Responsible Mechanisms for the Restriction of Heavy Metal Toxicity in Plants via the Co-Foliar Spraying of Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. As Content in Bamboo Organs (Roots, Stems, and Leaves)
2.3. Calculation of the Bioaccumulation Factor, Translocation Factor, and Tolerance Index
2.4. Determination of Dry Weight of Shoots and Roots
2.5. Antioxidant Enzyme Activities
2.6. Total Phenolics and Flavonoid Content
2.7. Hydrogen Peroxide Malondialdehyde, Superoxide Radical, and Electrolyte Leakage
2.8. Nitric Oxide and Relative Water Content
2.9. Proline Content and Spermidine and Putrescine
2.10. Gaseous Exchange and Photosynthetic Pigments
2.11. Statistics
3. Results
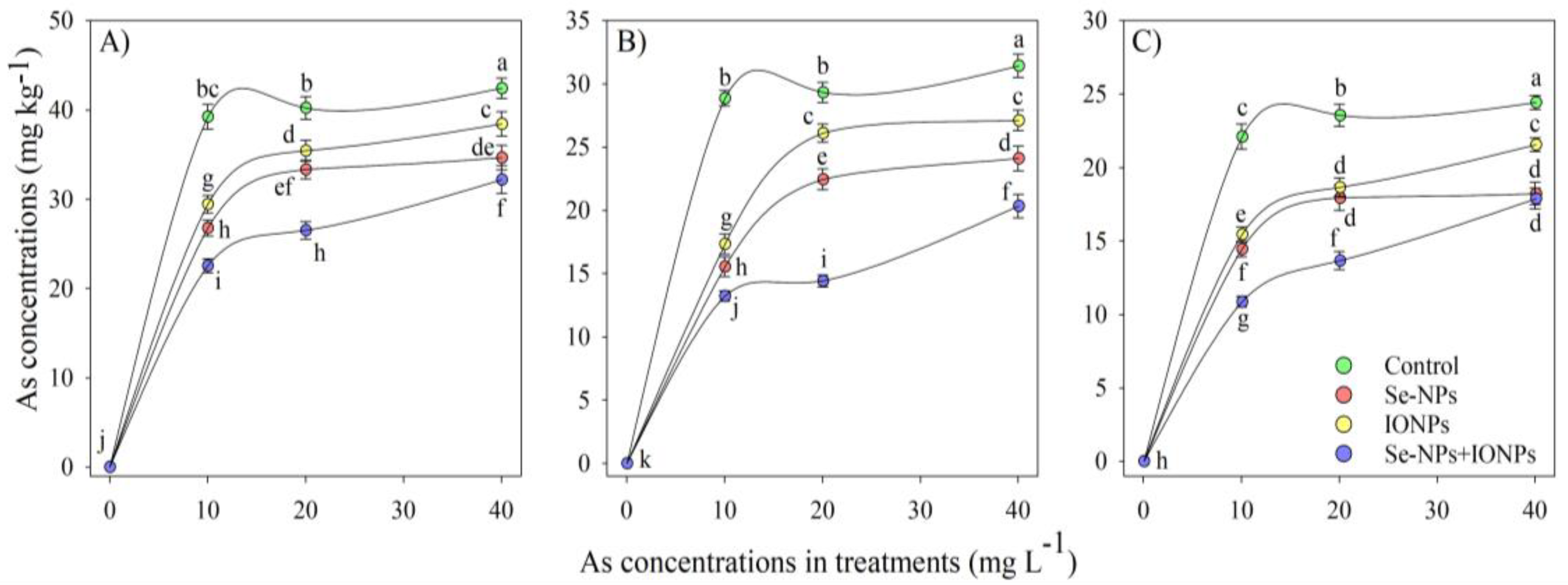
3.1. Impact of Se-NPs and IONPs on As Content in Bamboo Plant Organs
3.2. Impact of Se-NPs and IONPs on Bioaccumulation Factor, Translocation Factor, and Tolerance Index (TI) in Bamboo Exposed to As Toxicity
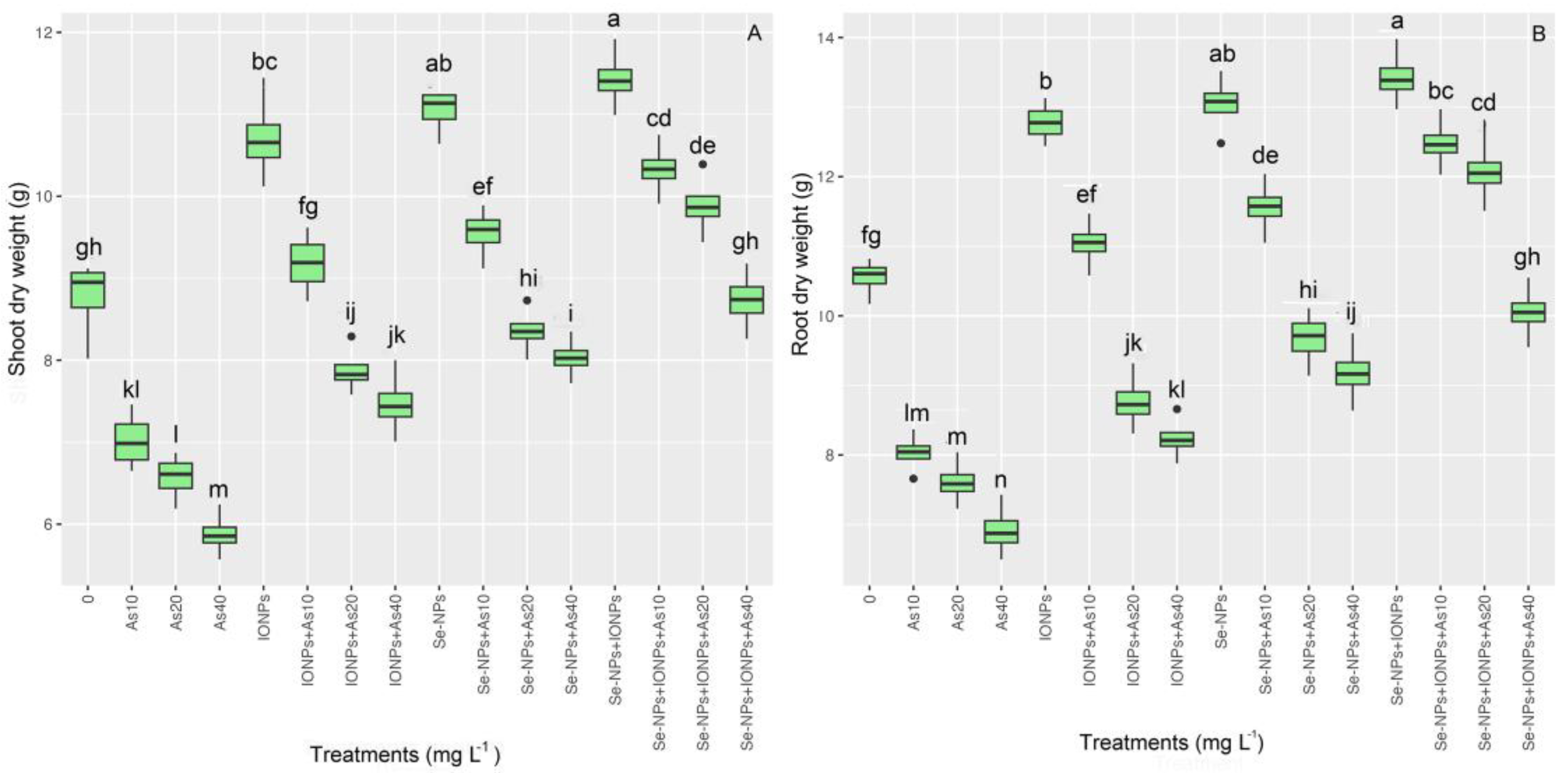
3.3. Impact of Se-NPs and IONPs on Root and Shoot Dry Weight in Bamboo Exposed to As Toxicity
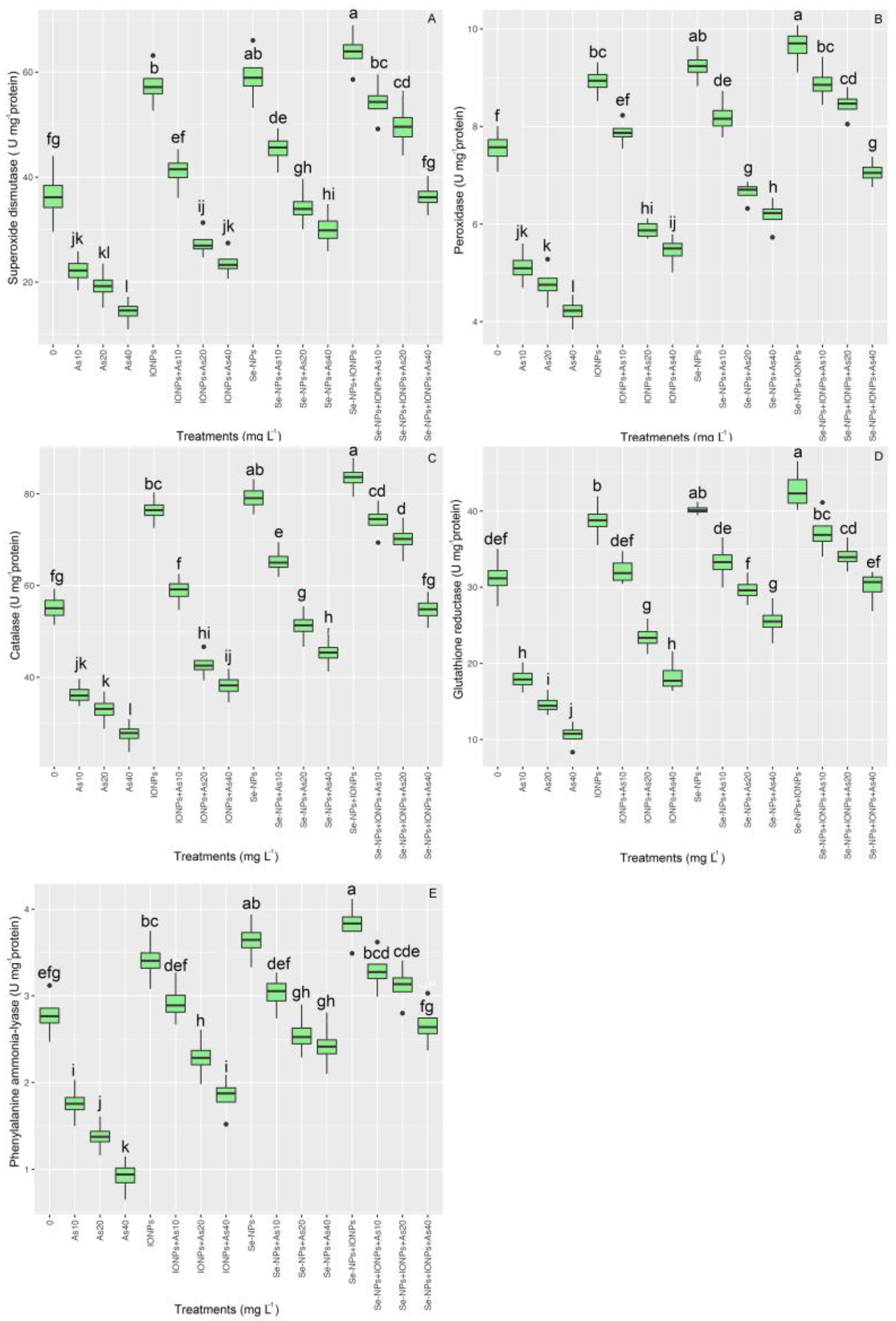
3.4. Effect of Se-NPs and IONPs on Antioxidant Enzyme Activities in Bamboo Exposed to As Toxicity
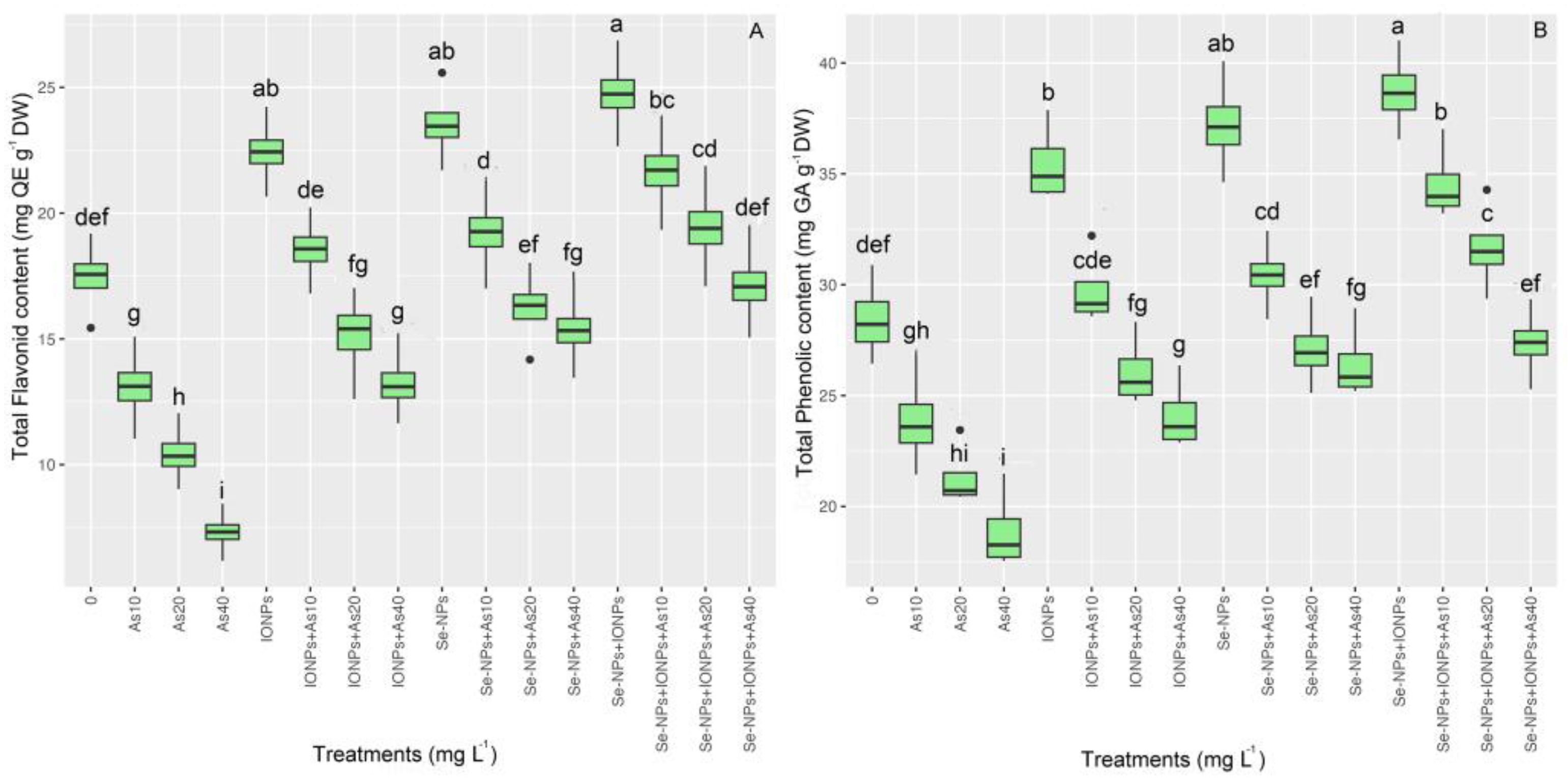
3.5. Impact of Se-NPs and IONPs on Flavonoid Content and Phenolics in Bamboo under As Toxicity
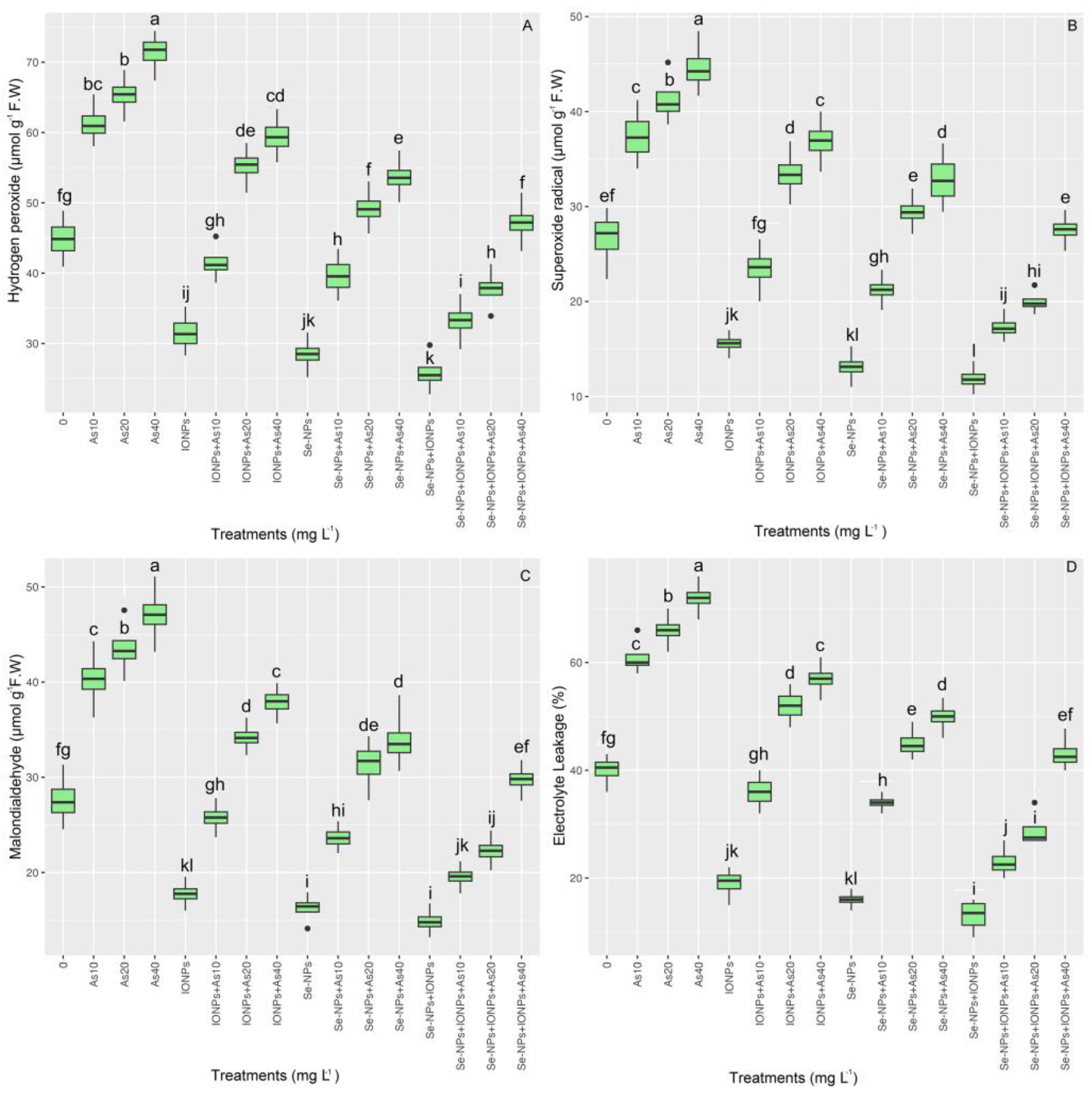
3.6. Impact of Se-NPs and IONPs on Hydrogen Peroxide, Malondialdehyde, Superoxide Radicals, and Electrolyte Leakage in Bamboo Exposed to As Toxicity
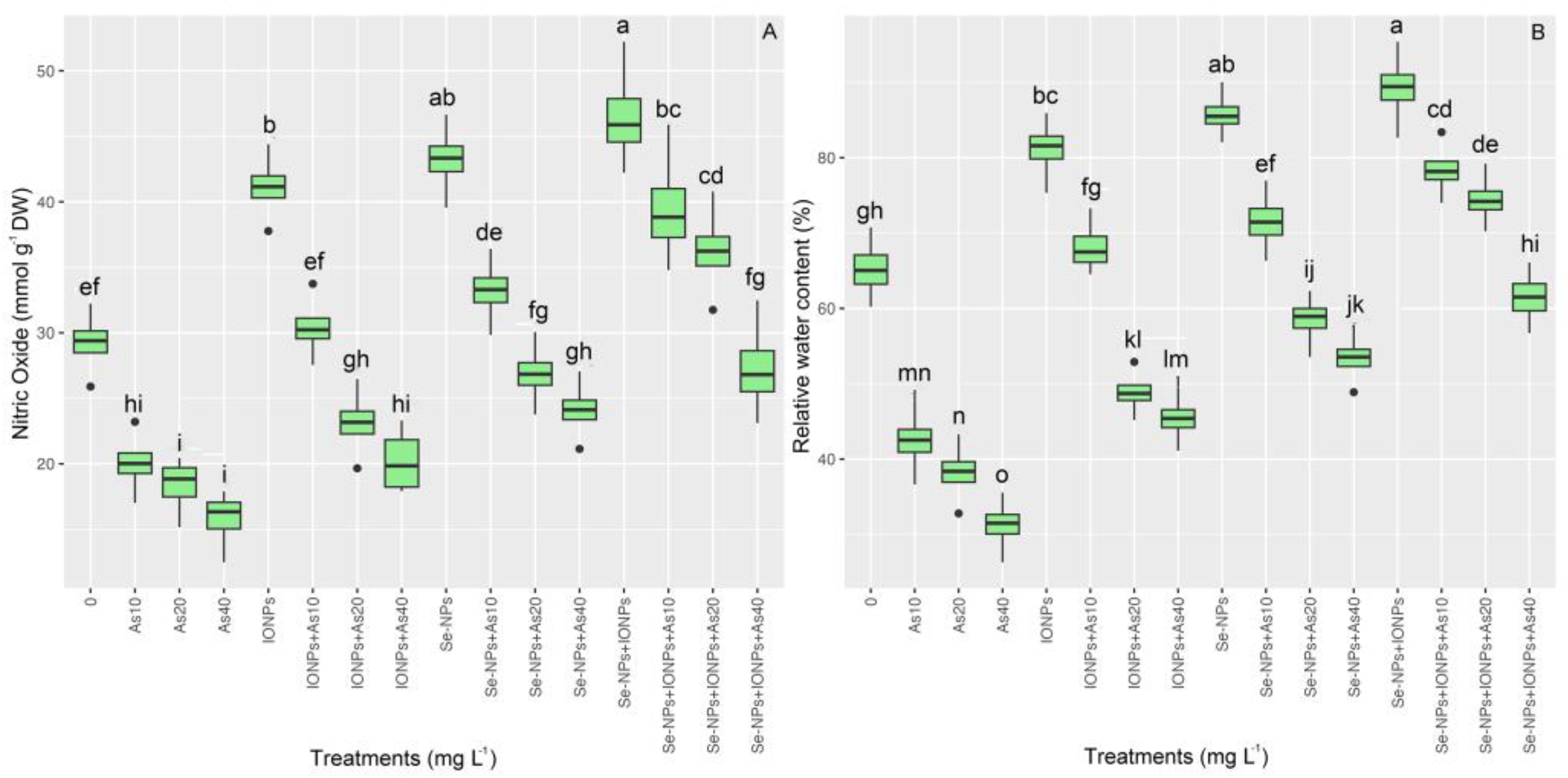
3.7. Impact of Se-NPs and IONPs on Nitric Oxide and Water Content in Bamboo Exposed to As Toxicity
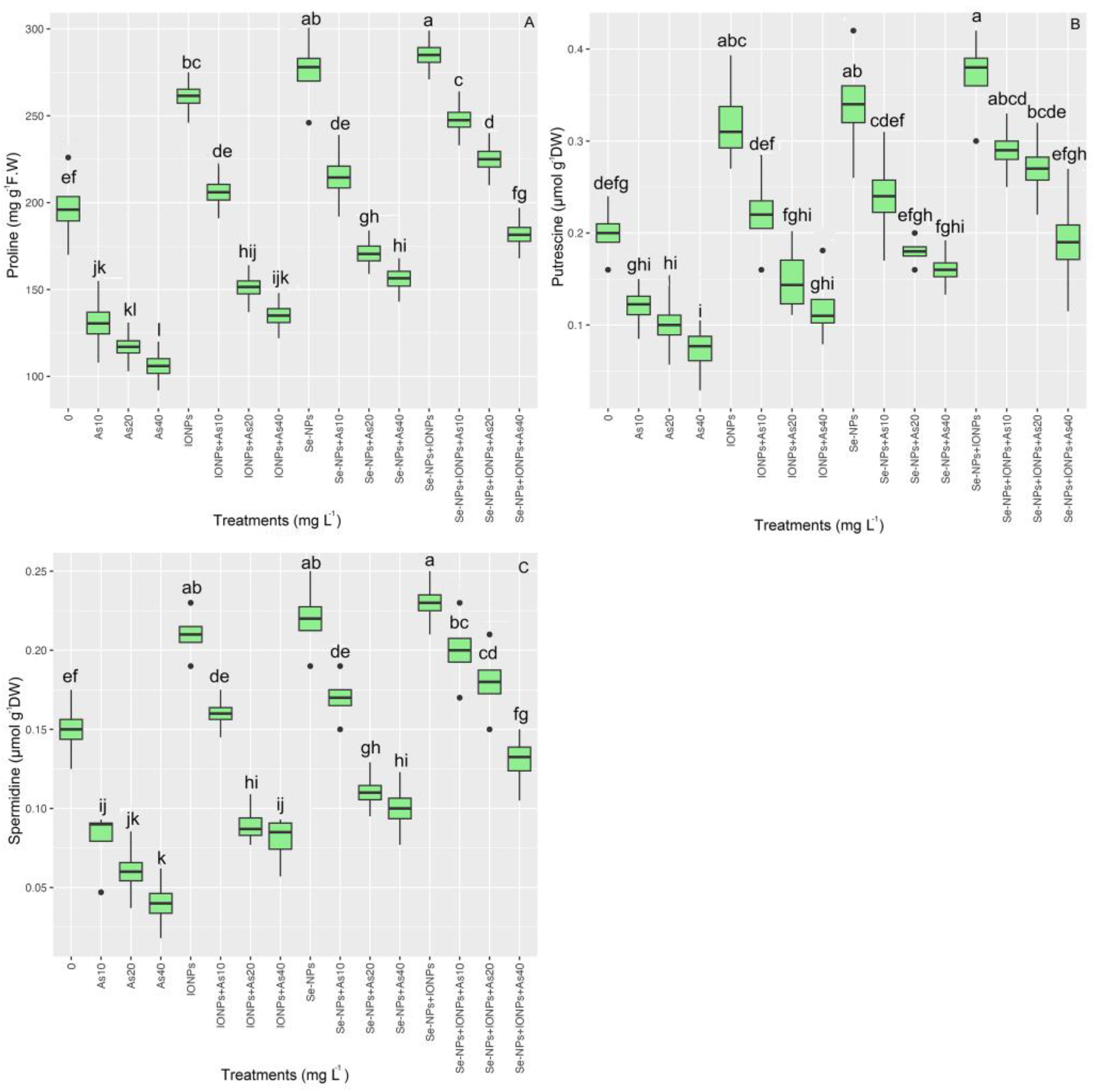
3.8. Impact of Se-NPs and IONPs on Proline, Spermidine (Spd), and Putrescine (Put) in Bamboo under As Toxicity
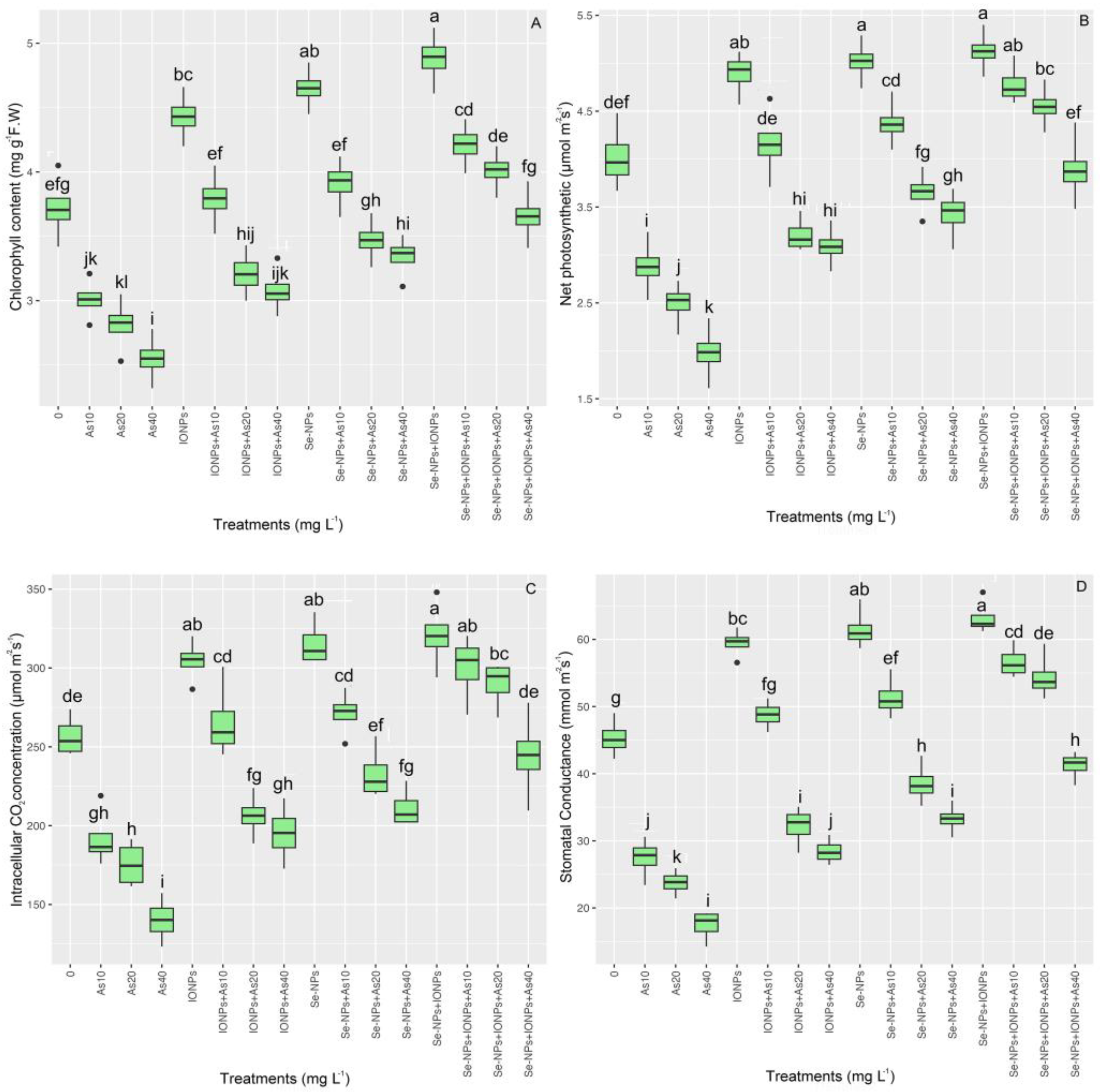
3.9. Effect of Se-NPs and IONPs on Gas Exchange and Photosynthetic Pigments in Bamboo Exposed to As Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Khan, M.B.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci. Total. Environ. 2020, 712, 136497. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Smith, E.; Owens, G.; Bhattacharya, P. Managing Arsenic in the Environment: From Soil to Human Health; CSIRO Publishing: Clayton, Australia, 2006. [Google Scholar]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Chauhan, R.; Srivastava, S.; Tripathi, R.D. The journeyof arsenic from soil to grain in rice. Front. Plant Sci. 2017, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar HMs uptake, toxicity and detoxification in plants: Acomparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, P.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Garg, N.; Singla, P. Arsenic toxicity in crop plants: Physiological effects and tolerance mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
- Pandey, C.; Augustine, R.; Panthri, M.; Zia, I.; Bisht, N.C.; Gupta, M. Arsenic affects the production of glucosinolate, thiol and phytochemical compounds: A comparison of two Brassica cultivars. Plant Physiol. Biochem. 2017, 111, 144–154. [Google Scholar] [CrossRef]
- El-Saadony, T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Hassan, M.A. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef]
- Fatemi, H.; Pour, B.E.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid and antioxidant enzyme activities of coriander (Coriandrumsativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 1417–1425. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Wang, C. Treatment of CrVI-Containing Mg(OH)2 Nanowaste. Angew. Chem. Int. Ed. 2008, 47, 5619–5622. [Google Scholar] [CrossRef]
- Rubio, L.; Pyrgiotakis, G.; Beltran-Huarac, Z.Y.; Gaurav, J.; Deloid, G.; Demokritou, P. Safer-by-design flame-sprayed silicon dioxide nanoparticles: The role of silanol content on ROS generation, surface activity and cytotoxicity. Part. Fibre Toxicol. 2019, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustain-able agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Emamverdian, A.; Hasanuzzaman, M.; Ding, Y.; Barker, J.; Mokhberdoran, F.; Liu, G. Zinc Oxide Nanoparticles Improve Pleioblastus pygmaeus Plant Tolerance to Arsenic and Mercury by Stimulating Antioxidant Defense and Reducing the Metal Accumulation and Translocation. Front. Plant Sci. 2022, 13, 841501. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Hasanuzzaman, M.; Barker, J.; Liu, G.; Li, Y.; Mokhberdoran, F. Insight into the biochemical and physiological mechanisms of nanoparticles-induced arsenic tolerance in bamboo. Front. Plant Sci. 2023, 14, 1121886. [Google Scholar] [CrossRef] [PubMed]
- Irmak, S. Effects of Selenium Application on Plant Growth and Some Quality Parameters in Peanut (Arachis hypogaea). Pak. J. Biol. Sci. 2017, 20, 92–99. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.U.R. Biomedical poten-tial of plant-based selenium nanoparticles: A comprehensive review on therapeutic and mechanistic aspects. Int. J. Nano Med. 2021, 16, 249. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wang, X.; Qing, X.; Wang, P.; Zhang, Y.; Zhao, X. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019, 173, 314–321. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar. Hakimi. Meybodi, N.; Peijnenburg, W. Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef]
- Askary, M.; Talebi, S.M.; Amini, F.; Bangan, D.B.A. Effect of NaCl and iron oxide nanoparticles on Mentha piperita essential oil composition. Environ. Exp. Bot. 2016, 14, 27–32. [Google Scholar] [CrossRef]
- Askary, M.; Talebi, S.M.; Amini, F.; Bangan, A.D. Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologia 2017, 63, 65–75. [Google Scholar] [CrossRef]
- Sheykhbaglou, R.; Sedghi, M.; Fathi-Achachlouie, B. The Effect of Ferrous Nano-oxide Particles on Physiological Traits and Nutritional Compounds of Soybean (Glycine max L.) Seed. An. Acad. Bras. Ciênc. 2018, 90, 485–494. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.H.; Ahmad, A. Brassino steroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Sowmya, R.; Sudhagar, R.; Moorthy, P.S.; Govindaraju, K.; Subramanian, K.S. Biogenic ZnO and Cu nanoparticles to improve seed germination quality in black gram (Vigna mungo). Mater. Lett. 2019, 235, 164–167. [Google Scholar] [CrossRef]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc bio fortification in maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef]
- Ahmad, Z.; Upadhyay, A.; Ding, Y.; Emamverdian, A.; Shahzad, A. Bamboo: Origin, Habitat, Distributions and Global Prospective. In Biotechnological Advances in Bamboo; Ahmad, Z., Ding, Y., Shahzad, A., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokberdoran, F.; Ahmad, Z.; Xie, Y. Determination of HMs tolerance threshold in a bamboo species (Arundinaria pygmaea) as treated with silicon dioxide nanoparticles. Glob. Ecol. Conserv. 2020, 24, e0130. [Google Scholar]
- Emamverdian, A.; Ding, Y.; Ranaei, F.; Ahmad, Z. Application of Bamboo Plants in Nine Aspects. Sci. World J. 2020, 2020, 7284203. [Google Scholar] [CrossRef]
- Bal, L.M.; Singhal, P.; Satya, S.; Naik, S.N.; Kar, A. Bamboo shoot preservation for enhancing its business potential and local economy: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 804–814. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X. Bamboo—An untapped plant resource for the phytoremediation of HMs contaminated soils. Chemosphere 2019, 246, 125750. [Google Scholar] [CrossRef]
- Liu, J.N.; Zhou, Q.X.; Sun, T.; Ma, L.Q.; Wang, S. Growth responses of three ornamental plants to Cd and Pb stress and their metal accumulation characteristics. J. Hazard. Mater. 2008, 151, 261–267. [Google Scholar] [CrossRef]
- Huang, W.; Olson, E.; Wang, S.H.; Shi, P. The growth and mortality of Pleioblastus pygmaeus under different light availability. Glob. Ecol. 2020, 24, e01262. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, M.; Wang, H.; Taneike, Y.; Zhang, X. Arsenic speciation in moso bamboo shoot—A terrestrial plant that contains organoarsenic species. Sci. Total Environ. 2006, 371, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Khosropour, E.; Attarod, P.; Shirvany, A.; Pypker, T.G.; Bayramzadeh, V.; Hakimi, L.; Moeinaddini, M. Response of Plat-anus orientalis leaves to urban pollution by HMss. J. For. Res. 2019, 30, 1437–1445. [Google Scholar] [CrossRef]
- Souri, Z.; Karimi, N. Enhanced Phytoextraction by as Hyperaccumulator Isatis cappadocica Spiked with Sodium Nitro-prusside. Soil. Sediment. Contam. Int. J. 2017, 26, 457–468. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of superoxide dismutase (SOD). In Plant-Microbe Interactions; Springer: Berlin/Heidelberg, Germany, 2021; pp. 117–118.49. [Google Scholar]
- Liu, N.; Lin, Z.; Guan, L.; Gaughan, G.; Lin, G. Antioxidant enzymes regulate reactive oxygen species during pod elongation in Pisum sativum and Brassica chinensis. PLoS ONE 2014, 9, e87588. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acidmetabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Berner, M.; Krug, D.; Bihlmaier, C.; Vente, A.; Müller, R.; Bechthold, A. Genes and enzymes involved in caffeic acid bio-synthesisin the actinomycete Saccharothrix espanaensis. J. Bacteriol. 2006, 188, 2666–2673. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug. Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenoliccontent and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protectiverole of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Li, C.; Bai, T.; Ma, F.; Han, M. Hypoxia tolerance and adaptation of anaerobic respiration to hypoxia stress in two Malus species. Sci. Hortic. 2010, 124, 274–279. [Google Scholar] [CrossRef]
- Rao, K.M.; Sresty, T. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Valentovic, P.; Luxova, M.; Kolarovic, L.; Gasparikova, O. Effect of osmotic stress on compatible solutes content, mem-brane stability and water relations in two maize cultivars. Plant Soil Environ. 2006, 52, 186–191. [Google Scholar] [CrossRef]
- Dhopte, A.M.; Manuel, L.M. Principles and techniques for plant scientists. In Updesh Purohit for Agrobios, 1st ed.; Agrobios: Jodhpur, India, 2002; Volume 81, p. 373. [Google Scholar]
- Bates, L.S.; Walden, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, G.; Li, S. Polyamines and plant stress resistance. J. South Agric. 2004, 35, 443–447. [Google Scholar]
- Holá, D.; Benešová, M.; Honnerová, J.; Hnilicka, F.; Rothová, O.; Kocová, M.; Hniličková, H. The evaluation of photo-synthetic parameters in maize inbred lines subjected to water deficiency: Can these parameters be used for the prediction of performance of hybrid progeny? Photosynthetica 2010, 48, 545–558. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphe-noloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Chung, J.Y.; Yu, S.D.; Hong, Y.S. Environmental source of arsenic exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectivenessof nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 858. [Google Scholar] [CrossRef]
- Mozafariyan, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M.M. Protective Role of Selenium on Pepper Exposed to Cadmium Stress During Reproductive Stage. Biol. Trace Elem. Res. 2014, 160, 97–107. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia Ur Rehman, M.; Waris, A.A. Zinc and iron oxide na-noparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, V.; Katanic Stankovic, J.S.; Selakovic, D.; Rosic, G. An Overview of the Beneficial Role of Antioxidants in the Treatment of Nanoparticle-Induced Toxicities. Oxid. Med. Cell. Longev. 2021, 15, 7244677. [Google Scholar] [CrossRef] [PubMed]
- Sardar, R.; Ahmed, S.; Yasin, N.A. Titanium dioxide nanoparticles mitigate cadmium toxicity in Coriandrum sativum L. through modulating antioxidant system, stress markers and reducing cadmium uptake. Environ. Pollut. 2022, 292 Pt A, 118373. [Google Scholar] [CrossRef]
- Babashpour-Asl, M.; Farajzadeh-Memari-Tabrizi, E.; Yousefpour-Dokhanieh, A. Foliar-applied selenium nanoparticles alleviate cadmium stress through changes in physio-biochemical status and essential oil profile of coriander (Coriandrum sativum L.) leaves. Environ. Sci. Pollut. Res. 2022, 29, 80021–80031. [Google Scholar] [CrossRef]
- Guo, K.; Hu, A.; Wang, K.; Wang, L.; Fu, D.; Hao, Y.; Wang, Y.; Ali, A.; Adeel, M.; Rui, Y.; et al. Effects of spraying nano-materials on the absorption of metal(loid)s in cucumber. IET Nanobiotechnol. 2019, 13, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Mobasherpour, I.; Salahi, E.; Pazoukib, M. Removal of nickel (II) from aqueous solutions by using nano-crystalline calcium hydroxyapatite. J. Saudi Chem. Soc. 2011, 15, 105–112. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional andnanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Riaz, M.; Adrees, M.; Hussain, A.; Zahir, Z.A.; Rinklebe, J. Effects of nanoparticles on trace element uptake and toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2021, 221, 112437. [Google Scholar] [CrossRef]
- Wani, K.I.; Naeem, M.; Castroverde, C.D.M.; Kalaji, H.M.; Albaqami, M.; Aftab, T. Molecular mechanisms of nitric oxide (NO) signaling and reactive oxygen species (ROS) homeostasis during abiotic stresses in plants. Int. J. Mol. Sci. 2021, 22, 9656. [Google Scholar] [CrossRef]
- Gechev, T.; Petrov, V. Reactive oxygen species and abiotic stress in plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Khan, S.; Rehman, S.U.; Rehman, Z.U.; Khatoon, A.; Rha, E.S.; Jamil, M. Synergistic Effects of Zinc Oxide Na-noparticles and Bacteria Reduce Heavy Metals Toxicity in Rice (Oryza sativa L.) Plant. Toxics 2021, 9, 113. [Google Scholar] [CrossRef]
- Jiang, D.; Hou, J.; Gao, W.; Tong, X.; Li, M.; Chu, X. Exogenous spermidine alleviates the adverse effects of aluminum toxicity on photosystem II through improved antioxidant system and endogenous polyamine contents. Ecotoxicol. Environ. Saf. 2021, 207, 111265. [Google Scholar] [CrossRef] [PubMed]
- Jurkow, R.; Pokluda, R.; Sękara, A.; Kalisz, A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biol. 2020, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Qiu, W.Y.; Sun, L.; Ding, Z.C.; Ya, J.K. Peparation, characterization, and antioxidant capacities of selenium nanoparticles stabilized using polysaccharide-protein complexes from Corbicula fluminea. Food Biosci. 2018, 26, 177–184. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Ahmad, Z.; Xie, Y. The Investigation of TiO2 NPs Effect as a Wastewater Treatment to Mitigate Cd Negative Impact on Bamboo Growth. Sustainability 2021, 13, 3200. [Google Scholar] [CrossRef]
- Ali-Arab, H.; Bahadori, F.; Mirza, M.; Badi, H.N.; Kalate-Jari, S. Variability in essential oil composition and phenolic acid profile of Thymus daenensis Celak. populations from Iran. Ind. Crops Prod. 2022, 178, 114345. [Google Scholar] [CrossRef]
- Memari-Tabrizi, E.F.; Yousefpour-Dokhanieh, A.; Babashpour-Asl, M. Foliar-applied silicon nanoparticles mitigate cadmium stress through physio-chemical changes to improve growth, antioxidant capacity, and essential oil profile of summer savory (Satureja hortensis L.). Plant Physiol. Biochem. 2021, 165, 71–79. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftarmanesh, A. Effect of foliarspray of zinc oxide on some antioxidant enzymes activity of sunflower under salt stress. J. Agric. Sci. Technol. 2016, 18, 1013–1025. Available online: http://jast.modares.ac.ir/article-23-5061-en.html (accessed on 1 January 2020).
- Bandurska, H. Does proline accumulated in leaves of water deficit stressed barley plants confine cell membrane injuries? II. Proline accumulation during hardening and its involvement in reducing membrane injuries in leaves subjected to severe osmotic stress. Acta Physiol. Plant. 2001, 23, 483–490. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Noureldeen, A.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles and 24-epibrassinolide alleviates Cu toxicity in tomato by regulating ROS scavenging, stomatal movement and photosynthesis. Ecotoxicol. Environ. Saf. 2021, 218, 112293. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef] [PubMed]
- Helaly, M.N.; El-Metwally, M.A.; El-Hoseiny, H.; Omar, S.A.; El-Sheery, N.I. Effect of nanoparticles on biological con-tamination of in vitro culturesand organogenic regeneration of banana. Aust. J. Crop. Sci. 2014, 8, 612–624. [Google Scholar]
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticleson crop plants: A perspective analysis. In Sustainable Agriculture Reviews 41; Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 83–99. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Merwad, A.R.M.; Desok, E.S.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Shirani Bidabadi, S.; Sabbatini, P.; Vander Weide, J. Iron oxide (IO) nanoparticles alleviate PEG-simulated drought stress in grape (Vitis vinifera L.) plants by regulating leaf antioxidants. Sci. Hortic. 2023, 312, 111847. [Google Scholar] [CrossRef]
- Sardar, R.; Ahmed, S.; Sha, A.A.; Yasin, N.A. Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere 2022, 287, 132332. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Barker, J.; Liu, G.; Li, Y.; Mokhberdoran, F. Sodium Nitroprusside Improves Bamboo Resistance under Mn and Cr Toxicity with Stimulation of Antioxidants Activity, Relative Water Content, and Metal Trans-location and Accumulation. Int. J. Mol. Sci. 2023, 24, 1942. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Hashem, A.M.; Batool, M.; Sherif, A.; Nishawy, E.; Ayaad, M.; Hassan, H.M.; Elrewainy, I.M.; Wang, J.; Kuai, J.; et al. Comparative efficacy of bio-selenium nanoparticles and sodium selenite on mor-pho-physiochemical attributes under normal and salt stress conditions, besides selenium detoxification pathways in Brassica napus L. J. Nanobiotechnology 2022, 20, 163. [Google Scholar] [CrossRef]
- Moloi, M.J.; Khoza, B.M. The Effect of Selenium Foliar Application on the Physiological Responses of Edamame under Different Water Treatments. Agronomy 2022, 12, 2400. [Google Scholar] [CrossRef]
- Malik, A.; Yadav, P.; Singh, S. Role of polyamines in HMs stressed plants. Plant Physiol. Rep. 2022, 27, 680–694. [Google Scholar] [CrossRef]
- Spormann, S.; Soares, C.; Teixeira, J.; Fidalgo, F. Polyamines as key regulatory players in plants under metal stress—A way for an enhanced tolerance. Ann. Appl. Biol. 2021, 178, 209–226. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Ijaz, M.; Ali, S.; Rizwan, M.; Ijaz, U.; Hameed, A.; Ahmad, U.; Wang, Y.; Sun, G.; et al. Current trends and future prospective in nanoremediation of HMs contaminated soils: A way forward towards sustainable agriculture. Ecotoxicol. Environ. Saf. 2021, 227, 112888. [Google Scholar] [CrossRef]
- Khalid, M.F.; Iqbal Khan, R.; Jawaid, M.Z.; Shafqat, W.; Hussain, S.; Ahmed, T.; Rizwan, M.; Ercisli, S.; Pop, O.L.; Alina Marc, R. Nanoparticles: The Plant Saviour under Abiotic Stresses. Nanomaterials 2022, 12, 3915. [Google Scholar] [CrossRef] [PubMed]
- Reddy Pullagurala, V.L.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense systemin plants. In Nanotechnology and Plant Sciences; Siddiqui, M., Al-Whaibi, M., Mo-hammad, F., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Salama, H.M.H. Effects of silver nanoparticles in some crop plants, Common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Smirnoff, N. Chapter 4—Vitamin C: The metabolism and functions of ascorbic acid in plants. In Advances in Botanical Research; Rébeillé, F., Douce, R., Eds.; Academic Press: Cambridge, UK, 2011; pp. 107–177. [Google Scholar]
- Ze, Y.; Liu, C.; Wang, L.; Hong, M.; Hong, F. The regulation of TiO2 nanoparticles on the expression of light-harvesting complex II and photosynthesis of chloroplasts of Arabidopsis thaliana. Biol. Trace Elem. Res. 2011, 143, 1131–1141. [Google Scholar] [CrossRef]
- Rai, P.; Samarth Sharma, S.; Tripathi, S.; Prakash, V.; Tiwari, K.; Suri, S.; Sharma, S. Nanoiron: Uptake, translocation and accumulation in plant systems. Plant Nano Biol. 2022, 2, 100017. [Google Scholar] [CrossRef]
- Sheykhbaglou, R.; Sedghi, M.; Shishevan, M.T.; Sharifi, R.S. Effects of nano-iron oxide particles on agronomic traits of soybean. Not. Sci. Biol. 2010, 2, 112–113. [Google Scholar] [CrossRef]
- Moussa, H.R.; El-Fatah, A.; Ahmed, M. Protective role of selenium on development and physiological responses of Viciafaba. Int. J. Veg. Sci. 2010, 16, 174–183. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Bano, I.; Skalickova, S.; Sajjad, H.; Skladanka, J.; Horky, P. Uses of Selenium Nanoparticles in the Plant Production. Agronomy 2021, 11, 2229. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, K.; Liu, Z.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on the subcellular distribution of cadmium and oxidative stress induced by cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2019, 26, 16220–16228. [Google Scholar] [CrossRef]
- Qi, W.Y.; Li, Q.; Chen, H.; Liu, J.; Xin, S.F.; Xu, M.; Wang, S.G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef] [PubMed]
- Bidi, H.; Fallah, H.; Niknejad, Y.; Tari, D.B. Iron Oxide Nanoparticles Alleviate Arsenic Phytotoxicity in Rice by Improving Iron Uptake, Oxidative Stress Tolerance and Diminishing Arsenic Accumulation. Plant Physiol. Biochem. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Koleva, L.; Umar, A.; Yasin, N.A.; Shah, A.A.; Siddiqui, M.H.; Alamri, S.; Riaz, L.; Raza, A.; Javed, T.; Shabbir, Z. Iron Oxide and Silicon Nanoparticles Modulate Mineral Nutrient Homeostasis and Metabolism in Cadmium-Stressed Phaseolus vulgaris. Front. Plant Sci. 2022, 13, 806781. [Google Scholar] [CrossRef]
- Pehlivan, N.; Gedik, K.; Eltem, R.; Terzi, E. Dynamic interactions of Trichoderma harzianum TS 143 from an old mining site in Turkey for potent metal(oid)s phytoextraction and bioenergy crop farming. J. Hazard. Mater. 2021, 403, 123609. [Google Scholar] [CrossRef]
| Treatments | Concentrations |
|---|---|
| Control | 0 |
| As | 10 mg L−1 As |
| As | 20 mg L−1 As |
| As | 40 mg L−1 As |
| Se-NPs | 60 mg L−1 Se-NPs |
| Se-NPs + As | 60 mg L−1 Se-NPs + 10 mg L−1 As |
| Se-NPs + As | 60 mg L−1 Se-NPs + 20 mg L−1 As |
| Se-NPs + As | 60 mg L−1 Se-NPs + 40 mg L−1 As |
| IONPs | 60 mg L−1 IONPs |
| IONPs + As | 60 mg L−1 IONPs + 10 mg L−1 As |
| IONPs + As | 60 mg L−1 IONPs + 20 mg L−1 As |
| IONPs + As | 60 mg L−1 IONPs + 40 mg L−1 As |
| Se-NPs + IONPs | 60 mg L−1 Se-NPs + IONPs |
| Se-NPs + IONPs + As | 60 mg L−1 Se-NPs + IONPs + 10 mg L−1 As |
| Se-NPs + IONPs + As | 60 mg L−1 Se-NPs + IONPs + 20 mg L−1 As |
| Se-NPs + IONPs + As | 60 mg L−1 Se-NPs + IONPs + 40 mg L−1 As |
| Treatments | BAF (Leaf) | BAF (Stem) | BAF (Root) | TF (Leaf) | TF (Stem) | TI (Shoot) | TI (Root) |
|---|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 | 0 | 1.00 ± 0.01 fg | 1.00 ± 0.02 fg |
| 10 mg L−1 As | 2.21 ± 0.01 a | 2.88 ± 0.02 a | 3.92 ± 0.03 a | 0.56 ± 0.01 ab | 0.73 ± 0.01 ab | 0.77 ± 0.01 k | 0.76 ± 0.02 jk |
| 20 mg L−1 As | 1.17 ± 0.01 d | 1.46 ± 0.02 d | 2.01 ± 0.03 e | 0.58 ± 0.01 a | 0.73 ± 0.01 ab | 0.73 ± 0.01 k | 0.72 ± 0.02 kl |
| 40 mg L−1 As | 0.61 ± 0.01 h | 2.88 ± 0.02 g | 1.06 ± 0.03 h | 0.57 ± 0.01 a | 0.74 ± 0.01 a | 0.65 ± 0.01 i | 0.65 ± 0.02 l |
| 60 mg L−1 Se-NPs | 0 | 0 | 0 | 0 | 0 | 1.22 ± 0.01 ab | 1.23 ± 0.02 ab |
| 60 mg L−1 Se-NPs + 10 mg L−1 As | 1.44 ± 0.01 c | 1.55 ± 0.02 c | 2.67 ± 0.03 c | 0.53 ± 0.01 bcd | 0.58 ± 0.01 e | 1.05 ± 0.01 de | 1.09 ± 0.02 de |
| 60 mg L−1 Se-NPs + 20 mg L−1 As | 0.89 ± 0.01 f | 1.12 ± 0.02 f | 1.66 ± 0.03 f | 0.53 ± 0.01 bcd | 0.67 ± 0.01 c | 0.92 ± 0.01 h | 0.91 ± 0.02 gh |
| 60 mg L−1 Se-NPs + 40 mg L−1 As | 0.45 ± 0.01 j | 0.60 ± 0.02 i | 0.86 ± 0.03 ij | 0.52 ± 0.01 cd | 0.69 ± 0.01 bc | 0.88 ± 0.01 i | 0.87 ± 0.02 hi |
| 60 mg L−1 IONPs | 0 | 0 | 0 | 0 | 0 | 1.18 ± 0.01 bc | 1.21 ± 0.02 abc |
| 60 mg L−1 IONPs + 10 mg L−1 As | 1.54 ± 0.01 b | 1.73 ± 0.02 b | 2.94 ± 0.03 b | 0.52 ± 0.01 cd | 0.58 ± 0.01 e | 1.01 ± 0.01 ef | 1.04.00 ± 0.02 ef |
| 60 mg L−1 IONPs + 20 mg L−1 As | 0.93 ± 0.01 f | 1.30 ± 0.02 e | 1.64 ± 0.03 f | 0.52 ± 0.01 cd | 0.73 ± 0.01 ab | 0.87 ± 0.01 i | 0.83 ± 0.03 ij |
| 60 mg L−1 IONPs + 40 mg L−1 As | 0.53 ± 0.01 i | 0.67 ± 0.02 h | 0.96 ± 0.03 hi | 0.56 ± 0.01 ab | 0.69 ± 0.01 abc | 0.82 ± 0.01 j | 0.78 ± 0.02 jk |
| 60 mg L−1 Se-NPs + IONPs | 0 | 0 | 0 | 0 | 0 | 1.26 ± 0.01 a | 1.27 ± 0.02 a |
| 60 mg L−1 Se-NPs + IONPs + 10 mg L−1 As | 1.08 ± 0.01 e | 1.32 ± 0.02 e | 2.25 ± 0.03 d | 0.47 ± 0.01 e | 0.58 ± 0.01 e | 1.14 ± 0.01 c | 1.18 ± 0.02 bc |
| 60 mg L−1 Se-NPs + IONPs + 20 mg L−1 As | 0.68 ± 0.01 g | 0.72 ± 0.02 gh | 1.32 ± 0.03 g | 0.51 ± 0.01 d | 0.54 ± 0.01 f | 1.09 ± 0.01 d | 1.14 ± 0.02 cd |
| 60 mg L−1 Se-NPs + IONPs + 40 mg L−1 As | 044 ± 0.01 j | 0.50 ± 0.02 j | 0.80 ± 0.03 j | 0.55 ± 0.01 abc | 0.63 ± 0.01 d | 0.96 ± 0.01 gh | 0.95 ± 0.02 gh |
| NP (mg L−1) | As (mg L−1) | SHDW (%) | RDW (%) |
|---|---|---|---|
| 0 | 10 mg L−1 | 19% ↓ | 24% ↓ |
| 0 | 20 mg L−1 | 25% ↓ | 21% ↓ |
| 0 | 40 mg L−1 | 32% ↓ | 27% ↓ |
| 60 mg L−1 Se-NPs | 0 | 26% ↑ | 33% ↑ |
| 60 mg L−1 Se-NPs | 10 mg L−1 | 9% ↑ | 18% ↑ |
| 60 mg L−1 Se-NPs | 20 mg L−1 | 4% ↓ | 0.5% ↓ |
| 60 mg L−1 Se-NPs | 40 mg L−1 | 8% ↓ | 9% ↓ |
| 60 mg L−1 IONPs | 0 | 22% ↑ | 29% ↑ |
| 60 mg L−1 IONPs | 10 mg L−1 | 4% ↑ | 12% ↑ |
| 60 mg L−1 IONPs | 20 mg L−1 | 10% ↓ | 8% ↓ |
| 60 mg L−1 IONPs | 40 mg L−1 | 14% ↓ | 15% ↓ |
| 60 mg L−1 Se-NPs + IONPs | 0 | 30% ↑ | 37% ↑ |
| 60 mg L−1 Se-NPs + IONPs | 10 mg L−1 | 18% ↑ | 27% ↑ |
| 60 mg L−1 Se-NPs + IONPs | 20 mg L−1 | 12% ↑ | 24% ↑ |
| 60 mg L−1 Se-NPs + IONPs | 40 mg L−1 | 0.3% ↓ | 6% ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emamverdian, A.; Ghorbani, A.; Li, Y.; Pehlivan, N.; Barker, J.; Ding, Y.; Liu, G.; Zargar, M. Responsible Mechanisms for the Restriction of Heavy Metal Toxicity in Plants via the Co-Foliar Spraying of Nanoparticles. Agronomy 2023, 13, 1748. https://doi.org/10.3390/agronomy13071748
Emamverdian A, Ghorbani A, Li Y, Pehlivan N, Barker J, Ding Y, Liu G, Zargar M. Responsible Mechanisms for the Restriction of Heavy Metal Toxicity in Plants via the Co-Foliar Spraying of Nanoparticles. Agronomy. 2023; 13(7):1748. https://doi.org/10.3390/agronomy13071748
Chicago/Turabian StyleEmamverdian, Abolghassem, Abazar Ghorbani, Yang Li, Necla Pehlivan, James Barker, Yulong Ding, Guohua Liu, and Meisam Zargar. 2023. "Responsible Mechanisms for the Restriction of Heavy Metal Toxicity in Plants via the Co-Foliar Spraying of Nanoparticles" Agronomy 13, no. 7: 1748. https://doi.org/10.3390/agronomy13071748
APA StyleEmamverdian, A., Ghorbani, A., Li, Y., Pehlivan, N., Barker, J., Ding, Y., Liu, G., & Zargar, M. (2023). Responsible Mechanisms for the Restriction of Heavy Metal Toxicity in Plants via the Co-Foliar Spraying of Nanoparticles. Agronomy, 13(7), 1748. https://doi.org/10.3390/agronomy13071748








