Abstract
Strawberry is a worldwide demanded edible fruit with high economic and nutritional value; however, a very short storage life largely limits its supply and marketing. In this study, strawberries were treated using sodium selenite with different concentrations (6, 12, 18, and 24 mg/L), and the postharvest fruit quality and resistance to Botrytis cinerea were substantially assessed. Among all concentrations, 12 mg/L Se was the most effective treatment, which maintained fruit skin brightness, reduced natural decay incidence, severity, and weight loss, increased the Se content in fruit, and thus maintained the postharvest fruit quality of the strawberry. Furthermore, strawberries treated with 12 mg/L Se had lower flavonoid, phenolic, anthocyanins, proanthocyanidins, H2O2, and O2− contents compared to the control and, correspondingly, lower antioxidant capacity; moreover, 12 mg/L Se treatment decreased the decay incidence and severity caused by the infection of B. cinerea. Collectively, our findings may provide a reference for developing safe and environmentally friendly alternative methods to sustain quality and manage gray mold in postharvest strawberries as well as other horticultural crops.
1. Introduction
Strawberry (Fragaria × ananassa Duch.) is an economically important fruit that is widely cultivated in the world, and it is also a prominent dietary source of essential nutrients for human beings, such as sugars (e.g., glucose–3% and fructose–3%), ascorbic acid (AsA) (around 40–70 mg/100 g strawberries), minerals (in 100 g strawberries: potassium~75–123 mg; calcium~22–30 mg; magnesium~6–10 mg; phosphorus~2 mg; and nitrogen~600–1400 mg), as well as phenolic compounds (catechins: 40–100 mg/100 g; anthocyanins: 50–100 mg/100 g) and other antioxidants. Although it can be processed into jams, wines, jellies, and juices, etc., strawberries are mainly consumed fresh due to their delicious taste; however, strawberry fruit displays a very short shelf-life in the postharvest storage period (normally 1–2 days at room temperature, 5–7 days in a refrigerator), owing to its thin epidermis and, thus, soft fruit, fast metabolism, and sensitivity to mechanical damage, as well as decay caused by infection of pathogens of bacteria (e.g., Xanthomonas feagariae), fungi (Botrytis cinerea), and viruses (strawberry vein-banding virus) [1]. It has been found that postharvest losses can reach up to 50%, while gray mold can result in losses of about 35%, or approximately USD 10–100 million per year [2]. Nowadays, various techniques have been developed to preserve strawberries, such as pre-cooling, controlled atmosphere storage (CO2 levels of 15–20% and O2 concentrations of 5–10%), edible coating (e.g., alginate, chitosan, and polysaccharide), chemical and physical treatments (salicylic acid, melatonin, and UVC radiation), or the combination of several treatments [2]. For the control of gray mold, the most-used method still is the application of synthetic fungicides. However, along with the increase in peoples’ requirements for life and health, the application of synthetic fungicides raises potential risks regarding food and environmental safety. Developing environmentally friendly and safe alternative procedures is therefore needed to control the postharvest quality and extend strawberry shelf-life. So far, a large number of such strategies have been proposed, including the application of melatonin [3,4], β-aminobutyric acid [5], and phytosulfokine [6]. Recently, it has been suggested that mineral nutrition is one of the major elements influencing numerous properties of fruit [7]; however, few reports are found on how such micronutrients affect the quality of strawberries.
Selenium (Se) is an important micronutrient that has various biological functions regarding plant development and growth, fruit quality, as well as stress-response regulation [8]; moreover, Se is a microelement necessary to humans and other animals, conferring multiple benefits [9]. According to the World Health Organization (WHO), the recommended dietary allowance (RDA) for Se is 20–45 μg per day for children (from 1 to 13 years old) and 55–70 μg per day for adolescents and adults (14 years old and above) [10]. The maximum limit is 400 μg of Se intake per day [11,12]; however, in most populations, the intake of Se is below the daily lowest requirement. The supplemental application of Se on plants is, therefore, of great interest in production and industry. Se can be used as a safe compostable alternative to chemicals for the management of plant diseases and fruit quality, and exogenous Se has been widely applied to various species in recent years [13]. Increasing evidence has suggested that Se treatments delay plant senescence and fruit ripening, maintain and improve the fruit quality, and therefore decrease postharvest losses [14]. In addition, Se has been proven to be capable of inhibiting ethylene production, hence enhancing the quality and extending the shelf-life of horticultural crops such as lettuce [15] and tomato [16]. One possible reason for this is that the incorporation of Se and methionine forms Se–methionine, thus reducing free methionine, which is the initial substrate of the ethylene biosynthesis pathway. Another reason is that Se treatment could repress the expression of ethylene biosynthetic key genes, including 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase. Meanwhile, the application of Se also has a positive effect on the control of tomato fruit gray mold during the postharvest period [17], revealing its strong role in fruit protection against abiotic stress; however, to our knowledge, little information is available about the effects of Se on postharvest fruit quality and the B. cinerea infection of strawberry, a typical non-climacteric fruit.
Considering the importance and essential nature of Se, this work was accomplished with the objective to explore the influence of an exogenous Se application on postharvest quality and controlling postharvest gray mold of strawberries. The important sensory properties, nutritional compounds, and antioxidant capacity were determined after the Se treatment. Additionally, the Se content and the tolerance of strawberry fruit to B. cinerea infection were estimated. Our findings in this work provide an effective and safe way for maintaining the postharvest quality and controlling the postharvest gray mold of strawberries, as well as other horticultural fruits and vegetables.
2. Materials and Methods
2.1. Experimental Design
Strawberry fruits at commercially ripe (around 75% surface area is red) stage were harvested from plants, which were cultivated in a greenhouse located at Sichuan Agricultural University. The fruit uniform in size (about 15 g per fruit), lacking defects, were selected and transported to the lab immediately. They were subsequently separated into different groups, randomly, with 30 fruit at least in each. Then, each group was dipped into sodium selenite solution at 6, 12, 18, 24 mg/L and distilled water (set as control) for 15 min. Thereafter, the fruits were dried for around 30 min in the air and kept in an incubator under the condition of 25/21 °C (day/night), 16/8 h photoperiod, and 85–90% RH for 6 days. The Se-treated or untreated fruit were sampled on 0, 2, 4, and 6 days after treatment, and stored at −80 °C for distant use. A minimum of ten fruit were combined as one biological replicate, and three independent replicates were detected in total.
2.2. Determination of Se Content
The content of Se was assessed according to the standard (GB 5009.93-2017). In brief, around 0.2 g of fruit was weighed and then added to 10 mL HNO3–HClO4 with a 9:1 ratio; the mixture was subsequently heated on a hot plate. After cooling it down, 6 M hydrochloric acid was added and mixed. When a clear and colorless solution was observed, it was used for Se-content determination through a hydride atomic fluorescence spectrophotometer (AFS-8230, Beijing, China). The Se content was quantified by comparison with external standards and represented as mg per kg fresh weight (FW).
2.3. Skin Color, Weight Loss, Titratable Acidity, and Total Soluble Sugars
The surface color in the center of the strawberry fruit was detected using a CR-400 chromometer (Konica Minolta, Tokyo, Japan). Color equals contained three parameters formulated by the International Commission on Illumination (CIE) and represented by L*, a*, and b*: the L* value ranges from 0 to 100, indicating darkness to lightness; the a* value ranges from greenness to redness; and the b* value represents blueness to yellowness.
A total of 10 strawberries from each repeat group were weighed before treatment and on different sampling days. The FW loss was calculated by comparing the weight on each sampling day to the initial weight, expressed as a percentage.
The determination of titratable acidity (TA) content was performed by titrating the fruit extract to 0.1 N sodium hydroxide (NaOH) to the end point of pH 8.2 and represented as a percentage of citric acid. The content of total soluble sugars was detected by the anthrone colorimetric method, as previously described, with slight modification [18]. About 0.1 g of frozen stored fruit was extracted in 1 mL of distilled water. Then, the extract was diluted into 2% (w/v) anthrone-ethyl acetate and concentrated sulfuric acid. After that, the mixed solution was subsequently put in a boiled water bath for 1 min. After cooling it down to room temperature, the absorbance of the mixture was recorded at 620 nm using a spectrophotometer. The soluble sugar content was computed by comparison to external standard.
2.4. The Incidence and Severity of Decay
The fruit that displayed rot, lesion, or obvious fungal growth on the surface was regarded as decayed. The incidence of decay was calculated as decayed fruit percentage compared to the total fruit amount. The decay severity was evaluated by observing the decayed area around the surface of strawberry fruit. A total of 6 decay scales (0–5) were used to determine the severity of decay [19], where 0 = no occurrence of infection on fruit surface; 1 = minor decay (the infected surface area is ≤20%); 2 = temperate decay (20–40%); 3 = severe decay (40–60%); 4 = very severe decay (60–80%); 5 = almost all (>80%).
2.5. Total Flavonoid, Phenolic, Anthocyanin, Proanthocyanidin, and Ascorbic Acid Content
As previously described, the measurement of total flavonoid and phenolic content was carried out using the methods described formerly [20]. A total of 3 g of fruit was weighed and added to 5 mL of 80% acetone for 1 h at room temperature. After centrifugation for 10 min at room temperature at 4500 rpm, the clear solution was collected for measurement. For total flavonoids detection, the photographic density of the sample was read at 415 nm, while the absorption value of extracted mixture at 650 nm was read to quantify the total phenolic content. The quercetin and gallic acid were used as external standards to make the calibration curves separately. The results were presented as mg of quercetin per kg of FW for total flavonoid content and g of gallic acid per kg of FW.
Total anthocyanins were determined using the previously demonstrated pH differential method [21]. Around 1 g sample was collected with extraction solution (acetic acid: water: acetone: methanol = 1:2:4:4). After 30 min incubation at room temperature, the solution was then placed into a water bath for 4 h at 40 °C. Thereafter, the extracts were centrifuged; the supernatant was used for determination. Potassium chloride at 0.025 M with pH 1.0 and sodium acetate at 0.4 M with pH 4.5 were used as buffer solutions, and the absorption values of 496 and 700 nm were recorded, respectively. The content of total anthocyanins was represented as g of pelargonidin 3-glucoside (Pg3G) per kg of FW.
Proanthocyanidin content was assayed by a previously demonstrated method [21]. A mix of acetone, water, and glacial acetic acid in 150:49:1 ratio was used as extract solution. Around 1.5 g sample was extracted for 1 h at room temperature. After centrifugation for 20 min at 12 °C, the mixture was stained with 0.1% 4-dimethylaminocinnamaldehyde (DMAC). After that, the absorption value of 640 nm was read, the proanthocyanidin content was quantified by comparison with an external standard (Procyanidin B2). The total proanthocyanidin content was presented as g of procyanidin B2 per kg of FW.
AsA content was detected based on the approach described previously [18]. The extract photographic density was read at 534 nm, and the result was expressed as g of AsA per kg of FW.
2.6. Total Antioxidant Activity
FRAP (ferric-reducing antioxidant power) and DPPH (2,2-diphenyl-1-picrylhydrazyl) assays were employed to estimate the total antioxidant activities. According to the previous study [18], around 0.1 g of sample was extracted for 2 h in methanol. After centrifugation, 500 μL supernatant was added with DPPH solution in methanol. The reaction solution endured for 30 min at room temperature without light. The absorption value of the solution was recorded at 517 nm. The scavenging activity was evaluated by the inhibition percentage of DPPH. The FRAP measurement was performed using the previous approach [20]. The solution, consisting of acetate buffer (300 mM pH 3.6), TPTZ (10 mM), and FeCl3·6H2O (20 mM) in the ratio of 10:1:1 (v/v/v), was used as working FRAP reagent. After blending with the sample extract and reacting for 30 min, the absorbance of the mixture was read at 593 nm. The result was denoted as mmol kg−1 FW.
2.7. Malondialdehyde, Hydrogen Peroxide, and Superoxide Anions
According to the procedure described formerly [18], the malondialdehyde (MDA) was assayed as follows: 0.5 g of frozen sample was thoroughly homogenized with 10% trichloroacetic acid; then, this underwent centrifugation for 10 min at 4 °C, the clear solution was gathered and blended with 0.67% 2-thiobarbituric acid; after that, the combination was boiled for 10 min, immediately cooled on ice, and centrifuged. Finally, the absorption values at 450 nm, 523 nm, and 600 nm were recorded separately. The result was represented as μmol per g FW.
The superoxide anion (O2−) was assayed following the procedure described in the previous study [18]. First, a total of 0.5 g sample was extracted in Tris–HCl solution at 3 mL of 0.05 mon/L (pH 8.2), the extracts were subsequently centrifuged at 12,000× g for 10 min at 4 °C. Next, 0.5 mL of the upper phase was added with hydroxylamine hydrochloride and incubated at room temperature for 30 min. Finally, p-aminobenzenesulfonic acid and α-naphthylamine were added to the mixture and left standing at 25 °C for further 30 min, reading the absorption value of the mixture at 530 nm at once. Sodium nitrite was engaged as external standard to quantify the O2− content, the result was expressed as nmol per g FW.
According to the previously described method [18] for the measurement of hydrogen peroxide (H2O2), the 0.5 g strawberry fruit was extracted in 3 mL of 80% acetone. After centrifuging at 4 °C, the supernatant was blended with ammonia and titanium sulfate. The solution was centrifuged again, and the supernatants were discarded. After that, the precipitate was dissolved in H2SO4 and the mixture was then centrifuged for 10 min. The clear solution was quantified at 410 nm for H2O2. The result was represented as μmol per g of FW.
2.8. Data Analysis
All experiments for each treatment were performed in three independent biological replicates. Experimental data were expressed as mean value ± SD (standard deviation). The statistical differences between treatments were analyzed using one-way ANOVA method of IBM SPSS Statistics software (version 23.0) and determined using LSD test. The result with p value below 0.05 was considered as statistically significantly different. The correlation and PCA (principal components analysis) were performed by R package.
3. Results
3.1. Skin Color, Water Loss, Decay Incidence, and Severity
As shown in Figure 1, the L* and b* values gradually decreased during the postharvest time regardless of treatment. Strawberry fruits treated with 6 mg/L Se showed a faster decrease than the control (CK) during the whole postharvest time; however, 12, 18, and 24 mg/L treatments displayed higher L* and b* values on days 2 and 4 postharvest, only 12 mg/L Se-treated fruits conferred higher L* and b* values eventually. The a* value reached its peak 2 days after harvest and then decreased and showed the lowest level until 6 days postharvest. Se treatments did not alter the a* value on the first 2 days, while 12 mg/L Se treatment exhibited the highest a* value on days 4 and 6 after harvest.
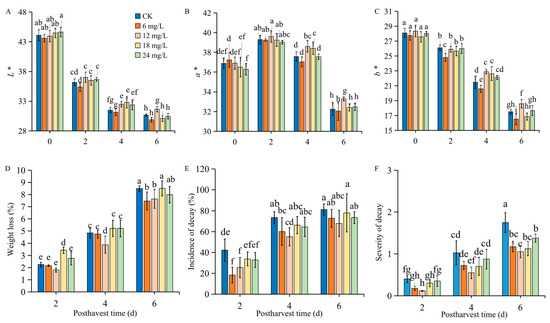
Figure 1.
The skin color, weight loss, decay incidence, and decay severity of strawberry fruit either untreated or treated with sodium selenite. L* (A), a* (B), and b*(C) values, weight loss (D), incidence of decay (E), and decay severity (F) of strawberry fruit during postharvest storage under Se treatment at different concentrations. All data were expressed as the mean values ± standard deviation. The letters indicate the statistically significant difference at p ≤ 0.05 based on the ANOVA analysis with LSD test.
In addition, the results presented in Figure 1E also demonstrate that 12 mg/L Se treatment could reduce the water loss, while the other treatments showed no significantly different—or even higher—water loss than the control group during postharvest storage. Strawberries treated with Se at 6, 12, 18, or 24 mg/L exhibited lower decay incidence and severity (Figure 1E,F). Among all of the treatments, 12 mg/L displayed the lowest decay incidence and severity during storage for 6 days, reducing the decay incidence by up to 20%; therefore, Se at 12 mg/L was selected for further determination and analysis in our study, in order to discover its positive effects on maintaining strawberry postharvest quality.
3.2. Se Content
At the initial stage (0 days), the Se content in strawberry fruit could not be detected. On day 2 postharvest, the content of Se was detected as 0.01 mg·kg−1 in the control group and 0.16 mg·kg−1 in the Se-treated group, which is 10 times higher compared to the control. Thereafter, although a progressively decreasing trend was observed for Se content in the Se-treated group, it still contained significantly higher Se levels than the control group. The highest Se content in the control group was detected on day 4 postharvest as 0.04 mg·kg−1, which was three times lower than that in the Se-treated group at the same postharvest stage (Figure 2).
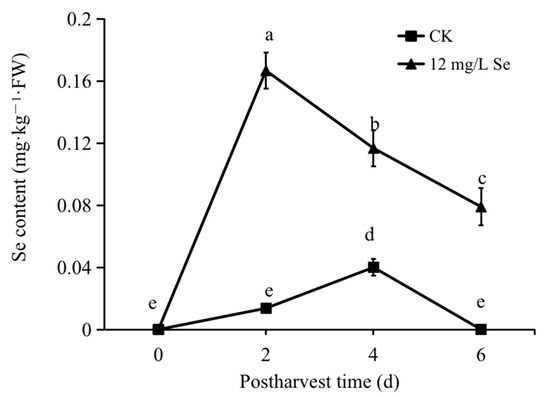
Figure 2.
The Se content in sodium selenite-treated or untreated strawberry fruit during storage. All data were expressed as the mean values ± standard deviation. The letters indicate the statistically significant difference at p ≤ 0.05 based on the ANOVA analysis with LSD test.
3.3. Soluble Sugars, Titratable Acid, and Ascorbic Acid
According to the results (Table 1), the AsA progressively increased during storage, while Se treatment did not have obvious effects on the AsA content; moreover, the TA content showed an increasing trend in the first 2 days but an opposite trend during the latter 4 storage days in the control fruit. In the Se-treated fruit, the TA content exhibited a stable level during the entire storage time. Although a slightly lower level of TA was found in the Se-treated fruit, no significant difference was found compared to the control (Table 1). Additionally, the soluble sugars declined during storage in both control and Se-treated fruit, and the Se treatment significantly increased the soluble sugar content (Table 1).

Table 1.
The effects of Se treatment on the nutritional qualities of strawberries during postharvest storage. The letters indicate a statistically significant difference at p ≤ 0.05 based on the ANOVA analysis with LSD test.
3.4. Total Phenolic, Flavonoid, Anthocyanin, and PA Content
As shown in Figure 3A, the TFC in both Se-treated and untreated strawberries showed gradually increasing trends after harvest, and an overall lower level of TFC in Se-treated fruit was found during the entire postharvest period compared to the control. Similarly, the TPC also displayed a gradually increasing trend during the postharvest period regardless of treatment. A slightly lower TPC level was detected in Se-treated fruit than that in untreated fruit but no statistically significant difference was found (Figure 3B). Anthocyanin content presented an increasing trend (Figure 3C), while proanthocyanidin content exhibited a decreasing trend (Figure 3D) in both control and Se-treated strawberry fruit during the storage period. The anthocyanins and proanthocyanidins were lower in the Se-treated fruit than that in the control fruit before 4 days; however, there was no remarkable difference between the Se-treated and untreated groups on day 6 of storage (Figure 3C,D).
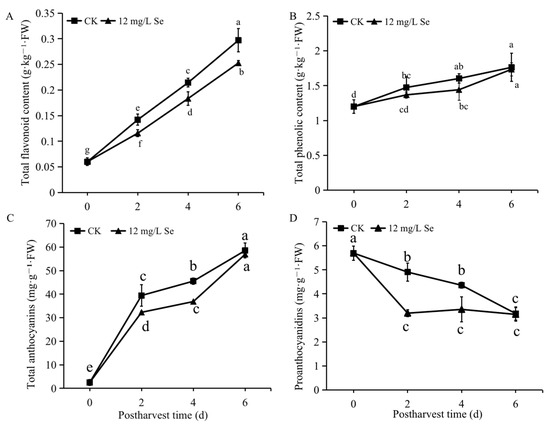
Figure 3.
The total flavonoid (A), phenolic (B), anthocyanins (C), and proanthocyanidins (D) contents in Se-treated or untreated strawberry fruit during postharvest storage. All data were expressed as the mean values ± standard deviation. The letters indicate the statistically significant difference at p ≤ 0.05 based on the ANOVA analysis with LSD test.
3.5. MDA, H2O2, and O2− Production and Antioxidant Capacity
Overall, the MDA, H2O2, and O2− production increased progressively from 0 to 6 days after harvest (Figure 3A–C). Specifically, no change in H2O2 content was found on day 2 postharvest (Figure 3A), while a notable increment in the levels of O2− and MDA was found in strawberry fruit treated with 12 mg/L Se (Figure 3B,C); lower levels of H2O2 and O2− were found after 2 days of storage but no remarkable change in MDA content was observed during this storage period. Additionally, the DPPH increased moderately in both Se-treated and untreated fruit during the entire storage period; no obvious difference in DPPH was found between the fruit treated or untreated with Se (Figure 4D). The FRAP showed an increasing trend during the postharvest storage in all samples (Figure 4E), which was inconsistent with the changing patterns of bioactive compounds including flavonoids, phenolics, anthocyanins, and proanthocyanidins in our results.
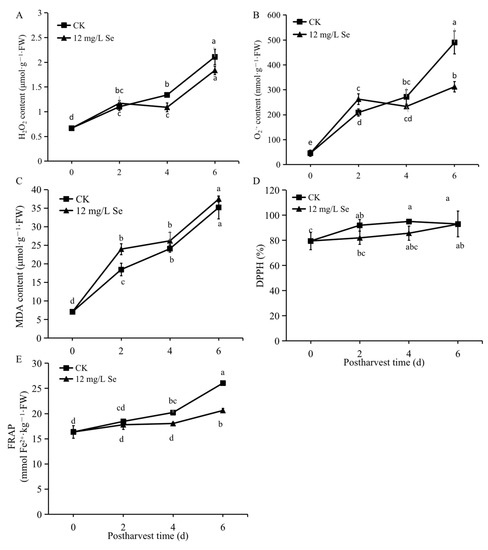
Figure 4.
The changes in H2O2 (A), O2− (B), MDA (C), DPPH (D), and FRAP (E) in Se-treated or untreated strawberry fruit during storage. All data were expressed as the mean values ± standard deviation. The letters indicate the statistically significant difference at p ≤ 0.05 based on the ANOVA analysis with LSD test.
3.6. Se Treatment Increased the Resistance of Strawberry to B. cinerea
The results of the B. cinerea infection experiment showed that Se treatment significantly reduced the occurrence of gray mold disease on postharvest strawberry fruit. Apparently, the symptoms were lighter and the lesions were smaller in the Se-treated fruit (Figure 5A). The incidence of decay after inoculation of B. cinerea was prominently decreased by Se biofortification, there was no significant difference in this parameter on the sixth day after inoculation (Figure 5B). Notably, the severity of decay was also largely cut down by Se treatment (Figure 5C), the largest severity degree of the control fruit was over three, while the largest severity degree of the Se-treated fruit was below two.
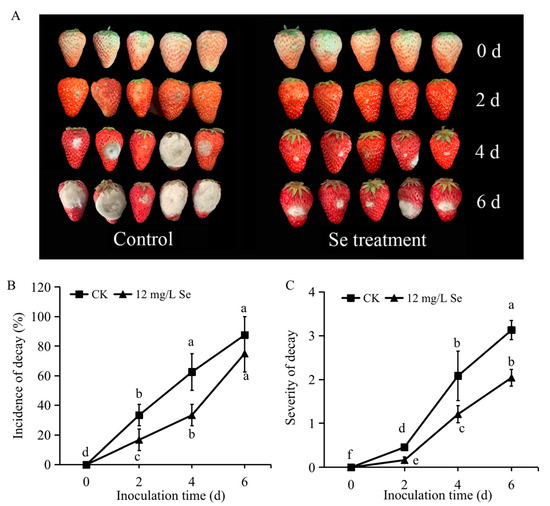
Figure 5.
The effects of Se treatment on the defense of strawberries against B. cinerea. (A), the symptoms of Se-treated or untreated strawberry fruit infected with B. cinerea; (B), the incidence of gray mold decay; (C), the severity of decay. All data were expressed as the mean values ± standard deviation. The letters indicate the statistically significant difference at p ≤ 0.05 based on the ANOVA analysis with LSD test.
3.7. Correlation and PCA Analysis
To better learn the relationship between nutritional variables during strawberry postharvest under Se treatment, a correlation analysis was carried out. As the results show (Figure 6A), the skin color indicators L*, a*, and b* showed significantly negative correlations with most other variables, such as AsA, hydrogen peroxide, FRAP, and the decay severity after B. cinerea inoculation. Similarly, the soluble sugar content showed a positive correlation with the b* value, with a negative correlation with other traits except for the a* value, Se content, TA, and proanthocyanidin content. On the contrary, the anthocyanins, H2O2, O2−, MDA, DPPH, and FRAP, and the incidence and severity after B. cinerea inoculation exhibited positive correlations with most variables but negative correlations with the L* and b* values. Interestingly, the content of Se and TA did not show any significant correlations with any other variables.
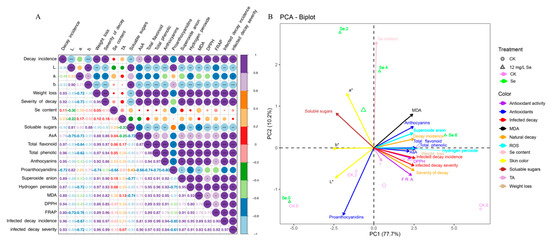
Figure 6.
Correlation (A) and PCA (B) analysis of all the measured variables during strawberry postharvest under Se treatment. * means significant differences at 0.05 level; ** mean significant differences at 0.01 level; *** mean significant differences at 0.001 level.
In addition, PCA was conducted to evaluate the change in quality indicators of strawberries subjected to treatments during postharvest storage. The results (Figure 6B) show us that all variables could be distinguished by the first (PC1) and second (PC2) principle components, which explained 77.7% and 10.2% of the total discrepancy, correspondingly. The Se-treated samples were clearly separated from the control samples along the PC2, and the postharvest time was obviously distinguished by PC1. The first 2 days were located on the negative quadrants while days 4 and 6 were interspersed in the positive quadrants of PC1. Some variables were evidently discerned from each other, suggesting a great difference between the CK and Se treatment. However, there is partial overlap, demonstrating that CK and Se treatment had comparable influences on the nutritional indexes of strawberries during postharvest. Among them, a* and soluble sugars were negatively related to Se treatment during postharvest, while MDA, anthocyanins, O2−, AsA, and natural decay incidence were positively related. The L* value and proanthocyanins content were negatively related to CK samples, while DPPH, FRAP, and the incidence and severity of decay after B. cinerea inoculation were positively related. However, the b* value is intervened between the first and third quadrants, while the total flavonoid content, total phenolic content, weight loss, and H2O2 were sprinkled in the second and fourth quadrants.
4. Discussion
4.1. Se Application Maintained the Postharvest Quality of Strawberry Fruit
Fruit skin color, an important trait attributing to the commercial value of strawberries, is generally reflected by numerical terms with the L*, a*, and b* coordinates. Among these, L* indicates brightness and the a* value indicates the darkness of the red color [22]. Our results showed that Se treatment at 12 mg/L conferred a lower lightness loss than other concentrations, according to its higher L* value during the entire postharvest storage (Figure 1A). Postharvest physiological loss in weight is mainly caused by the loss of water, which is a main determining factor for the quality loss of horticultural products. It has previously been reported that water loss in strawberry fruit increased progressively and significantly from around 10% to 40% as the storage period increased from 3 to 9 days [20]. Similarly, our results showed a gradually increasing trend of water loss during the postharvest storage of strawberries (Figure 1D) regardless of the treatment. The application of Se in delaying the loss of weight has been reported in guava, especially at lower concentrations [14]; however, our results did not show a more effective reduction in water loss at 6 mg/L than 12 mg/L Se treatment. This might be due to the species difference because strawberry fruit is more sensitive to rapid water loss due to their extraordinarily faint skin structure [23] compared to guava. More importantly, strawberries are too soft and suffer postharvest decay, and many techniques have been widely applied to extend the strawberry shelf life, such as spraying, coating, or dipping [24]. Here, we found that strawberries treated with Se exhibited lower decay incidence and severity (Figure 1E,F). This is similar to the previous study on tomatoes, in which Se treatment was shown to reduce the natural decay by around 18% [16]. It is possibly due to the lower oxidative stress in the Se-treated fruit, which may reduce the damage of cell membranes and thus reduce fruit decay.
AsA, TA, and soluble sugars are important traits of strawberry quality. Our findings indicated that exogenous Se did not affect the content of AsA in strawberry fruit (Table 1). This is different from the results in pear [25] and citrus [26], possibly due to the species difference. However, although the TA content was slightly reduced by Se treatment, no significant difference was observed compared to that of the control fruit (Table 1), which is inconsistent with the results in tomato [27]. Moreover, some studies have proved that the application of Se on leaves could increase the AsA and soluble sugar contents but reduce the organic acids in the fruit of pear-jujube and grape [28,29]. Similarly, according to our results (Table 1), the soluble sugar content was significantly improved by exogenous Se treatment in strawberries during postharvest storage.
4.2. The Application of Se Increased the Se Content in Strawberry Fruit
Se is an elemental micronutrient, and the adequate intake of Se is proven to be beneficial for maintaining human health, while the deficiency of Se (daily intake below 20 μg) will increase the risk of several diseases such as cancers, Alzheimer’s or Parkinson’s disease, and male infertility [30]. Under normal conditions, the Se content in strawberry fruit was detected as around 0.006 mg·kg−1 in the ripened fruit [31]. Here, it was too low to be detected at the initial stage (0 days), while up to 0.04 mg·kg−1 in the control group (Figure 2). This was probably due to the different cultivation environments and the soil in the area where we took the samples containing low concentrations of Se. As previously suggested, the Se concentrations in the soil varied in different areas [32,33]. Moreover, the addition of 10 and 100 μM Se in the cultivation solution could result in an average Se content in strawberry fruit of 3.95 and 46.04 μg·g−1 [34], respectively, while foliar spraying of 60 mg/L Se led to around 0.03 mg·kg−1·FW Se in the ripened strawberry fruit [31]. Our study largely increased the Se content up to 0.16 mg·kg−1·FW by dipping the strawberry fruit into 12 mg/L Se solution (Figure 2), developing a possibly more efficient method to increase the Se content in strawberry fruit. The RDA for Se is 20–45 μg per day for children and 55–70 μg per day for adults; however, amounts near 400 μg per day show signs of toxicity [11,12]. Our results showed that 100 g of Se-treated strawberry offered up to 16 μg Se, which may provide a rational Se supplementation for human nutrition.
4.3. Se Treatment Reduced the Oxidative Damage
Flavonoids such as anthocyanins are important antioxidants in plants. It was found that Se-treated fruit had an overall lower level of TFC compared to the control (Figure 3A), which might be the result of an inhibition of anthocyanins content by Se treatment (Figure 3C) and because anthocyanins are one of the most abundant flavonoids in strawberry. These results indicated that the fruit treated with Se might be exposed to less oxidative stress; however, these results are different from that in sweet basil, which showed no significant change in TFC under Se treatment [35], and Mechora et al. [36] and Zahedi et al. [37], who have shown different influences of Se on anthocyanins. It could be explained by the fact that the effects of Se on flavonoid content are related to the Se concentration and treated stages, as previously suggested [38].
In addition, both MDA and H2O2 are relevant standards to determine the oxidative force in plants. It was reported that MDA content declined at 6.0 mg·kg−1 of Se treatment, whereas 24 mg·kg−1 Se application significantly increased MDA content, indicating that MDA content could only be reduced by low-dose Se treatments [39,40]. Inconsistent with this, it was found that high-Se-concentration treatments at 10 and 20 mg/L led to high levels of MDA and H2O2, while 2.5 and 5 mg/L Se treatments resulted in depressed MDA and H2O2 accumulation [41]. Here, the comparatively high concentration of 12 mg/L Se treatment was shown to increase the MDA content on the first 2 days but decrease the H2O2 production during 4 to 6 days of the storage period in strawberry fruit (Figure 4A,C). This might be related to the ability of Se to delay senescence since H2O2 is a signal molecule that regulates senescence. Furthermore, the effects of Se on the antioxidant activity depend on the variety of strawberries and the biofortification conditions [42]. Our study showed lower antioxidant activity in strawberry fruit with Se biofortification due to the lower level of FRAP during the late storage period (Figure 4E). Combined with the fact that Se treatment decreased the content of H2O2 and O2− but did not affect the MDA content in the late storage period (Figure 4A–C), it was speculated that Se treatment might reduce the oxidative stress of strawberry fruit during postharvest storage.
4.4. Exogenous-Se Application Enhanced the Tolerance of Strawberry to B. cinerea Infection
Strawberries are extremely vulnerable to gray mold caused by B. cinerea during production, transportation, and storage, leading to more than 50% yield and economic loss in severe cases [1]. Se, an essential micronutrient for both humans and plants, is suggested to be involved in gray mold resistance, supported by increasing evidence. In vitro, it has been shown that 24 mg/L Se treatment markedly suppressed the spore germination of B. cinerea [17]; in vivo, Se treatment, alone or combined with other chemicals such as melatonin and methyl jasmonate, could effectively control tomato postharvest gray mold, either by increasing the antioxidant enzyme activities or damaging the membrane structure of the pathogen [43,44,45]. In our study, 12 mg/L treatment was shown to significantly control the gray mold disease of postharvest strawberry fruit. The incidence on the fourth day after inoculation was 33%, which was around half of the control. The symptoms and severity were also significantly reduced by Se treatment. This could be caused by the effects of the 12 mg/L Se treatment reducing the fruit decay (Figure 1) and oxidative stress (Figure 4), thus preventing the pathogen attack to some extent. Taken together, the application of 12 mg/L Se showed an extraordinarily declined susceptibility to B. cinerea infection in strawberry fruit; however, the potential molecular mechanism needs further research.
5. Conclusions
To summarize, our findings suggest that the 12 mg/L Se treatment could largely increase Se content in strawberries, which could rationally supplement Se and its benefits to human health; moreover, Se treatment improved the postharvest fruit quality by maintaining the fruit brightness, increasing the soluble sugars, decreasing TA content, and reducing the fruit decay. More importantly, 12 mg/L Se dipping could enhance the tolerance of strawberries to B. cinerea. The results may provide a new method for exploiting environmentally friendly approaches against B. cinerea. However, the molecular mechanism underlying the positive effects of Se on postharvest fruit quality and resistance to B. cinerea of strawberries needs to be further explored.
Author Contributions
Conceptualization, data analysis, writing—original draft preparation, review and editing: Y.L. (Yuanxiu Lin); data collecting and analysis: W.L.; data collecting and graphics presentation: S.C.; data collecting: R.T., Z.M., G.L. and S.Z.; software: Y.Z. (Yunting Zhang); formal analysis, writing—review and editing: M.L.; investigation, Y.W. and Q.C.; supervision, Y.L. (Ya Luo), X.W., Y.Z. (Yong Zhang) and H.T.; funding acquisition, Y.L. (Yuanxiu Lin). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Sichuan Province (Grant number: 2023NSFSC1239), and the Undergraduate Research Interest Cultivation Project of Sichuan Agricultural University (2022391).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef]
- Huma Qureshi, Q.; Waseem, A.; Rafia, A.; Nabila, C.-B.; Abdul, Q.; Asad, A. Post-Harvest Problems of Strawberry and Their Solutions. In Recent Studies on Strawberries; Nesibe Ebru, K., Ed.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Mansouri, S.; Sarikhani, H.; Sayyari, M.; Aghdam, M.S. Melatonin accelerates strawberry fruit ripening by triggering GAMYB gene expression and promoting ABA accumulation. Sci. Hortic. 2021, 281, 109919. [Google Scholar] [CrossRef]
- Pang, L.; Wu, Y.; Pan, Y.; Ban, Z.; Li, L.; Li, X. Insights into exogenous melatonin associated with phenylalanine metabolism in postharvest strawberry. Postharvest Biol. Technol. 2020, 168, 111244. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Aghdam, M.S.; Farmani, B.; Maggi, F.; Morshedloo, M.R. β-Aminobutyric acid treatment confers decay tolerance in strawberry fruit by warranting sufficient cellular energy providing. Sci. Hortic. 2018, 240, 249–257. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Alikhani-Koupaei, M. Exogenous phytosulfokine α (PSKα) applying delays senescence and relief decay in strawberry fruits during cold storage by sufficient intracellular ATP and NADPH availability. Food Chem. 2021, 336, 127685. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Shen, Y.; Huang, Y. Advances in Mineral Nutrition Transport and Signal Transduction in Rosaceae Fruit Quality and Postharvest Storage. Front. Plant Sci. 2021, 12, 620018. [Google Scholar] [CrossRef]
- Hasanuzzam, M.; Hossain, M.A.; Fujita, M. Selenium in Higher Plants: Physiological Role, Antioxidant Metabolism and Abiotic Stress Tolerance. J. Plant Sci. 2010, 5, 354–375. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Strand, T.A.; Lillegaard, I.T.L.; Frøyland, L.; Haugen, M.; Henjum, S.; Løvik, M.; Stea, T.H.; Holvik, K. Assessment of Selenium Intake in Relation to Tolerable Upper Intake Levels. Eur. J. Nutr. Food Saf. 2018, 8, 155–156. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- García Márquez, V.; Morelos Moreno, Á.; Benavides Mendoza, A.; Medrano Macías, J. Ionic Selenium and Nanoselenium as Biofortifiers and Stimulators of Plant Metabolism. Agronomy 2020, 10, 1399. [Google Scholar] [CrossRef]
- Choudhary, P.; Jain, V. Effect of post-harvest treatments of selenium on physico-chemical quality in guava (Psidium guajava L.) fruit. Hortic. Int. J. 2018, 2, 41–44. [Google Scholar] [CrossRef]
- Malorgio, F.; Diaz, K.E.; Ferrante, A.; Mensuali-Sodi, A.; Pezzarossa, B. Effects of selenium addition on minimally processed leafy vegetables grown in floating system. J. Sci. Food Agric. 2009, 89, 2243–2251. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Zhang, X.; Li, M. Effect of foliar treatment of sodium selenate on postharvest decay and quality of tomato fruits. Sci. Hortic. 2016, 198, 304–310. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, X.; Bañuelos, G.S.; Lin, Z.-Q.; Zhu, Z.; Liu, Y.; Yuan, L.; Li, M. Effect of Selenium on Control of Postharvest Gray Mold of Tomato Fruit and the Possible Mechanisms Involved. Front. Microbiol. 2016, 6, 1441. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, X.; Gu, X.; Deng, M.; Li, X.; Zhou, A.; Suo, M.; Gao, W.; Lin, Y.; Wang, Y.; et al. Light Quality and Sucrose-Regulated Detached Ripening of Strawberry with Possible Involvement of Abscisic Acid and Auxin Signaling. Int. J. Mol. Sci. 2023, 24, 5681. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria×anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Deng, M.; Gui, R.; Liu, Y.; Chen, X.; Lin, Y.; Li, M.; Wang, Y.; He, W.; et al. Blue light combined with salicylic acid treatment maintained the postharvest quality of strawberry fruit during refrigerated storage. Food Chem. X 2022, 15, 100384. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Peng, X.; Sun, B.; Wang, X.; Tang, H. Characterization of anthocyanin and proanthocyanidin biosynthesis in two strawberry genotypes during fruit development in response to different light qualities. J. Photochem. Photobiol. B 2018, 186, 225–231. [Google Scholar] [CrossRef]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Muñoz, P.; Almenar, E.; Del Valle, V.; Velez, D.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria×ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Shaghef, E.; Rana Naveed Ur, R.; Mumtaz, K.; Rashad, Q. Postharvest Quality Management of Strawberries. In Strawberry-Pre and Post-Harvest Management Techniques for Higher Fruit Quality; Toshiki, A., Md, A., Eds.; IntechOpen: Rijeka, Croatia, 2019; p. 22. [Google Scholar]
- Liu, Q.L.; Hao, Y.Y.; Hao, G.W.; Wu, G.L.; Niu, T.Q. Effects of Spraying Selemium on the Mineral Elements Content and the Storage Properties of the Pear Fruits. Plant Physiol. J. 2015, 51, 655–660. [Google Scholar]
- Wen, M.; Wang, P.; Gao, W.; Wu, S.; Huang, B. Effects of Foliar Spraying with Different Concentrations of Selenium Fertilizer on the Development, Nutrient Absorption, and Quality of Citrus Fruits. Hortscience 2021, 56, 1363–1367. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Rosellini, I.; Borghesi, E.; Tonutti, P.; Malorgio, F. Effects of Se-enrichment on yield, fruit composition and ripening of tomato (Solanum lycopersicum) plants grown in hydroponics. Sci. Hortic. 2014, 165, 106–110. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, P.; Wang, Y.; Feng, H. Different approaches for selenium biofortification of pear-jujube (Zizyphus jujuba M. cv. Lizao) and associated effects on fruit quality. J. Food Agric. Environ. 2013, 11, 529–534. [Google Scholar]
- Zhu, S.; Liang, Y.; Gao, D.; An, X.; Kong, F. Spraying foliar selenium fertilizer on quality of table grape (Vitis vinifera L.) from different source varieties. Sci. Hortic. 2017, 218, 87–94. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Lu, N.; Wu, L.; Zhang, X.; Zhang, Y.; Shan, C. Selenium improves the content of vitamin C in the fruit of strawberry by regulating the enzymes responsible for vitamin C metabolism. Plant, Soil Environ. 2022, 68, 205–211. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: New York, NY, USA, 2011; pp. 645–651. [Google Scholar]
- Fordyce, F. Selenium Geochemistry and Health. Ambio 2007, 36, 94–97. [Google Scholar] [CrossRef]
- Mimmo, T.; Tiziani, R.; Valentinuzzi, F.; Lucini, L.; Nicoletto, C.; Sambo, P.; Scampicchio, M.; Pii, Y.; Cesco, S. Selenium Biofortification in Fragaria × ananassa: Implications on Strawberry Fruits Quality, Content of Bioactive Health Beneficial Compounds and Metabolomic Profile. Front. Plant Sci. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of Phenolic Compounds, Essential Oil Content and Antioxidant Properties of Sweet Basil (Ocimum basilicum L.) Depending on Type and Concentration of Selenium Application. Plants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Mechora, Š.; Stibilj, V.; Radešček, T.; Gaberščik, A.; Germ, M. Impact of Se (VI) fertilization on Se concentration in different parts of red cabbage plants. J. Food Agric. Environ. 2011, 9, 357–361. [Google Scholar]
- Zahedi, S.M.; Hosseini, M.S.; Meybodi, N.D.H.; da Silva, J.A.T. Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. Malase Saveh) fruit yield and quality. S. Afr. J. Bot. 2019, 124, 350–358. [Google Scholar] [CrossRef]
- Skrypnik, L.; Styran, T.; Savina, T.; Golubkina, N. Effect of Selenium Application and Growth Stage at Harvest on Hydrophilic and Lipophilic Antioxidants in Lamb’s Lettuce (Valerianella locusta L. Laterr). Plants 2021, 10, 2733. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Shen, J.; Shao, F.; Li, T. Effects of selenium on the growth and photosynthetic characteristics of flue-cured tobacco (Nicotiana tabacum L.). Acta Soc. Bot. Pol. 2015, 84, 71–77. [Google Scholar] [CrossRef]
- Cartes, P.; Gianfreda, L.; Mora, M. Uptake of Selenium and its Antioxidant Activity in Ryegrass When Applied as Selenate and Selenite Forms. Plant Soil 2005, 276, 359–367. [Google Scholar] [CrossRef]
- Khalofah, A.; Migdadi, H.; El-Harty, E. Antioxidant Enzymatic Activities and Growth Response of Quinoa (Chenopodium quinoa Willd) to Exogenous Selenium Application. Plants 2021, 10, 719. [Google Scholar] [CrossRef]
- Groth, S.; Budke, C.; Neugart, S.; Ackermann, S.; Kappenstein, F.-S.; Daum, D.; Rohn, S. Influence of a Selenium Biofortification on Antioxidant Properties and Phenolic Compounds of Apples (Malus domestica). Antioxidants 2020, 9, 187. [Google Scholar] [CrossRef]
- Zang, H.; Ma, J.; Wu, Z.; Yuan, L.; Lin, Z.-Q.; Zhu, R.; Bañuelos, G.S.; Reiter, R.J.; Li, M.; Yin, X. Synergistic Effect of Melatonin and Selenium Improves Resistance to Postharvest Gray Mold Disease of Tomato Fruit. Front. Plant Sci. 2022, 13, 903936. [Google Scholar] [CrossRef]
- Li, C.; Hu, C.; Xie, J.; Shi, G.; Wang, X.; Yuan, X.; Li, K.; Chen, S.; Zhao, X.; Fan, G. Selenium Combined with Methyl Jasmonate to Control Tomato Gray Mold by Optimizing Microbial Community Structure in Plants. J. Fungi 2022, 8, 731. [Google Scholar] [CrossRef]
- Yuan, X.; Li, C.; Xie, J.; Li, K.; Chen, S.; Yuan, L.; Hu, C.; Wang, X.; Zhao, X. Combination of Selenium and Methyl Jasmonate Controls Postharvest Tomato Gray Mold by Damaging the Membrane System. Horticulturae 2022, 8, 782. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).