Abstract
Multi-environment field testing of chickpea accessions winter sown in Southern Spain showed that environmental effects on yield were more important than genotypic effects and GEI. The most detrimental factor on grain yield was ascochyta blight infection. We did not find a significant effect of low temperatures on yield in the environments studied, probably due to the mild winters in the area. On the contrary, we found detrimental effects of high temperatures at the reproductive stage, particularly with numbers of days with Tmax >30 °C. We found that genotypic effects were larger than the environmental on ascochyta infection as we included accessions previously selected for their levels of resistance or susceptibility. Biplots based on the WAASB/productivity ratio highlighted AS19, AS30, AS23, AS26, and AS18 accessions as the best for productivity and stability of yield, matching with those with a lower ascochyta blight infection. The MTSI index also identified these as the best accessions for the region.
1. Introduction
Chickpea (Cicer arietinum L.) is a cool season grain legume traditionally important in the human diet of Mediterranean and Asian countries that has been increasingly adopted as food globally. In Mediterranean environments, chickpea is typically grown as a rainfed crop sown in early spring, relying on the residual moisture of the soil. This practice facilitates the escape to cold temperatures and to ascochyta blight (Ascochyta rabiei (Pass.) Lab., teleomorph Didymella rabiei (Kov.) Arx) damage [1,2,3]. However, mild winter conditions of this area offer the possibility to sow chickpea in autumn, with increased yield and yield stability, profiting from winter rains and minimizing the effects of terminal heat and drought stress [4,5]. Unfortunately, winter sowing also increases the risk of ascochyta blight devastation. Therefore, control of ascochyta blight is essential to increase chickpea production and yield stability. Host resistance is the most practical and economic way to manage the ascochyta blight problem, but its level has to improve. Concern has also been commonly raised regarding the damage of low temperatures in winter-sown chickpea [2], however less attention was paid to heat stress. Even though high temperatures at the reproductive stage are known, it was assumed that winter sowings would escape from heat stress [2,4,5]. Long term breeding efforts has resulted in a number of breeding lines and cultivars resistant to ascochyta blight [2,3,6] whose performance and agronomic value must now by validated in different environments.
Under multi-environmental trials, yield, abiotic, or biotic stress resistance are influenced by the effects of the genotype (G), the environment (E), and their interactions (GEI). GEI can be studied by a number of methods such as AMMI (additive main effect and multiplicative interaction) analysis [7] or GGE biplot (genotype plus genotype-by-environment) [8]. However, as long as these methods assume genotypes as random variables, they are not appropriate for analyzing the structure of the linear mixed-effect model (LMM) [9]. WAASB (weighted average of absolute scores) has been proposed [9] to better characterize ideal genotypes and a superiority index, WAASBY, to select genotypes based on both yield performance and the WAASB stability score [9].
The objectives of this research were to evaluate the performance and stability of ascochyta blight resistance and of yield among chickpea breeding lines in Southern Spain under winter sowings.
2. Materials and Methods
2.1. Plant and Experimental Design
Performance of 10 chickpea accessions (Table 1) was studied at three Southern Spanish locations (Córdoba, Escacena, and Tomegil) over five consecutive field seasons (2008–2009, 2009–2010, 2010–2011, 2011–2012, and 2012–2013) (Table 2). Accessions studied were selected out of 80 Kabuli type accessions previously field studied at Córdoba and Escacena during 2007–2008 (data not presented). At each location, a randomized complete block design with three replications was used. The experimental unit consisted of small plots, with three 1 m long rows per accession separated 0.35 m, 10 plants per row. Sowing took place by the middle of December each season, according to local practice. Weeds were controlled by hand weeding. Ascochyta blight (Ascochyta rabiei) and any other occurring disease or pest were monitored, recording disease severity (DS), and estimated as a percentage of the canopy covered by lesions. The harvest of the plants took place by late May, depending on the environment. All plants were harvested, threshed, and grain yields recorded. Climatic data were obtained from Red de Información Agroclimática de Andalucía [10] and provided in Table 2.

Table 1.
Chickpea accessions included in the study.

Table 2.
Description of the environments (combination of location and season) of the trials for the multi-environment study. Climatic data.
2.2. Statistical Analysis
2.2.1. Variance Components
Each environment was a combination of the year and location; therefore, 6 environments were included for stability analysis of the 15 genotypes. All analyses were done by executing the ‘metan’ package on R Studio statistical Software version 4.1.0 [11]. For the percentage data (i.e., Ascochyta blight data), arcsine transformations of the square root of proportions were carried out, and statistical analyses were performed on the transform data. A combined analysis of variance (ANOVA) for randomized complete-block designs across environments was performed to deduce the variance components of different sources of variation and to detect the presence of GEIs by assuming all effects as random factors. The data from all the environments were subjected to Shapiro–Wilk test for normality and a Bartlett’s test for homogeneity of variances.
2.2.2. GEI Analysis
Means vs. Stability Biplot
Using the weighted average of absolute scores from the singular value decomposition of the matrix of BLUPs (WAASB), we estimated to evaluate the stability of grain yield or of ascochyta infection of genotypes across environments [9]. With the purpose of better characterizing ideal genotypes based on both high performance and stability, a biplot was rendered based on the WAASB and mean performance trait. In this biplot, four quadrants are seen [9]: the genotypes or environments placed in quadrant I are unstable or environments with high discrimination ability and low performance below the grand mean. In quadrant II, the performance of the genotype is above the grand mean but unstable and the environments were good discriminating environments with high magnitudes of the response variable. Genotypes in quadrant III have a low but stable performance due to the lower values of WAASB, whereas the environments are considered poor performing and with low discrimination ability. The genotypes in quadrant IV are high performance and broadly adapted due to the high magnitude of the response variable and the high stability [9].
Cluster Analysis
Based on the WAASBY superiority index [9], weights for WAASB/trait ratio (stability/performance trait) were allotted from the 50/50 to the 0/100 scenarios so that the performance of the trait had more weight than stability.
A Euclidean distance-based dendrogram is used for grouping the genotypes based on their ranks in scenario matrix from 50/50 to 0/100 for WAASB/trait ratio, so the groups formed were indicated as ellipses in the mean vs. stability biplots to identify groups of genotypes so that each group with a different color could have similar performance regarding stability and performance [9].
Multi-Trait Stability Index (MTSI)
The Multi-Trait Stability Index (MTSI) based on the WAASBY index [12] was used to allow for simultaneous selection of stability and mean performance based on several traits (grain yield and ascochyta blight) assigning a selection intensity of 25%. We drafted 11 scenarios, assigning different relative weights to performance vs. stability of the trait, starting from 50:50 to 100:0.
2.2.3. Correlations
Correlation analysis was applied to describe the impact on grain yield of ascochyta blight infection and of the various climatic parameters, including temperatures, humidity, and rain during pre-flowering and at the flowering and post-flowering period. This analysis was performed using the genotypic best linear unbiased prediction (BLUP) of each genotype in each environment for each trait. Analysis and visualization of the correlation matrix was made using the ggcorrplot function from the ggcorrplot package in R.
3. Results
3.1. Pooled ANOVA
Pooled ANOVA (Table 3) showed that all the main (E and G) and multiplicative (GEI) effects were highly significant (p < 0.0001) for grain yield and ascochyta response. E effects were more important for grain yield explaining 38% of total variation, with 26% explained by G, and only 11% by GEI. However, G effects were more important for ascochyta response explaining 44% of total variation, with 35% explained by E and only 13% by GEI.

Table 3.
Pooled analysis of variance and estimated variance components of grain yield (kg/ha) and ascochyta blight (transformed data) of a chickpea performance trial, consisting of 14 genotypes (G) grown in 10 environments (E).
3.2. Grain Yield
Average grain yield over accessions and environments was 1248 kg/ha, with great differences across environments (Table 2), being higher than 1100 kg/ha (Table 4) at Tom09, Cord09, Cord12, Cord13, Esc10, Esc11, Esc12, and Esc13 but only 644 Kg/ha at Cord12 and 113 kg/ha at Cord11, confirming the high effects of the E and of G*E, higher than those of G on grain yield (Table 3). The genotypes performing better across environments were AS19, As8, AS30, AS23, AS26, M38, and AS18 with an average yield over environments higher than 1500 kg/ha. The yield of Blanco Lechoso was the lowest of all the environments, with an average of 256 kg/ha. These yields are in line with the reported yield in the region, with Blanco Lechoso yielding very poorly (in the range of 300 kg/ha) in winter sowings and approximately 1400–1600 kg/ha for ascochyta resistant accessions [13].

Table 4.
Grain yield (kg/ha) of 14 chickpea accessions grown at 10 location–year environments.
Figure 1 shows the WAASB vs. GY biplot where we can see a simultaneous interpretation of productivity and stability. The lines perpendicular to these axes divide the biplot into four parts. Quadrant I contains the Blanco Lechoso and M7 genotypes and the Cord09 and Cord11 environments. These genotypes and environments performed lower than average grain yields and played the largest role in GEI. Quadrant II contains genotypes AS19, AS8, and M38 and environment Esc10, with a good grain yield but little stability, playing also a big role in GEI. So, this environment in quadrant II deserves special attention since it provides above-average production and a high ability to discriminate genotypes. Quadrant III contains M30, M35, M8, M42, and AS41 genotypes, with low performance although high stability, along with the Cord10, Cord12, Esc11, Esc12, and Tom09 environments, with the lowest WAASB values among all environments. The Tom09 environment was exactly on the frontier of the third and fourth quadrant, meaning that the grain yield was equal to the total average and low discriminate power. Finally, quadrant IV includes AS30, AS23, AS26, and AS18 genotypes, with high productivity and stability and Cord13 and Esc13 environments, which had a high production capacity and less WAASB (Figure 1).
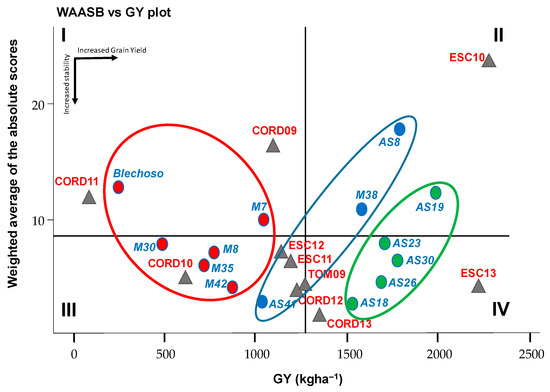
Figure 1.
Biplot of grain yield vs. WAASB of 14 chickpea genotypes evaluated in 10 environments. Horizontal black arrows indicate the direction of the increase in grain yield; vertical arrow indicate increase of stability. Triangle for environments and circle with different colors for genotypes. Groups of genotypes with similar performance regarding stability and performance in ellipses according to cluster analysis of the ranking matrix from the 50/50 to the 0/100 scenarios for the WAASB/GY ratio.
Biplots similar to that in Figure 1 are in fact a snapshot allowing for selection of the best genotypes based on productivity and stability. However, a reality might be that the breeders do not always have a clear criterion to pre-assign relative weights to stability versus performance for a given trait. In this case, one option [14] might be to rank the genotypes based on the WAASB/GY ratio, giving different weights for stability/productivity. After the cluster analysis (Supplemental Figure S1) based on the Euclidean distance of the ranking matrix of the genotypes based on the WAASB/productivity ratio with different weights for stability/productivity from the 50/50 to the 0/100, the three clusters formed (ellipses of different colors) are shown in Figure 1. Cluster 1 (ellipse marked in green) includes the best genotypes, more productive and more stable (AS19, AS30, AS23, AS26, and AS18). Note that these genotypes remained the firsts-ranked regardless of when the WAASB/GY ratio was low (greater weight for yield). Cluster 2 (marked in blue) includes AS8, M38, and AS41 genotypes, with very different productivity but showing similar profiles in the WAASB/GY ratio ranking. Cluster 3 (marked in red) includes genotypes with poor yields, well below the average. We can highlight the “Blanco lechoso” genotype with both the lowest stability and yield.
3.3. Ascochyta Blight
Ascochyta blight infection was the only significant biotic constraint observed at all sites, with only negligible levels of leaf miner infection at some sites (data not shown). Levels of ascochyta blight infection (Table 5) varied with genotypes (average infection over environments varying from 10% in AS19 to 73% in Blanco Lechoso) and environment (average over genotypes varying from 13,8% at Esc12 to 66.5% at Cord11).

Table 5.
Ascochyta blight (%) of 14 chickpea accessions grown at 10 location–year environments.
Quadrant I in the ascochyta blight biplot response (Figure 2) includes genotypes AS8 and AS23 with the lowest levels of ascochyta infection but with only moderate stability. Quadrant III includes genotypes AS18, AS19, AS26, AS30, and M38 genotypes with stably low ascochyta infections, being the most desirable ones. Quadrants II and IV include genotypes with above-average ascochyta infections, highlighting the genotype Blanco Lechoso with the highest infection and instability high infection (>25%). The cluster analysis (Supplemental Figure S2) highlighted three clusters. Cluster 1, in green includes AS8, AS23, AS18, AS19, AS30, and AS26 with the lower level of infection and higher stability based on ranking profiles for the WAASB/ascochyta infection. On the contrary, in red we highlight the genotypes with a high level of infection and medium to high instability.
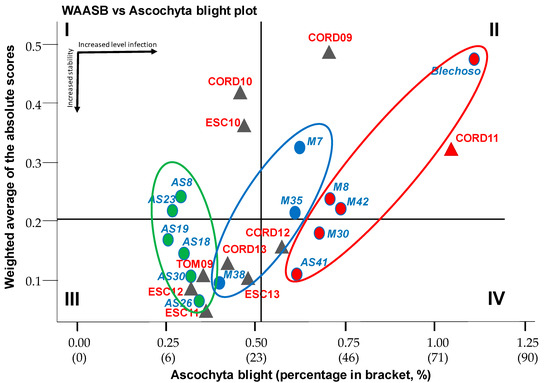
Figure 2.
Biplot of ascochyta blight vs. WAASB of 14 chickpea genotypes evaluated in 10 environments. Horizontal black arrows indicate the direction of the increase in level ascochyta infection; vertical arrow indicate increase of stability. Triangle for environments and circle with different colors for genotypes. Groups of genotypes with similar performance regarding stability and performance in ellipses according to cluster analysis of the ranking matrix from the 50/50 to the 0/100 scenarios for the WAASB/GY ratio.
3.4. MTSI Index
Previously described results show the most desirable genotypes in terms of grain yield (AS19, AS30, AS23, AS26, and AS18) or in terms of reduced ascochyta infection (AS8, AS23, AS18, AS19, AS30, and AS26). Currently, there are various indices to select the best genotypes based on several agronomic traits [15]. Thus, we have used the MTSI index [12] that allows for defining the weight to be assigned to the performance and stability. Figure 3 shows the selected genotypes in different scenarios already defined in previous paragraphs. In Figure 3, we can see how from the ratio 25/75 the selected genotypes (AS19, AS30, AS23, and AS26, based on a selection intensity of 25%) are maintained up to the ratio 5/95 where the weight assigned to stability is minimal (where the AS8 genotype was included and the AS26 genotype was excluded). Therefore, we can indicate that genotypes AS19, AS30, AS23, AS26, and AS8 would be selected for the next stage of the breeding program, based on the two agronomic traits studied.
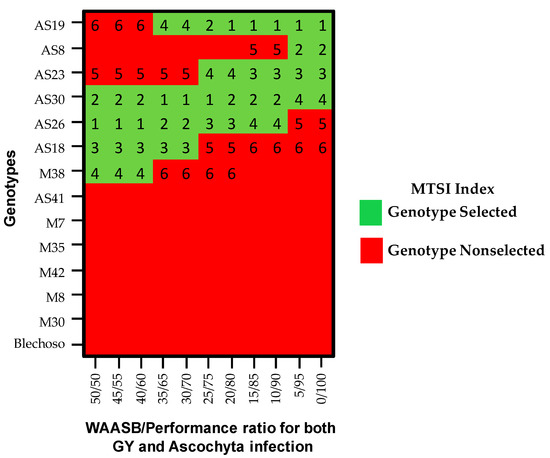
Figure 3.
Heatmap showing the MTSI index rank of 14 chickpea genotypes considering different weights for stability and both, GY and ascochyta infection.
An example of the rankings obtained of the MTSI index assigning a 25/75 ratio (25% stability, 75% performance) for grain yield and for ascochyta infection is shown in Figure 4, with AS30, AS19, AS26, and AS23 (red dots) marked as the most desirable to be selected.
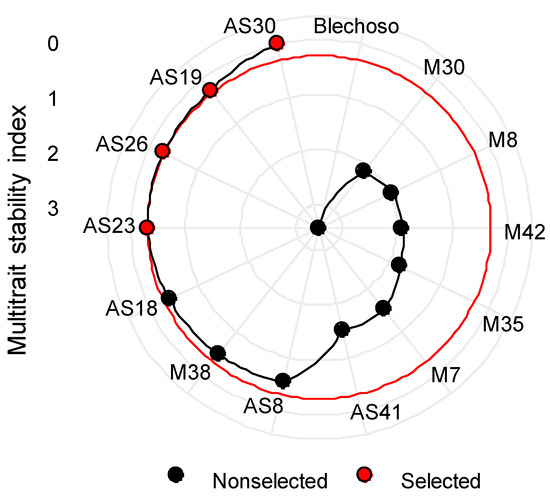
Figure 4.
Ranking of accessions based on MTSI index for GY and ascochyta infection assigning a selection intensity of 25/75 (25% stability, 75% performance).
The grain yield of the four selected genotypes (XS, 1787kg/ha) was higher than the original average of all genotypes (XO, 1248 kg/ha) (Table 6). Conversely, the average infection on the four selected genotypes (XO, 11.68%) was lower than the average on the whole set (XS, 26.8%). The magnitude of this difference is given by SD. The broad heritability (96%) and genetic gain (53.7%) were slightly higher for ascochyta infection than for grain yield (93% and 40.3%), revealing the feasibility of improving both traits but with better chances for ascochyta infection than for yield.

Table 6.
Estimates of genetic parameters for GY and Ascochyta infection based on MTSI for the 14 chickpea accessions tested in 10 environments.
3.5. Correlations among Traits and Environmental Factors
Pearson’s correlation between traits and climatic parameters (Figure 5) shows that grain yield was favored by a higher humidity at pre-flowering and flowering stages (r = 0.26 ** and r = 0.34 ***, respectively) and by rain at flowering (r = 0.29 ***), whereas rain after flowering was detrimental for yield (r = −0.36 ***). Similarly, high temperatures at all growing periods were detrimental but particularly at and after flowering (r = −0.25 **, r = −0.46 *** and r = −0.45 ***, for PreTmax, FlowTmax, and PostTmax, respectively). The most detrimental factor on grain yield was ascochyta blight infection (r = − 0.64 ***) paired with the number of days with Tmax >30 °C (T30, r = −0.61 ***). Ascochyta blight infection was favored by warm temperatures at flowering (r = 0.26 **) and by rain at post-flowering (r = 0.23 **) (Figure 5).
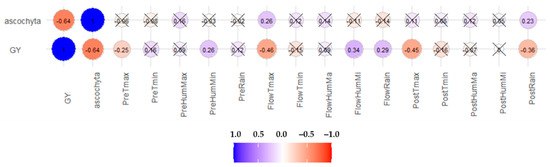
Figure 5.
Graphical representation of the effects of climatic parameters and of disease incidence on grain yield and among them. Size of the dot indicates its effect on the trait, blue when positive, red when negative. The not statistically significant crossed.
4. Discussions
Environmental effects were more important than genotypic effects and GEI for grain yield, which agrees with previous reports [16]. Indeed, climatic factors affected yield, with high temperatures at all growing periods being detrimental for yield but particularly at and after flowering. The most detrimental factor on grain yield was ascochyta blight infection, as widely acknowledged in winter-sown chickpea [3]. Ascochyta infection also varied with the environment, being favored by warm temperatures and rain after flowering. As a consequence, even when rain at flowering was beneficial for yield, rains after flowering were detrimental as they favored ascochyta infection. The devastating effect of ascochyta blight is in fact regarded as the reason driving delayed sowing from an autumn- to a spring-sown crop to escape infection, and this is suggested to have already occurred, specifically before the Early Bronze Age [17]. We found that genotypic effects were larger than the environmental on ascochyta infection as we included accessions previously selected for their levels of resistance or susceptibility. This is the result of long-term breeding efforts with ascochyta resistance as a major target [2,3,6]. As for Chandirasekaran et al. (2009) [18], the relative rank of resistant accessions was maintained across environments. As a result, the set of resistant accessions (AS8, AS18, AS19, AS23, AS30, AS26, and M38, with overall DS < 16%) yielded overall >1500 kg/ha, whereas the non-resistant yielded less than 1000 kg/ha.
We did not find any other significant biotic stress in any of the environments. This is not surprising for fusarium wilt or rust [19,20] as they both can be devastating in spring-sown chickpea in the area, but winter-sown chickpea escapes infection. There has been some concern regarding the risk of broomrape infection in winter chickpea [21]. However, we did not observe any infection even when the experimental sites are heavily infested and nearby fava bean, lentil, pea, or crops suffered from high damage [14,22,23]. This supports earlier reports, suggesting high resistance to broomrape in chickpea [24,25].
In addition to biotic stresses, chickpea production might face drought and low and high temperatures, which are becoming increasingly important with unpredictable climate change and the associated increased frequency of drought and extreme temperatures, which considerably reduces grain yield [26]. Winter-sown chickpea can be damaged by low temperatures at the vegetative and reproductive phase, when mean temperature of the day falls below 15 °C [27,28,29]. However, we did not find a significant effect of low temperatures on yield in the environments studied, probably due to the mild winters in the area. On the contrary, we found detrimental effects of high temperatures at all growing periods but particularly at and after flowering. Contrary to the long term concern regarding cold temperatures, awareness of heat stress is rather recent but widely acknowledged as a major constraint for chickpea productivity as temperatures >30 °C reduce grain weight and number due to reduced pollen viability and flower and pod abortion [30,31,32,33]. Chickpea genotypes tolerant to heat stress have been recently identified [34,35,36] and QTLs reported [37], which will facilitate chickpea heat tolerance breeding [38,39].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12092194/s1, Figure S1. Dendrogram of the cluster analysis applied to the Euclidean distance of the ranks matrix in scenario from 50/50 to 0/100 for the WAASB/GY ratio, grouped by the Complete-linkage fusion technique. Figure S2. Dendrogram of the cluster analysis applied to the Euclidean distance of the ranks matrix in scenario from 50/50 to 0/100 for the WAASB/ascochyta blight (data transformed) ratio, grouped by the Complete-linkage fusion technique.
Author Contributions
D.R. and A.M. designed and performed the trials; D.R. and F.F. analyzed the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript, except the late A.M. who participated in the analytical part of the work but deceased before the manuscript was submitted.
Funding
This research was funded by the Junta de Andalucía Project LEGAND P20_00986 and Spanish AEI project PID2020-114668RB-100.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are deeply indebted to ICARDA for providing chickpea accessions and to IFAPA-Tomegil and to Agrovegetal staff for facilitating access to Tomegil and Escacena fields.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mazid, A.; Shideed, K.; El-Abdullah, M.; Zyadeh, G.; Moustafa, J. Impacts of crop improvement research on farmers’ livelihoods: The case of winter-sown chickpea in Syria. Exp. Agric. 2013, 49, 336–351. [Google Scholar] [CrossRef]
- Rubio, J.; Gil, J.; Cobos, M.J.; Millán, T. Chickpea. In Genetics, Genomics and Breeding of Cool Season Grain Legumes; Pérez-de-la-Vega, M., Torres, A.M., Cubero, J.I., Kole, C., Eds.; CRC-Press: Boca Raton, FL, USA, 2011; pp. 205–236. [Google Scholar]
- Singh, R.; Kumar, K.; Purayannur, S.; Chen, W.; Verma, P.K. Ascochyta rabiei: A threat to global chickpea production. Mol. Plant Pathol. 2022, 23, 1241–1261. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Malhotra, R.S.; Saxena, M.C.; Bejiga, G. Superiority of Winter Sowing over Traditional Spring Sowing of Chickpea in the Mediterranean Region. Agron. J. 1997, 89, 112–118. [Google Scholar] [CrossRef]
- Iliadis, C. Evaluation of six chickpea varieties for seed yield under autumn and spring sowing. J. Agric. Sci. 2001, 137, 439–444. [Google Scholar] [CrossRef]
- Sharma, M.; Ghosh, R. An Update on Genetic Resistance of Chickpea to Ascochyta Blight. Agronomy 2016, 6, 18. [Google Scholar] [CrossRef]
- Zobel, R.W.; Wright, M.J.; Gauch, H.G. Statistical analysis of a yield trial. Agron. J. 1988, 80, 388–393. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C.; da Silva, J.A.G.; Marchioro, V.S.; de Souza, V.Q.; Jost, E. Mean Performance and Stability in Multi-Environment Trials I: Combining Features of AMMI and BLUP Techniques. Agron. J. 2019, 111, 2949–2960. [Google Scholar] [CrossRef]
- Red de Información Agroclimática de Andalucía (RIA). Available online: https://www.juntadeandalucia.es/agriculturaypesca/ifapa/riaweb/web/ (accessed on 2 March 2022).
- Olivoto, T.; Lúcio, A.D.C. Metan: An R package for multienvironment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.; da Silva, J.A.; Sari, B.G.; Diel, M.I. Mean performance and stability in multi-environment trials II: Selection based on multiple traits. Agron. J. 2019, 111, 2961–2969. [Google Scholar] [CrossRef]
- RAEA. Ensayos de Garbanzos y Habas, Campaña 2006–2007. 2007. Available online: https://www.juntadeandalucia.es/agriculturaypesca/ifapa/servifapa/registro-servifapa/b98168f1-d488-4695-a274-b297c69f9af5 (accessed on 2 August 2022).
- Rubiales, D.; Moral, A.; Flores, F. Agronomic performance of broomrape resistant and susceptible faba bean accession. Agronomy 2022, 12, 1421. [Google Scholar] [CrossRef]
- Sellami, M.H.; Pulvento, C.; Lavini, A. Selection of Suitable Genotypes of Lentil (Lens culinaris Medik.) under Rainfed Conditions in South Italy Using Multi-Trait Stability Index (MTSI). Agronomy 2021, 11, 1807. [Google Scholar] [CrossRef]
- Sellami, M.H.; Lavini, A.; Pulvento, C. Phenotypic and Quality Traits of Chickpea Genotypes under Rainfed Conditions in South Italy. Agronomy 2021, 11, 962. [Google Scholar] [CrossRef]
- Abbo, S.; Berger, J.; Turner, N. Evolution of cultivated chickpea: Four bottlenecks limit diversity and constrain adaptation. Funct. Plant Biol. 2003, 30, 1081–1087. [Google Scholar] [CrossRef]
- Chandirasekaran, R.; Warkentin, T.D.; Gan, Y.; Shirtliffe, S.; Gossen, B.D.; Taran, B.; Banniza, S. Improved sources of resistance to ascochyta blight in chickpea. Can. J. Plant Sci. 2009, 89, 107–118. [Google Scholar] [CrossRef]
- Landa, B.B.; Navas-Cortés, J.A.; Jiménez-Díaz, R. Integrated Management of Fusarium Wilt of Chickpea with Sowing Date, Host Resistance, and Biological Control. Phytopathology 2004, 94, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Sillero, J.C.; Moreno-Alías, I.; Rubiales, D. Identification and characterization of resistance to rust (Uromyces ciceris-arietini (Grognot) Jacz. & Boyd) in a germplasm collection of Cicer spp. Euphytica 2012, 188, 229–238. [Google Scholar] [CrossRef]
- Nefzi, F.; Trabelsi, I.; Amri, M.; Triki, E.; Kharrat, M.; Abbes, Z. Response of some chickpea (Cicer arietinum L.) genotypes to Orobanche foetida Poir. parasitism. Chil. J. Agric. Res. 2016, 76, 170–178. [Google Scholar] [CrossRef]
- Rubiales, D.; Osuna-Caballero, S.; González-Bernal, M.J.; Cobos, M.J.; Flores, F. Pea breeding lines adapted to autumn sowings in broomrape prone Mediterranean environments. Agronomy 2021, 11, 769. [Google Scholar] [CrossRef]
- Rubiales, D.; Moral, A.; Flores, F. Heat waves and broomrape are the major constraints for lentil cultivation in Southern Spain. Agronomy 2021, 11, 1871. [Google Scholar] [CrossRef]
- Rubiales, D.; Alcántara, C.; Pérez-de-Luque, A.; Gil, J.; Sillero, J.C. Infection of chickpea (Cicer arietinum) by crenate broomrape (Orobanche crenata) as influenced by sowing date and weather conditions. Agronomie 2003, 23, 359–362. [Google Scholar] [CrossRef]
- Rubiales, D.; Pérez-de-Luque, A.; Joel, D.M.; Alcantara, C.; Sillero, J.C. Characterisation of resistance in chickpea to crenate broomrape (Orobanche crenata). Weed Sci. 2003, 51, 702–707. [Google Scholar] [CrossRef]
- Kadiyala, M.D.M.; Kumara-Charyulu, D.; Nedumaran, S.; Shyam, M.; Gumma, M.K.; Bantilan, M.C.S. Agronomic management options for sustaining chickpea yield under climate change scenario. J. Agrometeorol. 2016, 18, 41–47. [Google Scholar] [CrossRef]
- Singh, K.B.; Malhotra, R.S.; Saxena, M.C. Relationship between cold severity and yield loss in chickpea (Cicer arietinum L.). J. Agron. Crop Sci. 1993, 170, 121–127. [Google Scholar] [CrossRef]
- Croser, J.S.; Clarke, H.J.; Siddique, K.H.M.; Khan, T.N. Low-temperature stress: Implications for chickpea (Cicer arietinum L.) improvement. CRC Crit. Rev. Plant Sci. 2003, 22, 185–219. [Google Scholar] [CrossRef]
- Nezami, A.; Bandara, M.S.; Gusta, L.V. An evaluation of freezing tolerance of winter chickpea (Cicer arietinum L.) using controlled freeze tests. Can. J. Plant Sci. 2012, 92, 155–161. [Google Scholar] [CrossRef]
- Wang, J.; Gan, Y.T.; Clarke, F.; McDonald, C.L. Response of chickpea yield to high temperature stress during reproductive development. Crop Sci. 2006, 46, 2171–2178. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.M.; Mallikarjuna, N.; Tokashishu, R.N.; Trethowan, R.M.; Tan, D.K.Y. Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant Biol. 2012, 39, 1009–1018. [Google Scholar] [CrossRef]
- Kaushal, N.; Awasthi, R.; Gupta, K.; Gaur, P.M.; Siddique, K.H.M.; Nayyar, H. Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct. Plant Biol. 2013, 40, 1334–1349. [Google Scholar] [CrossRef]
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.M.; Nayyar, H. Developing Climate-Resilient Chickpea Involving Physiological and Molecular Approaches with a Focus on Temperature and Drought Stresses. Front. Plant Sci. 2020, 10, 1759. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Gaur, P.M.; Basu, P.S.; Chaturvedi, S.K.; Tripathi, S.; Vadez, V.; Rathore, A.; Varshney, R.K.; Gowda, C.L.L. Large genetic variation for heat tolerance in the reference collection of chickpea (Cicer arietinum L.) germplasm. Plant Genet. Resour. 2011, 9, 59–69. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.M.; Raju, T.N.; Trethowan, R.M.; Tan, D.K.Y. Field response of chickpea (Cicer arietinum L.) to high temperature. Field Crop Res. 2015, 172, 59–71. [Google Scholar] [CrossRef]
- Kumar, A.; Agrawal, T.; Kumar, S.; Kumar, A.; Kumar, R.R.; Kumar, M.; Singh, C.K.P. Identification and evaluation of Heat Tolerant Chickpea genotypes for Enhancing its Productivity in Rice Fallow area of Bihar and Mitigating Impacts of Climate Change. J. Pharmacogn. Phytochem. 2017, SP1, 1105–1113. [Google Scholar]
- Paul, P.J.; Samineni, S.; Thudi, M.; Sajja, S.B.; Rathore, A.; Das, R.R.; Khan, A.W.; Chaturvedi, S.K.; Lavanya, G.R.; Varshney, R.K.; et al. Molecular Mapping of QTLs for Heat Tolerance in Chickpea. Int. J. Mol. Sci. 2018, 19, 2166. [Google Scholar] [CrossRef]
- Singh, S.; Singh, I.; Kapoor, K.; Gaur, P.M.; Chaturvedi, S.K.; Singh, N.P.; Sandhu, J.S. Chickpea. In Broadening the Genetic Base of Grain Legumes; Singh, M., Bisht, I., Dutta, M., Eds.; Springer: New Delhi, India, 2014; pp. 51–73. [Google Scholar] [CrossRef]
- Gaur, P.M.; Samineni, S.; Thudi, M.; Tripathi, S.; Sajja, S.B.; Jayalakshmi, V.; Mannur, D.M.; Vijayakumar, A.D.; Ganga Rao, N.V.P.R.; Ojiewo, C.; et al. Integrated breeding approaches for improving drought and heat adaptation in chickpea (Cicer arietinum L.). Plant Breed. 2019, 138, 389–400. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).