Scholar] [CrossRef]
- Zeffa, D.M.; Fantin, L.H.; Koltun, A.; de Oliveira, A.L.M.; Nunes, M.P.B.A.; Canteri, M.G.; Goncalves, L.S.A. Effects of plant growth-promoting rhizobacteria on co-inoculation with Bradyrhizobium in soybean crop: A meta-analysis of studies from 1987 to 2018. Peer J. 2020, 8, e7905. [Google Scholar] [CrossRef]



| Location | Year | Previous Crop | Soil Series A | Soil Taxonomy |
|---|---|---|---|---|
| Casselton | 2021 | Wheat B | Kindred-Bearden | Fine–silty, mixed, superactive, frigid Typic Endoaquolls |
| Fine–silty, mixed, superactive, frigid Aeric Calciaquolls | ||||
| Fargo (1 and 2) | 2021 and 2022 | Wheat | Fargo-Ryan | Fine, smectitic, frigid Typic Epiaquerts |
| Fine smectitic, frigid Typic Natraquerts | ||||
| Lisbon | 2021 and 2022 | Corn C | Barnes-Svea | Fine–loamy, mixed, superactive, frigid Calcic Hapludolls |
| Fine–loamy, mixed, superactive, frigid Pachic Hapludolls | ||||
| Prosper | 2022 | Wheat | Kindred-Bearden | Fine–silty, mixed, superactive, frigid Typic Endoaquolls |
| Fine–silty, mixed, superactive, frigid Aeric Calciaquolls |
| Environment | Depth | NO3-N | P | K | SO4-S | pH A | OM B | EC C |
|---|---|---|---|---|---|---|---|---|
| cm | kg ha−1 | mg kg−1 | kg ha−1 | % | mmhos cm−1 | |||
| 2021 | ||||||||
| Casselton | 0–15 | 45 | 16 | 445 | 5 | 7.7 | 4.9 | 0.97 |
| 15–60 | 47 | 4 | 225 | 72 | 7.9 | 3.6 | 1.58 | |
| Fargo 1 | 0–15 | 56 | 31 | 625 | 19 | 7.6 | 5.1 | 0.85 |
| 15–60 | 111 | 9 | 350 | 165 | 8.1 | 3.6 | 0.95 | |
| Fargo 2 | 0–15 | 41 | 32 | 575 | 12 | 7.6 | 5.4 | 0.77 |
| 15–60 | 101 | 14 | 355 | 30 | 8.0 | 3.7 | 0.85 | |
| Lisbon | 0–15 | 28 | 15 | 258 | 19 | 7.5 | 4.2 | 0.61 |
| 15–60 | 37 | 4 | 150 | 34 | 8.0 | 2.5 | 0.50 | |
| 2022 | ||||||||
| Fargo 1 | 0–15 | 10 | 26 | 497 | 16 | 8.0 | 5.4 | 0.40 |
| 15–60 | 20 | 3 | 425 | 81 | 7.9 | 3.5 | 0.71 | |
| Fargo 2 | 0–15 | 10 | 21 | 509 | 11 | 7.8 | 5.5 | 0.49 |
| 15–60 | 24 | 2 | 385 | 61 | 8.0 | 3.5 | 0.72 | |
| Lisbon | 0–15 | 4 | 20 | 285 | 13 | 6.2 | 4.8 | 0.24 |
| 15–60 | 30 | 4 | 230 | 40 | 7.2 | 2.6 | 0.37 | |
| Prosper | 0–15 | 1 | 39 | 241 | 11 | 6.4 | 4.1 | 0.20 |
| 15–60 | 10 | 4 | 193 | 34 | 7.9 | 2.2 | 0.35 | |
| Application Timing | Total N Applied | |
|---|---|---|
| Planting | R3 Growth Stage | |
| N Source | N Source | N Rate |
| kg ha−1 | kg ha−1 | |
| B. Japonicum | - | 0 |
| Azospirillum A | - | 0 |
| Zero N (0) B | - | 0 |
| AMS C (30) | - | 30 |
| Urea + AMS (28) | Urea + AMS (28) | 56 |
| Urea + AMS (56) | Urea + AMS (56) | 112 |
| Urea + AMS (168) | Urea + AMS (168) | 336 |
| Operation/Measurement | Casselton | Fargo 1 and 2 | Lisbon | Prosper |
|---|---|---|---|---|
| 2021 | ||||
| Soil test pre-plant characterization | 6 May | 10 May | 7 May | - |
| Planting/fertilizer application | 6 May | 10 May | 7 May | - |
| Stand count | 15 June | 16 June | 16 June | - |
| Nodule count R2 | - | 20 July | - | - |
| R3 fertilizer application | 15 July | 20 July | 20 July | - |
| Nodule count R6 | - | 30 Aug | - | - |
| Height measurement | 16 Sept | 23 Sept | 21 Sept | - |
| Harvest | 23 Sep | 29 Sep | 28 Sep | - |
| 2022 | ||||
| Soil test pre-plant characterization | - | 25 May | 24 May | 23 May |
| Planting/fertilizer application | - | 25 May | 24 May | 23 May |
| Stand count | - | 22 June | 21 June | 23 June |
| Nodule count R2 | - | 21 July | - | - |
| R3 fertilizer application | - | 27 July | 26 July | 25 July |
| Nodule count R6 | - | 5 Sept | - | - |
| Height measurement | - | 26 Sept | 23 Sept | 26 Sept |
| Harvest | - | 3 Oct | 5 Oct | 4 Oct |
| Average Air Temp | Precipitation | ||||||
|---|---|---|---|---|---|---|---|
| Month | 2021 | 2022 | Normal A | 2021 | 2022 | Normal | |
| °C | mm | ||||||
| Casselton and Prosper | |||||||
| May | 13 | 13 | 13 | 20 | 103 | 78 | |
| June | 22 | 21 | 19 | 48 | 93 | 109 | |
| July | 22 | 22 | 21 | 25 | 85 | 91 | |
| Aug | 21 | 20 | 20 | 47 | 56 | 67 | |
| Sept | 18 | 16 | 15 | 76 | 24 | 69 | |
| Average | 19 | 18 | 18 | Total | 216 | 361 | 413 |
| Fargo | |||||||
| May | 14 | 13 | 14 | 9 | 87 | 83 | |
| June | 23 | 21 | 19 | 89 | 67 | 109 | |
| July | 24 | 23 | 22 | 18 | 97 | 84 | |
| Aug | 22 | 21 | 20 | 76 | 68 | 66 | |
| Sept | 18 | 17 | 16 | 83 | 14 | 69 | |
| Average | 20 | 19 | 18 | Total | 276 | 332 | 411 |
| Lisbon | |||||||
| May | 13 | 13 | 13 | 28 | 105 | 79 | |
| June | 22 | 21 | 19 | 108 | 53 | 86 | |
| July | 23 | 22 | 21 | 23 | 65 | 73 | |
| Aug | 22 | 21 | 20 | 52 | 45 | 67 | |
| Sept | 18 | 16 | 15 | 44 | 9 | 65 | |
| Average | 20 | 19 | 18 | Total | 255 | 276 | 371 |
| N | PP A | HT | PC | OC | Yield | SWT | PY | Partial Profit B |
|---|---|---|---|---|---|---|---|---|
| kg ha−1 | Plants ha−1 | cm | g kg−1 | g kg−1 | kg ha−1 | g | kg ha−1 | USD/ha−1 |
| B. Japonicum | 291,398 a | 69.6 b | 340 b | 186.3 bc | 3398 c | 137.2 c | 1158 b | 1767 a |
| Azospirillum C | 279,742 ab | 69.6 b | 338 bc | 187.6 ab | 3340 c | 134.9 c | 1132 b | 1729 a |
| 0 | 285,907 a | 70.5 b | 339 bc | 187.4 ab | 3319 c | 135.7 c | 1127 b | 1726 a |
| 30 | 265,061 b | 71.4 ab | 337 c | 188.3 a | 3410 c | 137.1 c | 1152 b | 1740 a |
| 56 | 288,933 a | 70.9 b | 339 bc | 186.3 bc | 3472 bc | 138.0 bc | 1180 b | 1744 a |
| 112 | 293,080 a | 73.1 a | 340 b | 186.5 bc | 3616 ab | 141.4 b | 1234 a | 1757 a |
| 336 | 235,248 c | 70.8 b | 345 a | 184.9 c | 3684 a | 146.1 a | 1274 a | 1546 b |
| ANOVA Significance | ** | * | ** | ** | ** | *** | *** | *** |
| LSD (0.05) | 15,267 | 2.1 | 3 | 1.7 | 159 | 3.7 | 54 | 83 |
| Treatment | Small | Medium | Large | Total | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | R6 | R2 | R6 | R2 | R6 | R2 | R6 | |
| ----------------------------Number of nodules----------------------------- | ||||||||
| B. Japonicum | 6.3 a | 4.4 a | 24.6 a | 36.3 a | 1.8 b | 3.6 b | 32.8 a | 44.3 a |
| Azospirillum A | 5.8 a | 4.7 a | 20.8 b | 34.5 a | 4.8 a | 6.5 a | 31.4 a | 45.6 a |
| 56 or 112 B kg N ha−1 | 3.8 a | 5.4 a | 17.3 c | 28.0 b | 0.1 c | 0.8 c | 21.2 b | 34.2 b |
| ANOVA Significance | NS | NS | *** | *** | * | *** | *** | *** |
| LSD (0.05) | - | - | 3.2 | 5.1 | 0.9 | 1.4 | 4.9 | 5.6 |
| Treatment | Small | Medium | Large | Total | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | R6 | R2 | R6 | R2 | R6 | R2 | R6 | |
| ----------------------------mm−3----------------------------- | ||||||||
| B. Japonicum | 3 a | 2 a | 201 a | 297 a | 60 b | 119 b | 264 b | 418 b |
| Azospirillum A | 3 a | 2 a | 170 b | 282 a | 161 a | 218 a | 334 a | 502 a |
| 56 or 112 B kg N ha−1 | 2 a | 3 a | 142 c | 229 b | 3 c | 28 c | 147 c | 260 c |
| ANOVA Significance | NS | NS | *** | *** | *** | *** | *** | *** |
| LSD (0.05) | - | - | 26 | 41 | 30 | 46 | 45 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bais, J.; Kandel, H.; DeSutter, T.; Deckard, E.; Keene, C. Soybean Response to N Fertilization Compared with Co-Inoculation of Bradyrhizobium japonicum and Azospirillum brasilense. Agronomy 2023, 13, 2022. https://doi.org/10.3390/agronomy13082022
Bais J, Kandel H, DeSutter T, Deckard E, Keene C. Soybean Response to N Fertilization Compared with Co-Inoculation of Bradyrhizobium japonicum and Azospirillum brasilense. Agronomy. 2023; 13(8):2022. https://doi.org/10.3390/agronomy13082022
Chicago/Turabian StyleBais, Jose, Hans Kandel, Thomas DeSutter, Edward Deckard, and Clair Keene. 2023. "Soybean Response to N Fertilization Compared with Co-Inoculation of Bradyrhizobium japonicum and Azospirillum brasilense" Agronomy 13, no. 8: 2022. https://doi.org/10.3390/agronomy13082022
APA StyleBais, J., Kandel, H., DeSutter, T., Deckard, E., & Keene, C. (2023). Soybean Response to N Fertilization Compared with Co-Inoculation of Bradyrhizobium japonicum and Azospirillum brasilense. Agronomy, 13(8), 2022. https://doi.org/10.3390/agronomy13082022






