Synthesis of Zinc Oxide Nanoparticles and Their Applications in Enhancing Plant Stress Resistance: A Review
Abstract
:1. Introduction
2. Preparation of ZnO Nanoparticles
2.1. Physical Methods
2.1.1. Physical Vapor Deposition (PVD)
2.1.2. Ball Milling
2.2. Chemical Methods
2.2.1. Hydrothermal Synthesis
2.2.2. Solvothermal Synthesis
2.2.3. Precipitation Method
2.2.4. Microwave Synthesis
2.3. Biological Methods
2.3.1. Microbial Synthesis
2.3.2. Plant-Mediated Synthesis
3. Absorption and Transfer of ZnO Nanoparticles in the Plant
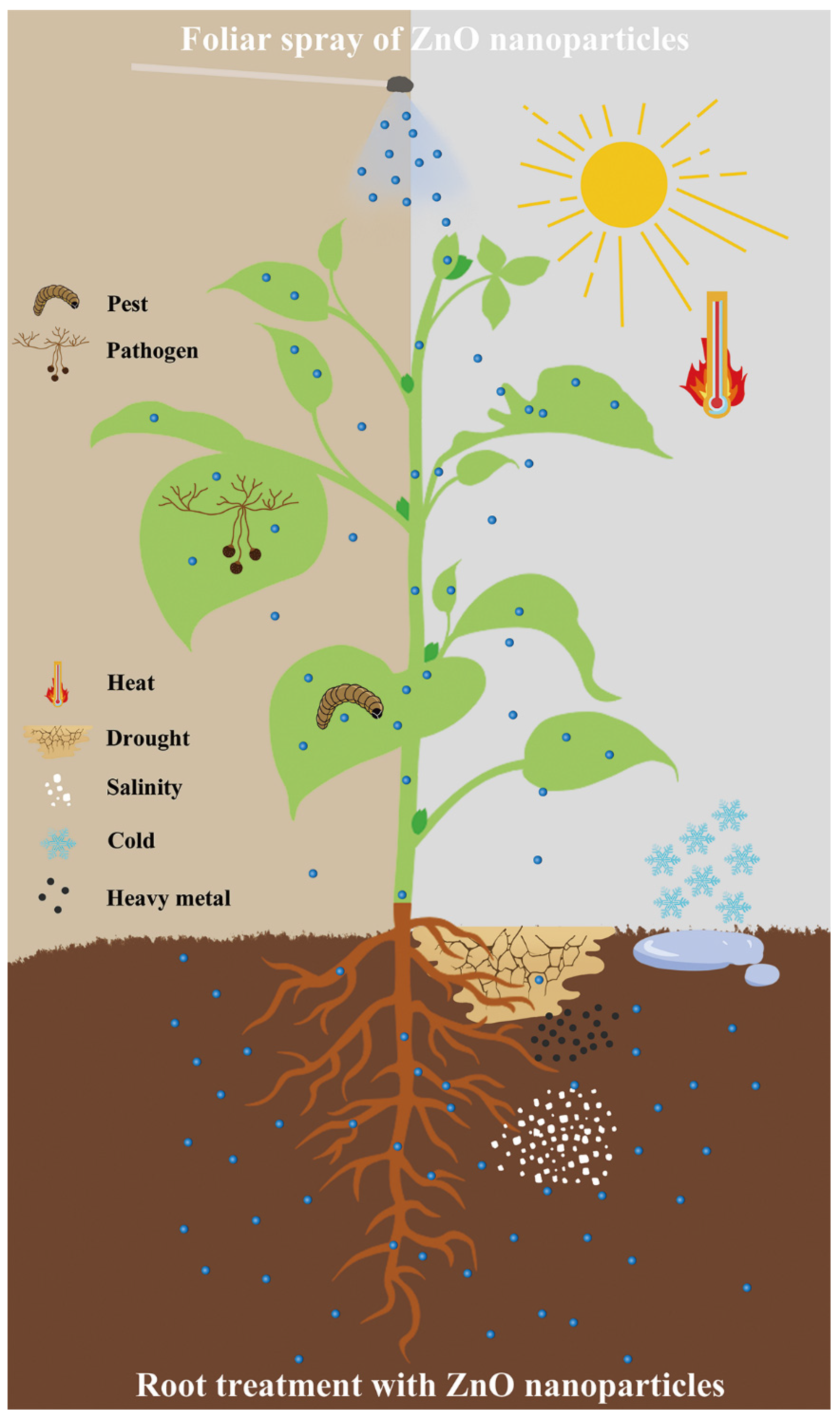
4. Impact of ZnO Nanoparticles against Biotic and Abiotic Stress
4.1. Impact of ZnO Nanoparticles against Biotic Stress
4.1.1. Pests
4.1.2. Plant Pathogens
4.2. Impact of ZnO Nanoparticles against Abiotic Stress
4.2.1. Drought Stress
4.2.2. Heat Stress
4.2.3. Salinity Stress
4.2.4. Cold Stress
4.2.5. Heavy Metal Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gowdy, J. Our hunter-gatherer future: Climate change, agriculture and uncivilization. Futures 2020, 115, 102488. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, J.-W.; Li, J.; Han, B. Designing future crops: Challenges and strategies for sustainable agriculture. Plant J. 2021, 105, 1165–1178. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Zsögön, A.; Peres, L.E.P.; Xiao, Y.; Yan, J.; Fernie, A.R. Enhancing crop diversity for food security in the face of climate uncertainty. Plant J. 2022, 109, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A.; Ignacimuthu, S. Management of phosphorus nutrient amid climate change for sustainable agriculture. J. Environ. Qual. 2021, 50, 1303–1324. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Grote, U.; Neubacher, F.; Rahut, D.B.; Do, M.H.; Paudel, G.P. Security risks from climate change and environmental degradation: Implications for sustainable land use transformation in the Global South. Glob. Chang. Biol. 2023, 63, 101322. [Google Scholar] [CrossRef]
- Miller, R.N.G.; Costa Alves, G.S.; Van Sluys, M.-A. Plant immunity: Unravelling the complexity of plant responses to biotic stresses. Ann. Bot. 2017, 119, 681–687. [Google Scholar] [CrossRef]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide research on plant defense against biotic stresses as improvement for sustainable agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W.J.E. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, Y.; Zhang, L.; Zhang, J.; Zhou, Y.; Huang, H.; Luo, S.; Luo, L. Silicon-based additive on heavy metal remediation in soils: Toxicological effects, remediation techniques, and perspectives. Environ. Res. 2022, 205, 112244. [Google Scholar] [CrossRef]
- Islam, M.; Sandhi, A. Heavy metal and drought stress in plants: The role of microbes—A review. Gesunde Pflanz. 2023, 75, 695–708. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a reduced reliance on conventional pesticides in European agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Schröder, P.; Sauvêtre, A.; Gnädinger, F.; Pesaresi, P.; Chmeliková, L.; Doğan, N.; Gerl, G.; Gökçe, A.; Hamel, C.; Millan, R.; et al. Discussion paper: Sustainable increase of crop production through improved technical strategies, breeding and adapted management—A European perspective. Sci. Total Environ. 2019, 678, 146–161. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.-J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown gold of marginal soil: Plant growth promoting bacteria to overcome plant abiotic stress for agriculture, biofuels and carbon sequestration. Sci. Total Environ. 2020, 711, 135062. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Siddiqui, A.J.; Elkhalifa, A.E.O.; Khan, M.I.; Patel, M.; Alreshidi, M.; Moin, A.; Singh, R.; Snoussi, M.; Adnan, M. Innovations in nanoscience for the sustainable development of food and agriculture with implications on health and environment. Sci. Total Environ. 2021, 768, 144990. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Wang, D.; Saleh, N.B.; Byro, A.; Zepp, R.; Sahle-Demessie, E.; Luxton, T.P.; Ho, K.T.; Burgess, R.M.; Flury, M.; White, J.C.; et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022, 17, 347–360. [Google Scholar] [CrossRef]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Tripathi, D.K.; Yadav, S.; Kalaji, H.M.; Brestic, M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Arora, S.; Murmu, G.; Mukherjee, K.; Saha, S.; Maity, D. A comprehensive overview of nanotechnology in sustainable agriculture. J. Biotechnol. 2022, 355, 21–41. [Google Scholar] [CrossRef]
- Saritha, G.N.G.; Anju, T.; Kumar, A. Nanotechnology-big impact: How nanotechnology is changing the future of agriculture? J. Agric. Food Res. 2022, 10, 100457. [Google Scholar] [CrossRef]
- Prasad, A.R.; Williams, L.; Garvasis, J.; Shamsheera, K.O.; Basheer, S.M.; Kuruvilla, M.; Joseph, A. Applications of phytogenic ZnO nanoparticles: A review on recent advancements. J. Mol. Liq. 2021, 331, 115805. [Google Scholar] [CrossRef]
- Gomez, J.L.; Tigli, O. Zinc oxide nanostructures: From growth to application. J. Mater. Sci. 2013, 48, 612–624. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A 2021, 404, 112866. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Singh, A.K. Bio-waste and natural resource mediated eco-friendly synthesis of zinc oxide nanoparticles and their photocatalytic application against dyes contaminated water. Chem. Eng. J. Adv. 2023, 16, 100536. [Google Scholar] [CrossRef]
- Ragavendran, C.; Kamaraj, C.; Jothimani, K.; Priyadharsan, A.; Anand Kumar, D.; Natarajan, D.; Malafaia, G. Eco-friendly approach for ZnO nanoparticles synthesis and evaluation of its possible antimicrobial, larvicidal and photocatalytic applications. Sustain. Mater. Technol. 2023, 36, e00597. [Google Scholar] [CrossRef]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Akhtar, N.; Wani, A.K.; Dhanjal, D.S.; Mukherjee, S. Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: Resilience to biotic and abiotic stresses. World J. Microbiol. Biotechnol. 2022, 38, 79. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.; Bello, S.K. Piriformospora indica colonization reprograms plants to improved P-uptake, enhanced crop performance, and biotic/abiotic stress tolerance. Physiol. Mol. Plant Pathol. 2019, 106, 232–237. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, S. Evaluation of Pseudomonas sp. for its multifarious plant growth promoting potential and its ability to alleviate biotic and abiotic stress in tomato (Solanum lycopersicum) plants. Sci. Rep. 2020, 10, 20951. [Google Scholar] [CrossRef]
- Kashyap, B.; Kumar, R. Sensing methodologies in agriculture for monitoring biotic stress in plants due to pathogens and pests. Inventions 2021, 6, 29. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, H.; Chen, S.; Yu, D.; Reiter, R.J. Phytomelatonin: An emerging regulator of plant biotic stress resistance. Trends Plant Sci. 2021, 26, 70–82. [Google Scholar] [CrossRef]
- Gupta, A.; Bano, A.; Rai, S.; Mishra, R.; Singh, M.; Sharma, S.; Pathak, N. Mechanistic insights of plant-microbe interaction towards drought and salinity stress in plants for enhancing the agriculture productivity. Plant Stress 2022, 4, 100073. [Google Scholar] [CrossRef]
- Vancostenoble, B.; Blanchet, N.; Langlade, N.B.; Bailly, C. Maternal drought stress induces abiotic stress tolerance to the progeny at the germination stage in sunflower. Environ. Exp. Bot. 2022, 201, 104939. [Google Scholar] [CrossRef]
- Wang, X.; Komatsu, S. The role of phytohormones in plant response to flooding. Int. J. Mol. Sci. 2022, 23, 6383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of proline function in higher plants under extreme temperatures. Plant Biol. J. 2023, 25, 379–395. [Google Scholar] [CrossRef]
- Reddy Pullagurala, V.L.; Adisa, I.O.; Rawat, S.; Kim, B.; Barrios, A.C.; Medina-Velo, I.A.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Finding the conditions for the beneficial use of ZnO nanoparticles towards plants-A review. Environ. Pollut. 2018, 241, 1175–1181. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Harish; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Silva, S.; Dias, M.C.; Silva, A.M.S. Titanium and zinc based nanomaterials in agriculture: A promising approach to deal with (a)biotic stresses? Toxics 2022, 10, 172. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Hussain, S.A. Foliar application of zinc oxide nanoparticles improves rice yield under biotic stress posed by Magnaporthe oryzae. Arch. Phytopathol. Plant Prot. 2023, 56, 1093–1111. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A 2012, 90, 78–84. [Google Scholar] [CrossRef]
- Cai, L.; Liu, C.; Fan, G.; Liu, C.; Sun, X. Preventing viral disease by ZnONPs through directly deactivating TMV and activating plant immunity in Nicotiana benthamiana. Environ. Sci. Nano 2019, 6, 3653–3669. [Google Scholar] [CrossRef]
- Das, S.; Yadav, A.; Debnath, N. Entomotoxic efficacy of aluminium oxide, titanium dioxide and zinc oxide nanoparticles against Sitophilus oryzae (L.): A comparative analysis. J. Stored Prod. Res. 2019, 83, 92–96. [Google Scholar] [CrossRef]
- Mosquera-Sánchez, L.P.; Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Guerra–Sierra, B.E.; Muñoz-Florez, J.E.; Rodríguez-Páez, J.E. Antifungal effect of zinc oxide nanoparticles (ZnO-NPs) on Colletotrichum sp., causal agent of anthracnose in coffee crops. Biocatal. Agric. Biotechnol. 2020, 25, 101579. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Dacrory, S.; Hashem, A.H.; Attia, M.S.; Hasanin, M.; Fouda, H.M.; Kamel, S.; ElSaied, H. Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal. Agric. Biotechnol. 2021, 35, 102083. [Google Scholar] [CrossRef]
- Zudyte, B.; Luksiene, Z. Visible light-activated ZnO nanoparticles for microbial control of wheat crop. J. Photochem. Photobiol. B 2021, 219, 112206. [Google Scholar] [CrossRef] [PubMed]
- Bouqellah, N.A.; El-Sayyad, G.S.; Attia, M.S. Induction of tomato plant biochemical immune responses by the synthesized zinc oxide nanoparticles against wilt-induced Fusarium oxysporum. Int. Microbiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, M.; Rajini, S.B.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Shetty, H.S.; Prakash, H.S. Biofabricated zinc oxide nanoparticles as an eco-friendly alternative for growth promotion and management of downy mildew of pearl millet. Crop Prot. 2019, 121, 103–112. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Noureldeen, A.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles and 24-epibrassinolide alleviates Cu toxicity in tomato by regulating ROS scavenging, stomatal movement and photosynthesis. Ecotoxicol. Environ. Saf. 2021, 218, 112293. [Google Scholar] [CrossRef]
- Ali, B.; Saleem, M.H.; Ali, S.; Shahid, M.; Sagir, M.; Tahir, M.B.; Qureshi, K.A.; Jaremko, M.; Selim, S.; Hussain, A.; et al. Mitigation of salinity stress in barley genotypes with variable salt tolerance by application of zinc oxide nanoparticles. Front. Plant Sci. 2022, 13, 973782. [Google Scholar] [CrossRef]
- Ajmal, M.; Ullah, R.; Muhammad, Z.; Khan, M.N.; Kakar, H.A.; Kaplan, A.; Okla, M.K.; Saleh, I.A.; Kamal, A.; Abdullah, A.; et al. Kinetin capped zinc oxide nanoparticles improve plant growth and ameliorate resistivity to polyethylene glycol (PEG)-induced drought stress in Vigna radiata (L.) R. Wilczek (Mung Bean). Molecules 2023, 28, 5059. [Google Scholar] [CrossRef]
- Markarian, S.; Shariatmadari, H.; Shirvani, M.; Mirmohammady Maibody, S.A.M. Impacts of ZnO nanoparticles and dissolved zinc (ZnSO4) on low temperature induced responses of wheat. J. Plant Nutr. 2023, 46, 3435–3449. [Google Scholar] [CrossRef]
- Rukhsar Ul, H.; Kausar, A.; Hussain, S.; Javed, T.; Zafar, S.; Anwar, S.; Hussain, S.; Zahra, N.; Saqib, M. Zinc oxide nanoparticles as potential hallmarks for enhancing drought stress tolerance in wheat seedlings. Plant Physiol. Biochem. 2023, 195, 341–350. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the mobilization of native phosphorus in the mung bean rhizosphere using ZnO nanoparticles synthesized by soil fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, R.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, transcriptomic, and metabolomic analyses reveal zinc oxide nanoparticles modulate plant growth in tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Wu, F.; Fang, Q.; Yan, S.; Pan, L.; Tang, X.; Ye, W. Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): Germination, early growth, and arsenic uptake. Environ. Sci. Pollut. Res. 2020, 27, 26974–26981. [Google Scholar] [CrossRef] [PubMed]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 738, 140240. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci. Nano 2020, 7, 3901–3913. [Google Scholar] [CrossRef]
- Pejam, F.; Ardebili, Z.O.; Ladan-Moghadam, A.; Danaee, E. Zinc oxide nanoparticles mediated substantial physiological and molecular changes in tomato. PLoS ONE 2021, 16, e0248778. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Wojnarowicz, J. Zinc oxide and zinc oxide nanoparticles impact on in vitro germination and seedling growth in Allium cepa L. Materials 2020, 13, 2784. [Google Scholar] [CrossRef]
- Lyu, S.C.; Zhang, Y.; Lee, C.J.; Ruh, H.; Lee, H.J. Low-temperature growth of ZnO nanowire array by a simple physical vapor-deposition method. Chem. Mater. 2003, 15, 3294–3299. [Google Scholar] [CrossRef]
- Thangadurai, P.; Zergioti, I.; Saranu, S.; Chandrinou, C.; Yang, Z.; Tsoukalas, D.; Kean, A.; Boukos, N. ZnO nanoparticles produced by novel reactive physical deposition process. Appl. Surf. Sci. 2011, 257, 5366–5369. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Glushenkov, A.; Zhang, H.-Z.; Chen, Y. Reactive ball milling to produce nanocrystalline ZnO. Mater. Lett. 2008, 62, 4047–4049. [Google Scholar] [CrossRef]
- Asjadi, F.; Yaghoobi, M. Characterization and dye removal capacity of green hydrothermal synthesized ZnO nanoparticles. Ceram. Int. 2022, 48, 27027–27038. [Google Scholar] [CrossRef]
- Lee, C.Y.; Tseng, T.Y.; Li, S.Y.; Lin, P. Effect of phosphorus dopant on photoluminescence and field-emission characteristics of Mg0.1 Zn0.9O nanowires. J. Appl. Phys. 2006, 99, 024303. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Conventional and microwave hydrothermal synthesis and application of functional materials: A review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.K.; Rana, S. Microwave reactors: A brief review on its fundamental aspects and applications. Open Acc. Lib. J 2014, 1, 1–20. [Google Scholar] [CrossRef]
- Strachowski, T.; Baran, M.; Małek, M.; Kosturek, R.; Grzanka, E.; Mizeracki, J.; Romanowska, A.; Marynowicz, S. Hydrothermal synthesis of zinc oxide nanoparticles using different chemical reaction stimulation methods and their influence on process kinetics. Materials 2022, 15, 7661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y. Zinc oxide nanoparticles: Synthesis, characterization, modification, and applications in food and agriculture. Prosses 2023, 11, 1193. [Google Scholar] [CrossRef]
- Ruba, A.A.; Johny, L.M.; Jothi, N.N.; Sagayaraj, P. Solvothermal synthesis, characterization and photocatalytic activity of ZnO nanoparticle. Mater. Today Proc. 2019, 8, 94–98. [Google Scholar] [CrossRef]
- Šarić, A.; Despotović, I.; Štefanić, G. Solvothermal synthesis of zinc oxide nanoparticles: A combined experimental and theoretical study. J. Mol. Struct. 2019, 1178, 251–260. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N. Structural and vibrational properties of ZnO nanoparticles synthesized by the chemical precipitation method. Phys. E 2014, 58, 130–137. [Google Scholar] [CrossRef]
- Kahouli, M.; Barhoumi, A.; Bouzid, A.; Al-Hajry, A.; Guermazi, S. Structural and optical properties of ZnO nanoparticles prepared by direct precipitation method. Superlattices Microstruct. 2015, 85, 7–23. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.; Liu, P.; Yu, B. Investigation of photocatalytic degradation of methyl orange by using nano-sized ZnO catalysts. Adv. Chem. Eng. Sci. 2011, 1, 9. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef] [PubMed]
- López-Cuenca, S.; Aguilar-Martínez, J.; Rabelero-Velasco, M.; Hernández-Ibarra, F.; López-Ureta, L.; Pedroza-Toscano, M. Spheroidal zinc oxide nanoparticles synthesized by semicontinuous precipitation method at low temperatures. Rev. Mex. Ing. Quim. 2019, 18, 1179–1187. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Nokhodchi, A. Synthesis and modification of bio-derived antibacterial Ag and ZnO nanoparticles by plants, fungi, and bacteria. Drug Discovery Today 2021, 26, 1953–1962. [Google Scholar] [CrossRef]
- Murali, M.; Gowtham, H.G.; Shilpa, N.; Singh, S.B.; Aiyaz, M.; Sayyed, R.Z.; Shivamallu, C.; Achar, R.R.; Silina, E.; Stupin, V.; et al. Zinc oxide nanoparticles prepared through microbial mediated synthesis for therapeutic applications: A possible alternative for plants. Front. Microbiol. 2023, 14, 1227951. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.S.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 2019, 102, 212–220. [Google Scholar] [CrossRef]
- Shayegan Mehr, E.; Sorbiun, M.; Ramazani, A.; Taghavi Fardood, S. Plant-mediated synthesis of zinc oxide and copper oxide nanoparticles by using ferulago angulata (schlecht) boiss extract and comparison of their photocatalytic degradation of Rhodamine B (RhB) under visible light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 1333–1340. [Google Scholar] [CrossRef]
- Venkateasan, A.; Prabakaran, R.; Sujatha, V. Phytoextract-mediated synthesis of zinc oxide nanoparticles using aqueous leaves extract of Ipomoea pes-caprae (L). R. br revealing its biological properties and photocatalytic activity. Nanotechnol. Environ. Eng. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Parajuli, D.; Kawakita, H.; Inoue, K.; Ohto, K.; Kajiyama, K. Persimmon peel gel for the selective recovery of gold. Hydrometallurgy 2007, 87, 133–139. [Google Scholar] [CrossRef]
- Xiong, Y.; Adhikari, C.R.; Kawakita, H.; Ohto, K.; Inoue, K.; Harada, H. Selective recovery of precious metals by persimmon waste chemically modified with dimethylamine. Bioresour. Technol. 2009, 100, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Adam, V.; Rizvi, T.F.; Zhang, B.; Ahamad, F.; Jośko, I.; Zhu, Y.; Yang, M.; Mao, C. Nanoparticle–plant interactions: Two-way traffic. Small 2019, 15, 1901794. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Paulraj, T.; Wennmalm, S.; Wieland, D.C.F.; Riazanova, A.V.; Dėdinaitė, A.; Günther Pomorski, T.; Cárdenas, M.; Svagan, A.J. Primary cell wall inspired micro containers as a step towards a synthetic plant cell. Nat. Commun. 2020, 11, 958. [Google Scholar] [CrossRef]
- Zhang, H.; Goh, N.S.; Wang, J.W.; Pinals, R.L.; González-Grandío, E.; Demirer, G.S.; Butrus, S.; Fakra, S.C.; Del Rio Flores, A.; Zhai, R.; et al. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 2022, 17, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Z. Nano-enabled agriculture: How do nanoparticles cross barriers in plants? Plant Commun. 2022, 3, 100346. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, E.; Dehshahri, A.; Izadpanah, K.; Ahmadi, F. Plant virus nanoparticles: Novel and robust nanocarriers for drug delivery and imaging. Colloids Surf. B 2018, 167, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.u.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, J.; Fan, W.; Liu, S.; Kamruzzaman, M. Production of reactive oxygen species via nanobubble water improves radish seed water absorption and the expression of aquaporin genes. Langmuir 2022, 38, 11724–11731. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Yang, X.; Alidoust, D.; Wang, C. Effects of iron oxide nanoparticles on the mineral composition and growth of soybean (Glycine max L.) plants. Acta Physiol. Plant. 2020, 42, 128. [Google Scholar] [CrossRef]
- Gao, X.; Kundu, A.; Bueno, V.; Rahim, A.A.; Ghoshal, S. Uptake and translocation of mesoporous SiO2-coated ZnO nanoparticles to solanum lycopersicum following foliar application. Environ. Sci. Technol. 2021, 55, 13551–13560. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Hassan, Z.U.; Akram, M.A.; Tariq, R.; Brestic, M.; Xie, W. Nanoparticle’s uptake and translocation mechanisms in plants via seed priming, foliar treatment, and root exposure: A review. Environ. Sci. Pollut. Res. 2022, 29, 89823–89833. [Google Scholar] [CrossRef]
- Capaldi Arruda, S.C.; Diniz Silva, A.L.; Moretto Galazzi, R.; Antunes Azevedo, R.; Zezzi Arruda, M.A. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A.; et al. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Avellan, A.; Yun, J.; Morais, B.P.; Clement, E.T.; Rodrigues, S.M.; Lowry, G.V. Critical review: Role of inorganic nanoparticle properties on their foliar uptake and in planta translocation. Environ. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.S.A.; Borah, K.K.; Goswami, Y.; Hakeem, K.R.; Chakrabartty, I. The potential exposure and hazards of metal-based nanoparticles on plants and environment, with special emphasis on ZnO NPs, TiO2 NPs, and AgNPs: A review. Environ. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Yadav, V.; Arif, N.; Kováč, J.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Vaculík, M. Structural modifications of plant organs and tissues by metals and metalloids in the environment: A review. Plant Physiol. Biochem. 2021, 159, 100–112. [Google Scholar] [CrossRef]
- Shi, J.; Yang, B.; Wang, H.; Wu, Y.; He, F.; Dong, J.; Qin, G. The combined contamination of nano-polystyrene and nanoAg: Uptake, translocation and ecotoxicity effects on willow saplings. Sci. Total Environ. 2023, 905, 167291. [Google Scholar] [CrossRef]
- Dong, S.; Jing, X.; Lin, S.; Lu, K.; Li, W.; Lu, J.; Li, M.; Gao, S.; Lu, S.; Zhou, D.; et al. Root hair apex is the key site for symplastic delivery of graphene into plants. Environ. Sci. Technol. 2022, 56, 12179–12189. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Etxeberria, E.; Gonzalez, P.; Pozueta, J. Evidence for two endocytic transport pathways in plant cells. Plant Sci. 2009, 177, 341–348. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Mukarram, M.; Petrik, P.; Mushtaq, Z.; Khan, M.M.A.; Gulfishan, M.; Lux, A. Silicon nanoparticles in higher plants: Uptake, action, stress tolerance, and crosstalk with phytohormones, antioxidants, and other signalling molecules. Environ. Pollut. 2022, 310, 119855. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dou, R.; Yang, Z.; You, T.; Gao, X.; Wang, L. Phytotoxicity and bioaccumulation of zinc oxide nanoparticles in rice (Oryza sativa L.). Plant Physiol. Biochem. 2018, 130, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Palacios, J.T.; Henry, D.; Penrose, B.; Bell, R. Formulation of zinc foliar sprays for wheat grain biofortification: A review of current applications and future perspectives. Front. Plant Sci. 2023, 14, 1247600. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef]
- Dutta, T.; Bagchi, D.; Bera, A.; Das, S.; Adhikari, T.; Pal, S.K. Surface engineered ZnO-humic/citrate interfaces: Photoinduced charge carrier dynamics and potential application for smart and sustained delivery of Zn micronutrient. ACS Sustain. Chem. Eng. 2019, 7, 10920–10930. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Maurer, L.L.; Harms, V.M.; Meyer, J.N.; Laromaine, A.; Roig, A. Materials and toxicological approaches to study metal and metal-oxide nanoparticles in the model organism Caenorhabditis elegans. Mater. Horiz. 2017, 4, 719–746. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Avellan, A.; Rodrigues, S.; Salvador, D.; Rodrigues, S.M.; Trindade, T. Composites of biopolymers and ZnO NPs for controlled release of zinc in agricultural soils and timed delivery for maize. ACS Appl. Nano Mater. 2020, 3, 2134–2148. [Google Scholar] [CrossRef]
- Wang, P.; Menzies, N.W.; Lombi, E.; McKenna, B.A.; Johannessen, B.; Glover, C.J.; Kappen, P.; Kopittke, P.M. Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ. Sci. Technol. 2013, 47, 13822–13830. [Google Scholar] [CrossRef]
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Andrews, J.C.; Cotte, M.; Rico, C.; Peralta-Videa, J.R.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 2013, 7, 1415–1423. [Google Scholar] [CrossRef]
- Thangavel, P.; Long, S.; Minocha, R. Changes in phytochelatins and their biosynthetic intermediates in red spruce (Picea rubens Sarg.) cell suspension cultures under cadmium and zinc stress. Plant Cell Tiss. Organ. Cult. 2007, 88, 201–216. [Google Scholar] [CrossRef]
- Zhang, Q.; Ying, Y.; Ping, J. Recent advances in plant nanoscience. Adv. Sci. 2022, 9, 2103414. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current research on zinc oxide nanoparticles: Synthesis, characterization, and biomedical applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Pittarate, S.; Rajula, J.; Rahman, A.; Vivekanandhan, P.; Thungrabeab, M.; Mekchay, S.; Krutmuang, P. Insecticidal effect of zinc oxide nanoparticles against Spodoptera frugiperda under laboratory conditions. Insects 2021, 12, 1017. [Google Scholar] [CrossRef]
- Pittarate, S.; Perumal, V.; Kannan, S.; Mekchay, S.; Thungrabeab, M.; Suttiprapan, P.; Sengottayan, S.-N.; Krutmuang, P. Insecticidal efficacy of nanoparticles against Spodoptera frugiperda (J.E. Smith) larvae and their impact in the soil. Heliyon 2023, 9, e16133. [Google Scholar] [CrossRef] [PubMed]
- Malaikozhundan, B.; Vaseeharan, B.; Vijayakumar, S.; Thangaraj, M.P. Bacillus thuringiensis coated zinc oxide nanoparticle and its biopesticidal effects on the pulse beetle, Callosobruchus maculatus. J. Photochem. Photobiol. B 2017, 174, 306–314. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Vinodhini, J. Nanopesticidal effects of Pongamia pinnata leaf extract coated zinc oxide nanoparticle against the Pulse beetle, Callosobruchus maculatus. Mater. Today Commun. 2018, 14, 106–115. [Google Scholar] [CrossRef]
- Jameel, M.; Shoeb, M.; Khan, M.T.; Ullah, R.; Mobin, M.; Farooqi, M.K.; Adnan, S.M. Enhanced insecticidal activity of thiamethoxam by zinc oxide nanoparticles: A novel nanotechnology approach for pest control. ACS Omega 2020, 5, 1607–1615. [Google Scholar] [CrossRef]
- Velsankar, K.; Parvathy, G.; Mohandoss, S.; Sudhahar, S. Effect of green synthesized ZnO nanoparticles using Paspalum scrobiculatum grains extract in biological applications. Microsc. Res. Tech. 2022, 85, 3069–3094. [Google Scholar] [CrossRef]
- Shukla, G.; Gaurav, S.S.; Singh, A. Synthesis of mycogenic zinc oxide nanoparticles and preliminary determination of its efficacy as a larvicide against white grubs (Holotrichia sp.). Int. Nano Lett. 2020, 10, 131–139. [Google Scholar] [CrossRef]
- Iqbal, H.; Fatima, A.; Khan, H.A.A. ZnO nanoparticles produced in the culture supernatant of Bacillus thuringiensis ser. israelensis affect the demographic parameters of Musca domestica using the age-stage, two-sex life table. Pest Manag. Sci. 2022, 78, 1640–1648. [Google Scholar] [CrossRef]
- Asghar, M.S.; Sarwar, Z.M.; Almadiy, A.A.; Shami, A.; El Hadi Mohamed, R.A.; Ahmed, N.; Waghulade, M.S.; Alam, P.; Abd Al Galil, F.M. Toxicological effects of silver and zinc oxide nanoparticles on the biological and life table parameters of Helicoverpa armigera (Noctuidae: Lepidoptera). Agriculture 2022, 12, 1744. [Google Scholar] [CrossRef]
- Thakur, P.; Thakur, S.; Kumari, P.; Shandilya, M.; Sharma, S.; Poczai, P.; Alarfaj, A.A.; Sayyed, R.Z. Nano-insecticide: Synthesis, characterization, and evaluation of insecticidal activity of ZnO NPs against Spodoptera litura and Macrosiphum euphorbiae. Appl. Nanosci. 2022, 12, 3835–3850. [Google Scholar] [CrossRef]
- Helmy, E.A.M.; San, P.P.; Zhang, Y.Z.; Adarkwah, C.; Tuda, M. Entomotoxic efficacy of fungus-synthesized nanoparticles against immature stages of stored bean pests. Sci. Rep. 2023, 13, 8508. [Google Scholar] [CrossRef]
- Siddique, M.A.; Hasan, M.u.; Sagheer, M.; Sahi, S.T. Comparative toxic effects of Eucalyptus globulus L. (Myrtales: Myrtaceae) and its green synthesized zinc oxide nanoparticles (ZnONPs) against Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae). Int. J. Trop. Insect Sci. 2022, 42, 1697–1706. [Google Scholar] [CrossRef]
- Biradar, W.; Nadagouda, S.; Aralimarad, P.; Hiregoudar, S. Entomotoxic effect of green nanoparticle an alternate strategy for stored grain pest management. Int. J. Trop. Insect Sci. 2021, 41, 2829–2840. [Google Scholar] [CrossRef]
- Hamdy, E.; Al-Askar, A.A.; El-Gendi, H.; Khamis, W.M.; Behiry, S.I.; Valentini, F.; Abd-Elsalam, K.A.; Abdelkhalek, A. Zinc oxide nanoparticles biosynthesized by Eriobotrya japonica leaf extract: Characterization, insecticidal and antibacterial properties. Plants 2023, 12, 2826. [Google Scholar] [CrossRef]
- Rebora, M.; Del Buono, D.; Piersanti, S.; Salerno, G. Reduction in insect attachment ability by biogenic and non-biogenic ZnO nanoparticles. Environ. Sci. Nano 2023, 10, 3062–3071. [Google Scholar] [CrossRef]
- Murugan, K.; Roni, M.; Panneerselvam, C.; Aziz, A.T.; Suresh, U.; Rajaganesh, R.; Aruliah, R.; Mahyoub, J.A.; Trivedi, S.; Rehman, H.; et al. Sargassum wightii-synthesized ZnO nanoparticles reduce the fitness and reproduction of the malaria vector Anopheles stephensi and cotton bollworm Helicoverpa armigera. Physiol. Mol. Plant Pathol. 2018, 101, 202–213. [Google Scholar] [CrossRef]
- Keerthana, P.; Vijayakumar, S.; Vidhya EV, N.P.; Punitha, V.N.; Nilavukkarasi, M.; Praseetha, P.K. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti -infection and growth promotion in plants. Ind. Crops Prod. 2021, 170, 113762. [Google Scholar] [CrossRef]
- Abdallah, Y.; Liu, M.; Ogunyemi, S.O.; Ahmed, T.; Fouad, H.; Abdelazez, A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. Bioinspired green synthesis of chitosan and zinc oxide nanoparticles with strong antibacterial activity against rice pathogen Xanthomonas oryzae pv. oryzae. Molecules 2020, 25, 4795. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Lee, B.; Ozcan, A.; Rawal, T.B.; Young, M.; Mendis, H.C.; Rajasekaran, P.; Washington, T.; Pingali, S.V.; O’Neill, H.; et al. Engineered zinc oxide-based nanotherapeutics boost systemic antibacterial efficacy against phloem-restricted diseases. Environ. Sci. Nano 2022, 9, 2869–2886. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Liu, Z.; Wen, H.; Jiang, N.; Shi, H.; Kou, Y. The application of zinc oxide nanoparticles: An effective strategy to protect rice from rice blast and abiotic stresses. Environ. Pollut. 2023, 331, 121925. [Google Scholar] [CrossRef]
- Li, J.; Sang, H.; Guo, H.; Popko, J.T.; He, L.; White, J.C.; Parkash Dhankher, O.; Jung, G.; Xing, B. Antifungal mechanisms of ZnO and Ag nanoparticles to Sclerotinia homoeocarpa. Nanotechnology 2017, 28, 155101. [Google Scholar] [CrossRef]
- Luksiene, Z.; Rasiukeviciute, N.; Zudyte, B.; Uselis, N. Innovative approach to sunlight activated biofungicides for strawberry crop protection: ZnO nanoparticles. J. Photochem. Photobiol. B 2020, 203, 111656. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Soren, S.; Kumar, S.; Mishra, S.; Jena, P.K.; Verma, S.K.; Parhi, P. Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method. Microb. Pathog. 2018, 119, 145–151. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Vani, C.; Sergin, G.; Annamalai, A. A study on the effect of zinc oxide nanoparticles in Staphylococcus aureus. Int. J. Adv. Pharm. Biol. Sci. 2011, 2, 326–335. [Google Scholar]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem.-Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Sharma, D.; Rajput, J.; Kaith, B.; Kaur, M.; Sharma, S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films 2010, 519, 1224–1229. [Google Scholar] [CrossRef]
- Kairyte, K.; Kadys, A.; Luksiene, Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. B 2013, 128, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 1–21. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar]
- Fowsiya, J.; Asharani, I.V.; Mohapatra, S.; Eshapula, A.; Mohi, P.; Thakar, N.; Monad, S.; Madhumitha, G. Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen. Green Process. Synth. 2019, 8, 488–495. [Google Scholar] [CrossRef]
- Şahin, B.; Soylu, S.; Kara, M.; Türkmen, M.; Aydin, R.; Çetin, H. Superior antibacterial activity against seed-borne plant bacterial disease agents and enhanced physical properties of novel green synthesized nanostructured ZnO using Thymbra spicata plant extract. Ceram. Int. 2021, 47, 341–350. [Google Scholar] [CrossRef]
- Jaithon, T.; Ruangtong, J.; T-Thienprasert, J.; T-Thienprasert, N.P. Effects of waste-derived ZnO nanoparticles against growth of plant pathogenic bacteria and epidermoid carcinoma cells. Crystals 2022, 12, 779. [Google Scholar] [CrossRef]
- Badar, R.; Ahmed, A.; Munazir, M.; Asghar, M.; Bashir, F. Wheat leaf rust control through biofabricated zinc oxide nanoparticles. Australasian Plant Pathol. 2023, 52, 609–612. [Google Scholar] [CrossRef]
- Sun, L.; Song, F.; Guo, J.; Zhu, X.; Liu, S.; Liu, F.; Li, X. Nano-ZnO-induced drought tolerance Is associated with melatonin synthesis and metabolism in maize. Int. J. Mol. Sci. 2020, 21, 782. [Google Scholar] [CrossRef]
- El-Zohri, M.; Al-Wadaani, N.A.; Bafeel, S.O. Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidative stress in tomato. Plants 2021, 10, 2400. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Raeisi Sadati, S.Y.; Jahanbakhsh Godehkahriz, S.; Ebadi, A.; Sedghi, M. Zinc oxide nanoparticles enhance drought tolerance in wheat via physio-biochemical changes and stress genes expression. Iran. J. Biotechnol. 2022, 20, 12–24. [Google Scholar] [CrossRef]
- Sun, L.; Song, F.; Zhu, X.; Liu, S.; Liu, F.; Wang, Y.; Li, X. Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Arch. Agron. Soil Sci. 2021, 67, 245–259. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Tomar, R.S.; Jajoo, A. H2O2 signaling regulates seed germination in ZnO nanoprimed wheat (Triticum aestivum L.) seeds for improving plant performance under drought stress. Environ. Exp. Bot. 2021, 189, 104561. [Google Scholar] [CrossRef]
- Ullah, A.; Romdhane, L.; Rehman, A.; Farooq, M. Adequate zinc nutrition improves the tolerance against drought and heat stresses in chickpea. Plant Physiol. Biochem. 2019, 143, 11–18. [Google Scholar] [CrossRef]
- Thakur, S.; Asthir, B.; Kaur, G.; Kalia, A.; Sharma, A. Zinc oxide and titanium dioxide nanoparticles influence heat stress tolerance mediated by antioxidant defense system in wheat. Cereal Res. Commun. 2022, 50, 385–396. [Google Scholar] [CrossRef]
- Kareem, H.A.; Saleem, M.F.; Saleem, S.; Rather, S.A.; Wani, S.H.; Siddiqui, M.H.; Alamri, S.; Kumar, R.; Gaikwad, N.B.; Guo, Z.; et al. Zinc oxide nanoparticles interplay with physiological and biochemical attributes in terminal heat stress alleviation in mungbean (Vigna radiata L.). Front. Plant Sci. 2022, 13, 842349. [Google Scholar] [CrossRef]
- Kareem, H.A.; Hassan, M.U.; Zain, M.; Irshad, A.; Shakoor, N.; Saleem, S.; Niu, J.; Skalicky, M.; Chen, Z.; Guo, Z.; et al. Nanosized zinc oxide (n-ZnO) particles pretreatment to alfalfa seedlings alleviate heat-induced morpho-physiological and ultrastructural damages. Environ. Pollut. 2022, 303, 119069. [Google Scholar] [CrossRef] [PubMed]
- Elshoky, H.A.; Yotsova, E.; Farghali, M.A.; Farroh, K.Y.; El-Sayed, K.; Elzorkany, H.E.; Rashkov, G.; Dobrikova, A.; Borisova, P.; Stefanov, M.; et al. Impact of foliar spray of zinc oxide nanoparticles on the photosynthesis of Pisum sativum L. under salt stress. Plant Physiol. Biochem. 2021, 167, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Siddiqui, H.; Sami, F.; Zaidi, R.; Azam, A.; Alam, P.; Hayat, S. Nanoparticles enhances the salinity toxicity tolerance in Linum usitatissimum L. by modulating the antioxidative enzymes, photosynthetic efficiency, redox status and cellular damage. Ecotoxicol. Environ. Saf. 2021, 213, 112020. [Google Scholar] [CrossRef]
- Yasmin, H.; Mazher, J.; Azmat, A.; Nosheen, A.; Naz, R.; Hassan, M.N.; Noureldeen, A.; Ahmad, P. Combined application of zinc oxide nanoparticles and biofertilizer to induce salt resistance in safflower by regulating ion homeostasis and antioxidant defence responses. Ecotoxicol. Environ. Saf. 2021, 218, 112262. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef]
- Singh, A.; Sengar, R.S.; Rajput, V.D.; Minkina, T.; Singh, R.K. Zinc oxide nanoparticles improve salt tolerance in rice seedlings by improving physiological and biochemical indices. Agriculture 2022, 12, 1014. [Google Scholar] [CrossRef]
- Lalarukh, I.; Zahra, N.; Al Huqail, A.A.; Amjad, S.F.; Al-Dhumri, S.A.; Ghoneim, A.M.; Alshahri, A.H.; Almutari, M.M.; Alhusayni, F.S.; Al-Shammari, W.B.; et al. Exogenously applied ZnO nanoparticles induced salt tolerance in potentially high yielding modern wheat (Triticum aestivum L.) cultivars. Environ. Technol. Innov. 2022, 27, 102799. [Google Scholar] [CrossRef]
- Sarkar, R.D.; Kalita, M.C. Alleviation of salt stress complications in plants by nanoparticles and the associated mechanisms: An overview. Plant Stress 2023, 7, 100134. [Google Scholar] [CrossRef]
- Ahmed, R.; Zia-ur-Rehman, M.; Sabir, M.; Usman, M.; Rizwan, M.; Ahmad, Z.; Alharby, H.F.; Al-Zahrani, H.S.; Alsamadany, H.; Aldhebiani, A.Y.; et al. Differential response of nano zinc sulphate with other conventional sources of Zn in mitigating salinity stress in rice grown on saline-sodic soil. Chemosphere 2023, 327, 138479. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef]
- Manasa, S.L.; Panigrahy, M.; Panigrahi, K.C.S.; Rout, G.R. Overview of cold stress regulation in plants. Plants. Bot. Rev. 2022, 88, 359–387. [Google Scholar] [CrossRef]
- Soualiou, S.; Duan, F.; Li, X.; Zhou, W. Crop production under cold stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; Najafi-Kakavand, S.; Abbas, S.; Shoaib, Y.; Anwar, S.; Sharifi, S.; Lu, G.; Siddique, K.H.M. Role of phytohormones in regulating cold stress tolerance: Physiological and molecular approaches for developing cold-smart crop plants. Plant Stress 2023, 8, 100152. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Sunoj, V.S.J.; Wen, Y.; Zhu, J.J.; Muralidharan, G.; Cao, K.F. Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiol. Biochem. 2020, 149, 50–60. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc oxide nanoparticles alleviate chilling stress in rice (Oryza Sativa L.) by regulating antioxidative system and chilling response transcription factors. Molecules 2021, 26, 2196. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Li, Y.C.; Peng, Y.; Wen, X.; Ni, X. Distribution, accumulation, and potential risks of heavy metals in soil and tea leaves from geologically different plantations. Ecotoxicol. Environ. Saf. 2020, 195, 110475. [Google Scholar] [CrossRef]
- Pescatore, A.; Grassi, C.; Rizzo, A.M.; Orlandini, S.; Napoli, M. Effects of biochar on berseem clover (Trifolium alexandrinum, L.) growth and heavy metal (Cd, Cr, Cu, Ni, Pb, and Zn) accumulation. Chemosphere 2022, 287, 131986. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, M.; Zong, D.; Li, W.; Li, X.; Wang, Z.; Zhang, Y.; Niu, Y.; Xiang, P. Are high-risk heavy metal(loid)s contaminated vegetables detrimental to human health? A study of incorporating bioaccessibility and toxicity into accurate health risk assessment. Sci. Total Environ. 2023, 897, 165514. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, D.; Ma, J.; Jin, B.; Peng, J.; He, X. Assessing the influence of immobilization remediation of heavy metal contaminated farmland on the physical properties of soil. Sci. Total Environ. 2021, 781, 146773. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, L.; Li, W.; Ashraf, U.; Ma, L.; Tang, X.; Pan, S.; Tian, H.; Mo, Z. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J. Nanobiotechnol. 2021, 19, 75. [Google Scholar] [CrossRef]
- Ghouri, F.; Shahid, M.J.; Liu, J.; Lai, M.; Sun, L.; Wu, J.; Liu, X.; Ali, S.; Shahid, M.Q. Polyploidy and zinc oxide nanoparticles alleviated Cd toxicity in rice by modulating oxidative stress and expression levels of sucrose and metal-transporter genes. J. Hazard. Mater. 2023, 448, 130991. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.; Alghanem, S.M.S.; Al-Huqail, A.A.; Nazir, M.M.; Zulfiqar, F.; Ahmed, T.; Ali, S.H.A.; Abeed, A.; Siddique, K.H.M.; Jin, X. Zinc oxide nanoparticles mitigated the arsenic induced oxidative stress through modulation of physio-biochemical aspects and nutritional ions homeostasis in rice (Oryza sativa L.). Chemosphere 2023, 338, 139566. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Hasanuzzaman, M.; Ding, Y.; Barker, J.; Mokhberdoran, F.; Liu, G. Zinc oxide nanoparticles improve Pleioblastus pygmaeus plant tolerance to arsenic and mercury by stimulating antioxidant defense and reducing the metal accumulation and translocation. Front. Plant Sci. 2022, 13, 841501. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.A.; Faizan, M.; Bhat, M.A.; Sharma, G.; Rinklebe, J.; Alyemeni, M.N.; Bajguz, A.; Ahmad, P. Mitigation of arsenic stress in Brassica juncea L. using zinc oxide-nanoparticles produced by novel hydrothermal synthesis. S. Afr. J. Bot. 2023, 163, 389–400. [Google Scholar] [CrossRef]
- Banerjee, S.; Islam, J.; Mondal, S.; Saha, A.; Saha, B.; Sen, A. Proactive attenuation of arsenic-stress by nano-priming: Zinc oxide nanoparticles in Vigna mungo (L.) Hepper trigger antioxidant defense response and reduce root-shoot arsenic translocation. J. Hazard. Mater. 2023, 446, 130735. [Google Scholar] [CrossRef]
- Hussain, F.; Hadi, F.; Rongliang, Q. Effects of zinc oxide nanoparticles on antioxidants, chlorophyll contents, and proline in Persicaria hydropiper L. and its potential for Pb phytoremediation. Environ. Sci. Pollut. Res. 2021, 28, 34697–34713. [Google Scholar] [CrossRef]
- Raghib, F.; Naikoo, M.I.; Khan, F.A.; Alyemeni, M.N.; Ahmad, P. Interaction of ZnO nanoparticle and AM fungi mitigates Pb toxicity in wheat by upregulating antioxidants and restricted uptake of Pb. J. Biotechnol. 2020, 323, 254–263. [Google Scholar] [CrossRef]
- Azim, Z.; Singh, N.B.; Khare, S.; Singh, A.; Amist, N.; Niharika; Yadav, R.K.; Hussain, I. Potential role of biosynthesized zinc oxide nanoparticles in counteracting lead toxicity in Solanum lycopersicum L. Plant Nano Biol. 2022, 2, 100012. [Google Scholar] [CrossRef]
- Wang, R.; Sun, L.; Zhang, P.; Wan, J.; Wang, Y.; Xu, J. Zinc oxide nanoparticles alleviate cadmium stress by modulating plant metabolism and decreasing cadmium accumulation in Perilla frutescents. Plant Growth Regul. 2023, 100, 85–96. [Google Scholar] [CrossRef]
- Zhang, W.; Long, J.; Li, J.; Zhang, M.; Xiao, G.; Ye, X.; Chang, W.; Zeng, H. Impact of ZnO nanoparticles on Cd toxicity and bioaccumulation in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2019, 26, 23119–23128. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jayaraj, M.; Manikandan, R.; Geetha, N.; Rene, E.R.; Sharma, N.C.; Sahi, S.V. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: A physiochemical analysis. Plant Physiol. Biochem. 2017, 110, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, N.; Geetha, N.; Manish, T.; Sahi, S.V.; Venkatachalam, P. Zinc oxide nanocatalyst mediates cadmium and lead toxicity tolerance mechanism by differential regulation of photosynthetic machinery and antioxidant enzymes level in cotton seedlings. Toxicol. Rep. 2021, 8, 295–302. [Google Scholar] [CrossRef]
- Pishkar, L.; Yousefi, S.; Iranbakhsh, A. Foliar application of Zinc oxide nanoparticles alleviates cadmium toxicity in purslane by maintaining nutrients homeostasis and improving the activity of antioxidant enzymes and glyoxalase system. Ecotoxicology 2022, 31, 667–678. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Mir, A.R.; Hayat, S. Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J. Plant Growth Regul. 2021, 40, 101–115. [Google Scholar] [CrossRef]
- Basit, F.; Nazir, M.M.; Shahid, M.; Abbas, S.; Javed, M.T.; Naqqash, T.; Liu, Y.; Yajing, G. Application of zinc oxide nanoparticles immobilizes the chromium uptake in rice plants by regulating the physiological, biochemical and cellular attributes. Physiol. Mol. Biol. Plants 2022, 28, 1175–1190. [Google Scholar] [CrossRef]
- Basit, F.; Shahid, M.; Abbas, S.; Naqqash, T.; Akram, M.S.; Tahir, M.; Azeem, M.; Cai, Y.; Jia, S.; Hu, J.; et al. Protective role of ZnO nanoparticles in soybean seedlings growth and stress management under Cr-enriched conditions. Plant Growth Regul. 2023, 100, 703–716. [Google Scholar] [CrossRef]
- Mehmood, S.; Ou, W.; Ahmed, W.; Bundschuh, J.; Rizwan, M.; Mahmood, M.; Sultan, H.; Alatalo, J.M.; Elnahal, A.S.M.; Liu, W.; et al. ZnO nanoparticles mediated by Azadirachta indica as nano fertilizer: Improvement in physiological and biochemical indices of Zea mays grown in Cr-contaminated soil. Environ. Pollut. 2023, 339, 122755. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Naz, G.; shah, A.A.; Parveen, M.; Jamil, M.; Gill, S.; Sharif, H.M.A. Synthesis of phytostabilized zinc oxide nanoparticles and their effects on physiological and anti-oxidative responses of Zea mays (L.) under chromium stress. Plant Physiol. Biochem. 2023, 196, 130–138. [Google Scholar] [CrossRef] [PubMed]


| Method of Synthesis | Particle Size (nm) | Target Pest | References |
|---|---|---|---|
| Commercial purchase | 25–50 nm | Spodoptera frugiperda | [136] |
| Synthesized using Paspalum scrobiculatum grains extract | 15–30 nm | Tribolium castaneum | [141] |
| Synthesized using Aspergillus niger biomass | 76.2–183.8 nm | Holotrichia sp. | [142] |
| Synthesized using Pongamia pinnata leaf extract | 21.3 nm | Callosobruchus maculatus | [139] |
| Synthesized using Bacillus thuringiensis | 300 nm–1 μm | Musca domestica | [143] |
| Synthesized using Azadirachta indica leaf extract | 10–70 nm | Helicoverpa armigera | [144] |
| Synthesized using Zingiber officinale rhizome extract | 50–100 nm | Spodoptera litura and Macrosiphum euphorbiae | [145] |
| Synthesized using fungus Fusarium solani extract | 8–33 nm | Callosobruchus | [146] |
| ZnO nanoparticles with a thiamethoxam nanocomposite | 30 nm | Spodoptera litura | [140] |
| Synthesized using Eucalyptus globulus leaf extract | 186.7 nm | Rhyzopertha dominica | [147] |
| Synthesized using Spinacia oleracea leaf extract | 87.94 nm | Corcyra cephalonica | [148] |
| Synthesized using Eriobotrya japonica leaf extract | 5–27 nm | Sitophilus oryzae and Tribolium castaneum | [149] |
| Synthesized using Lemna minor hydroalcoholic extract | 10–20 nm | Nezara viridula | [150] |
| Synthesized using Sargassum wightii leaf extract | 20–62 nm | Helicoverpa armigera | [151] |
| Method of Synthesis | Particle Size (nm) | Target Pathogen | References |
|---|---|---|---|
| Synthesized using Citrus medica aqueous peel extract | 29 nm | Streptomyces sannanesis, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella enterica, Candida albicans, and Aspergillus niger | [152] |
| Commercial purchase | 300–800 nm | Botrytis cinerea | [157] |
| Synthesized using lycopersicon esculentum aqueous extract | 31.3–88.9 nm | Xanthomonas oryzae pv. oryzae | [153] |
| Synthesized using Aegle marmelos leaf extract | 18 ± 2 nm | Aspergillus flavus and Aspergillus niger | [169] |
| Synthesized using one-pot wet-chemical method | 2.5–5 nm | Candidatus Liberibacter asiaticus | [154] |
| Synthesized using Thymbra spicata plant extract | 426–540 nm | C. michiganensis subsp. Michiganensis, Pseudomonas cichorii, Pseudomonas syringae pv. Phaseolicola and Pectobacterium carotovorum subsp. Carotovorum | [170] |
| Synthesized using Garcinia mangostana or Eichhornia crassipes extract | 50–100 nm | Xanthomonas oryzae pv. oryzae, Xanthomonas axonopodis pv. citri, and Ralstonia solanacearum | [171] |
| Commercial purchase | 30 nm | Sclerotinia homeocarpa | [156] |
| Commercial purchase | 30 nm | Magnaporthe oryzae | [155] |
| Synthesized using Azadirachta indica leaf extract | 101.6 nm | Puccini triticina | [172] |
| Heavy Metal | Plant | Alteration in Plant Parameters | References |
|---|---|---|---|
| As | Oryza sativa | Enhanced plant growth parameters, gas exchange parameters, chlorophyll content (SPAD value), fluorescence efficiency (Fv/m), and antioxidant enzyme activities | [205] |
| As and Hg | Pleioblastus pygmaeus | Increased antioxidant activity, proline content, glycine betaine content, tyrosine ammonia-lyase activity, phenylalanine ammonia-lyase activity, chlorophyll indices, and plant biomass | [206] |
| As | Brassica juncea | Enhanced plant growth, photosynthesis-related parameters, protein content, carbonic anhydrase, nitrate reductase, and RuBisCO | [207] |
| As | Vigna mungo | Enhanced seed germination rate, germination rate, seedling vigor, plant biomass, shoot length, root length, antioxidant enzymes activity (SOD, CAT, POX, APX), and osmoregulators | [208] |
| Pb | Persicaria hydropiper | Enhanced plant growth, chlorophyll content, carotenoid content, free proline, phenolics, flavonoids, and antioxidative enzymes (CAT, GR, GST, SOD) | [209] |
| Pb | Triticum aestivum | Increased plant height, fresh weight, dry weight, total chlorophyll content, proline content, SOD content, CAT content, H2O2 content, and lipid peroxidation content | [210] |
| Pb | Solanum lycopersicum | Increased germination rate, seedling vigor index, relative water content, chlorophyll content, protein, sugars, nitrate reductase, SOD, POD, and APX activity | [211] |
| Cd | Perilla frutescens | Increased SOD, POD, nutrient elements contents, organic acids (citric acid, malic acid and maleic acid), and amino acids (arginine, glutamate and phenylalanine), root and leaf dry weight | [212] |
| Cd | Oryza sativa | Increased plant height, biomass, photosynthetic attributes, oxidative stress (MDA, H2O2), antioxidant enzymes (SOD, POD, CAT, GSH. APX) | [204,213] |
| Cd and Pb | Gossypium hirsutum and Leucaena leucocephala | Enhanced plant growth and biomass, level photosynthetic pigments, MDA, protein content, and oxidative enzymes (POD, SOD, POX, and APX) | [214,215] |
| Cd | Portulaca oleracea | Improved the activity of antioxidant enzymes, the glyoxalase system, photosynthetic pigments, and the glyoxalase cycle | [216] |
| Cd | Triticum aestivum | Increased the growth of wheat, chlorophyll content, zinc content, POD, and SOD | [217] |
| Cd | Oryza sativa | Enhanced mean root fresh weight, root-shoot length, SOD, POD, metallothionein content, α-amylase, and total amylase activity | [203] |
| Cd | Lycopersicon esculentum | Enhanced plant height, fresh and dry weight of plant, leaf area, SPAD chlorophyll, photosynthetic attributes, protein content, and activities of nitrate reductase and carbonic anhydrase | [218] |
| Cr | Oryza sativa | Increased biomass accumulation, antioxidants (SOD, CAT, POD), nutrient acquisition (zinc, ferrum), and brassinosteroids | [219] |
| Cr | Glycine max | Enhanced biomass, antioxidant system, altered enzymatic (SOD, POD, CAT) and non-enzymatic antioxidant activities (GR, GSH, GSSH), and nutrient uptake | [220] |
| Cr | Zea mays | Increased fresh shoot weight, fresh root weight, shoot length, root length, chlorophyll content, total soluble sugars, proline content, POD, CAT, and APX enzyme activities | [221,222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, S.; Ma, T.; Liang, Y.; Huo, Z.; Yang, F. Synthesis of Zinc Oxide Nanoparticles and Their Applications in Enhancing Plant Stress Resistance: A Review. Agronomy 2023, 13, 3060. https://doi.org/10.3390/agronomy13123060
Wang Z, Wang S, Ma T, Liang Y, Huo Z, Yang F. Synthesis of Zinc Oxide Nanoparticles and Their Applications in Enhancing Plant Stress Resistance: A Review. Agronomy. 2023; 13(12):3060. https://doi.org/10.3390/agronomy13123060
Chicago/Turabian StyleWang, Zijun, Sijin Wang, Tingting Ma, You Liang, Zhongyang Huo, and Fengping Yang. 2023. "Synthesis of Zinc Oxide Nanoparticles and Their Applications in Enhancing Plant Stress Resistance: A Review" Agronomy 13, no. 12: 3060. https://doi.org/10.3390/agronomy13123060
APA StyleWang, Z., Wang, S., Ma, T., Liang, Y., Huo, Z., & Yang, F. (2023). Synthesis of Zinc Oxide Nanoparticles and Their Applications in Enhancing Plant Stress Resistance: A Review. Agronomy, 13(12), 3060. https://doi.org/10.3390/agronomy13123060






