Greenhouse Gas Emissions and Bacterial Community Structure as Influenced by Biodegradable Film Mulching in Eastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Experimental Design and Treatments
2.3. Sample Collection and Analysis
2.3.1. CH4 and N2O Sampling and Analysis
2.3.2. Soil Sample Collection and Analysis
2.3.3. Plant Sample Collection and Analysis
2.4. Methane and Nitric Oxide Data Calculations
2.5. Statistical Analyses
3. Results
3.1. The Dynamics of CH4 Emission Fluxes
3.2. The Dynamics of N2O Emission Fluxes
3.3. Yield and Greenhouse Gas Intensity
3.4. Abundance and Diversity of Soil Bacteria
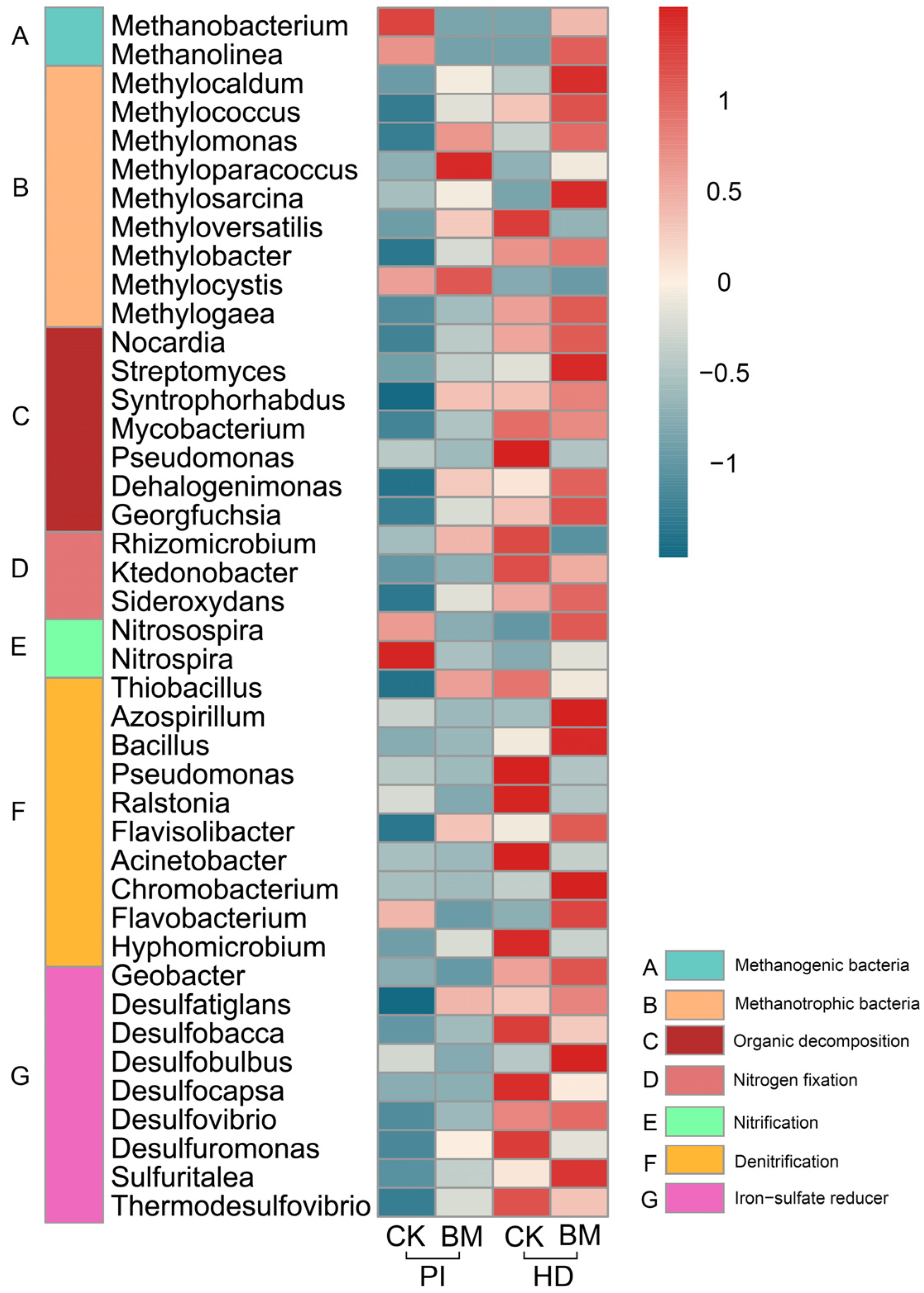
3.5. Structure and Composition of the Soil Bacterial Community
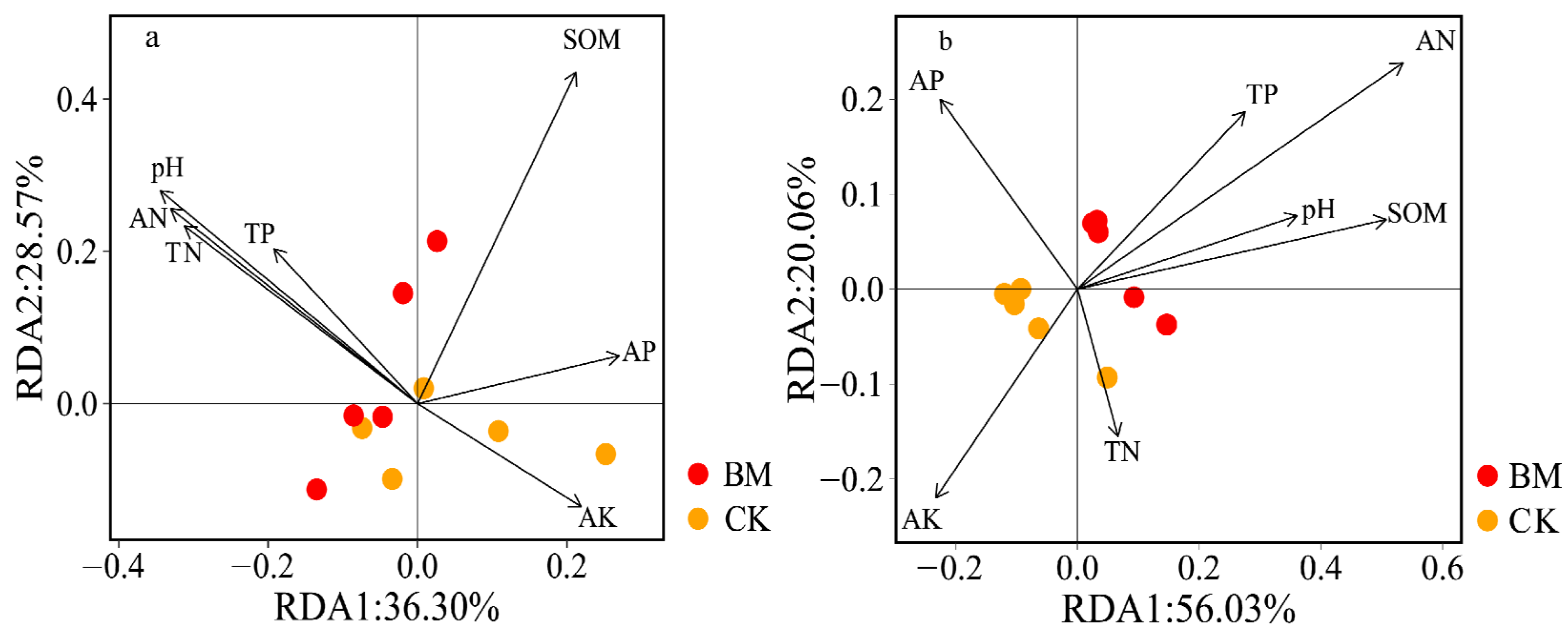
3.6. Relationships between the Soil Properties and Bacterial Community Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klimchuk, E.F.; Tarasov, V.F. On the critical mass of greenhouse gas. Geofiz. Zhurnal 2018, 40, 70–77. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K. The global methane budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Carlson, K.M.; Gerber, J.S.; Mueller, N.D.; Herrero, M.; MacDonald, G.K.; Brauman, K.A.; Havlik, P.; O’Connell, C.S.; Johnson, J.A.; Saatchi, S. Greenhouse gas emissions intensity of global croplands. Nat. Clim. Chang. 2016, 7, 63–68. [Google Scholar] [CrossRef]
- Kritee, K.; Nair, D.; Zavala-Araiza, D.; Proville, J.; Rudek, J.; Adhya, T.K.; Loecke, T.; Esteves, T.; Balireddygari, S.; Dava, O. High nitrous oxide fluxes from rice indicate the need to manage water for both long- and short-term climate impacts. Proc. Natl. Acad. Sci. USA 2018, 115, 9720–9725. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Z.; Wang, E.; Zhang, W. Reducing greenhouse gas emissions while maintaining yield in the croplands of Huang-Huai-Hai Plain, China. Agric. For. Meteorol. 2018, 260–261, 80–94. [Google Scholar] [CrossRef]
- Cardoso, A.d.S.; Oliveira, S.C.; Janusckiewicz, E.R.; Brito, L.F.; Morgado, E.d.S.; Reis, R.A.; Ruggieri, A.C. Seasonal effects on ammonia, nitrous oxide, and methane emissions for beef cattle excreta and urea fertilizer applied to a tropical pasture. Soil Till. Res. 2019, 194, 104341. [Google Scholar] [CrossRef]
- Guardia, G.; Aguilera, E.; Vallejo, A.; Sanz-Cobena, A.; Alonso-Ayuso, M.; Quemada, M. Effective climate change mitigation through cover cropping and integrated fertilization: A global warming potential assessment from a 10-year field experiment. J. Clean. Prod. 2019, 241, 118307. [Google Scholar] [CrossRef]
- Wu, J.; Guo, W.; Feng, J.; Li, L.; Yang, H.; Wang, X.; Bian, X. Greenhouse gas emissions from cotton field under different irrigation methods and fertilization regimes in arid northwestern China. Sci. World J. 2014, 2014, 407832. [Google Scholar] [CrossRef]
- Riya, S.; Zhou, S.; Watanabe, Y.; Sagehashi, M.; Terada, A.; Hosomi, M. CH4 and N2O emissions from different varieties of forage rice (Oryza sativa L.) treating liquid cattle waste. Sci. Total Environ. 2012, 419, 178–186. [Google Scholar] [CrossRef]
- Linquist, B.; Groenigen, K.J.; Adviento-Borbe, M.A.; Pittelkow, C.; Kessel, C. An agronomic assessment of greenhouse gas emissions from major cereal crops. Glob. Chang. Biol. 2012, 18, 194–209. [Google Scholar] [CrossRef]
- Shakoor, A.; Ashraf, F.; Shakoor, S.; Mustafa, A.; Rehman, A.; Altaf, M.M. Biogeochemical transformation of greenhouse gas emissions from terrestrial to atmospheric environment and potential feedback to climate forcing. Environ. Sci. Pollut. Res. Int. 2020, 27, 38513–38536. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Chang. Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef]
- Pareja-Sánchez, E.; Cantero-Martínez, C.; Álvaro-Fuentes, J.; Plaza-Bonilla, D. Tillage and nitrogen fertilization in irrigated maize: Key practices to reduce soil CO2 and CH4 emissions. Soil Till. Res. 2019, 191, 29–36. [Google Scholar] [CrossRef]
- Mitra, S.; Wassmann, R.; Jain, M.C.; Pathak, H. Properties of rice soils affecting methane production potentials: 1. Temporal patterns and diagnostic procedures. Nutr. Cycl. Agroecosys. 2002, 64, 169–182. [Google Scholar] [CrossRef]
- Angel, R.; Matthies, D.; Conrad, R. Activation of methanogenesis in arid biological soil crusts despite the presence of oxygen. PLoS ONE 2011, 6, e20453. [Google Scholar] [CrossRef]
- Cicerone, R.J.; Oremland, R.S. Biogeochemical aspects of atmospheric methane. Glob. Biogeochem. Cycles 1988, 2, 299–327. [Google Scholar] [CrossRef]
- Hu, H.W.; Chen, D.; He, J.Z. Microbial regulation of terrestrial nitrous oxide formation: Understanding the biological pathways for prediction of emission rates. FEMS Microbiol. Rev. 2015, 39, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, W.; Xu, H.; Cai, Z.; Yagi, K. Effect of timing and duration of midseason aeration on CH4 and N2O emissions from irrigated lowland rice paddies in China. Nutr. Cycl. Agroecosystems 2011, 91, 293–305. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, G.; Zheng, X.; Wang, R.; Liu, C.; Butterbach-Bahl, K. Reducing N2O and NO emissions while sustaining crop productivity in a Chinese vegetable-cereal double cropping system. Environ. Pollut. 2017, 231, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.L.; Wang, D.; Xing, X.Y.; Tang, Y.F.; Wei, X.M.; Chen, X.B.; Zhang, W.Z.; Chen, A.L.; Li, L.L.; Liu, Y. A few key nirK- and nosZ-denitrifier taxa play a dominant role in moisture-enhanced N2O emissions in acidic paddy soil. Geoderma 2021, 385, 114917. [Google Scholar] [CrossRef]
- Ishii, S.; Ikeda, S.; Minamisawa, K.; Senoo, K. Nitrogen cycling in rice paddy environments: Past achievements and future challenges. Microbes Environ. 2011, 26, 282–292. [Google Scholar] [CrossRef]
- He, G.; Wang, Z.; Li, S.; Malhi, S.S. Plastic mulch: Tradeoffs between productivity and greenhouse gas emissions. J. Clean. Prod. 2018, 172, 1311–1318. [Google Scholar] [CrossRef]
- Lee, J.G.; Cho, S.R.; Jeong, S.T.; Hwang, H.Y.; Kim, P.J. Different response of plastic film mulching on greenhouse gas intensity (GHGI) between chemical and organic fertilization in maize upland soil. Sci. Total Environ. 2019, 696, 133827. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Liang, W.L.; Qu, H.; Zhi, G.Y.; Chen, Q.X.; Gong, Y.S.; Butterbach-Bahl, K.; Lin, S. Ground cover rice production systems are more adaptable in cold regions with high content of soil organic matter. Field Crops Res. 2014, 164, 74–81. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Wang, R.; Liu, C.; Lin, S.; Butterbach-Bahl, K. Benefits of integrated nutrient management on N2O and NO mitigations in water-saving ground cover rice production systems. Sci. Total Environ. 2019, 646, 1155–1163. [Google Scholar] [CrossRef]
- Zhang, G.B.; Ma, J.; Yang, Y.T.; Yu, H.Y.; Song, K.F.; Dong, Y.J.; Lv, S.H.; Xu, H. Achieving low methane and nitrous oxide emissions with high economic incomes in a rice-based cropping system. Agric. For. Meteorol. 2018, 259, 95–106. [Google Scholar] [CrossRef]
- Yao, Z.S.; Zheng, X.H.; Liu, C.Y.; Lin, S.; Zuo, Q.; Butterbach-Bahl, K. Improving rice production sustainability by reducing water demand and greenhouse gas emissions with biodegradable films. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef]
- Cuello, J.P.; Hwang, H.Y.; Gutierrez, J.; Kim, S.Y.; Kim, P.J. Impact of plastic film mulching on increasing greenhouse gas emissions in temperate upland soil during maize cultivation. Appl. Soil Ecol. 2015, 91, 48–57. [Google Scholar] [CrossRef]
- Yu, Y.X.; Zhang, Y.X.; Xiao, M.; Zhao, C.Y.; Yao, H.Y. A meta-analysis of film mulching cultivation effects on soil organic carbon and soil greenhouse gas fluxes. Catena 2021, 206, 9. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Shi, Y.; Zhang, L.; Wu, Z. Do fallow season cover crops increase N2O or CH4 emission from paddy soils in the mono-rice cropping system? Agronomy 2021, 11, 199. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Xie, X.; Yin, C.; Hou, H.; Yan, W.; Wang, G. Net global warming potential and greenhouse gas intensity as affected by different water management strategies in Chinese double rice-cropping systems. Sci. Rep. 2018, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Sha, L.; Cao, M.; Zheng, Z.; Tang, J.; Wang, Y.; Zhang, Y.; Wang, R.; Liu, G.; Wang, Y. Fluxes of CH4 and N2O from soil under a tropical seasonal rain forest in Xishuangbanna, Southwest China. J. Environ. Sci. 2008, 20, 207–215. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, X.; Sun, J.; Tang, W.; Wang, K.; Li, W.; Yang, G. Effects of different winter covering crops cultivation on methane and nitrous oxide emission fluxes and soil microorganism in double-cropping paddy field. Ecol. Environ. Sci. 2014, 23, 736–742, (In Chinese with English abstract). [Google Scholar]
- Watanabe, T.; Kimura, M.; Asakawa, S. Dynamics of methanogenic archaeal communities based on rRNA analysis and their relation to methanogenic activity in Japanese paddy field soils. Soil Biol. Biochem. 2007, 39, 2877–2887. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M.; Lu, Y.; Chidthaisong, A. Methanogenic pathway and archaeal communities in three different anoxic soils amended with rice straw and maize straw. Front. Microbiol. 2012, 3, 4. [Google Scholar] [CrossRef]
- Yuan, Y.; Conrad, R.; Lu, Y. Responses of methanogenic archaeal community to oxygen exposure in rice field soil. Environ. Microbiol. Rep. 2009, 1, 347–354. [Google Scholar] [CrossRef]
- Ma, K.; Conrad, R.; Lu, Y. Responses of methanogen mcrA genes and their transcripts to an alternate dry/wet cycle of paddy field soil. Appl. Environ. Microbiol. 2012, 78, 445–454. [Google Scholar] [CrossRef]
- Bedard, C.; Knowles, R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol. Rev. 1989, 53, 68–84. [Google Scholar] [CrossRef]
- Henckel, T.; Roslev, P.; Conrad, R. Effects of O2 and CH4 on presence and activity of the indigenous methanotrophic community in rice field soil. Environ. Microbiol. 2000, 2, 666–679. [Google Scholar] [CrossRef]
- Noll, M.; Frenzel, P.; Conrad, R. Selective stimulation of type I methanotrophs in a rice paddy soil by urea fertilization revealed by RNA-based stable isotope probing. FEMS Microbiol. Ecol. 2008, 65, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Flessa, H.; Dorsch, P.; Beese, F. Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany. J. Geophys. Res. Atmos. 1995, 100, 23115–23124. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Wang, X.; Chen, F.; Lai, D.; Tian, C. Effects of plastic film mulching with drip irrigation on N2O and CH4 emissions from cotton fields in arid land. J. Agric. Sci. 2013, 152, 534–542. [Google Scholar] [CrossRef]
- Hou, P.; Li, G.; Wang, S.; Jin, X.; Yang, Y.; Chen, X.; Ding, C.; Liu, Z.; Ding, Y. Methane emissions from rice fields under continuous straw return in the middle-lower reaches of the Yangtze River. J. Environ. Sci. 2013, 25, 1874–1881. [Google Scholar] [CrossRef]
- Hu, A.; Lu, Y. The differential effects of ammonium and nitrate on methanotrophs in rice field soil. Soil Biol. Biochem. 2015, 85, 31–38. [Google Scholar] [CrossRef]
- Karbin, S.; Hagedorn, F.; Dawes, M.A.; Niklaus, P.A. Treeline soil warming does not affect soil methane fluxes and the spatial micro-distribution of methanotrophic bacteria. Soil Biol. Biochem. 2015, 86, 164–171. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, H.; Fan, X.; Ma, J.; Xu, H. Carbon isotope fractionation reveals distinct process of CH4 emission from different compartments of paddy ecosystem. Sci. Rep. 2016, 6, 27065. [Google Scholar] [CrossRef]
- Paustian, K.; Six, J.; Elliott, E.T.; Hunt, H.W. Management options for reducing CO2 emissions from agricultural soils. Biogeochemistry 2000, 48, 147–163. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Chotte, J.-L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Cai, Z.; Shen, G.; Yan, X.; Tsuruta, H.; Yagi, K.; Minami, K. Effects of soil texture, soil temperature and Eh on methane emissions from rice paddy fields. Acta Pedol. Sin. 1998, 35, 145–154, (In Chinese with English abstract). [Google Scholar]
- Zou, J.; Huang, Y.; Zheng, X.; Wang, Y. Quantifying direct N2O emissions in paddy fields during rice growing season in mainland China: Dependence on water regime. Atmos. Environ. 2007, 41, 8030–8042. [Google Scholar] [CrossRef]
- Huang, B.; Chen, G.X. Effects of cultivation on N2O emission and seasonal quantitative variations of related microbes in a temperate grassland soil. J. Environ. Sci. 2001, 13, 376–379. [Google Scholar]
- Hu, G.; Wang, J.; Wang, Y.; Zhu, D.; Chen, H.; Xiang, J.; Zhang, Y.; Zhang, Y. Effect of biodegradable film mulching on greenhouse gas emission and yield of rice(Oryza sativa L.). Ecol. Environ. Sci. 2020, 29, 977–986, (In Chinese with English abstract). [Google Scholar]
- Chen, J.; Li, C.; Kong, D.; Geng, Y.; Wang, H.; Fang, X.; Li, S.; Hu, Z.; Liu, S.; Zou, J. Incorporating DNA-level microbial constraints helps decipher methane emissions from Chinese water-saving ground cover rice production systems. Field Crops Res. 2021, 260, 107992. [Google Scholar] [CrossRef]






| Year | Treatment | CH4 Emissions | N2O Emissions | Shoot Biomass | Grain Yield | GWP | GHGI |
|---|---|---|---|---|---|---|---|
| (kg ha−1) | (kg ha−1) | (t ha−1) | (t ha−1) | (kg ha−1) | (kg·kg−1) | ||
| 2019 | CK | 204.68 ± 13.03 a | 0.74 ± 0.04 a | 28.51 ± 2.45 a | 10.59 ± 0.04 b | 7331.81 ± 220.53 a | 0.69 ± 0.02 a |
| BM | 112.86 ± 10.74 b | 0.31 ± 0.01 b | 30.26 ± 0.83 a | 11.02 ± 0.07 a | 3753.44 ± 228.41 b | 0.34 ± 0.02 b | |
| 2020 | CK | 249.87 ± 2.18 a | 0.75 ± 0.08 a | 24.01 ± 0.10 b | 11.44 ± 0.15 b | 8503.57 ± 199.38 a | 0.74 ± 0.01 a |
| BM | 145.37 ± 4.11 b | 0.47 ± 0.05 a | 28.23 ± 0.2 a | 12.12 ± 0.07 a | 5041.98 ± 175.66 b | 0.42 ± 0.02 b |
| TN | TP | AP | AN | AK | SOM | pH | |
|---|---|---|---|---|---|---|---|
| (g kg−1) | (g kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (g kg−1) | ||
| CK-PI | 2.40 ± 0.01 b | 0.75 ± 0.01 a | 23.25 ± 2.14 a | 58.80 ± 1.21 b | 151.04 ± 1.42 a | 52.58 ± 0.49 a | 4.98 ± 0.02 a |
| BM-PI | 2.80 ± 0.04 a | 0.76 ± 0.01 a | 23.61 ± 0.86 a | 99.98 ± 1.19 a | 149.23 ± 1.73 a | 53.33 ± 0.46 a | 5.04 ± 0.02 a |
| CK-HD | 2.87 ± 0.02 a | 0.84 ± 0.01 a | 18.53 ± 0.49 a | 106.54 ± 2.42 b | 152.72 ± 1.84 a | 53.84 ± 0.40 b | 4.98 ± 0.02 a |
| BM-HD | 2.85 ± 0.02 a | 0.86 ± 0.01 a | 18.62 ± 0.44 a | 132.68 ± 2.50 a | 149.14 ± 1.44 a | 54.90 ± 0.21 a | 5.03 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J.; Ye, T.; Sun, K.; Gao, Y.; Chen, H.; Xiang, J.; Wang, Y.; Wang, Z.; Zhang, Y.; Zhang, Y. Greenhouse Gas Emissions and Bacterial Community Structure as Influenced by Biodegradable Film Mulching in Eastern China. Agronomy 2023, 13, 1535. https://doi.org/10.3390/agronomy13061535
Xiong J, Ye T, Sun K, Gao Y, Chen H, Xiang J, Wang Y, Wang Z, Zhang Y, Zhang Y. Greenhouse Gas Emissions and Bacterial Community Structure as Influenced by Biodegradable Film Mulching in Eastern China. Agronomy. 2023; 13(6):1535. https://doi.org/10.3390/agronomy13061535
Chicago/Turabian StyleXiong, Jiahuan, Tiancheng Ye, Kaixuan Sun, Yizhuo Gao, Huizhe Chen, Jing Xiang, Yaliang Wang, Zhigang Wang, Yuping Zhang, and Yikai Zhang. 2023. "Greenhouse Gas Emissions and Bacterial Community Structure as Influenced by Biodegradable Film Mulching in Eastern China" Agronomy 13, no. 6: 1535. https://doi.org/10.3390/agronomy13061535
APA StyleXiong, J., Ye, T., Sun, K., Gao, Y., Chen, H., Xiang, J., Wang, Y., Wang, Z., Zhang, Y., & Zhang, Y. (2023). Greenhouse Gas Emissions and Bacterial Community Structure as Influenced by Biodegradable Film Mulching in Eastern China. Agronomy, 13(6), 1535. https://doi.org/10.3390/agronomy13061535






