Abstract
Dry soil conditions at soybean planting results in poor stand establishment, which often necessitates replanting. We conducted a study to identify soybean genotypes that can maintain germination rates and possess better root morphology under water stress. We tested 41 Plant Introductions (PI) for germination and seedling root traits under controlled environmental conditions at five water potentials: 0.00, −0.27, −0.54, −0.82, and −1.09 MPa (no, low, mild, severe, and extreme water stress, respectively). The same genotypes were tested for emergence and seedling root traits under field conditions in South Carolina (2021 and 2022) and North Carolina (2022). Among the 41 genotypes evaluated, PI 398566 and PI 424605A maintained higher germination percentages (≥63%) under water stress. The same genotypes were ranked among the top 15 genotypes for root traits (total-root and fine-root (diameter between 0.25 and 0.50 mm) length, surface area, and/or volume) under water stress. Furthermore, they had relatively higher emergence percentages under field conditions (≥35% under dry soil conditions). The superior genotypes identified in this study (PI 398566 and PI 424605A) that had better germination and root morphology under water-stress and no-stress conditions and better emergence would be useful for developing varieties with drought tolerance during the emergence phase.
1. Introduction
Soybean (Glycine max L. Merr.) is an important legume crop globally with Brazil (38% of total global production in 2021), USA (31%), and Argentina (13%) being the major producers [1]. Soybean is widely grown as an oilseed and a protein source worldwide [1,2,3]. Because of its high nutritional quality and protein content, soybean is a raw material in a variety of food products and is used as an important feed material. Recently, soy foods and their bioactive compounds have received significant attention because of their human health benefits, and they are also known as the ‘functional foods of the century’ [4]. In USA, soybean is the second most planted field crop after maize (Zea mays L.) [1], and the second most important crop that contributes to total crop cash receipts [5]. Changing precipitation patterns and rising temperatures create significant challenges to soybean production, especially under rainfed production conditions worldwide [6,7].
Soybean is the most drought-sensitive species among the legumes [8]. Drought stress negatively affects the growth and metabolism of the shoot and root tissues in soybean [9,10]. The soybean crop is most sensitive to drought stress at two stages: at emergence and from flowering through pod-fill [11,12,13]. Limited knowledge is available about the effects of water stress during the emergence phase as most drought research has been conducted during the reproductive phases. In arid and semiarid environments, soil moisture deficiency during the planting time often affects seed germination, resulting in poor seedling emergence and crop stand establishment. Drought tolerance during seed germination is an important characteristic to ensure normal seedling emergence and plant density. In the southeastern USA, soybean varieties with drought tolerance at planting time could expand a relatively new but limited farming practice, double cropping after corn (Zea mays L.). Double cropping is typically done using soybean but is limited to irrigated land. In the double cropping system, as soybean is planted in hot, dry summer, poor germination and emergence limit yields if irrigation is not provided.
Drought stress can have significant impacts on soybean root systems [10]. For example, drought stress can significantly decrease root length and root biomass [14] and alter root architecture by altering rooting depth, root branching density, and root angle [15,16]. Previous studies have identified specific root traits that would be advantageous for plants under drought conditions, e.g., better root penetration [17,18], large xylem vessels in roots, and/or larger lateral root systems with more root hairs [19,20]. Such root systems may possess a larger surface area that helps enhance water and nutrient absorption [17,21,22]. Lynch [23] reported that a shallow basal root growth angle would reduce nitrate and water uptake, while a deeper basal root growth angle would improve it. A soybean seed must absorb at least 50% of its weight in water to begin germination, and more is needed for emergence through the soil surface [24]. Root system architecture can influence soybean seedling emergence under dry soil conditions as it strongly influences water uptake from the soil.
Exploring genetic variability for germination, emergence, and putative root traits that could improve emergence under varying levels of water stress may identify contrasting genotypes that can be used to develop varieties with drought tolerance at the emergence phase. The objective of this study was to characterize a population of 41 soybean Plant Introductions (PI) for germination and seedling root traits under varying levels of water stress. The soybean PIs used in this study were collected from five different countries based on sustained nitrogen fixation under drought, water use efficiency, and canopy wilting. Previous studies have shown that drought tolerance of soybean can be associated with sustained nitrogen fixation under drought [25,26,27,28], water use efficiency [29], and canopy wilting [30,31,32]. Increasing genetic diversity in the breeding populations by including exotic genotypes has been a successful approach for the soybean breeding programs in the USA for drought tolerance [29,33]. Thus, any superior genotypes identified in this study will be useful for soybean breeding programs for drought tolerance at the emergence phase.
2. Materials and Methods
2.1. Plant Material
The designation of all 41 soybean PIs that were tested in this study are given in Table S1. All genotypes were of cultivated soybean (Glycine max) type, and they belonged to maturity group IV. Most genotypes originated from South Korea (n = 30). Three genotypes originated from China, six from Japan, and one each from Russia and North Korea. All genotypes possessed good agronomic characteristics of height, lodging, and shattering. All PIs were previously genotyped using genome-wide association analysis to identify single nucleotide polymorphism (SNP) markers associated with canopy wilting, and carbon isotope ratio (a surrogate measure of water use efficiency), and traits related to nitrogen fixation [26,27,31,34]. More information regarding the genotypes is available from the Germplasm Resources Information Network [35].
2.2. Seed Source
Seeds were originally obtained from the USDA Germplasm Resource Information Network (GRIN) and were grown under field conditions in the summer of 2016, 2018, and 2020 to increase seed quantity and prevent seed degradation. Each year, the seed increases were rouged to remove any off-types and harvested using an Almaco Large plot Thresher. In between each genotype, the combine was cleaned using an air compressor. After harvest, the soybean seeds were cleaned and stored in a cold room at 7.2 °C and 50% relative humidity and maintained around 12% moisture content until use. The seed quality of all genotypes was determined using the protocol given in the ‘Uniform Soybean Tests- Southern States’ [36]. Visual ratings were used to assess seed quality according to the amount and degree of wrinkled, cracked, greenish, or moldy seeds, using a scale of 1 = very good, 2 = good, 3 = fair, 4 = poor, and 5 = very poor (only ‘very good’-quality seeds were used for the present study). The ragdoll method was used to determine the germination rate of each genotype [37]. Briefly, non-toxic paper toweling was obtained from the Seedburo Equipment Company. The paper towel was moistened and placed on a clean, flat surface and 100 seeds were placed on one half of the towel. The towel was folded over and rolled into a moderately tight tube. The rolled-up ragdoll was then placed upright in a plastic container with no lid. The cylinder was placed in the greenhouse, which was kept between 23 and 30 °C. After five days, the towel was unrolled and the number of seeds that germinated was counted. The germination rate of genotypes varied between 92 and 98%.
2.3. Laboratory Experiment to Measure Seed Germination
To measure seed germination, a laboratory experiment was conducted following the “Rules for Testing Seeds” of the Association of Official Seed Analysts (AOSA) [38]. Clean, healthy, and undamaged seeds of each genotype were selected to test for germination. The selected seeds were first disinfected by treating them with 2% (v/v) sodium hypochlorite solution for 10 min. After that, seeds were rinsed with deionized water and airdried on filter papers. Twenty seeds of each genotype were placed on top of two layers of Whatman No.1 filter paper in a sterile petri dish with an internal diameter of 9 cm. Seed germination was tested under five different water potentials: 0.00, −0.27, −0.54, −0.82, and −1.09 MPa using polyethylene glycol (PEG) 6000 at a concentration of 0, 5, 10, 15, and 20% (w/v), respectively [39]. Deionized water was used to prepare the PEG solutions. Zero percent PEG concentration represented the control treatment (no-water stress) for which just 10 mL of deionized water was added to petri dishes containing seeds. Similarly, for the other water stress treatments [−0.27 MPa (low water stress), −0.54 MPa (mild water stress), −0.82 MPa (severe water stress), and −1.09 MPa (extreme water stress)], 10 mL of corresponding PEG solutions was added to respective petri dishes containing the seeds. There were three Petri dishes (replications) per genotype in each water stress treatment; thus, a total of 60 seeds were sampled per genotype in each water stress treatment. Petri dishes with seeds were placed at 25 °C in an incubator under dark conditions. Petri dishes were arranged in an incomplete block design. Seeds with a radicle length, ≥2 mm were considered germinated [39]. Each petri dish was scored for germination from day 3 to day 7 after placing the seeds in it. Germination percentage, germination potential [40], and germination rate index [41] were calculated as given below:
where G1 is the germination percentage on day 1, G2 is the germination percentage on day 2, and so on.
Germination percentages = (Number of seeds germinated in 7 days)/(Total number of seeds) × 100
Germination potential (%) = (Number of seeds germinated in 3 days)/(Total number of seeds) × 100
Germination rate index (GRI) = G1/1 + G2/2 + … + G7/7;
2.4. Greenhouse Experiment to Measure Root Morphological Traits
A greenhouse experiment was conducted to measure root morphological traits of soybean genotypes under different levels of water stress. Thirty seeds of each genotype were germinated in a Petri dish containing Whatman No.1 filter paper, moistened with 0, 5, 10, 15, or 20% (w/v) PEG 6000 solution (corresponding to water potentials of 0.00 MPa (no water stress), −0.27 MPa (low water stress), −0.54 MPa (mild water stress), −0.82 MPa (severe water stress), and −1.09 MPa (extreme water stress)). Deionized water was used to prepare the PEG solutions. Petri dishes with seeds were placed at 25 °C in an incubator under dark conditions. Germinated seeds with a radicle length, ≥1 cm were carefully placed in opaque plastic cups (0.5 L) containing full-strength Hoagland’s solution with PEG 6000 at one of the five concentrations (0, 5, 10, 15, and 20% (w/v)) [42,43]. Three seedlings of the same genotype were grown in each plastic cup. The plastic cups were arranged in a split-plot design in a greenhouse with water stress as the main plot factor and genotype as the sub-plot factor. There were four cups (replications) per genotype in each water stress level. The environmental conditions in the greenhouse included daytime maximum/night-time minimum temperatures of 30/20 °C, 13 h light/11 h dark, ambient CO2 levels (414 ppm), and relative humidity of 70%. Fourteen days after starting the seeds, one of the three seedlings (subsamples) in each cup (replication) was randomly chosen to measure root traits, and its root system was gently separated from the shoot using sharp scissors. Each root system was washed with distilled water to remove the growth solution and then blotted with tissue paper [44]. The cleaned roots were scanned using an Epson Perfection V600 scanner at 300 dpi resolution (Epson, Long Beach, CA, USA) [45,46]. The scanned images were analyzed using WinRHIZO Pro image analysis system (Regent Instruments, Inc., Quebec City, QC, Canada) to estimate the length, surface area, and volume of the whole root system and that of fine roots (diameter <0.25 mm or between 0.25 and 0.5 mm) [47].
2.5. Field Experiment to Measure Seedling Emergence and Root Morphological Traits
2.5.1. Experimental Site and Plant Husbandry
The field experiments were conducted at the Pee Dee Research and Education Center of Clemson University in Florence, SC, USA (38 m above sea level) in 2021 (34°17′31.4″ N, 79°44′38.0″ W) and 2022 (34°17′30″ N, 79°44′13″ W) and JACK—Sandhills Research Station in Jackson Springs, NC, USA in 2022 (35°11′03″ N, 79°40′50″ W and 183 m above sea level). The soil type at Florence was Emporia loamy fine sand (Fine-loamy, siliceous, sub-active, thermic Typic Hapludults) and Bonneau sand (Loamy, siliceous, sub-active, thermic Arenic Paleudults) in the experimental fields used in 2021 and 2022, respectively. The soil type in the experimental field at Jackson Springs was Candor sand (Sandy, kaolinitic, thermic Grossarenic Kandiudults). The previous crop in the 2021 experimental field at Florence was corn during the summer seasons in 2020 and 2019. The same field was covered by a wheat (Triticum aestivum L.) cover crop during the winter seasons in 2020–2021 and 2019–2020. The previous crop in the 2022 experimental field at Florence was winter wheat in 2020. In the same field, no crop was grown in 2021. At Jackson Springs, cotton and corn were grown during the summer seasons in 2020 and 2021, respectively, and no cover crops were grown during the winter seasons in both years. A soil test was conducted prior to planting in all experimental fields. The results of soil analysis are presented in Table S2. At Florence, no fertilizer was added to the experimental fields in 2021 and 2022. At Jackson Springs, K2O and S were applied at a rate of 112 kg ha−1 and 28 kg ha−1 in 2022 as recommended. The land was strip-tilled using a disk plow on the day of planting at Florence in 2021 and 2022. At Jackson Springs, the land was prepared using a disc harrow five days before planting in 2022. At Florence, soybean genotypes were planted on May 21st in 2021 and May 31st in 2022 using a four-row dynamic disc planter (Wintersteiger, Salt Lake City, UT, USA). At Jackson Springs, soybean genotypes were planted on 7 June 2022 using a four-row cone plot planter (Almaco, Nevada, IA, USA). Planting depth was about 4 cm in all fields. Each genotype was planted in four plots (four replications). Plots were arranged in a randomized complete block design. Each plot was of 3 m × 3 m size and consisted of four rows. Row length was 3 m and row spacing was 0.76 m. A total of 100 seeds were sown in each row.
In the 2021 experimental site at Florence, the cumulative precipitation for a 21-day period prior to planting was 3.4 cm, which was just half that compared to historical precipitation data (30-year climate normal) (Figure S1). No rain was recorded in the 7-day period after planting (Figure S1). A total of 5 cm of rain was recorded in the 7-day period thereafter (Figure S1). In the 2022 experimental site at Florence, a total of 1.9 cm of rain was recorded in the last 8 d prior to planting. In the same site, a total of 0.84 cm rain was recorded within 3 d after planting (DAP), but no rain was recorded thereafter until the emergence was counted at 7 DAP. At Jackson Springs in 2022, no rain was recorded in the last 9 d prior to planting, however, a total of 1.12 cm rain was recorded within 5 DAP. No irrigation was provided in any fields as we wanted to measure emergence and root morphology under rainfed conditions. Weeds were controlled through hand-weeding whenever needed. No pest or pathogen problems were observed during the experiments.
2.5.2. Measurement of Seedling Emergence and Root Morphology
A seedling was considered to have emerged when cotyledons were visible over the soil surface [48]. Emerged seedlings in each of the four rows in each plot were counted at 7 DAP. In each plot (where a total of 400 seeds were sown), emergence was estimated as the number of emerged seedlings divided by 400 and was expressed in percentage.
Root morphology of the seedlings was measured at Florence at 14 DAP in 2021 and at 7 and 14 DAP in 2022. Root morphology was not measured at Jackson Springs. To measure root morphology, root systems of 10–15 consecutive seedlings were excavated using a shovel from the second row of each plot to a depth of 25 to 30 cm and a distance of 25 to 30 cm on either side of the row [49]. The excavated root system was carefully shaken to remove adhering soil. After that, three intact root systems from each plot were selected for further analysis. The selected root systems were washed and placed between two paper towels before storing them at 4 °C in sealed Ziploc bags (S.C. Johnson & Sons, Inc. Racine, WI, USA). Afterward, the root systems were gently separated from the shoots using sharp scissors, and each of the three root systems was scanned separately using an Epson Perfection V600 scanner (Epson, Long Beach, CA, USA) at 300 dpi resolution [45,46] in 2021 and at 800 dpi resolution in 2022. The scanning process was completed within three days after root excavation. The scanned root images were analyzed using WinRHIZO Pro image analysis system to estimate the length, surface area, and volume of the whole root system and that of fine roots (diameter <0.25 mm or between 0.25 and 0.5 mm). Root traits of all three seedlings (subsamples) in each plot (replication) were measured and averaged to get a single value per replication.
2.6. Statistical Analyses
A statistical model was developed for germination, emergence, and root trait data including genotype, water stress level, and the interaction as fixed effects, and replication as a random effect. Analysis of variance to test the effects of genotype, water stress level, and the interaction was performed using the GLIMMIX procedure in SAS (Version 9.4, SAS Institute, Cary, NC, USA). There were three replications per genotype in each water stress treatment for germination percentage, germination rate index, and germination potential measured under laboratory conditions; four replications per genotype in each water stress treatment for root morphological traits measured under greenhouse conditions, and four replications per genotype for emergence percentage and root morphological traits measured under field conditions. The probability threshold level (α) for statistical significance was set at 0.05. Separation of least-squares means was performed using Fisher’s LSD test. Ward’s hierarchical cluster analysis was performed using the JMP program (Version 16.0.0, SAS Institute, Cary, NC, USA) to cluster the genotypes based on germination traits at different water stress levels in the laboratory experiment and emergence percentage under field conditions.
3. Results
3.1. Germination and Emergence of Soybean Genotypes
Water stress, genotype, and their interaction had significant effects on germination percentage, germination potential, and germination rate of soybean genotypes evaluated in the laboratory experiment (Table 1). Water stress significantly reduced soybean germination percentage (Figure 1 and Figure S3 and Table S3). Germination percentage was reduced by 53 to 97% under extreme water stress of −1.09 MPa, compared to no-water stress conditions, except for genotypes PI 398566, PI 398532, and PI 424605A (Table S3). Genotypes PI 398566, PI 398532, and PI 424605A demonstrated only 7, 32, and 37% reductions in germination percentage under extreme water stress. Five genotypes (PI 398237, PI 398201, PI 407848, PI 408169D, and PI 507382) did not germinate under extreme water stress. At −0.82 MPa of water potential (severe water stress), the germination percentage was less than 60% for half of the genotypes. However, genotypes PI 398319, PI 398566, PI 424605A, and PI 442012B demonstrated >95% germination under −0.82 MPa of water potential. Genotypes PI 398532, PI 398566, PI 424605A, which had better germination percentages under extreme and severe water stress levels, also possessed high values for germination percentage under mild, low, and no water stress conditions (−0.54, −0.27, and 0.00 MPa, respectively) (Figure 1). Germination of genotypes PI 398532, PI 398566, and PI 424605A were 95, 98, and 98%, respectively, under mild water stress and 95, 100, and 100%, respectively, under low and no water stress (Table S3).

Table 1.
Analysis of variance results on the effects of water stress, genotype, and their interaction on germination percentage, germination potential, germination index, and root traits of soybean under controlled environmental conditions.
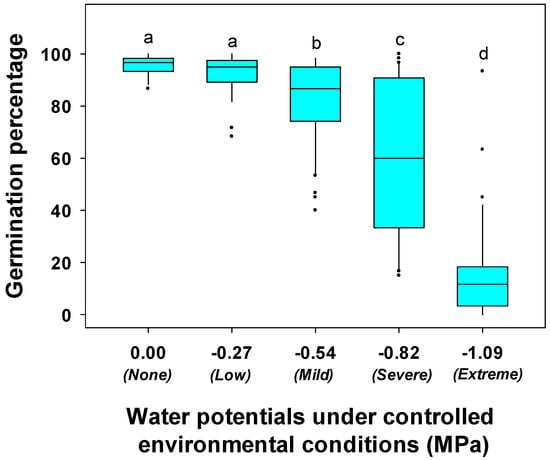
Figure 1.
Box plot showing germination percentage of soybean genotypes under controlled environmental conditions. Germination percentage was measured at water potentials of 0.00, −0.27, −0.54, −0.82 and −1.09 MPa induced by polyethylene glycol (PEG 6000) concentrations of 0, 5, 10, 15, and 20% (w/v), respectively. In the box, the upper bound represents the 75th percentile and lower bound represents the 25th percentile. The line bisecting the box represents the median (50th percentile). The dots outside the whiskers represent the genotypes that are not within 1.5 times of the interquartile range (IQR). Different letters above bars indicate significant differences among treatments at α = 0.05.
Genotypic differences were significant for emergence percentage at Florence, SC and Jackson Springs, NC (Table 2). The emergence percentage of genotypes ranged between 4 and 39 at Florence in 2021, 2 and 65 at Florence in 2022, and 23 and 77 at Jackson Springs in 2022 (Figure 2 and Table S3). Genotypes PI 398566, PI 398532, and PI 424605A, which demonstrated a higher germination percentage under severe and extreme water stress (−0.82 and −1.09 MPa, respectively) in the laboratory experiment, also exhibited a relatively greater emergence percentage (≥35% at Florence and ≥54% at Jackson Springs) under field conditions (Table S3). Similarly, genotypes PI 398237 and PI 603171, which demonstrated poor germination percentage under severe and extreme water stress in the laboratory experiment, also exhibited a poor emergence percentage (≤4% at Florence and ≤22% at Jackson Springs) under field conditions (Table S3).

Table 2.
Analysis of variance results on the effects of genotypes on emergence percentage and root traits of soybean grown at Florence, SC in 2021 and 2022 and Jackson Springs, NC in 2022. Emergence percentage was measured at 7 days after planting (DAP) at both locations. Root traits were measured at Florence at 14 DAP in 2021 and at 7 and 14 DAP in 2022. Root traits were not measured at Jackson Springs.
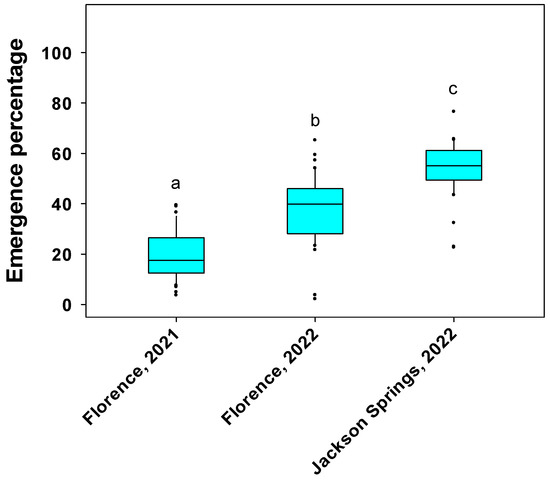
Figure 2.
Box plot showing emergence percentage of soybean genotypes at Florence, SC in 2021 and 2022 and Jackson Springs, NC in 2022. Emergence percentage was recorded at 7 d after planting. In the box, the upper bound represents the 75th percentile and lower bound represents the 25th percentile. The line bisecting the box represents the median (50th percentile). The dots outside the whiskers represent the genotypes that are not within 1.5 times of the interquartile range (IQR). Different letters above bars indicate significant differences among environments at α = 0.05.
Based on germination percentages under the controlled environmental conditions and emergence percentages under field conditions, a hierarchical cluster analysis was performed using Ward’s linkage method (Figure 3a). Genotypes PI 398532, PI 398566, and PI 424605A, which demonstrated a higher germination percentage under severe and extreme water stress under the controlled environmental conditions and a relatively greater emergence percentage under field conditions, formed a distinct cluster on the dendrogram (Figure 3a). Genotypes PI 398237, PI 603171, PI 398704, PI 408169D, and PI 408175, which had lower germination/emergence percentages under all the tested conditions, formed another distinct cluster (Figure 3a). Genotypes PI 398237 and PI 603171 had the lowest values for emergence percentage under all three field environments (Table S3). A similar trend was observed when the genotypes were clustered based on germination index and germination potentials (both traits demonstrate the germination rigor) under laboratory conditions (Figure 3b,c).
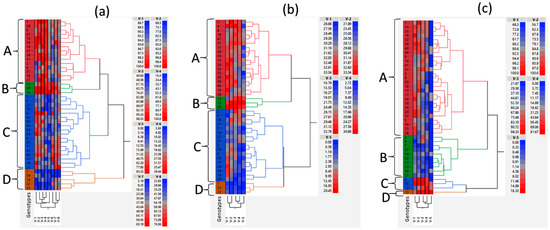
Figure 3.
Dendrograms showing Ward’s hierarchical clustering of soybean genotypes based on germination/emergence percentage (a); germination index (b); and germination potential (c). The genotypes were grouped into four distinct clusters: A through D. Labels V-1 through V-5 indicate germination percentage (a), germination index (b), or germination potential (c) at water potentials of 0.00, −0.27, −0.54, −0.82 and −1.09 MPa, respectively, under controlled environmental conditions. Labels V-6, V-7, and V-8 indicate emergence percentages measured at Florence, SC in 2021; Florence, SC in 2022; and Jackson Springs, NC in 2022, respectively. Genotypes 1–41 are marked on the dendrograms; e.g., # 7 indicates PI 398237, # 12, PI 398532; # 13, PI 398566; # 14, PI 398704; # 22, PI 408169D: # 23, PI 408175, # 30, PI 424605A; and # 39, PI 603171. Please see Table S1 for other genotype names corresponding to the numbers. The color bars right to the dendrograms represent values for the traits presented in the dendrograms, with colors changing from blue (lowest) to red (highest).
3.2. Root Morphological Traits of Soybean Genotypes at the Seedling Stage
The main effect of water stress was significant on all measured root traits (length, surface area, and volume of the total root system, fine roots with diameter <0.25 mm, and fine roots with diameter between 0.25 and 0.5 mm) under controlled environmental conditions (Table 1). Similarly, the main effect of genotypes was significant on all root traits except total root volume under controlled environmental conditions (Table 1). However, the ‘water stress-by-genotype’ interaction effect was not significant on any root traits. This suggests that the relative ranking of genotypes for root traits was similar at different levels of water stress (no stress, low, mild, severe, and extreme). Under field conditions at Florence, SC, genotypic differences were observed for total root surface area and total root volume (Table 2).
Under controlled environmental conditions, the magnitude of all measured root traits decreased with increasing water stress (Figure 4). When genotypes were ranked based on the numerical values of root traits under extreme and severe water stress conditions, PI 424605A and PI 398566 that showed better germination under water stress conditions (extreme, severe, mild, and low stress) were ranked among the top 15 (Table S4). Between those two genotypes, PI 424605A was superior; it was ranked among the top three under extreme water stress conditions and among the top 10 under severe water stress conditions for all measured root traits. PI 398532, another genotype with better germination under water stress conditions (extreme, severe, mild, and low stress), was ranked among the top 15 for all measured root traits under extreme water stress conditions.
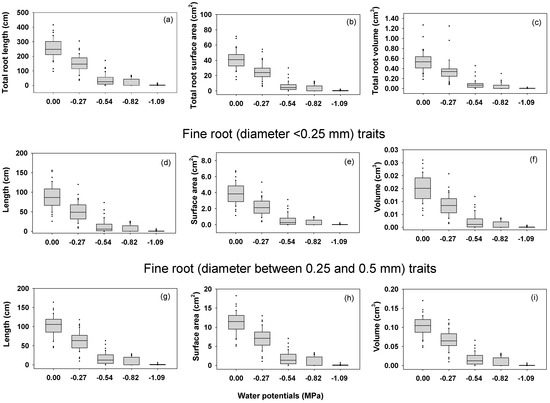
Figure 4.
(a–i) Box plot showing root traits of soybean genotypes at different water potentials. Under controlled environmental conditions, plants were hydroponically grown at five different water potentials [0.00 MPa (no water stress), −0.27 MPa (low water stress), −0.54 MPa (mild water stress), −0.82 MPa (severe water stress), and −1.09 MPa (extreme water stress)] and root traits were measure at 14 days after sowing.
The correlation analysis between the germination percentage and root traits under controlled environmental conditions showed that no root traits were correlated with germination percentage in the absence of water stress (Figure 5a). However, with the increasing severity of water stress, the correlation between root traits and germination became more prominent. For example, under low water stress (−0.27 MPa), germination was correlated with length, surface area, and volume of fine roots with diameter between 0.25 and 0.5 mm. Under mild water stress (−0.54 MPa), germination was correlated with all measured root traits, except total root volume. However, under low and mild water stress, the values of the correlation coefficient among root traits and germination were always less than 0.40. Under severe and extreme water stress (−0.82 or −1.09 MPa, respectively), germination was correlated with all measured root traits with a correlation coefficient greater than 0.40 in most cases. The strongest correlation of germination with root traits was observed under severe water stress (correlation coefficient ≥0.60). Though the values of correlation coefficient between germination and any root trait steadily increased with increasing severity of water stress, they decreased under extreme water stress (Figure 5a). This may be because of the apparent damage of water stress on germination and root morphology when the stress intensity is too high. We also evaluated the relationship of root traits with the emergence percentage of the soybean genotypes under field conditions at Florence, SC in 2021 and 2022 (Figure 5b). We found that fine root (diameter between 0.25 and 0.5 mm) length had a positive relationship with emergence percentage (correlation coefficient ≥0.32) in both years.
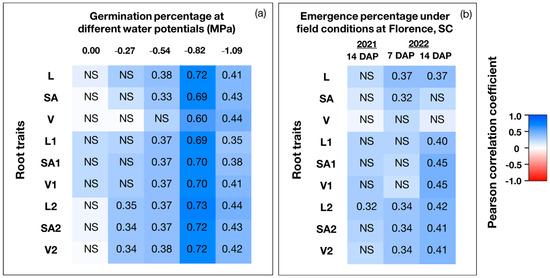
Figure 5.
Heatmap representing the correlation between root morphological traits and germination percentage under controlled environmental conditions (a), and between root morphological traits and emergence percentage under field conditions (b). Under controlled environmental conditions, plants were grown at five different water potentials (0.00 MPa (no water stress), −0.27 MPa (low water stress), −0.54 MPa (mild water stress), −0.82 MPa (severe water stress), and −1.09 MPa (extreme water stress)). The value in each cell represents the Pearson correlation coefficient (r). DAP = Days after planting; NS = Not significant at α = 0.05. L = Total root length (cm); SA =Total root surface area (cm2); V = Total root volume (cm3); L1 = Length of fine roots with diameter <0.25 mm (cm); SA1 = Surface area of fine roots with diameter <0.25 mm (cm2); V1 = Volume of fine roots with diameter <0.25 mm (cm3); L2 = Length of fine roots with diameter between 0.25 and 0.5 mm (cm); SA2 = Surface area of fine roots with diameter between 0.25 and 0.5 mm (cm2); V2 = Volume of fine roots with diameter between 0.25 and 0.5 mm (cm3).
4. Discussion
Significant genetic variability was observed in germination percentage across all water stress levels under laboratory conditions and emergence percentage under field conditions. Overall, based on germination percentage under no, low, mild, severe, and extreme water stresses (0.00, −0.27, −0.54, −0.82, and −1.09 MPa) and emergence percentage under three environments (Florence, SC in 2021 and 2022 and Jackson Springs, NC in 2022), PI 398566, PI 424605A, and PI 398532 were the best genotypes, and PI 398237, PI 603171, PI 408169D, PI 408175, and PI 398704 were the poor genotypes. Among those genotypes, PI 398566 and PI 424605A were the top two, and PI 398237 and PI 603171 were the bottom two according to ranking based on numerical values of the above traits. A principal component analysis (PCA) using germination percentage at different water stress conditions in the laboratory experiment and emergence percentage under field conditions confirmed these results (PI 398566 and PI 424605A as the best and PI 398237 and PI 603171 as the worst) (Figure 6). On the PCA biplot, PI 398566 and PI 424605A formed a cluster (Cluster-1), and PI 398237 and PI 603171 formed another cluster (Cluster-4), which were clearly distinct from other clusters (Figure 6). Since it would be advantageous to develop new varieties with good germination percentages, not only in the presence of water stress but in the absence of it, PI 398566 and PI 424605A which had high germination percentages under no, low, mild, severe, and extreme water stress would be good materials to include in variety development programs.
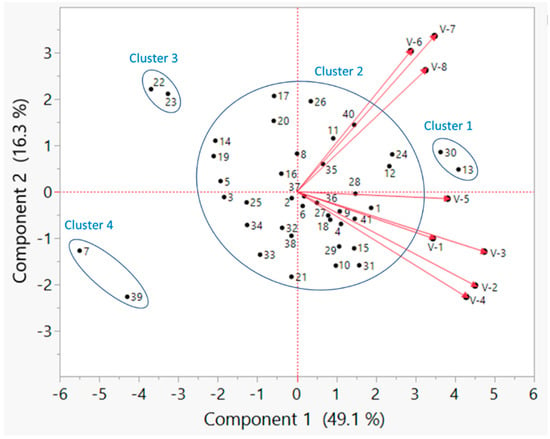
Figure 6.
Principal component analysis biplot demonstrating clustering of soybean genotypes based on germination percentage under controlled environmental conditions and emergence percentage under field conditions. Vectors V-1 through V-5 indicate germination percentage at water potentials of 0.00, −0.27, −0.54, −0.82 and −1.09 MPa, respectively, under controlled environmental conditions. Vectors V-6, V-7, and V-8 indicate emergence percentages measured at Florence, SC in 2021; Florence, SC in 2022; and Jackson Springs, NC in 2022, respectively. Genotypes 1–41 are marked on the biplot; e.g., # 13 indicates PI 398566; # 30, PI 424605A; # 7, PI 398237; and # 39, PI 603171. Please see Table S1 for other genotype names corresponding to the numbers.
With increasing intensity of water stress, germination percentage decreased, and the range widened (i.e., the difference between the lowest and highest values of germination percentage increased) (Figure 1). This result indicates that the germination of soybean is significantly affected by water stress. The above result is supported by our previous research that demonstrated decreasing emergence rate with increasing levels of water stress when soybean plants were grown at 100, 80, 60, 40, and 20% pot-water holding capacity [50]. In the present study, while most genotypes suffered from a drastic reduction in germination percentage under water stress conditions, the genotypes PI 398566 and PI 424605A could maintain better germination under water stress. These genotypes were more rigorous in germination which is reflected in their better germination potential and germination rate index (Figure 3).
Often, seed size is believed to influence soybean emergence, though there are contradicting reports regarding this [51,52,53]. In the present study, we did not find any significant relationship between the seed size and germination percentage under different water stress levels and between seed size and emergence percentage under three field environments (Figure S2). These results are supported by our previous research in which we did not find any correlation between soybean seed size and emergence percentage when seeds were sown in pots maintained at 100, 80, 60, 40, and 20% pot-water holding capacity [50]. A lack of the relation between seed size and emergence percentage has also been reported for cereal grains such as wheat, oat, and barley [54,55,56,57,58].
Emergence percentage was measured at Florence and Jackson Springs, two major soybean growing areas in SC and NC, respectively. Though germination percentage ranged between 87 and 100% under no-water stress conditions in the laboratory experiment (Figure 1 and Table S3), emergence was considerably lower for most genotypes under field conditions (between 3 and 39% at Florence in 2021; 2 and 65% at Florence in 2022; and 22 and 77% at Jackson Springs in 2022) (Figure 2 and Table S3). This may be because of the dry soil conditions that existed during the planting time. The experimental sites did not receive any rain in the last 7 to 8 d prior to planting. The cumulative precipitation values at Florence in a month prior to planting in 2021 and 2022 were lower than what is typical for this region (based on the 30-year cumulative normal precipitation) (Figure S1). In 2021, only 3.5 cm rainfall was recorded in a month prior to planting at Florence. Emergence was recorded at 7 DAP in all experimental fields. Florence experimental field did not receive any rainfall till 7 DAP in 2021, and it received only 0.84 cm rainfall in the first week of planting in 2022. Jackson Springs experimental field received 1.12 cm rainfall in the first week of planting in 2022. The dry soil conditions at Florence in 2021 might have led to the relatively worst emergence percentage in that environment, compared to that in the other two environments.
We also evaluated whether emergence percentage was related to root traits, and found that the length of fine roots with a diameter between 0.25 and 0.5 mm was positively related to emergence percentage in 2021 and 2022. Interestingly, the finest root (diameter <0.25 mm) traits did not appear to influence emergence (no correlation between emergence and L1, SA1, or V1 at 14 DAP in 2021 and at 7 DAP in 2022; Table 2). In other dicot species, it has been reported that instead of the finest parts in the root system (such as root tip), the first 2–3 inches behind the root tip represents the most active site of water and nutrient uptake [59]. Our results provide a background to investigate the role of root morphology in supporting emergence.
When root traits measured at Florence in 2022 were further evaluated, the length, surface area, and volume of the total root system and fine roots with diameters between 0.25 and 0.5 mm measured at 7 DAP were correlated with the same traits measured at 14 DAP with correlation coefficient values ≥0.38 (Table S5). Furthermore, in 2021, at the same location, the length, surface area, and volume of the total root system and fine roots (diameters between 0.25 and 0.5 mm) measured at the seedling stage (14 DAP) were correlated with the same traits measured at the late vegetative stage (74 DAP), with correlation coefficients ≥0.37 (Table S5). This implies that genotypes that possess a better root system in the early seedling stage could maintain doing so later in their life cycle. However, it should be noted that after root emergence, further development of the root system and the final root system architecture could be influenced by soil physical conditions and biotic and abiotic factors (e.g., temperature, water, oxygen, soil nutrient availability, and soil biota) [60].
Germination and emergence can be affected by different biotic and abiotic factors. Among the biotic factors, soil-borne pathogens and pests are major concerns, though in some cases birds and other vertebrate pests also impact soybean emergence [61,62]. In the present study, we did not evaluate the effect of biotic factors on germination and emergence. However, we dug up the ungerminated seeds in the experimental fields and did not find any disease symptoms on those seeds. Abiotic factors such as soil temperature, soil compaction, and soil surface crusting may also affect soybean germination and emergence [63,64] and should be evaluated in future studies.
5. Conclusions
In the present study, we found that soybean germination and root morphology are significantly affected by water stress. Among the 41 soybean PI’s evaluated, PI 424605A and PI 398566 could maintain germination percentage and better root morphology (greater length, surface area, and volume of the whole root system and those of fine roots) under water stress. PI 424605A and PI 398566 had better germination and root morphology not only under water-stress but under no-stress conditions as well. Additionally, the same genotypes possessed better emergence. These genotypes would be useful for breeding programs in developing new varieties with drought tolerance at the emergence phase. The best (PI 424605A and PI 398566) and worst (PI 398237 and PI 603171) genotypes identified in this study could be further characterized to identify any seed-related or root-related compounds that may improve germination and emergence from dry as well as optimally moist soils. Furthermore, the above contrasting genotypes could be used to develop mapping populations to elucidate the genetic mechanism behind better germination and emergence and the genetic loci regulating traits related to improved germination and emergence.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12081944/s1. Figure S1: Cumulative precipitation and daily average temperature from one month prior to planting to one month after planting at Florence, SC (a,b) and Jackson Spring, NC (c,d) in comparison with the historic weather data. Figure S2: Relationship of seed size (measured through 200-seed weight) with germination percentage under controlled environmental conditions (a–e) and with emergence percentage under field conditions (f–h). Figure S3: Distribution of germination percentage values among 41 soybean genotypes under controlled environmental conditions (a–e) and distribution of emergence percentage among the same genotypes under field conditions (f–h). Table S1: List of soybean genotypes used in the study, country of origin, and 200-seed weight. Table S2: Results of soil analysis conducted before soybean planting. Table S3: Germination traits of soybean genotypes under controlled environmental conditions and emergence percentage under field conditions. Table S4: Ranking of soybean genotypes for root traits under severe and extreme water stress. Table S5: Correlation between root traits measured at multiple times [14 and 74 days after planting (DAP) in 2021 and 7 and 14 DAP in 2022] during soybean growth at Florence, SC.
Author Contributions
Conceptualization: S.N., B.F. Data Curation: J.P.K. Investigation: J.P.K. Formal analysis: J.P.K., S.N., W.B. Funding acquisition: S.N., B.F. Methodology: J.P.K., S.N. Validation: J.P.K., S.N. Project administration: S.N. Resources: S.N., B.F. Supervision: S.N. Visualization: J.P.K., S.N. Writing—original draft: J.P.K. Writing—review & editing: J.P.K., S.N., B.F., W.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the South Carolina Soybean Board Grant # 2011346 and the Clemson University Pee Dee Research and Education Center Graduate Support Award # 2280074.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data are available upon request to Sruthi Narayanan. Data have not been archived in a repository.
Acknowledgments
We thank Ricardo St. Aime, Enoch Noh, Om Prakash Ghimire, Luvina Madrid, Binaya Parajuli, Pratima Poudel, Victoria Burgess, Andrew Rabon, Vince Cantrell, Sarah Spivey, Naomi Higgins, Taylor Sherer, and Michael Cromer for their assistance with fieldwork and data collection, and Taylor Martin for his assistance with the greenhouse study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- SoyStats. A Reference Guide to Important Soybean Facts and Figures. American Soybean Association. 2022. Available online: http://soystats.com/ (accessed on 28 July 2022).
- Chen, L.M.; Zhou, X.A.; Li, W.B.; Chang, W.; Zhou, R.; Wang, C.; Sha, A.H.; Shan, Z.H.; Zhang, C.J.; Qiu, D.Z.; et al. Genome-Wide Transcriptional Analysis of Two Soybean Genotypes under Dehydration and Rehydration Conditions. BMC Genom. 2013, 14, 687. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, V.; George, P.; Aher, L.; Ramesh, S.V.; Thangasamy, A.; Anandan, S.; Raina, S.K.; Kumar, M.; Rane, J.; Annapurna, K.; et al. Comparative Conventional and Phenomics Approaches to Assess Symbiotic Effectiveness of Bradyrhizobia Strains in Soybean (Glycine Max L. Merrill) to drought. Sci. Rep. 2017, 7, 6958. [Google Scholar] [CrossRef] [PubMed]
- Shea, Z.; Singer, M.W.; Zhang, B. Soybean Production, Versatility, and Improvement. In Legume Crops-Prospects, Production and Uses; Hasanuzzaman, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- USDA-ERS, Farming and Farm Income. 2022. Available online: https://www.ers.usda.gov/data-products/ag-and-food-statistics-charting-the-essentials/farming-and-farm-income/ (accessed on 12 August 2022).
- Jin, Z.; Zhuang, Q.; Wang, J.; Archontoulis, S.V.; Zobel, Z.; Kotamarthi, V.R. The combined and separate impacts of climate extremes on the current and future US rainfed maize and soybean production under elevated CO2. Glob. Chang. Biol. 2017, 23, 2687–2704. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, M.F.; Gava, R.; Campos, C.N.S.; De David, C.H.O.; Reis, I.D.A.; Teodoro, L.P.R.; Teodoro, P.E. Physiological performance of soybean genotypes grown under irrigated and rainfed conditions. J. Agron. Crop Sci. 2021, 207, 34–43. [Google Scholar] [CrossRef]
- Clement, M.; Lambert, A.; Herouart, D.; Boncompagni, E. Identification of New Up-Regulated Genes under Drought Stress in Soybean Nodules. Gene 2008, 426, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of Drought Stress on Photosynthetic Gas Exchange, Chlorophyll Fluorescence and Stem Diameter of Soybean Plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Kunert, K.J.; Vorster, B.J.; Fenta, B.A.; Kibido, T.; Dionisio, G.; Foyer, C.H. Drought Stress Responses in Soybean Roots and Nodules. Front. Plant Sci. 2016, 7, 1015. [Google Scholar] [CrossRef]
- Senaratna, T.; McKersie, B.D. Dehydration Injury in Germinating Soybean (Glycine Max L. Merr.) Seeds. Plant Physiol. 1983, 72, 620–624. [Google Scholar] [CrossRef]
- Sionit, N.; Kramer, P.J. Effect of Water Stress During Different Stages of Growth of Soybean. J. Agron. 1977, 69, 274–278. [Google Scholar] [CrossRef]
- Jha, P.K.; Kumar, S.N.; Ines, A.V.M. Responses of Soybean to Water Stress and Supplemental Irrigation in Upper Indo-Gangetic Plain: Field Experiment and Modeling Approach. Field Crops Res. 2018, 219, 76–86. [Google Scholar] [CrossRef]
- Thu, N.B.A.; Nguyen, Q.T.; Hoang, X.L.T.; Thao, N.P.; Tran, L.S.P. Evaluation of Drought Tolerance of the Vietnamese Soybean Cultivars Provides Potential Resources for Soybean Production and Genetic Engineering. BioMed Res. Int. 2014, 2014, 809736. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.A.; Bañón, S.; Vicente, M.J.; Miralles, J.; Martínez-Sánchez, J.J. Root Development in Horticultural Plants Grown under Abiotic Stress Conditions—A Review. J. Hortic. Sci. 2011, 86, 543–556. [Google Scholar]
- Fenta, B.; Beebe, S.; Kunert, K.; Burridge, J.; Barlow, K.; Lynch, J.; Foyer, C. Field Phenotyping of Soybean Roots for Drought Stress Tolerance. Agronomy 2014, 4, 418–435. [Google Scholar] [CrossRef]
- Lopes, M.S.; Araus, J.L.; Heerden, P.D.R.; Foyer, C.H. Enhancing Drought Tolerance in C4 Crops. J. Exp. Bot. 2011, 62, 3135. [Google Scholar] [CrossRef]
- Ali, M.L.; Luetchens, J.; Singh, A.; Shaver, T.M.; Kruger, G.R.; Lorenz, A.J. Greenhouse Screening of Maize Genotypes for Deep Root Mass and Related Root Traits and Their Association with Grain Yield under Water-Deficit Conditions in the Field. Euphytica 2016, 207, 79–94. [Google Scholar] [CrossRef]
- Tanaka, N.; Kato, M.; Tomioka, R.; Kurata, R.; Fukao, Y.; Aoyama, T.; Maeshima, M. Characteristics of a Root Hair-Less Line of Arabidopsis Thaliana under Physiological Stresses. J. Exp. Bot. 2014, 65, 1497–1512. [Google Scholar] [CrossRef]
- Vadez, V. Root Hydraulics: The Forgotten Side of Roots in Drought Adaptation. Field Crops Res. 2014, 165, 15–24. [Google Scholar] [CrossRef]
- Blum, A. Drought Resistance Is It Really a Complex Trait? Funct. Plant Biol. 2011, 38, 753–757. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root Traits Contributing to Plant Productivity under Drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Lynch, J.P. Rightsizing Root Phenotypes for Drought Resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef]
- Bryant, C. Soybean Growth and Development. In Soybean Production in Georgia; Bryant, C., Ed.; University of Georgia Cooperative Extension: Athens, GA, USA, 2021; pp. 9–10. [Google Scholar]
- Sinclair, T.R.; Purcell, L.C.; King, C.A.; Sneller, C.H.; Chen, P.; Vadez, V. Drought Tolerance and Yield Increase of Soybean Resulting from Improved Symbiotic N2 Fixation. Field Crops Res. 2007, 101, 68–71. [Google Scholar] [CrossRef]
- Dhanapal, A.P.; Ray, J.D.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; Andy King, C.; Cregan, P.B.; Song, Q.; Fritschi, F.B. Genome-Wide Association Study (GWAS) of Carbon Isotope Ratio (Δ13C) in Diverse Soybean [Glycine Max (L.) Merr.] Genotypes. Theor. Appl. Genet. 2014, 128, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, A.P.; Ray, J.D.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; King, C.A.; Fritschi, F.B. Genome-Wide Association Analysis of Diverse Soybean Genotypes Reveals Novel Markers for Nitrogen Traits. Plant Genome 2015, 8, plantgenome2014.11.0086. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Sneller, C.H.; Purcell, L.C.; Sinclair, T.R.; King, C.A.; Ishibashi, T. Registration of Soybean Germplasm Lines R01-416F and R01-581F for Improved Yield and Nitrogen Fixation under Drought Stress. J. Plant Regist. 2007, 1, 166–167. [Google Scholar] [CrossRef]
- Hufstetler, E.V.; Boerma, H.R.; Carter, T.E.; Earl, H.J. Genotypic Variation for Three Physiological Traits Affecting Drought Tolerance in Soybean. Crop Sci. 2007, 47, 25–35. [Google Scholar] [CrossRef]
- King, C.A.; Purcell, L.C.; Brye, K.R. Differential Wilting among Soybean Genotypes in Response to Water Deficit. Crop Sci. 2009, 49, 290–298. [Google Scholar] [CrossRef]
- Kaler, A.S.; Ray, J.D.; Schapaugh, W.T.; King, C.A.; Purcell, L.C. Genome-Wide Association Mapping of Canopy Wilting in Diverse Soybean Genotypes. Theor. Appl. Genet. 2017, 130, 2203–2217. [Google Scholar] [CrossRef]
- Sadok, W.; Gilbert, M.E.; Raza, M.A.S.; Sinclair, T.R. Basis of Slow-Wilting Phenotype in Soybean PI 471938. Crop Sci. 2012, 52, 1261–1269. [Google Scholar] [CrossRef]
- Lee, G.J.; Lee, S.; Carter, T.E.; Shannon, G.; Boerma, H.R. Identification of Soybean Yield QTL in Irrigated and Rain-Fed Environments. Agronomy 2021, 11, 2207. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Germplasm Resources Information Network. Available online: https://npgsweb.ars-grin.gov/gringlobal/search. (accessed on 17 August 2022).
- Gillen, A.M.; Shelton, G.W. Uniform Soybean Tests Southern States 2017 USDA—Agricultural Research Service Crop Genetics Research Unit, Stoneville, MS. Available online: https://www.ars.usda.gov/ARSUserFiles/60661000/UniformSoybeanTests/2017SoyBook%20lockedREV.pdf (accessed on 3 February 2022).
- Newman, Y.C.; Vendramini, J. Seed Germination Testing (Rag-Doll Test). 2014. Available online: http://edis.ifas.ufl.edu/pdffiles/AG/AG18200.pdf (accessed on 3 February 2022).
- Association of Official Seed Analysts (AOSA). Rules for Testing Seeds; Association of Official Seed Analysts (AOSA): Wichita, KS, USA, 2017. [Google Scholar]
- Hellal, F.A.; El-Shabrawi, H.M.; Abd El-Hady, M.; Khatab, I.A.; El-Sayed, S.A.A.; Abdelly, C. Influence of PEG Induced Drought Stress on Molecular and Biochemical Constituents and Seedling Growth of Egyptian Barley Cultivars. J. Genet. Eng. Biotechnol. 2018, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, S.; Qin, B.; Jin, X.; Wang, M.; Ren, C. Exogenous Melatonin Reduces the Inhibitory Effect of Osmotic Stress on Antioxidant Properties and Cell Ultrastructure at Germination Stage of Soybean. PLoS ONE 2020, 12, e0243537. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, F.; Ksiksi, T. A Quantitative Assessment of Germination Parameters: Crotalaria Persica and Tephrosia Apollinea. Open J. Ecol. 2016, 9, 13–21. [Google Scholar] [CrossRef]
- Kpoghomou, B.K.; Sapra, V.T.; Beyl, C.A. Screening for Drought Tolerance: Soybean Germination and Its Relationship to Seedling Responses. J. Agron. Crop. Sci. 1990, 164, 153–159. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Bashir, W.; Anwar, S.; Zhao, Q.; Hussain, I.; Xie, F. Interactive Effect of Drought and Cadmium Stress on Soybean Root Morphology and Gene Expression. Ecotoxicol. Environ. Saf. 2019, 175, 90–101. [Google Scholar] [CrossRef]
- Hu, L.; Xie, Y.; Fan, S.; Wang, Z.; Wang, F.; Zhang, B.; Li, H.; Song, J.; Kong, L. Comparative Analysis of Root Transcriptome Profiles between Drought-Tolerant and Susceptible Wheat Genotypes in Response to Water Stress. Plant Sci. 2018, 272, 276–293. [Google Scholar] [CrossRef]
- Himmelbauer, M.L.; Loiskandl, W.; Kastanek, F. Estimating Length, Average Diameter and Surface Area of Roots Using Two Different Image Analyses Systems. Plant Soil. 2004, 260, 111–120. [Google Scholar] [CrossRef]
- Fried, H.G.; Narayanan, S.; Fallen, B. Evaluation of Soybean [Glycine Max (L.) Merr.] Genotypes for Yield, Water Use Efficiency, and Root Traits. PLoS ONE 2019, 14, e0212700. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development. Spec. Rep. 1977, 80, 11. [Google Scholar]
- Jochua, C.N.; Strock, C.F.; Lynch, J.P. Root Phenotypic Diversity in Common Bean Reveals Contrasting Strategies for Soil Resource Acquisition among Gene Pools and Races. Crop Sci. 2020, 60, 3261–3277. [Google Scholar] [CrossRef]
- Narayanan, S.; Fallen, B. Evaluation of Soybean Plant Introductions for Traits That Can Improve Emergence under Varied Soil Moisture Levels. Agronomy 2019, 9, 118. [Google Scholar] [CrossRef]
- Mangena, P. Analysis of Correlation between Seed Vigour, Germination and Multiple Shoot Induction in Soybean (Glycine Max L. Merr.). Heliyon 2021, 7, e07913. [Google Scholar] [CrossRef]
- Moshtaghi-Khavaran, A.; Khomari, S.; Zare, N. Soybean Seed Germination And Seedling Growth In Response To Deterioration And Priming: Effect Of Seed Size. Plant Breed. 2018, 70, 55–67. [Google Scholar] [CrossRef]
- Kering, M.K.; Zhang, B. Effect of Priming and Seed Size on Germination and Emergence of Six Food-Type Soybean Varieties. Int. J. Agron. 2015, 2015, 859212. [Google Scholar] [CrossRef]
- Mohan, A.; Schillinger, W.F.; Gill, K.S. Wheat Seedling Emergence from Deep Planting Depths and Its Relationship with Coleoptile Length. PLoS ONE 2013, 8, e73314. [Google Scholar] [CrossRef]
- Kaufmann, M.L. Coleoptile Length and Emergence in Varieties of Barley, Oats, and Wheat. Can. J. Plant Sci. 1968, 48, 357–361. [Google Scholar] [CrossRef]
- Mian, A.R.; Nafziger, E.D. Seed Size Effects on Emergence, Head Number and Grain Yield of Winter Wheat. J. Prod. Agric. 1992, 5, 265–268. [Google Scholar] [CrossRef]
- Chastain, T.G.; Ward, K.J.; Wysocki, D.J. Stand Establishment Responses of Soft White Winter Wheat to Seedbed Residue and Seed Size. Crop Sci. 1995, 35, 213–218. [Google Scholar] [CrossRef]
- Narayanan, S.; Mohan, A.; Gill, K.S.; Vara Prasad, P.V. Variability of Root Traits in Spring Wheat Germplasm. PLoS ONE 2014, 9, e100317. [Google Scholar] [CrossRef]
- Hake, K.; Cassman, K.; Whisler, F.; Upchurch, D. Root Physiology and Management. Physiology Today. In Newsletter of the Cotton Physiology Education Program; National Cotton Council: Cordova, TN, USA, 1990; Volume 1, pp. 1–3. [Google Scholar]
- Rich, S.M.; Watt, M. Soil Conditions and Cereal Root System Architecture: Review and Considerations for Linking Darwin and Weaver. J. Exp. Bot. 2013, 64, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Debaeke, P.; Steinberg, C.; You, M.P.; Barbetti, M.J.; Aubertot, J.N. Abiotic and biotic factors affecting crop seed germination and seedling emergence: A conceptual framework. Plant Soil. 2018, 432, 1–28. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Constantin, J.; Schoving, C.; Maury, P.; Debaeke, P.; Aubertot, J.N.; Dürr, C. Analysis of soybean germination, emergence, and prediction of a possible northward establishment of the crop under climate change. Eur. J. Agron. 2020, 113, 125972. [Google Scholar] [CrossRef]
- Hyatt, J.; Wendroth, O.; Egli, D.B.; TeKrony, D.M. Soil Compaction and Soybean Seedling Emergence. Crop Sci. 2007, 47, 2495–2503. [Google Scholar] [CrossRef]
- Rathore, T.R.; Ghildyal, B.P.; Sachan, R.S. Germination and Emergence of Soybean under Crusted Soil Conditions. Plant Soil. 1982, 65, 73–77. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).