Soybean Crop Rotation Stability in Rainfed Agroforestry System through GGE Biplot and EBLUP
Abstract
:1. Introduction
2. Materials and Methods
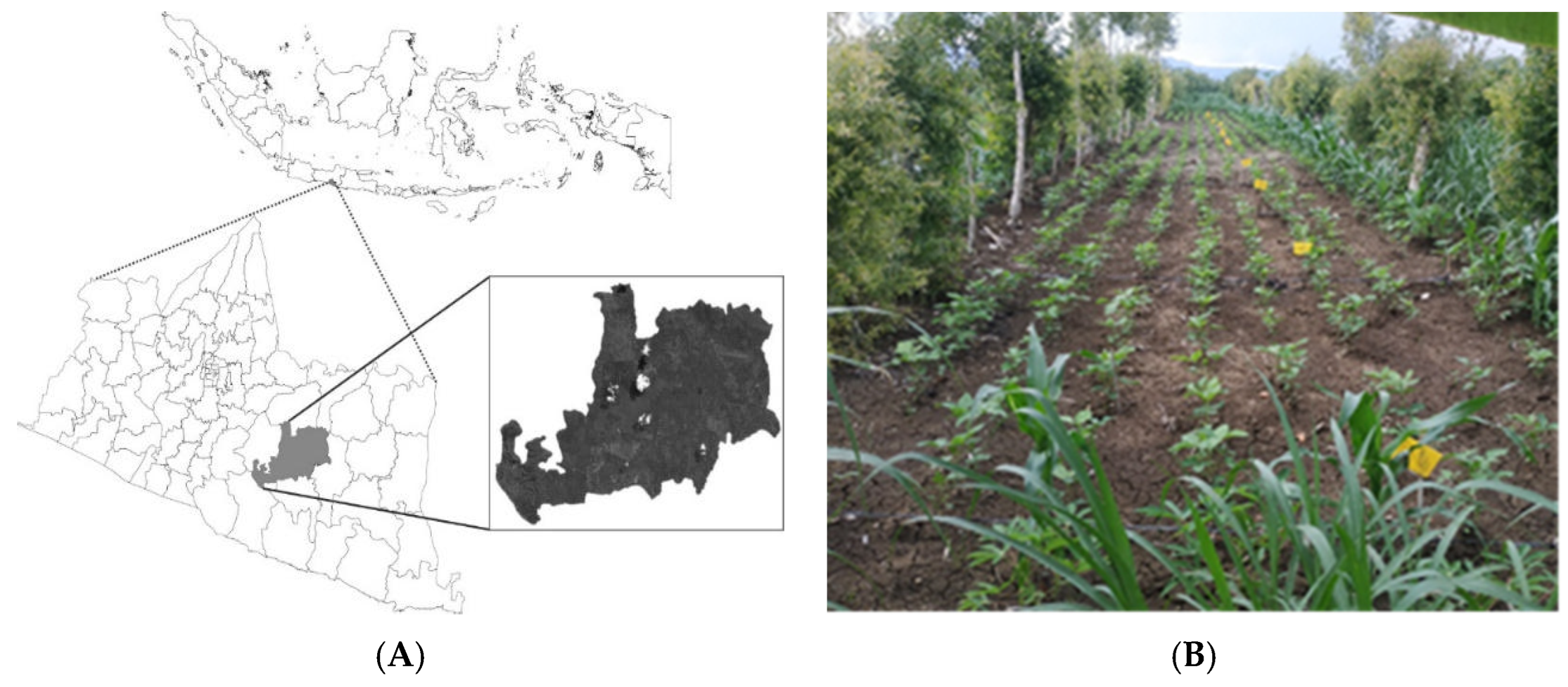
2.1. Study Sites
2.2. Multi-Environmental Trial Setup and Crop Management
2.3. Data Collection
2.3.1. Soil Characteristics
2.3.2. Soybean Yield
2.4. Statistical Analysis
- i
- The covariance structure for replicate (R) is , where is a diagonal matrix with diagonal elements . A certain soil type variance was assumed.
- ii
- The covariance structure for the cultivar effect is the identity structure, that is, .
- iii
- The residual covariance structure is heterogeneous with soil-type-specific , where is a diagonal matrix with .
| Factors | Total | Symbol |
|---|---|---|
| Cultivar | 15 | C |
| Crop rotation model | 4 | M |
| Replicate | 3 | R |
| No. | Cultivars | Pedigree | Yield Potential (tons ha−1) | Harvest Age (dap) | Pest or Disease Resistance | Specific Features |
|---|---|---|---|---|---|---|
| 1. | Anjasmoro | Mass selection for ‘Mansuria’ pure line | 2.03–2.25 | 82.5–92.5 | Moderate resistance to leaf rust | Resistance to pod shattering |
| 2. | Argomulyo | Introduction from Thailand | 1.5–2.0 | 80–82 | Tolerant to leaf rust | Suitable for soy milk ingredient |
| 3. | Baluran | AVRDC Cross | 2.5–3.5 | 80 | – | – |
| 4. | Biosoy I | The pedigree selection from a population of mutant strains from crosses of Chinese soybeans with Japanese soybeans irradiated with a dose of 250 Gray gamma rays | 3.3 | 83 | Resistance to leaf rust, pod borer, and army worm | Resistance to pod shattering |
| 5. | Burangrang | Pure-line selection from Jember landrace | 1.6–2.5 | 80–82 | Tolerant to leaf rust | Suitable for soy milk, tempeh, and tofu |
| 6. | Dega I | Single cross of ‘Grobogan’ and ‘Malabar’ | 3.82 | 69–73 | Moderate resistance to leaf rust and not resistant to army worm | Adaptive in paddy fields |
| 7. | Dena I | Single cross of ‘Agromulyo’ × IAC 100 | 2.9 | 78 | Resistance to leaf rust, not resistant to pod borer and army worm | Tolerant to 50% shade |
| 8. | Dena II | Single cross of IAC 100 × ‘Ijen’ | 2.8 | 81 | Resistance to leaf rust and pod borer, moderate resistance to army worm | Very tolerant to 50% shade |
| 9. | Dering I | Single cross of ‘Davros’ × MLG 2984 | 2.8 | 81 | Resistance to pod borer and resistance to leaf rust | Resistance to drought in reproductive phase |
| 10. | Dering II | Single cross of Arg/GCP–335 × ‘Baluran’ | 3.32 | 70–76 | Moderate resistance to leaf, army worm, and leaf rust | Resistance to drought in reproductive phase |
| 11. | Dering III | Single cross of ‘Dering I’ × ‘Malabar’ | 2.99 | 70–76 | Moderate resistance to leaf, army worm, and leaf rust | Resistance to drought in reproductive phase |
| 12. | Devon I | Derived from ‘Kawi’ × IAC100 | 2.75 | 83 | Resistance to leaf rust and moderate resistance to pod sucker | High isoflavone content (2219.8 µg g−1) |
| 13. | Grobogan | Pure-line selection from ‘Malabar’ in Grobogan | 2.77 | 76 | – | Less pod shattering |
| 14. | Mahameru | Mass selection for ‘Man–suria’ pure line | 2.04–2.16 | 83.5–94.8 | Moderate resistance to leaf rust | Resistance to pod shattering |
| 15. | Tanggamus | Hibrida (single cross): ‘Kerinci’ × No. 3911 | 1.22 | 85 | Moderate resistance to leaf rust | Resistance to pod shattering, adaptive in acid dry land |
3. Results
3.1. Soil Characteristic in Study Sites
3.2. Ranking and EBLUP of 15 Soybean Cultivars in Each Crop Rotation Model
| No. | Soil Characteristics | Unit | Crop Rotation Models | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry Season | Wet Season | |||||||||
| F−S | M−S | R−S | S−S | F−S | M−S | R−S | S−S | |||
| Soil Physical | ||||||||||
| 1. | Soil Texture | – | Clay | Clay | Clay | Clay | Clay | Clay | Clay | Clay |
| 2. | Bulk Density | g cm−3 | 1.16 | 1.12 | 1.11 | 1.12 | 1.11 | 1.12 | 1.15 | 1.09 |
| 3. | Soil Moisture Content | mm cm−1 | 16.45 | 17.18 | 19.21 | 19.77 | 25.35 | 26.46 | 27.14 | 27.53 |
| 4. | Permeability | cm h−1 | 0.001 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Soil Chemical | ||||||||||
| 1. | pH H2O | – | 8.4 | 8.3 | 8.2 | 8.1 | 8.3 | 8.1 | 8.0 | 8.0 |
| 2. | Soil Organic Carbon | % | 1.6 | 1.5 | 1.6 | 1.4 | 1.7 | 1.6 | 1.6 | 1.5 |
| 3. | Cation Exchange Capacity | cmol(+) kg−1 | 62.23 | 58.48 | 59.72 | 59.92 | 64.71 | 63.72 | 64.82 | 64.51 |
| 4. | Electrical Conductivity | dS m−1 | 1.682 | 1.689 | 1.614 | 1.647 | 1.711 | 1.742 | 1.691 | 1.708 |
| 5. | Total Nitrogen | % | 0.09 | 0.16 | 0.18 | 0.25 | 0.22 | 0.19 | 0.16 | 0.29 |
| 6. | Soil Nutrient Availability: | |||||||||
| mg L | 9 | 11 | 11 | 12 | 11 | 18 | 8 | 17 | |
| cmol(+) kg−1 | 0.18 | 0.12 | 0.15 | 0.11 | 0.26 | 0.22 | 0.22 | 0.24 | |
| cmol(+) kg−1 | 0.64 | 0.62 | 0.61 | 0.59 | 0.74 | 0.69 | 0.67 | 0.65 | |
| cmol(+) kg−1 | 29.72 | 24.46 | 25.67 | 24.89 | 23.11 | 23.01 | 21.38 | 22.71 | |
| cmol(+) kg−1 | 1.27 | 1.16 | 1.18 | 1.11 | 1.34 | 1.26 | 1.62 | 1.42 | |
| mg L−1 | 1.14 | 2.22 | 1.19 | 2.16 | 1.92 | 1.12 | 1.93 | 1.11 | |
| mg L−1 | 1.28 | 1.13 | 1.22 | 1.16 | 1.19 | 1.11 | 1.16 | 1.08 | |
| mg L−1 | 1.54 | 1.42 | 1.32 | 1.31 | 1.27 | 1.19 | 1.13 | 1.09 | |
| Soil Biological | ||||||||||
| 1. | Total Bacteria | cfu | 1.32 × 105 | 1.74 × 105 | 1.92 × 105 | 1.82 × 105 | 1.99 × 105 | 2.53 × 105 | 1.64 × 105 | 2.31 × 105 |
| 2. | Total Fungi | cfu | 1.46 × 103 | 1.61 × 103 | 1.71 × 103 | 1.68 × 103 | 1.83 × 103 | 1.94 × 103 | 1.57 × 103 | 1.90 × 103 |
| Model | Akaike Information Criterion | |
|---|---|---|
| Dry Season | Wet Season | |
| Identity | 1292.7 | 1400.8 |
| Compound symmetry | 1312.7 | 1420.8 |
| Heteroscedastic compound symmetry | 1314.1 | 1422.1 |
| Unstructured | 1319.8 | 1427.9 |
| Effect † | Group | Variance Estimate | |
|---|---|---|---|
| Dry Season | Wet Season | ||
| R | F–S | 0.00107 | 0.00565 |
| M–S | 0.00078 | 0.00645 | |
| R–S | 0.00089 | 0.00383 | |
| S–S | 0.00105 | 0.00494 | |
| C•M ‡ | Genetic variance (C) | 0.15450 | 0.20173 |
| Genetic correlation § | 0.08066 | 0.11877 | |
| E | F–S | 0.00003 | 0.00005 |
| M–S | 0.00002 | 0.00007 | |
| R–S | 0.00316 | 0.00005 | |
| S–S | 0.00003 | 0.00026 | |
3.3. Stability Variance Estimates
| Ranking | Fallow–Soybean (F–S) | Maize–Soybean (M–S) | Rice–Soybean (R–S) | Soybean–Soybean (S–S) | ||||
|---|---|---|---|---|---|---|---|---|
| Cultivars | EBLUP | Cultivars | EBLUP | Cultivars | EBLUP | Cultivars | EBLUP | |
| 1 | Dering I | 1.267 | Grobogan | 1.200 | Dering I | 1.375 | Grobogan | 1.349 |
| 2 | Dega I | 1.250 | Dering I | 1.174 | Grobogan | 1.334 | Dering I | 1.346 |
| 3 | Dena I | 1.222 | Devon I | 1.155 | Anjasmoro | 1.306 | Dering III | 1.210 |
| 4 | Devon I | 1.204 | Anjasmoro | 1.153 | Burangrang | 1.279 | Dena II | 1.179 |
| 5 | Grobogan | 1.096 | Argomulyo | 1.144 | Dega I | 1.270 | Burangrang | 1.065 |
| 6 | Dering II | 1.093 | Dega I | 1.080 | Dena I | 1.269 | Dena I | 1.021 |
| 7 | Dering III | 1.084 | Dering II | 1.069 | Biosoy I | 1.164 | Devon I | 1.021 |
| 8 | Tanggamus | 1.077 | Mahameru | 1.049 | Baluran | 1.138 | Dering II | 1.001 |
| 9 | Mahameru | 0.974 | Dering III | 1.015 | Dering III | 1.117 | Biosoy I | 0.969 |
| 10 | Argomulyo | 0.935 | Tanggamus | 0.939 | Dena II | 1.113 | Argomulyo | 0.966 |
| 11 | Biosoy I | 0.921 | Biosoy I | 0.908 | Argomulyo | 1.066 | Anjasmoro | 0.934 |
| 12 | Burangrang | 0.906 | Dena II | 0.880 | Dering II | 1.034 | Dega I | 0.931 |
| 13 | Anjasmoro | 0.853 | Dena I | 0.853 | Mahameru | 1.009 | Mahameru | 0.886 |
| 14 | Baluran | 0.758 | Burangrang | 0.838 | Tanggamus | 0.994 | Tanggamus | 0.844 |
| 15 | Dena II | 0.754 | Baluran | 0.736 | Devon I | 0.984 | Baluran | 0.789 |
| Ranking | Fallow–Soybean (F–S) | Maize–Soybean (M–S) | Rice–Soybean (R–S) | Soybean–Soybean (S–S) | ||||
|---|---|---|---|---|---|---|---|---|
| Cultivars | EBLUP | Cultivars | EBLUP | Cultivars | EBLUP | Cultivars | EBLUP | |
| 1 | Grobogan | 2.187 | Grobogan | 2.435 | Dega I | 2.049 | Grobogan | 2.247 |
| 2 | Dega I | 2.175 | Anjasmoro | 2.388 | Grobogan | 1.895 | Anjasmoro | 2.233 |
| 3 | Burangrang | 2.128 | Dena I | 2.354 | Argomulyo | 1.772 | Dega I | 2.202 |
| 4 | Dering I | 2.024 | Dega I | 2.206 | Anjasmoro | 1.761 | Tanggamus | 2.163 |
| 5 | Dena I | 2.019 | Tanggamus | 2.159 | Tanggamus | 1.756 | Burangrang | 2.162 |
| 6 | Devon I | 1.989 | Dering II | 2.158 | Dering III | 1.755 | Mahameru | 2.145 |
| 7 | Biosoy I | 1.981 | Biosoy I | 2.063 | Baluran | 1.596 | Dering II | 2.125 |
| 8 | Dena II | 1.941 | Dering III | 1.970 | Dena I | 1.586 | Dering III | 2.107 |
| 9 | Anjasmoro | 1.816 | Dering I | 1.967 | Burangrang | 1.578 | Dena I | 2.093 |
| 10 | Dering III | 1.806 | Argomulyo | 1.953 | Dering I | 1.535 | Devon I | 2.091 |
| 11 | Argomulyo | 1.794 | Burangrang | 1.943 | Biosoy I | 1.524 | Dena II | 2.073 |
| 12 | Mahameru | 1.786 | Devon I | 1.922 | Devon I | 1.521 | Argomulyo | 1.892 |
| 13 | Baluran | 1.697 | Dena II | 1.891 | Dena II | 1.510 | Biosoy I | 1.853 |
| 14 | Tanggamus | 1.669 | Baluran | 1.776 | Dering II | 1.485 | Dering I | 1.853 |
| 15 | Dering II | 1.611 | Mahameru | 1.733 | Mahameru | 1.449 | Baluran | 1.851 |
| Cultivars | Stability Variance Estimate For C•S | |
|---|---|---|
| Dry Season | Wet Season | |
| Anjasmoro | 2.742 | 7.684 |
| Argomulyo | 0.630 | 3.330 |
| Baluran | 6.454 | 3.706 |
| Biosoy I | 1.161 | 2.129 |
| Burangrang | 3.085 | 11.214 |
| Dega I | 1.887 | 9.584 |
| Dena I | 2.892 | 4.768 |
| Dena II | 5.120 | 0.000 |
| Dering I | 0.176 | 1.708 |
| Dering II | 0.708 | 4.789 |
| Dering III | 1.393 | 1.983 |
| Devon I | 1.114 | 0.537 |
| Grobogan | 4.054 | 0.026 |
| Mahameru | 0.210 | 1.695 |
| Tanggamus | 5.163 | 5.053 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture. The Strategic Plan of Ministry of Agriculture for 2015–2019; Ministry of Agriculture: Jakarta, Indonesia, 2016. Available online: http://sakip.pertanian.go.id/admin/file/Renstra%20Kementan%202015–2019%20(edisi%20revisi).pdf (accessed on 25 May 2022).
- FAO. The Future of Food and Agriculture–Trends and Challenges; The Food and Agriculture Organization (FAO): Rome, Italy, 2017; Available online: https://www.fao.org/3/i6583e/i6583e.pdf (accessed on 25 May 2022).
- Ministry of Agriculture. Indonesian Soybean Production Projection (2020–2024); Ministry of Agriculture: Jakarta, Indonesia, 2021. Available online: https://databoks.katadata.co.id/datapublish/2021/06/04/produksi–kedelai–diproyeksi–turun–hingga–2024#:~:text=Proyeksi%20Produksi%20Kedelai%20Indonesia%20(2020%2D2024)&text=Pada%20tahun%20ini%2C%20proyeksi%20kedelai,6%20ribu%20ton%20pada%202022 (accessed on 25 May 2022).
- Statistics Indonesia. The Harvested Area and Rice Production in Indonesia 2019; Statistics Indonesia: Jakarta, Indonesia, 2020. Available online: https://www.bps.go.id/pressrelease/2020/02/04/1752/luas–panen–dan–produksi–padi–pada–tahun–2019–mengalami–penurunan–dibandingkan–tahun–2018–masing–masing–sebesar–6–15–dan–7–76–persen.html (accessed on 25 May 2022).
- Mulyani, A.; Nursyamsi, D.; Syakir, M. Land resource utilization strategy to achieve sustainable rice self–sufficiency. J. Sumberd. Lahan 2017, 11, 11–22. [Google Scholar] [CrossRef]
- Alam, T.; Suryanto, P.; Kurniasaih, B.; Kastono, D.; Supriyanta; Widyawan, M.H.; Muttaqin, A.S.; Taryono. Kayu putih forest: Window opportunity for food estate development in Indonesia. In Appropriate Technology 75th Faculty of Agriculture Serving; Deviyanti, J., Ed.; Lily Publisher: Yogyakarta, Indonesia, 2021; pp. 144–146. [Google Scholar]
- Alam, T.; Suryanto, P.; Susyanto, N.; Kurniasih, B.; Basunanda, P.; Putra, E.T.S.; Kastono, D.; Respatie, D.W.; Widyawan, M.H.; Nurmansyah; et al. Performance of 45 non–linear models for determining critical period of weed control and acceptable yield loss in soybean agroforestry systems. Sustainability 2022, 14, 7636. [Google Scholar] [CrossRef]
- Suryanto, P.; Faridah, E.; Nurjanto, H.H.; Putra, E.T.S.; Kastono, D.; Handayani, S.; Boy, R.; Widyawan, M.H.; Alam, T. Short–term effect of in situ biochar briquettes on nitrogen loss in hybrid rice grown in an agroforestry system for three years. Agronomy 2022, 12, 564. [Google Scholar] [CrossRef]
- FAO. Drought Characteristics and Management in Central Asia and Turkey; The Food and Agriculture Organization (FAO) Sub–regional Office for Central Asia, Ankara, Turkey and Land and Water Division: Rome, Italy, 2017; Available online: https://www.fao.org/3/i6738e/i6738e.pdf (accessed on 25 May 2022).
- Bowles, T.M.; Mooshammer, M.; Socolar, Y.; Calderón, F.; Cavigelli, M.A.; Culman, S.W.; Deen, W.; Drury, C.F.; Garcia, A.G.Y.; Gaudin, A.C.M.; et al. Long–term evidence shows that crop–rotation diversification increases agricultural resilience to adverse growing conditions in North America. One Earth 2020, 2, 284–293. [Google Scholar] [CrossRef]
- Neupane, A.; Bulbul, I.; Wang, Z.; Lehman, R.M.; Nafziger, E.; Marzano, S.Y.L. Long term crop rotation effect on subsequent soybean yield explained by soil and root-associated microbes and soil health indicators. Sci. Rep. 2021, 11, 9200. [Google Scholar] [CrossRef]
- Dolijanović, Z.; Milena, S. The role of the crop rotation in maize agroecosystem sustainability. In Zea mays L.: Molecular Genetics, Potential Environmental Effects and Impact on Agricultural Practices; Barnes, L., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2016. [Google Scholar]
- Piepho, H.P.; Nazir, M.F.; Qamar, M.; Rattu, A.U.R.; Hussain, M.; Ahmad, G.; Ahmad, J.; Laghari, K.B.; Vistro, I.A.; Kakar, M.S.; et al. Stability analysis for a countrywide series of wheat trials in Pakistan. Crop Sci. 2016, 56, 2465–2475. [Google Scholar] [CrossRef]
- Eberhart, S.A.; Russell, W.A. Stability parameters for comparing varieties. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE–biplot vs. AMMI analysis of genotype–by–environment data. Crop Sci. 2007, 47, 643–655. [Google Scholar] [CrossRef]
- Alam, T.; Suryanto, P.; Supriyanta; Basunanda, P.; Wulandari, R.A.; Kastono, D.; Widyawan, M.H.; Nurmansyah, T. Rice cultivar selection in an agroforestry system through GGE biplot and EBLUP. Biodiversitas 2021, 22, 4750–4757. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists and Agronomists, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; p. 286. [Google Scholar]
- Bilgin, O.; Guzmán, C.; Baser, I.; Crossa, J.; Korkut, K.Z.; Balkan, A. Evaluation of grain yield and quality traits of bread wheat genotypes cultivated in Northwest Turkey. Crop Sci. 2015, 56, 73–84. [Google Scholar] [CrossRef]
- Gerrano, A.S.; van Rensburg, W.S.J.; Mathew, I.; Shayanowako, A.I.T.; Bairu, M.W.; Venter, S.L.; Swart, W.; Mofokeng, A.; Mellem, J.; Labuschagne, M. Genotype and genotype × environment interaction effects on the grain yield performance of cowpea genotypes in dryland farming system in South Africa. Euphytica 2020, 216, 80. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Magaji, U.; Miah, G.; Hussin, G.; Ramli, A. Genotype × environment interaction and stability analyses of yield and yield components of established and mutant rice genotypes tested in multiple locations in Malaysia. Acta Agric. Scand. Sect. B–Soil Plant Sci. 2017, 67, 590–606. [Google Scholar] [CrossRef]
- Buntaran, H.; Forkman, J.; Piepho, H.P. Projecting results of zoned multi-environment trials to new locations using environmental covariates with random coefcient models: Accuracy and precision. Theor. App. Genet. 2021, 134, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Buntaran, H.; Piepho, H.P.; Hagman, J.; Forkman, J. A cross–validation of statistical models for zoned–based prediction in cultivar testing. Crop Sci. 2019, 59, 1544–1553. [Google Scholar] [CrossRef]
- Buntaran, H.; Piepho, H.P.; Schmidt, P.; Ryden, J.; Halling, M.; Forkman, J. Cross–validation of stagewise mixed–model analysis of Swedish variety trials with winter wheat and spring barley. Crop Sci. 2020, 60, 2221–2240. [Google Scholar] [CrossRef]
- Kleinknecht, K.; Möhring, J.; Singh, K.P.; Zaidi, P.H.; Atlin, G.N.; Piepho, H.P. Comparison of the performance of BLUE and BLUP for zoned Indian maize data. Crop Sci. 2013, 53, 1384–1391. [Google Scholar] [CrossRef]
- Alam, T.; Kurniasih, B.; Suryanto, P.; Basunanda, P.; Supriyanta; Ambarwati, E.; Widyawan, M.H.; Handayani, S.; Taryono. Stability analysis for soybean in agroforestry system with kayu putih. SABRAO J. Breed. Genet. 2019, 51, 405–418. [Google Scholar]
- Alam, T.; Suryanto, P.; Nurmalasari, A.I.; Kurniasih, B. GGE Biplot analysis for the suitability of soybean varieties in an agroforestry system based on kayu putih (Melaleuca cajuputi) stands. Caraka Tani J. Sustain. Agric. 2019, 34, 213–222. [Google Scholar] [CrossRef]
- Alam, T.; Suryanto, P.; Handayani, S.; Kastono, D.; Kurniasih, B. Optimizing application of biochar, compost and nitrogen fertilizer in soybean intercropping with kayu putih (Melaleuca cajuputi). Rev. Bras. Cienc. Solo 2020, 44, e0200003. [Google Scholar] [CrossRef]
- Indonesian Center for Agricultural Biotechnology and Genetic Resource Research. Biosoy 1 Soybean; Indonesian Center for Agricultural Biotechnology and Genetic Resource Research, Ministry of Agriculture: West Java, Indonesia, 2018. Available online: http://biogen.litbang.pertanian.go.id/biosoy–1/ (accessed on 25 May 2022).
- Mejaya, M.J.; Harnowo, D.; Adie, M.M. Technical Guidelines for Soybean Cultivation in Various Agroecosystems; Indonesian Legume and Tuber Crops Research Institute, Ministry of Agriculture: East Java, Indonesia, 2015. Available online: https://opac.perpusnas.go.id/DetailOpac.aspx?id=956412 (accessed on 25 May 2022).
- Bandyopadhyay, K.K.; Aggarwal, P.; Chakraborty, D.; Pradhan, S.; Garg, R.N.; Singh, R. Practical Manual on Measurement of Soil Physical Properties; Division of Agricultural Physics, Indian Agricultural Research Institute: New Delhi, India, 2012; p. 62. [Google Scholar]
- Peters, J. Wisconsin Procedures for Soil Testing, Plant Analysis and Feed & Forage Analysis; Soil Science Department, College of Agriculture and Life Sciences, University of Wisconsin: Madison, WI, USA, 2013. Available online: https://datcp.wi.gov/Documents/NMProcedures.pdf (accessed on 29 June 2022).
- David, A.B.; Davidson, C.E. Estimation method for serial dilution experiments. J. Microbiol Methods 2014, 107, 214–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroup, W.W.; Milliken, G.A.; Claassen, E.A.; Wolfinger, R.D. SAS® for Mixed Models: Introduction and Basic Applications; SAS Institute Inc.: Cary, NC, USA, 2018; pp. 307–338. [Google Scholar]
- Shukla, G.K. Some statistical aspects of partitioning genotype–environmental components of variability. Heredity 1972, 29, 237–245. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. Step–by–Step Programming with Base SAS® 9.4, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Goedhart, P.W.; Thissen, J.T.N.M. Biometris GenStat Procedure Library Manual, 18th ed.; Wageningen University and Research Center: Wageningen, The Netherlands, 2016. [Google Scholar]
- Boettinger, J.; Chiaretti, J.; Ditzler, C.; Galbraith, J.; Kerschen, K.; Loerch, C.; McDanie, P.; McVey, S.; Monger, C.; Owens, P.; et al. Illustrated Guide to Soil Taxonomy, Version 2; U.S. Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2015.
- Piepho, H.P.; Möhring, J. Best linear unbiased prediction for subdivided target regions. Crop Sci. 2005, 45, 1151–1159. [Google Scholar] [CrossRef]
- Smith, A.B.; Cullis, B.R.; Thompson, R. The analysis of crop cultivar breeding and evaluation trials: An overview of current mixed model approaches. J. Agric. Sci. 2005, 143, 449–462. [Google Scholar] [CrossRef]
- Kasno, A.; Trustinah. Genotype–environment interaction analysis of peanut in Indonesia. SABRAO J. Breed. Genet. 2015, 47, 482–492. [Google Scholar]
- Giller, K.E.; Tittonell, P.; Rufino, M.C.; van Wijk, M.T.; Zingore, S.; Mapfumo, P.; Adjeinsiah, S.; Herrero, M.; Chikowo, R.; Corbeels, M.; et al. Communicating complexity: Integrated assessment of trade–offs concerning soil fertility management within African farming system to support innovation and development. Agric. Syst. 2011, 104, 191–203. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. Genetic challenges of flavor improvement in tomato. Trends Genet. 2013, 29, 257–267. [Google Scholar] [CrossRef]
- Alam, T.; Muhartini, S.; Suryanto, P.; Ambarwati, E.; Kastono, D.; Nurmalasari, A.I.; Kurniasih, B. Soybean varieties suitability in agroforestry system with kayu putih under influence of soil quality parameters. Rev. Ceres 2020, 67, 410–418. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Allen, F.L.; Saxton, A.M.; Tyler, D.D. Impact of crop rotations and soil amendments on long–term no–tilled soybean yield. Agron. J. 2017, 109, 938–946. [Google Scholar] [CrossRef]
- Agomoh, I.V.; Drury, C.F.; Yang, X.; Phillips, L.A.; Reynolds, W.D. Crop rotation enhances soybean yields and soil health indicators. Soil Sci. Soc. Am. J. 2021, 5, 1185–1195. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taryono; Suryanto, P.; Supriyanta; Basunanda, P.; Wulandari, R.A.; Handayani, S.; Nurmansyah; Alam, T. Soybean Crop Rotation Stability in Rainfed Agroforestry System through GGE Biplot and EBLUP. Agronomy 2022, 12, 2012. https://doi.org/10.3390/agronomy12092012
Taryono, Suryanto P, Supriyanta, Basunanda P, Wulandari RA, Handayani S, Nurmansyah, Alam T. Soybean Crop Rotation Stability in Rainfed Agroforestry System through GGE Biplot and EBLUP. Agronomy. 2022; 12(9):2012. https://doi.org/10.3390/agronomy12092012
Chicago/Turabian StyleTaryono, Priyono Suryanto, Supriyanta, Panjisakti Basunanda, Rani Agustina Wulandari, Suci Handayani, Nurmansyah, and Taufan Alam. 2022. "Soybean Crop Rotation Stability in Rainfed Agroforestry System through GGE Biplot and EBLUP" Agronomy 12, no. 9: 2012. https://doi.org/10.3390/agronomy12092012
APA StyleTaryono, Suryanto, P., Supriyanta, Basunanda, P., Wulandari, R. A., Handayani, S., Nurmansyah, & Alam, T. (2022). Soybean Crop Rotation Stability in Rainfed Agroforestry System through GGE Biplot and EBLUP. Agronomy, 12(9), 2012. https://doi.org/10.3390/agronomy12092012






